Effects of Pre-Harvest Supplemental UV-A Light on Growth and Quality of Chinese Kale
Abstract
:1. Introduction
2. Results
2.1. Effects of Different Pre-Harvest Supplemental UV-A LED Periods on the Growth and Quality of Chinese Kale
2.1.1. Growth
2.1.2. Photosynthetic Pigments Content
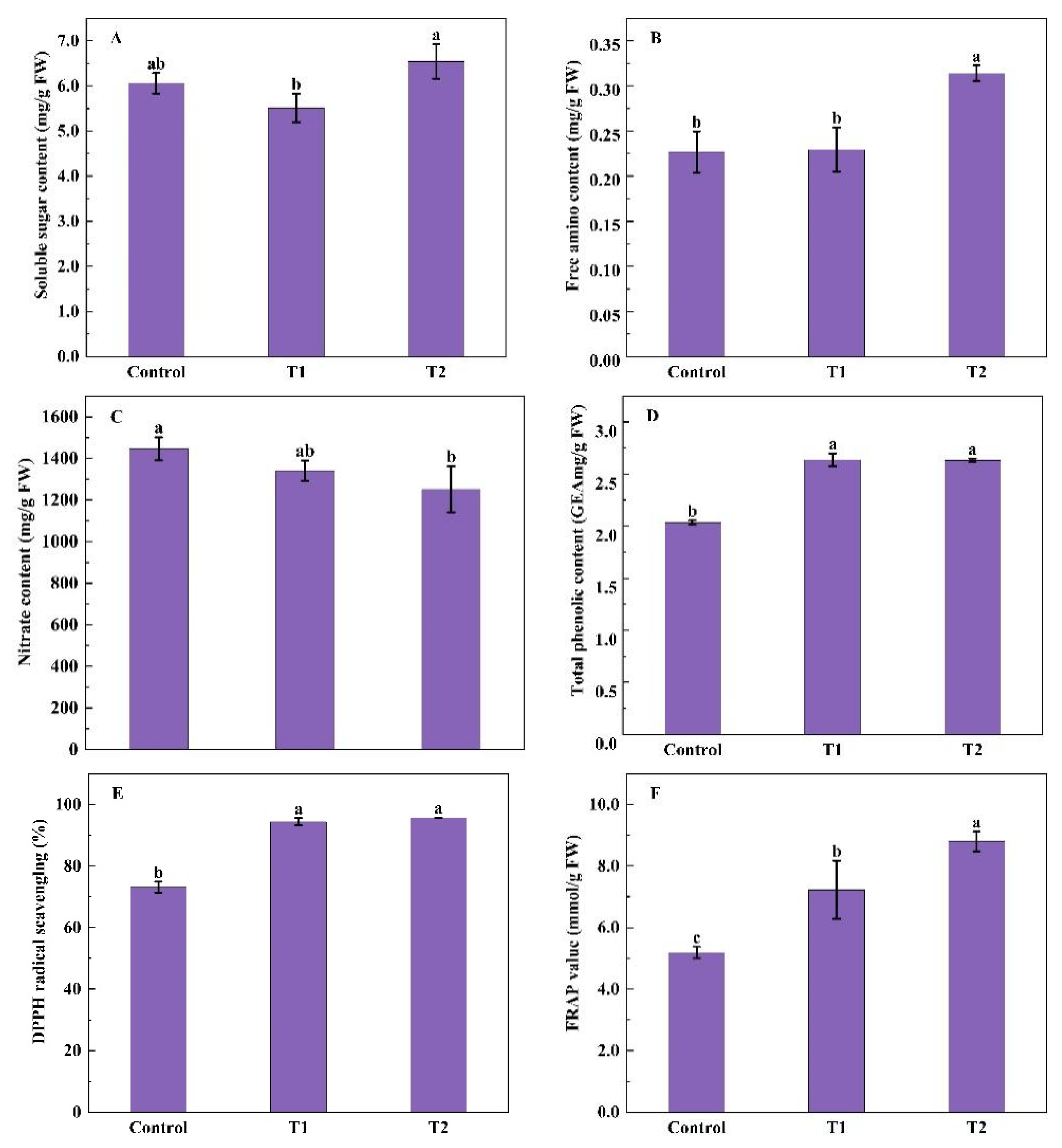
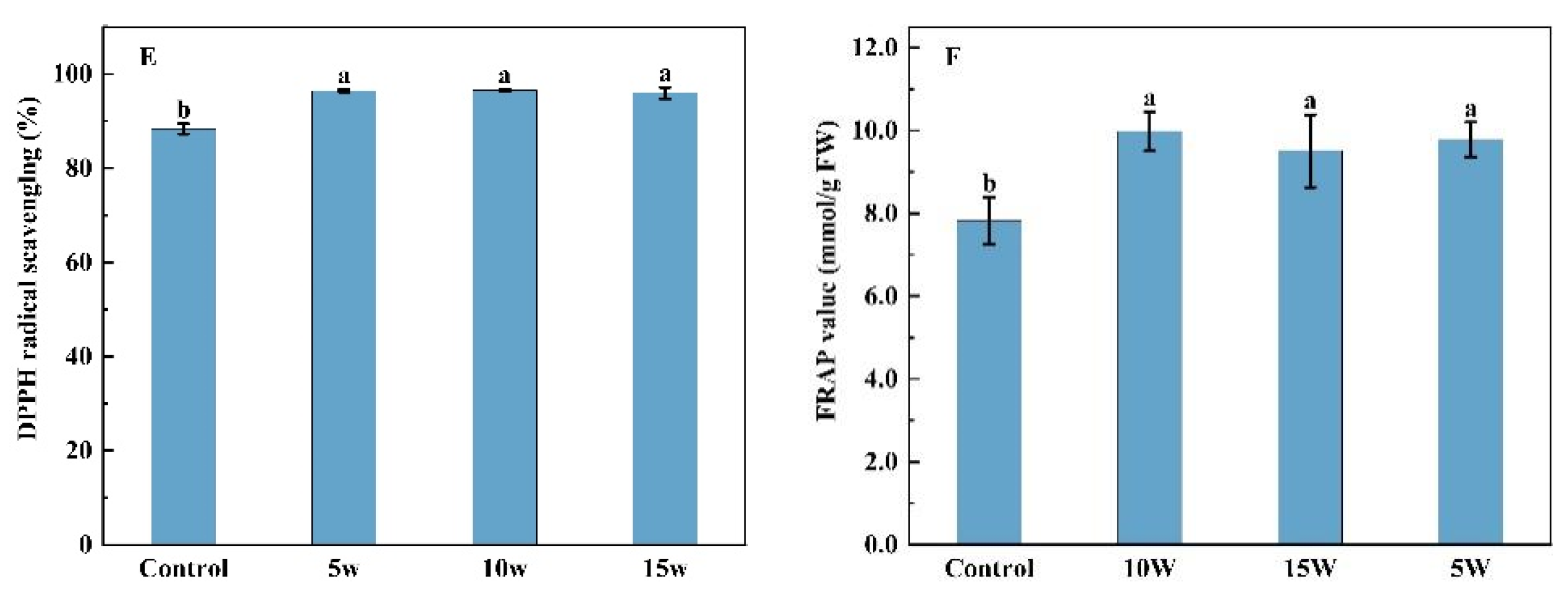
2.1.3. Contents of Soluble Sugars, Free Amino Acids, Nitrate, Total Phenolic and Antioxidant Capacity
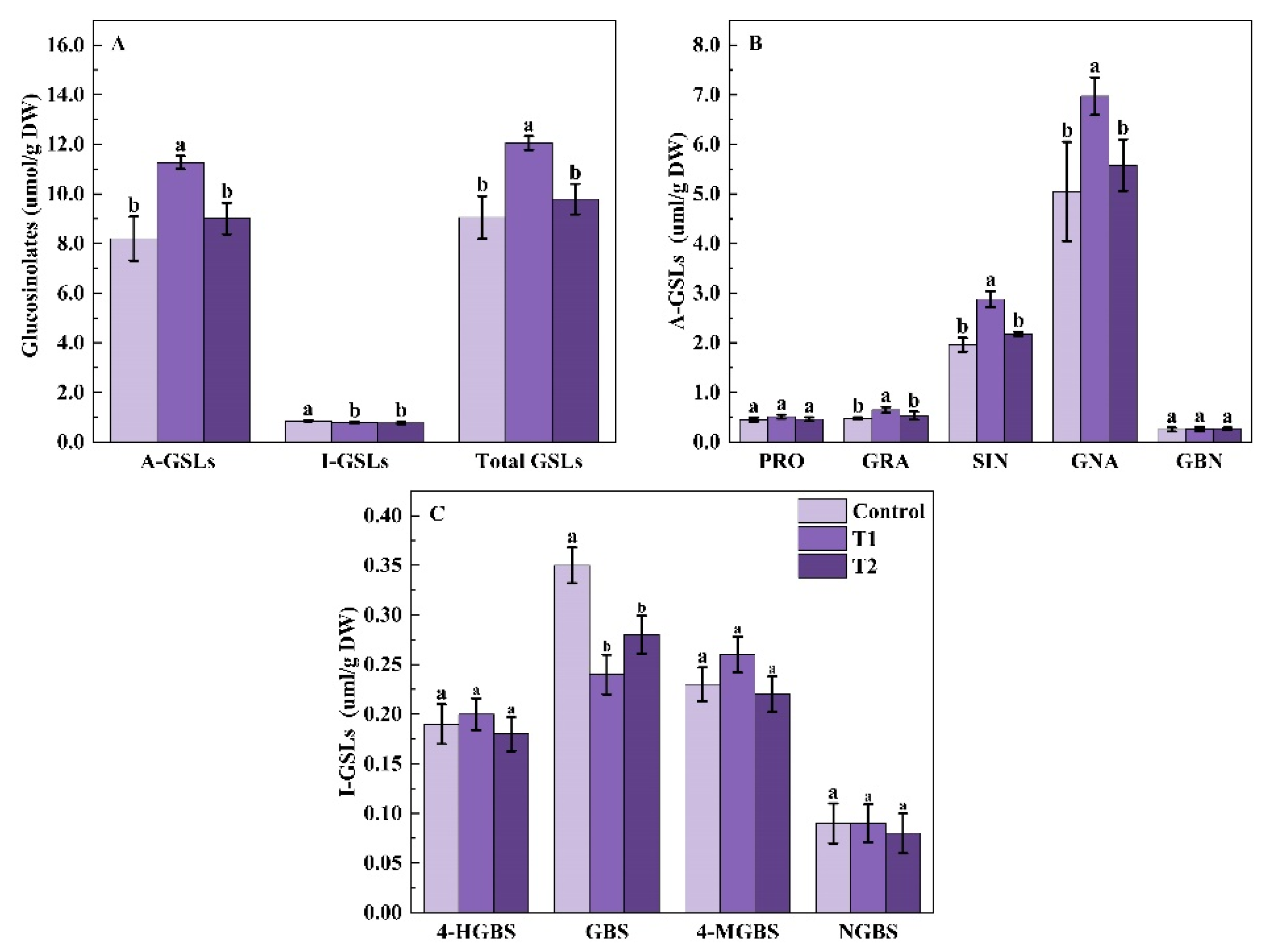
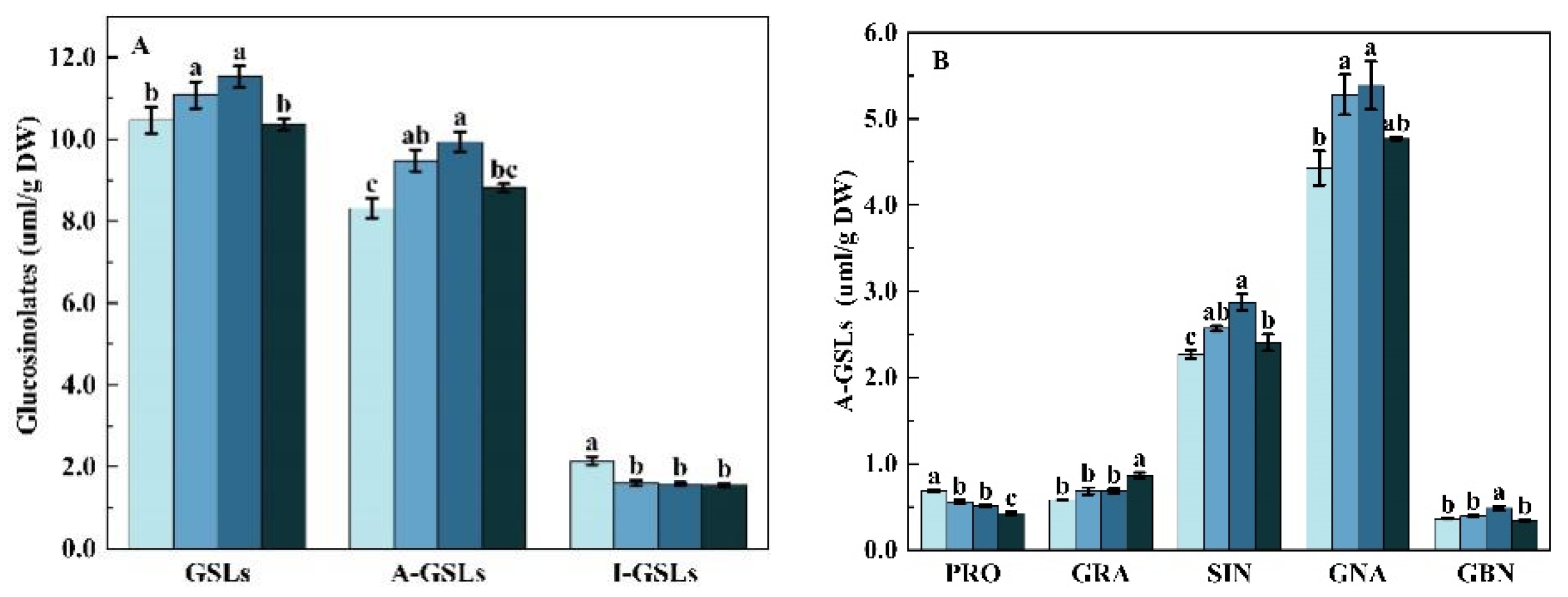
2.1.4. Glucosinolates Profile and Content
2.2. Effects of Different Pre-Harvest Supplemental UV-A LED Intensities on Growth and Quality of Chinese Kale
2.2.1. Growth
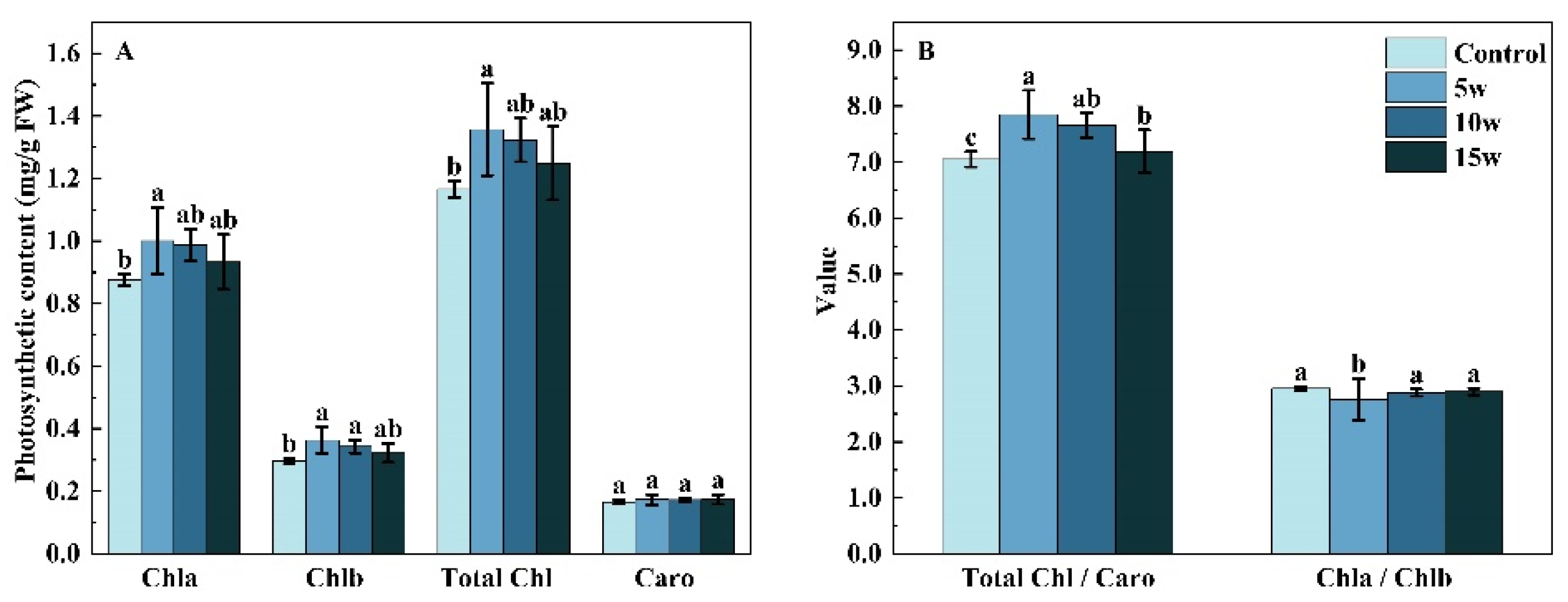
2.2.2. Photosynthetic Pigments Content
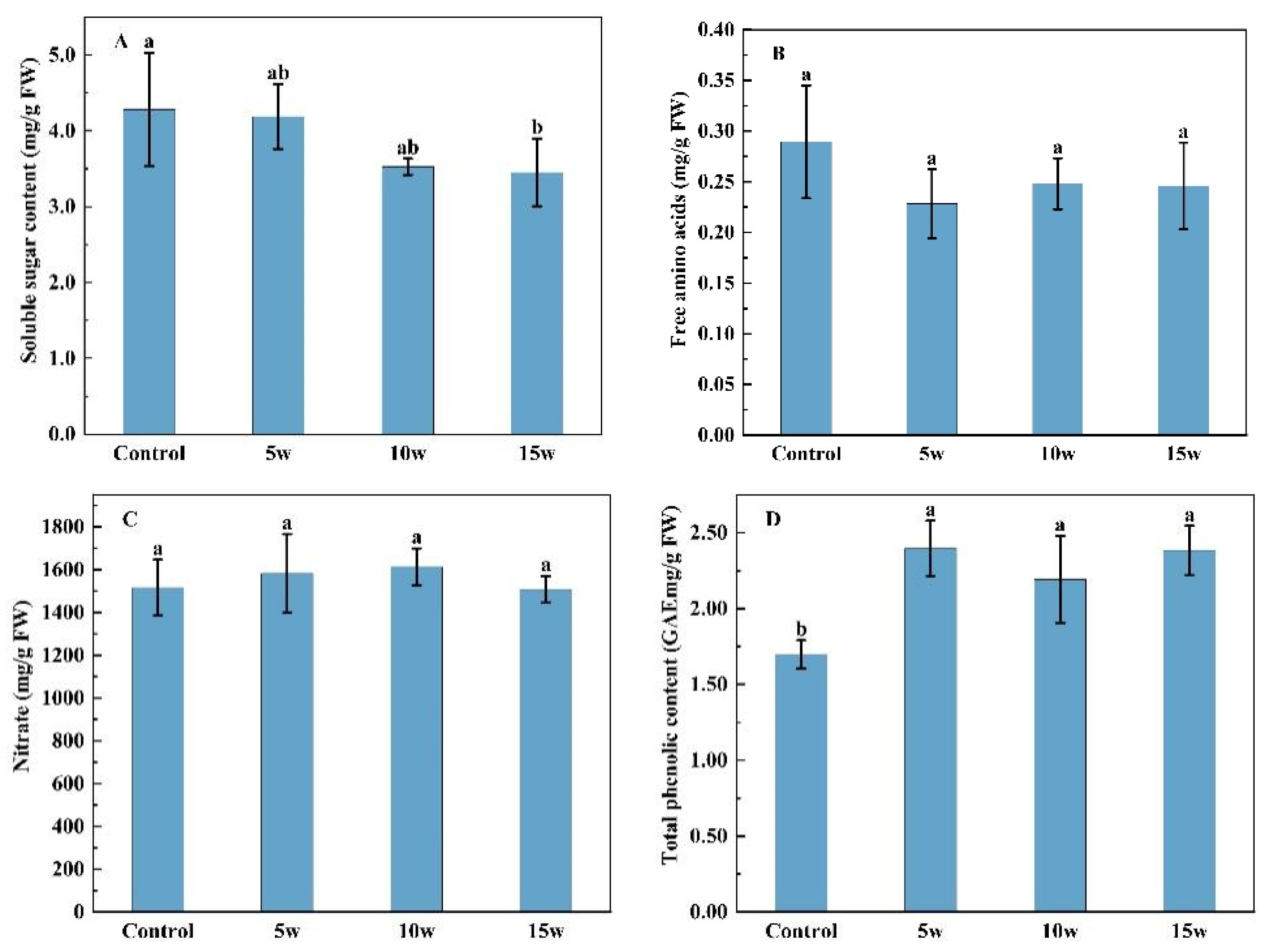
2.2.3. The Content of Soluble Sugars, Free Amino Acids, Nitrate, Total Phenolic and Antioxidant Capacity
2.2.4. Glucosinolates Profile
3. Discussion
3.1. Pre-Harvest Lower UV-A Dosage Was More Beneficial to the Growth of Chinese Kale
3.2. Pre-Harvest UV-A Effected the Phytochemical Contents of Chinese Kale
4. Materials and Methods
4.1. Plant Material and Cultivation Conditions
4.2. Supplemental UV-A LED Treatment
4.3. Growth Measurements
4.4. Photosynthetic Pigments Content
4.5. Contents of Soluble Sugar, Amino Acid, and Nitrate Measurements
4.6. Antioxidant Content and Antioxidant Activity
4.7. Extraction and Determination of Glucosinolates
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yuan, J.J.; Liu, H.C.; Song, S.W.; Sun, G.W.; Chen, R.Y. Srap Markers for Flower Stalk Color in Chinese Kale. J. Anim. Plant Sci. 2015, 25, 55–58. [Google Scholar]
- Khoshimkhujaev, B.; Kwon, J.K.; Park, K.S.; Choi, H.G.; Lee, S.Y. Effect of Monochromatic UV-A LED Irradiation on the Growth of Tomato Seedlings. Hortic. Env. Biotechnol. 2014, 55, 287–292. [Google Scholar] [CrossRef]
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Zou, J.; Bian, Z.; Yang, Q.; Li, T. UVA Radiation Promotes Tomato Growth through Morphological Adaptation Leading to Increased Light Interception. Env. Exp. Bot. 2020, 176, 104073. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tabuchi, T. Tomato Cultivation in a Plant Factory with Artificial Light: Effect of UV-A Irradiation During the Growing Period on Yield and Quality of Ripening Fruit. Hort. J. 2022, 91, 16–23. [Google Scholar] [CrossRef]
- Mao, P.; Duan, F.; Zheng, Y.; Yang, Q. Blue and UV-A Light Wavelengths Positively Affected Accumulation Profiles of Healthy Compounds in Pak-Choi. J. Sci. Food Agric. 2021, 101, 1676–1684. [Google Scholar] [CrossRef]
- Heinze, M.; Hanschen, F.S.; Wiesner-Reinhold, M.; Baldermann, S.; Gräfe, J.; Schreiner, M.; Neugart, S. Effects of Developmental Stages and Reduced UVB and Low UV Conditions on Plant Secondary Metabolite Profiles in Pak Choi (Brassica Rapa Subsp. Chinensis). J. Agric. Food Chem. 2018, 66, 1678–1692. [Google Scholar] [CrossRef]
- Choi, D.-S.; Nguyen, T.K.L.; Oh, M.-M. Growth and Biochemical Responses of Kale to Supplementary Irradiation with Different Peak Wavelengths of UV-A Light-Emitting Diodes. Hortic. Environ. Biotechnol. 2022, 63, 65–76. [Google Scholar] [CrossRef]
- Kang, S.; Kim, J.E.; Zhen, S.; Kim, J. Mild-Intensity UV-A Radiation Applied Over a Long Duration Can Improve the Growth and Phenolic Contents of Sweet Basil. Front. Plant. Sci. 2022, 13, 858433. [Google Scholar] [CrossRef]
- Gao, M.; Li, Y.; Jiang, H.; He, R.; Shi, R.; Song, S.; Liu, H. UVA-Radiation Exposure of Different Durations Promoted the Growth, Phytochemicals and Glucosinolate Biosynthesis of Chinese Kale. Int. J. Mol. Sci. 2022, 23, 7619. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Schreiner, M. UVB and UVA as Eustressors in Horticultural and Agricultural Crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
- Jia, L.; Tian, J.; Wei, S.; Zhang, X.; Xu, X.; Shen, Z.; Shen, W.; Cui, J. Hydrogen Gas Mediates Ascorbic Acid Accumulation and Antioxidant System Enhancement in Soybean Sprouts under UV-A Irradiation. Sci. Rep. 2017, 7, 16366. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR Irradiation Improves Growth and Nutritional Properties of Lettuce Grown in an Artificial Light Plant Factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef]
- He, R.; Li, Y.; Ou, S.; Gao, M.; Zhang, Y.; Song, S.; Liu, H. Regulation of Growth and Main Health-Promoting Compounds of Chinese Kale Baby-Leaf by UV-A and FR Light. Front. Plant Sci. 2021, 12, 799376. [Google Scholar] [CrossRef]
- Gao, M.; He, R.; Shi, R.; Li, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Combination of Selenium and UVA Radiation Affects Growth and Phytochemicals of Broccoli Microgreens. Molecules 2021, 26, 4646. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Oh, M.-M.; Son, K.-H. Short-Term Ultraviolet (UV)-A Light-Emitting Diode (LED) Radiation Improves Biomass and Bioactive Compounds of Kale. Front. Plant Sci. 2019, 10, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Wichansawakun, S.; Chupisanyarote, K.; Wongpipathpong, W.; Kaur, G.; Buttar, H. Antioxidant Diets and Functional Foods Attenuate Dementia and Cognition in Elderly Subjects. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Elsevier: Amsterdam, The Netherlands, 2022; pp. 533–549. ISBN 978-0-12-819815-5. [Google Scholar]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The Chemical Diversity and Distribution of Glucosinolates and Isothiocyanates among Plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [Green Version]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and Flavor Perceptions of Glucosinolates, Isothiocyanates, and Related Compounds. Mol. Nutr. Food Res. 2018, 62, e1700990. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Gao, M.; Li, Y.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Supplemental UV-A Affects Growth and Antioxidants of Chinese Kale Baby-Leaves in Artificial Light Plant Factory. Horticulturae 2021, 7, 294. [Google Scholar] [CrossRef]
- Viršilė, A.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Miliauskienė, J.; Jankauskienė, J.; Novičkovas, A.; Laužikė, K.; Samuolienė, G. The Distinct Impact of Multi-Color LED Light on Nitrate, Amino Acid, Soluble Sugar and Organic Acid Contents in Red and Green Leaf Lettuce Cultivated in Controlled Environment. Food Chem. 2020, 310, 125799. [Google Scholar] [CrossRef]
- Jeon, Y.-M.; Son, K.-H.; Kim, S.-M.; Oh, M.-M. Growth of Dropwort Plants and Their Accumulation of Bioactive Compounds after Exposure to UV Lamp or LED Irradiation. Hortic Environ. Biotechnol 2018, 59, 659–670. [Google Scholar] [CrossRef]
- Lee, M.-J.; Son, J.E.; Oh, M.-M. Growth and Phenolic Content of Sowthistle Grown in a Closed-Type Plant Production System with a UV-A or UV-B Lamp. Hortic. Environ. Biotechnol. 2013, 54, 492–500. [Google Scholar] [CrossRef]
- Tezuka, T.; Hotta, T.; Watanabe, I. Growth Promotion of Tomato and Radish Plants by Solar UV Radiation Reaching the Earth’s Surface. J. Photochem. Photobiol. B 1993, 19, 61–66. [Google Scholar] [CrossRef]
- Tezuka, T.; Yamaguchi, F.; Ando, Y. Physiological Activation in Radish Plants by UV-A Radiation. J. Photochem. Photobiol. B 1994, 24, 33–40. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA Radiation Is Beneficial for Yield and Quality of Indoor Cultivated Lettuce. Front. Plant Sci. 2019, 10, 1563. [Google Scholar] [CrossRef] [Green Version]
- Seedapalee, T.; Inkham, C.; Ruamrungsri, S.; Jogloy, S.; Hongpakdee, P. Physiological Responses of Sun Choke’s Seedlings under Different Wavelength LED Lighting. Sci. Hortic. 2021, 282, 110029. [Google Scholar] [CrossRef]
- Vass, I.; Turcsányi, E.; Touloupakis, E.; Ghanotakis, D.; Petrouleas, V. The Mechanism of UV-A Radiation-Induced Inhibition of Photosystem II Electron Transport Studied by EPR and Chlorophyll Fluorescence. Biochemistry 2002, 41, 10200–10208. [Google Scholar] [CrossRef]
- Mantha, S.V.; Johnson, G.A.; Day, T.A. Evidence from Action and Fluorescence Spectra That UV-Induced Violet–Blue–Green Fluorescence Enhances Leaf Photosynthesis. Photochem. Photobiol. 2001, 73, 249–256. [Google Scholar] [CrossRef]
- Helsper, J.P.F.G.; Ric de Vos, C.H.; Maas, F.M.; Jonker, H.H.; van den Broeck, H.C.; Jordi, W.; Pot, C.S.; Keizer, L.C.P.; Schapendonk, A.H.C.M. Response of Selected Antioxidants and Pigments in Tissues of Rosa Hybrida and Fuchsia Hybrida to Supplemental UV-A Exposure. Physiol. Plant. 2003, 117, 171–178. [Google Scholar] [CrossRef]
- Hooks, T.; Masabni, J.; Sun, L.; Niu, G. Effect of Pre-Harvest Supplemental UV-A/Blue and Red/Blue LED Lighting on Lettuce Growth and Nutritional Quality. Horticulturae 2021, 7, 80. [Google Scholar] [CrossRef]
- Lee, M.; Kim, J.; Oh, M.-M.; Lee, J.-H.; Rajashekar, C.B. Effects of Supplemental UV-A LEDs on the Nutritional Quality of Lettuce: Accumulation of Protein and Other Essential Nutrients. Horticulturae 2022, 8, 680. [Google Scholar] [CrossRef]
- Kadur, G.; Swapan, B.; Sunita, K.; Sanjeev, Y.; Arjun, T.; Sanjay, B.; Abhinav, R.; Mohanty, P. Growth Enhancement of Soybean (Glycine max) upon Exclusion of UV-B and UV-B/A Components of Solar Radiation: Characterization of Photosynthetic Parameters in Leaves. Photosynth. Res. 2007, 94, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Lillo, C. Signalling Cascades Integrating Light-Enhanced Nitrate Metabolism. Biochem. J. 2008, 415, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, Y.; He, R.; Tan, J.; Liu, K.; Chen, Y.; Liu, H. Effect of Supplemental UV-A Intensity on Growth and Quality of Kale under Red and Blue Light. Int. J. Mol. Sci. 2022, 23, 6819. [Google Scholar] [CrossRef] [PubMed]
- Viñegla, B.; Segovia, M.; Figueroa, F.L. Effect of Artificial UV Radiation on Carbon and Nitrogen Metabolism in the Macroalgae Fucus Spiralis L. and Ulva Olivascens Dangeard. Hydrobiologia 2006, 560, 31–42. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble Sugars—Metabolism, Sensing and Abiotic Stress. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [Green Version]
- Agati, G.; Tattini, M. Multiple Functional Roles of Flavonoids in Photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Martins, S.; Gonçalves, A.; Correia, C.M.; Ribeiro, C.; Dias, M.C.; Santos, C. The Potential Use of the UV-A and UV-B to Improve Tomato Quality and Preference for Consumers. Sci. Hortic. 2019, 246, 777–784. [Google Scholar] [CrossRef]
- Ibdah, M.; Krins, A.; Seidlitz, H.K.; Heller, W.; Strack, D.; Vogt, T. Spectral Dependence of Flavonol and Betacyanin Accumulation in Mesembryanthemum crystallinum under Enhanced Ultraviolet Radiation. Plant Cell Environ. 2002, 25, 1145–1154. [Google Scholar] [CrossRef]
- Christie, J.M.; Jenkins, G.I. Distinct UV-B and UV-A/Blue Light Signal Transduction Pathways Induce Chalcone Synthase Gene Expression in Arabidopsis Cells. Plant Cell 1996, 8, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Feinbaum, R.L.; Storz, G.; Ausubel, F.M. High Intensity and Blue Light Regulated Expression of Chimeric Chalcone Synthase Genes in Transgenic Arabidopsis Thaliana Plants. Mol. Gen. Genet. MGG 1991, 226, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Son, J.E.; Oh, M.-M. Growth and Phenolic Compounds of Lactuca Sativa L. Grown in a Closed-Type Plant Production System with UV-A, -B, or -C Lamp. J. Sci. Food Agric. 2014, 94, 197–204. [Google Scholar] [CrossRef]
- Guo, J.; Wang, M.-H. Ultraviolet A-Specific Induction of Anthocyanin Biosynthesis and PAL Expression in Tomato (Solanum lycopersicum L.). Plant Growth Regul. 2010, 62, 1–8. [Google Scholar] [CrossRef]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of Light Quality on Main Health-Promoting Compounds and Antioxidant Capacity of Chinese Kale Sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Zheng, D.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Effects of Supplementary Blue and UV-A LED Lights on Morphology and Phytochemicals of Brassicaceae Baby-Leaves. Molecules 2020, 25, 5678. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Kohyama, K.; Nishinari, K. Effect of Soluble Sugars on Gelatinization and Retrogradation of Sweet Potato Starch. J. Agric. Food Chem. 1991, 39, 1406–1410. [Google Scholar] [CrossRef]
- Inada, H.; Nagao, M.; Fujikawa, S.; Arakawa, K. Influence of Simulated Acid Snow Stress on Leaf Tissue of Wintering Herbaceous Plants. Plant Cell Physiol. 2006, 47, 504–512. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Rahman, M.d.J.; Costa de Camargo, A.; Shahidi, F. Phenolic Profiles and Antioxidant Activity of Defatted Camelina and Sophia Seeds. Food Chem. 2018, 240, 917–925. [Google Scholar] [CrossRef]
- Tadolini, B.; Juliano, C.; Piu, L.; Franconi, F.; Cabrini, L. Resveratrol Inhibition of Lipid Peroxidation. Free Radic. Res. 2000, 33, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybarczyk-Plonska, A.; Hagen, S.F.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.K.; Wold, A.-B. Glucosinolates in Broccoli (Brassica oleracea L. Var. Italica) as Affected by Postharvest Temperature and Radiation Treatments. Postharvest Biol. Technol. 2016, 116, 16–25. [Google Scholar] [CrossRef]


| Plant Height (cm) | Stem Diameter (mm) | Fresh Weight (g/Plant) | Dry Weight (g/Plant) | |||
|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |||
| Control | 19.07 ± 0.76 a | 12.38 ± 0.9 b | 51.89 ± 6.51 b | 5.45 ± 0.42 a | 3.44 ± 0.12 b | 0.80 ± 0.03 a |
| T1 | 19.33 ± 0.9 a | 13.77 ± 0.55 a | 64.35 ± 4.21 a | 5.67 ± 0.75 a | 4.52 ± 0.23 a | 0.95 ± 0.07 a |
| T2 | 19.73 ± 1.32 a | 12.99 ± 0.36 ab | 66.01 ± 3.88 a | 6.20 ± 0.55 a | 4.42 ± 0.33 a | 0.88 ± 0.09 a |
| Relative Content (%) | Treatments | ||
|---|---|---|---|
| Control | T1 | T2 | |
| PRO | 5.04 ± 0.57 a | 4.21 ± 0.36 a | 4.67 ± 14 a |
| GRA | 5.26 ± 0.21 a | 5.42 ± 0.43 a | 5.39 ± 0.52 a |
| SIN | 21.86 ± 3.1 a | 23.88 ± 1.54 a | 22.28 ± 1.42 a |
| GNA | 55.52 ± 5.21 a | 57.81 ± 1.65 a | 56.94 ± 1.58 a |
| GBN | 2.83 ± 0.57 a | 2.15 ± 0.24 a | 2.79 ± 0.12 a |
| A-GSL | 90.51 ± 1.02 c | 93.47 ± 0.16 a | 92.06 ± 0.73 b |
| 4-HGBS | 2.12 ± 0.24 a | 1.67 ± 0.11 a | 1.87 ± 0.24 a |
| GBS | 3.86 ± 0.5 a | 2.00 ± 0.23 c | 2.92 ± 0.38 b |
| 4-MGBS | 2.55 ± 0.35 a | 2.13 ± 0.07 a | 2.29 ± 0.19 a |
| NGBS | 0.96 ± 0.12 a | 0.75 ± 0.07 b | 0.85 ± 0.05 ab |
| I-GSLs | 9.49 ± 1.02 a | 6.53 ± 0.16 b | 7.94 ± 0.73 b |
| Treatment | Plant Height (cm) | Stem Diameter (mm) | Fresh Weight (g/Plant) | Dry Weight (g/Plant) | ||
|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |||
| Control | 12.27 ± 0.69 b | 11.91 ± 0.47 b | 58.81 ± 1.94 b | 4.84 ± 0.31 b | 3.03 ± 0.28 b | 0.67 ± 0.035 a |
| 5 w | 14.99 ± 0.47 a | 13.00 ± 0.40 a | 69.07 ± 2.86 a | 5.87 ± 0.17 a | 3.59 ± 0.19 a | 0.74 ± 0.03 a |
| 10 w | 14.80 ± 0.69 a | 12.15 ± 0.433 ab | 65.70 ± 3.22 a | 5.31 ± 0.42 ab | 3.13 ± 0.40 b | 0.75 ± 0.05 a |
| 15 w | 14.03 ± 0.52 a | 12.13 ± 0.5 ab | 65.81 ± 3.45 a | 5.09 ± 0.5 b | 3.24 ± 0.36 b | 0.74 ± 0.05 a |
| Relative Content (%) | Treatments | |||
|---|---|---|---|---|
| Control | 5 w | 10 w | 15 w | |
| PRO | 6.52 ± 0.30 a | 5.01 ± 0.12 b | 4.42 ± 0.31 c | 4.09 ± 0.26 c |
| GRA | 5.53 ± 0.31 b | 6.15 ± 0.36 b | 5.92 ± 0.23 b | 8.33 ± 0.61 a |
| SIN | 21.67 ± 0.50 a | 23.23 ± 1.58 a | 24.99 ± 2.36 a | 23.17 ± 1.11 a |
| GNA | 42.27 ± 1.39 b | 47.61 ± 1.3 a | 46.69 ± 2.41 a | 46.04 ± 1.21 a |
| GBN | 3.46 ± 0.14 b | 3.55 ± 0.35 b | 4.20 ± 0.21 a | 3.30 ± 0.10 b |
| total A-GSL | 79.44 ± 0.74 c | 85.56 ± 0.38 ab | 86.21 ± 0.47 a | 84.94 ± 0.28 b |
| 4-HGBS | 2.24 ± 0.14 b | 2.80 ± 0.19 a | 1.97 ± 0.12 c | 1.85 ± 0.07 c |
| GBS | 8.61 ± 0.31 a | 5.22 ± 0.33 c | 4.78 ± 0.33 c | 6.87 ± 0.36 b |
| 4-MGBS | 8.38 ± 0.31 a | 5.24 ± 0.21 b | 5.86 ± 0.26 b | 5.03 ± 0.66 b |
| NGBS | 1.33 ± 0.14 a | 1.18 ± 0.09 a | 1.17 ± 0.10 a | 1.32 ± 0.05 a |
| total I-GSL | 20.56 ± 0.74 a | 14.44 ± 0.38 bc | 13.79 ± 0.47 c | 15.06 ± 0.28 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Li, X.; He, X.; He, R.; Li, Y.; Liu, X.; Liu, H. Effects of Pre-Harvest Supplemental UV-A Light on Growth and Quality of Chinese Kale. Molecules 2022, 27, 7763. https://doi.org/10.3390/molecules27227763
Hu Y, Li X, He X, He R, Li Y, Liu X, Liu H. Effects of Pre-Harvest Supplemental UV-A Light on Growth and Quality of Chinese Kale. Molecules. 2022; 27(22):7763. https://doi.org/10.3390/molecules27227763
Chicago/Turabian StyleHu, Youzhi, Xia Li, Xinyang He, Rui He, Yamin Li, Xiaojuan Liu, and Houcheng Liu. 2022. "Effects of Pre-Harvest Supplemental UV-A Light on Growth and Quality of Chinese Kale" Molecules 27, no. 22: 7763. https://doi.org/10.3390/molecules27227763







