Analysis of Phenolic Compounds in Buckwheat (Fagopyrum esculentum Moench) Sprouts Modified with Probiotic Yeast
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phenolic Compounds
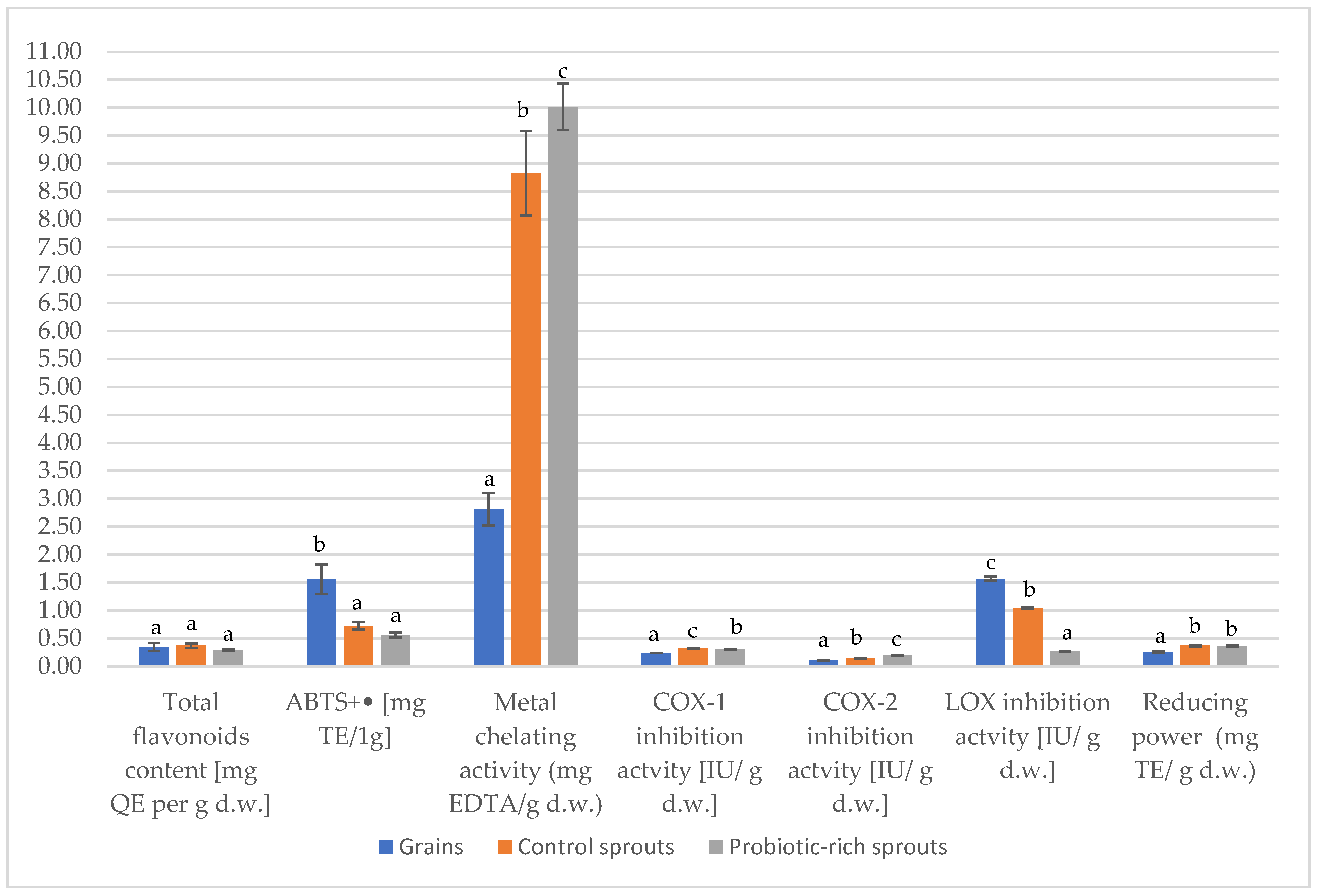
2.2. Antioxidant Activity
2.3. Anti-Inflammatory Activity
2.4. Saccharomyces cerevisiae var. boulardii
2.5. Dietary Fiber
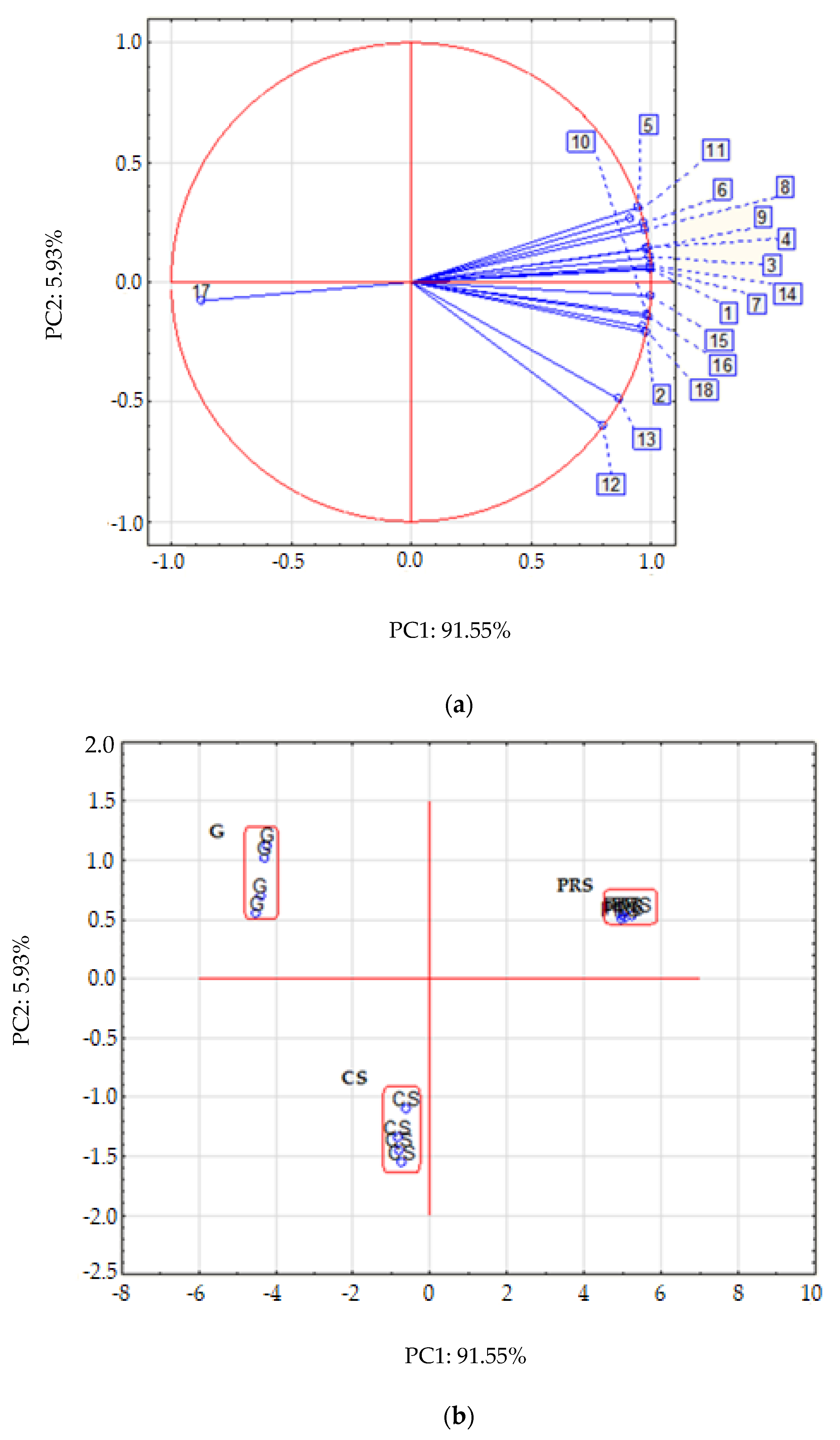
2.6. Principal Component Analysis
3. Materials and Methods
3.1. Buckwheat Sprouting
3.2. Phenolic Content
3.2.1. Extraction Procedure
Solid: Solvent Extraction
In Vitro Digestion
3.2.2. Qualitative–Quantitative Analysis of Phenolic Compounds
3.2.3. Quantitative Analysis of Total Flavonoids Content (TFC)
3.3. Pro-Health Properties
3.3.1. Antiradical Activity (ABTS+•)
3.3.2. Reducing Power (RP)
3.3.3. Metal Chelating Activity (CHP)
3.3.4. Ability to Inhibit the Activity of Cyclooxygenases (COX-1 and COX-2)
3.3.5. Ability to Inhibit the Activity of Lipoxygenase (LOX)
3.4. Theoretical Approaches
3.5. Dietary Fiber
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.-L.; Zhou, M.-L.; Tang, Y.; Li, F.-L.; Tang, Y.-X.; Shao, J.-R.; Xue, W.-T.; Wu, Y.-M. Bioactive Compounds in Functional Buckwheat Food. Food Res. Int. 2012, 49, 389–395. [Google Scholar] [CrossRef]
- Molska, M.; Reguła, J. Cultivation and Technological Value of Pseudocereals—Nutritional and Functional Aspect in the Context of a Gluten-Free Diet. J. Res. Appl. Agric. Technol. 2022, 67, 29–38. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Piątkowska, E.; Leszczyńska, T.; Pisulewska, E.; Witkowicz, R.; Bystrowska, B.; Francik, R. Identification of Polyphenolic Compounds and Determination of Antioxidant Activity in Extracts and Infusions of Buckwheat Leaves. Eur. Food Res. Technol. 2018, 244, 333–343. [Google Scholar] [CrossRef]
- Karki, R.; Park, C.-H.; Kim, D.-W. Extract of Buckwheat Sprouts Scavenges Oxidation and Inhibits Pro-Inflammatory Mediators in Lipopolysaccharide-Stimulated Macrophages (RAW264.7). J. Integr. Med. 2013, 11, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Živković, A.; Polak, T.; Cigić, B.; Požrl, T. Germinated Buckwheat: Effects of Dehulling on Phenolics Profile and Antioxidant Activity of Buckwheat Seeds. Foods 2021, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and Antiproliferative Activities in Different Maturation Stages of Broccoli (Brassica Oleracea Italica) Biofortified with Selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-L.; Kim, S.-K.; Park, C.-H. Introduction and Nutritional Evaluation of Buckwheat Sprouts as a New Vegetable. Food Res. Int. 2004, 37, 319–327. [Google Scholar] [CrossRef]
- Arendt, E.K.; Zannini, E. 11—Buckwheat. In Cereal Grains for the Food and Beverage Industries; Arendt, E.K., Zannini, E., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2013; pp. 369–408. ISBN 978-0-85709-413-1. [Google Scholar]
- Rivière, C.; Krisa, S.; Péchamat, L.; Nassra, M.; Delaunay, J.-C.; Marchal, A.; Badoc, A.; Waffo-Téguo, P.; Mérillon, J.-M. Polyphenols from the Stems of Morus Alba and Their Inhibitory Activity against Nitric Oxide Production by Lipopolysaccharide-Activated Microglia. Fitoterapia 2014, 97, 253–260. [Google Scholar] [CrossRef]
- Zeković, Z.; Kaplan, M.; Pavlić, B.; Olgun, E.O.; Vladić, J.; Canlı, O.; Vidović, S. Chemical Characterization of Polyphenols and Volatile Fraction of Coriander (Coriandrum Sativum L.) Extracts Obtained by Subcritical Water Extraction. Ind. Crops Prod. 2016, 87, 54–63. [Google Scholar] [CrossRef]
- Kosiorek, A.; Oszmiański, J.; Golański, J. Podstawy do zastosowania polifenoli roślinnych jako nutraceutyków o właściwościach przeciwpłytkowych [Rationale for the use of plant polyphenols as antiplatelet nutraceuticals]. Postępy Fitoter. 2013, 10, 108–117. [Google Scholar]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Suzuki, T.; Hara, T.; Katsu, K. Breeding of Buckwheat for Usage of Sprout and Pre-Harvest Sprouting Resistance. Plants 2021, 10, 997. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, P.; Baquero, L.P.; Larrota, H.R. Chapter 14—Flavonoids: Potential Therapeutic Agents by Their Antioxidant Capacity. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 265–288. ISBN 978-0-12-814774-0. [Google Scholar]
- Matsui, K.; Walker, A.R. Biosynthesis and Regulation of Flavonoids in Buckwheat. Breed. Sci. 2020, 70, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Zawadzka, A.; Kobus-Cisowska, J.; Stachowiak, B. Bioaktywne metabolity gryki (Fagopyrum Mill.) [Buckwheat bioactive metabolites (Fagopyrum Mill.)]. Zagadnienia Doradz. Rol. 2021, 103, 58–66. [Google Scholar]
- Lee, L.-S.; Choi, E.-J.; Kim, C.-H.; Sung, J.-M.; Kim, Y.-B.; Seo, D.-H.; Choi, H.-W.; Choi, Y.-S.; Kum, J.-S.; Park, J.-D. Contribution of Flavonoids to the Antioxidant Properties of Common and Tartary Buckwheat. J. Cereal Sci. 2016, 68, 181–186. [Google Scholar] [CrossRef]
- Rauf, M.; Yoon, H.; Lee, S.; Hyun, D.Y.; Lee, M.-C.; Oh, S.; Choi, Y.-M. Evaluation of Sprout Growth Traits and Flavonoid Content in Common and Tartary Buckwheat Germplasms. Plant Breed. Biotechnol. 2019, 7, 375–385. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.S.; Laufer, S.; Lima, J.L.F.C.; Fernandes, E. Flavonoids Inhibit COX-1 and COX-2 Enzymes and Cytokine/Chemokine Production in Human Whole Blood. Inflammation 2015, 38, 858–870. [Google Scholar] [CrossRef]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L.; Lu, Y.; Zhang, Z.; Wang, Z. Molecular Basis of Fas and Cytochrome c Pathways of Apoptosis Induced by Tartary Buckwheat Flavonoid in HL-60 Cells. Methods Find Exp. Clin. Pharmacol. 2003, 25, 431–436. [Google Scholar] [CrossRef]
- Ishii, S.; Katsumura, T.; Shiozuka, C.; Ooyauchi, K.; Kawasaki, K.; Takigawa, S.; Fukushima, T.; Tokuji, Y.; Kinoshita, M.; Ohnishi, M.; et al. Anti-Inflammatory Effect of Buckwheat Sprouts in Lipopolysaccharide-Activated Human Colon Cancer Cells and Mice. Biosci. Biotechnol. Biochem. 2008, 72, 3148–3157. [Google Scholar] [CrossRef] [Green Version]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from Garden: Bioactive Compounds of Buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef]
- Mansur, A.R.; Lee, S.G.; Lee, B.-H.; Han, S.G.; Choi, S.-W.; Song, W.-J.; Nam, T.G. Phenolic Compounds in Common Buckwheat Sprouts: Composition, Isolation, Analysis and Bioactivities. Food Sci. Biotechnol. 2022, 31, 935–956. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Świeca, M.; Kordowska-Wiater, M.; Pytka, M.; Gawlik-Dziki, U.; Bochnak, J.; Złotek, U.; Baraniak, B. Lactobacillus Plantarum 299V Improves the Microbiological Quality of Legume Sprouts and Effectively Survives in These Carriers during Cold Storage and in Vitro Digestion. PLoS ONE 2018, 13, e0207793. [Google Scholar] [CrossRef] [PubMed]
- Swieca, M.; Kordowska-Wiater, M.; Pytka, M.; Gawlik-Dziki, U.; Seczyk, L.; Złotek, U.; Kapusta, I. Nutritional and Pro-Health Quality of Lentil and Adzuki Bean Sprouts Enriched with Probiotic Yeast Saccharomyces cerevisiae var. boulardii. LWT 2019, 100, 220–226. [Google Scholar] [CrossRef]
- Witkowicz, R.; Biel, W.; Skrzypek, E.; Chłopicka, J.; Gleń-Karolczyk, K.; Krupa, M.; Prochownik, E.; Galanty, A. Microorganisms and Biostimulants Impact on the Antioxidant Activity of Buckwheat (Fagopyrum Esculentum Moench) Sprouts. Antioxidants 2020, 9, 584. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Zieliński, H. Buckwheat as a Functional Food and Its Effects on Health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef] [PubMed]
- Molska, M.; Reguła, J.; Rudzińska, M.; Świeca, M. Fatty Acids Profile, Atherogenic and Thrombogenic Health Lipid Indices of Lyophilized Buckwheat Sprouts Modified with the Addition of Saccharomyces cerevisiae var. boulardii. Acta Sci. Pol. Technol. Aliment. 2020, 19, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Molska, M.; Reguła, J.; Grygier, A.; Muzsik-Kazimierska, A.; Rudzińska, M.; Gramza-Michałowska, A. Effect of the Addition of Buckwheat Sprouts Modified with the Addition of Saccharomyces cerevisiae var. boulardii to an Atherogenic Diet on the Metabolism of Sterols, Stanols and Fatty Acids in Rats. Molecules 2022, 27, 4394. [Google Scholar] [CrossRef]
- Brajdes, C.; Vizireanu, C. Sprouted Buckwheat an Important Vegetable Source of Antioxidants. Ann. Univ. Dunarea De Jos Galati 2012, 36, 53–60. [Google Scholar]
- Molska, M.; Reguła, J.; Zielińska-Dawidziak, M.; Tomczak, A.; Świeca, M. Starch and Protein Analysis in Buckwheat (Fagopyrum Esculentum Moench) Sprouts Enriched with Probiotic Yeast. LWT 2022, 168, 113903. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, T.-G.; Lee, S.M.; Park, J.-H.; Kim, D.-O.; Baek, N.; Eom, S.H. Flavonoid Analysis of Buckwheat Sprouts. Food Chem. 2015, 170, 97–101. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Dębski, H.; Mitrus, J.; Horbowicz, M. Comparison of Flavonoids Profile in Sprouts of Common Buckwheat Cultivars and Wild Tartary Buckwheat. Int. J. Food Sci. Technol. 2014, 49, 1977–1984. [Google Scholar] [CrossRef]
- Džafić, A.; Žuljević, S.O. The Importance of Buckwheat as a Pseudocereal: Content and Stability of Its Main Bioactive Components; IntechOpen: London, UK, 2022; ISBN 978-1-80355-181-4. [Google Scholar]
- Kim, S.-J.; Zaidul, I.S.M.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of Phenolic Compositions between Common and Tartary Buckwheat (Fagopyrum) Sprouts. Food Chem. 2008, 110, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Burczynski, F.; Campbell, C.; Pierce, G.; Austria, J.A.; Briggs, C.J. Rutin and Flavonoid Contents in Three Buckwheat Species Fagopyrum Esculentum, F. Tataricum, and F. Homotropicum and Their Protective Effects against Lipid Peroxidation. Food Res. Int. 2007, 40, 356–364. [Google Scholar] [CrossRef]
- Qin, P.; Wang, Q.; Shan, F.; Hou, Z.; Ren, G. Nutritional Composition and Flavonoids Content of Flour from Different Buckwheat Cultivars. Int. J. Food Sci. Technol. 2010, 45, 951–958. [Google Scholar] [CrossRef]
- Rekha, C.R.; Vijayalakshmi, G. Bioconversion of Isoflavone Glycosides to Aglycones, Mineral Bioavailability and Vitamin B Complex in Fermented Soymilk by Probiotic Bacteria and Yeast. J. Appl. Microbiol. 2010, 109, 1198–1208. [Google Scholar] [CrossRef]
- Değirmencioğlu, N.; Gurbuz, O.; Şahan, Y. The Monitoring, Via an In Vitro Digestion System, of the Bioactive Content of Vegetable Juice Fermented with Saccharomyces Cerevisiae and Saccharomyces Boulardii. J. Food Process. Preserv. 2016, 40, 798–811. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chadchawan, S. Biostimulant Activity of Chitosan in Horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Limón, R.I.; Peñas, E.; Martínez-Villaluenga, C.; Frias, J. Role of Elicitation on the Health-Promoting Properties of Kidney Bean Sprouts. LWT—Food Sci. Technol. 2014, 56, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Świeca, M.; Dziki, D. Improvement in Sprouted Wheat Flour Functionality: Effect of Time, Temperature and Elicitation. Int. J. Food Sci. Technol. 2015, 50, 2135–2142. [Google Scholar] [CrossRef]
- Liu, H.; Kang, Y.; Zhao, X.; Liu, Y.; Zhang, X.; Zhang, S. Effects of Elicitation on Bioactive Compounds and Biological Activities of Sprouts. J. Funct. Foods 2019, 53, 136–145. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skałecki, P.; Domaradzki, P.; Florek, M. Relationships between the Content of Phenolic Compounds and the Antioxidant Activity of Polish Honey Varieties as a Tool for Botanical Discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Selyutina, O.Y.; Polyakov, N.E.; Didichenko, V.; Kontoghiorghes, G.J. Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine. Int. J. Mol. Sci. 2022, 23, 1247. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Czeczot, H. Flawonoidy w profilaktyce i terapii [Flavonoids in prevention and therapy]. Farm. Pol. 2009, 65, 369–377. [Google Scholar]
- Cooper, G.J.S. Therapeutic Potential of Copper Chelation with Triethylenetetramine in Managing Diabetes Mellitus and Alzheimer’s Disease. Drugs 2011, 71, 1281–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-J.; Wei, H.; Frei, B. The Iron Chelator, Desferrioxamine, Reduces Inflammation and Atherosclerotic Lesion Development in Experimental Mice. Exp. Biol. Med. 2010, 235, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Cyclooxygenase-2: A Therapeutic Target—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/11818462/ (accessed on 14 July 2022).
- Baraniak, B.M.; Szymanowska, U. Lipooksygenaza w zywnosci pochodzenia roslinnego [Lipooxygenase in food of plant origin]. Żywność Nauka Technol. Jakość 2006, 13, 29–45. [Google Scholar]
- Bhaskar, S.; Shalini, V.; Helen, A. Quercetin Regulates Oxidized LDL Induced Inflammatory Changes in Human PBMCs by Modulating the TLR-NF-ΚB Signaling Pathway. Immunobiology 2011, 216, 367–373. [Google Scholar] [CrossRef]
- Nam, T.G.; Lim, T.-G.; Lee, B.H.; Lim, S.; Kang, H.; Eom, S.H.; Yoo, M.; Jang, H.W.; Kim, D.-O. Comparison of Anti-Inflammatory Effects of Flavonoid-Rich Common and Tartary Buckwheat Sprout Extracts in Lipopolysaccharide-Stimulated RAW 264.7 and Peritoneal Macrophages. Oxidative Med. Cell Longev. 2017, 2017, e9658030. [Google Scholar] [CrossRef] [Green Version]
- Almuhayawi, M.S.; Hassan, A.H.A.; Abdel-Mawgoud, M.; Khamis, G.; Selim, S.; Al Jaouni, S.K.; AbdElgawad, H. Laser Light as a Promising Approach to Improve the Nutritional Value, Antioxidant Capacity and Anti-Inflammatory Activity of Flavonoid-Rich Buckwheat Sprouts. Food Chem. 2021, 345, 128788. [Google Scholar] [CrossRef] [PubMed]
- Liyana-Pathirana, C.M.; Shahidi, F. Importance of Insoluble-Bound Phenolics to Antioxidant Properties of Wheat. J. Agric. Food Chem. 2006, 54, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Ferraretto, A.; De Noni, I.; Bottani, M.; Cattaneo, S.; Galli, S.; Brandolini, A. Bioactive Compounds and Antioxidant Properties of Pseudocereals-Enriched Water Biscuits and Their in Vitro Digestates. Food Chem. 2018, 240, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz, M. Saccharomyces cerevisiae var. boulardii—Probiotic Yeast. In Probiotics; IntechOpen: London, UK, 2012; pp. 385–398. [Google Scholar]
- Champagne, C.P.; Gardner, N.J.; Roy, D. Challenges in the Addition of Probiotic Cultures to Foods. Crit. Rev. Food Sci. Nutr. 2005, 45, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeasts in Foods and Beverages: Impact on Product Quality and Safety. Curr. Opin Biotechnol. 2007, 18, 170–175. [Google Scholar] [CrossRef]
- Ryan, E.P.; Heuberger, A.L.; Weir, T.L.; Barnett, B.; Broeckling, C.D.; Prenni, J.E. Rice Bran Fermented with Saccharomyces Boulardii Generates Novel Metabolite Profiles with Bioactivity. J. Agric. Food Chem. 2011, 59, 1862–1870. [Google Scholar] [CrossRef]
- Datta, S.; Timson, D.; Annapure, U. Antioxidant Properties and Global Metabolite Screening of the Probiotic Yeast Saccharomyces cerevisiae var. boulardii. J. Sci. Food Agric. 2016, 97, 3039–3049. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Pacheco, P.; Pintado, C.; Briones Pérez, A.; Arévalo-Villena, M. Potential Probiotic Strains of Saccharomyces and Non-Saccharomyces: Functional and Biotechnological Characteristics. J. Fungi 2021, 7, 177. [Google Scholar] [CrossRef]
- Górecka, D.; Marzanna, H.; Szymandera-Buszka, K.; Krzysztof, D. Contents of Selected Bioactive Components in Buckwheat Groats. Acta Sci. Pol. Technol. Aliment. 2009, 8, 75–83. [Google Scholar]
- Sofi, S.; Ayoub, A.; Jan, A. Resistant Starch as Functional Ingredient: A Review. Food Res. Int. 2017, 2, 2455–4898. [Google Scholar]
- Bzducha-Wróbel, A.; Kieliszek, M.; Błażejak, S. Chemical Composition of the Cell Wall of Probiotic and Brewer’s Yeast in Response to Cultivation Medium with Glycerol as a Carbon Source. Eur. Food Res. Technol. 2013, 237, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Hozova, B.; Kuniak, L.; Moravcikova, P.; Gajdosova, A. Determination of Water-Insoluble Beta-D-Glucan in the Whole-Grain Cereals and Pseudocereals. Czech J. Food Sci. UZPI 2007, 25, 316. [Google Scholar] [CrossRef] [Green Version]
- Ragaee, S.; Guzar, I.; Dhull, N.; Seetharaman, K. Effects of Fiber Addition on Antioxidant Capacity and Nutritional Quality of Wheat Bread. LWT Food Sci. Technol. 2011, 44, 2147–2153. [Google Scholar] [CrossRef]
- Siemińska-Kuczer, A.; Szymańska-Chargot, M.; Zdunek, A. Recent Advances in Interactions between Polyphenols and Plant Cell Wall Polysaccharides as Studied Using an Adsorption Technique. Food Chem. 2022, 373, 131487. [Google Scholar] [CrossRef] [PubMed]
- Holasova, M.; Fiedlerova, V.; Smrcinova, H.; Orsak, M.; Lachman, J.; Vavreinova, S. Buckwheat—The Source of Antioxidant Activity in Functional Foods. Food Res. Int. 2002, 35, 207–211. [Google Scholar] [CrossRef]
- Hung, P.V.; Morita, N. Distribution of Phenolic Compounds in the Graded Flours Milled from Whole Buckwheat Grains and Their Antioxidant Capacities. Food Chem. 2008, 109, 325–331. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Swieca, M.; Gawlik-Dziki, U. Nutritional and Health-Promoting Properties of Bean Paste Fortified with Onion Skin in the Light of Phenolic-Food Matrix Interactions. Food Funct. 2015, 6, 3560–3566. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Baraniak, B.; Sikora, M.; Jakubczyk, A.; Kapusta, I.; Świeca, M. Potentially Bioaccessible Phenolic and Antioxidant Potential of Fresh and Stored Lentil Sprouts—Effect of Lactobacillus Plantarum 299v Enrichment. Molecules 2021, 26, 2109. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Antioxidant Activity, Total Polyphenol, Flavonoid and Tannin Contents of Fermented Green Coffee Beans with Selected Yeasts. Fermentation 2019, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols as Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.-T.; Lee, H.-L.; Chiang, S.-H.; Lin, F.-I.; Chang, C.-Y. Antioxidant Properties of the Extracts from Different Parts of Broccoli in Taiwan. J. Food Drug Anal. 2001, 9, 96–101. [Google Scholar] [CrossRef]
- Szymanowska, U.; Jakubczyk, A.; Baraniak, B.; Kur, A. Characterisation of Lipoxygenase from Pea Seeds (Pisum sativum var. Telephone L.). Food Chem. 2009, 116, 906–910. [Google Scholar] [CrossRef]
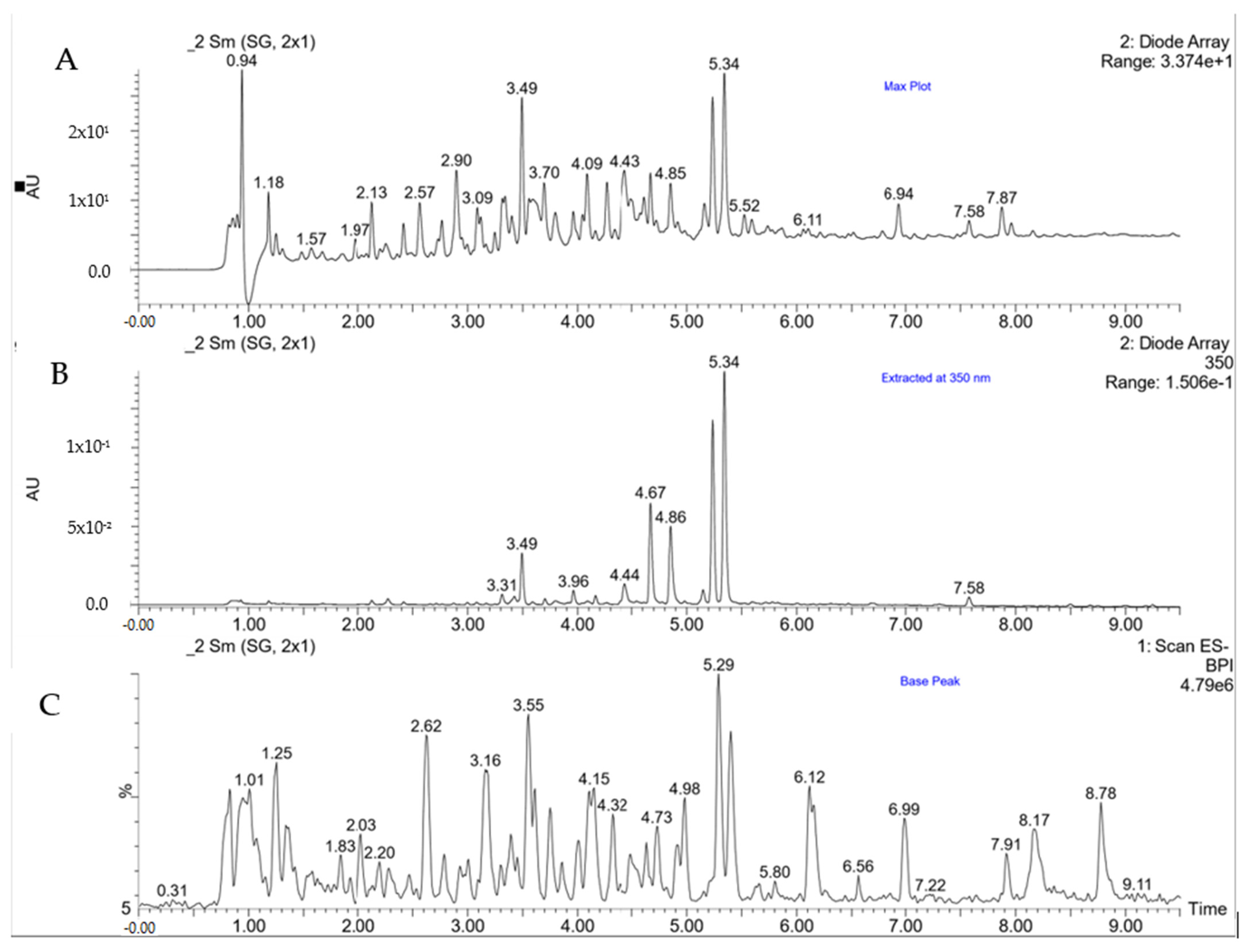

| Compound | Rt | λmax | (M-H) m/z | Sample | ||||
|---|---|---|---|---|---|---|---|---|
| min | nm | MS | MS/MS | Seeds (G) | Control Sprouts (CS) | Probiotic-Rich Sprouts (PRS) | ||
| Phenolic acids | ||||||||
| 1 | Caffeoyl-glucoside | 2.19 | 288 | 341 | 179. 143 | 47.29 ± 0.41 a | 53.23 ± 0.06 a,b | 55.48 ± 0.52 b |
| 2 | Caffeoyl-rhamnopyranosyl-glucopyranosyl-glucopyranoside | 2.47 | 289 | 649 | 487. 179 | 24.07 ± 0.54 a | 27.80 ± 0.25 a | 29.57 ± 0.92 a |
| 3 | Caffeoyl-rhamnopyranosyl-glucopyranosyl | 3.50 | 288 | 487 | 179 | 79.80 ± 0.30 a | 92.04 ± 0.05 b | 118.23 ± 0.04 c |
| Flavan-3-ols | ||||||||
| 1 | Unknown catechin derivate | 2.57 | 272 | 535 | 515. 267 | 73.52 ± 0.01 c | 61.92 ± 0.23 b | 61.89 ± 0.08 a |
| 2 | (Epi) afzelechin-(epi)-catechin | 2.76 | 277 | 561 | 289. 245 | 9.47 ± 0.25 a | 38.24 ± 0.89 b | 56.80 ± 0.13 c |
| 3 | Catechin-glucoside | 2.91 | 276 | 451 | 289 | 39.06 ± 0.43 a | 62.08 ± 0.94 b | 113.90 ± 0.71 c |
| 4 | Catechin-3-O-glucoside-6-O-rutinoside | 3.09 | 278 | 719 | 451. 289 | 29.55 ± 0.07 a | 32.60 ± 0.29 b | 46.19 ± 0.92 c |
| 5 | Caffeoyl-glucoside | 3.31 | 288 | 341 | 179 | 2.31 ± 0.99 a | 17.55 ± 0.5 b | 33.51 ± 0.38 c |
| 6 | (+)Catechin | 3.34 | 276 | 289 | - | 11.59 ± 0.11 a | 27.85 ± 0.1 b | 45.14 ± 0.75 c |
| 7 | Catechin-glucoside | 3.40 | 279 | 451 | 289 | 18.35 ± 0.11 a | 29.78 ± 0.2 b | 42.66 ± 0.21 c |
| 8 | Epicatechin-(4-8)-epicatechin | 3.61 | 277 | 577 | 289 | 107.36 ± 0.19 a | 100.88 ± 0.05 a | 115.94 ± 0.62 b |
| 9 | Epicatechin-(4-8)-epigallocatechin-gallate | 3.70 | 279 | 729 | 577. 407. 289 | 15.79 ± 0.54 a | 34.96 ± 0.76 b | 57.89 ± 0.40 c |
| 10 | Epicatechin gallate dimethyl derivative | 3.81 | 284 | 469 | 425. 137 | 3.45 ± 0.42 a | 49.86 ± 0.48 b | 63.37 ± 0.93 c |
| 11 | Epicatechin gallate | 3.97 | 317 | 883 2 [M-H]− | 441. 289 | 27.17 ± 0.805 a | 28.55 ± 0.45 a | 70.88 ± 0.34 b |
| 12 | (-)Epicatechin | 4.14 | 277 | 289 | - | 14.01 ± 0.74 a | 39.60 ± 0.62 b | 66.55 ± 0.07 c |
| 13 | Catechin trimer | 4.27 | 279 | 865 | 577. 289 | 11.26 ± 0.01 a | 19.80 ± 0.32 b | 32.73 ± 0.71 c |
| 14 | Epicatechin gallate methyl derivative | 4.44 | 292 | 455 | 441. 289 | 7.20 ± 0.86 a | 113.27 ± 0.02 b | 138.25 ± 0.98 c |
| 15 | Epicatechin trimer | 4.62 | 279 | 865 | 577. 289 | 60.67 ± 0.18 b | 22.61 ± 0.11 a | 57.14 ± 0.3 b |
| 16 | Epiafzelechin-epicatechin-gallate dimethyl derivative | 6.92 | 279 | 741 | 605. 469. 271 | 20.29 ± 0.22 a | 10.75 ± 0.35 a | 19.21 ± 0.98 a |
| 17 | Epiafzelechin-epicatechin-gallate methyl derivative | 7.88 | 271 | 727 | 601. 407. 289 | 21.39 ± 0.2 b | 5.68 ± 0.16 a | 12.32 ± 0.62 a |
| Flavonols | ||||||||
| 1 | Orientin | 4.67 | 269. 347 | 447 | 285 | 7.31 ± 0.43 a | 3.44 ± 0.54 a | 43.51 ± 0.42 b |
| 2 | Isorientin | 4.87 | 270. 312 | 447 | 285 | ND | 6.42 ± 0.56 a | 35.83 ± 0.25 b |
| 3 | Quercetin-3-O-rutinoside | 5.24 | 255. 352 | 609 | 301 | 41.28 ± 0.88 a | 52.48 ± 0.34 b | 88.37 ± 0.42 c |
| 4 | Vitexin | 5.35 | 269. 329 | 431 | 269 | ND | 20.09 ± 0.87 a | 121.03 ± 0.58 b |
| Total phenols compounds (µg/g d.w.) | 672.14 ± 0.92 a | 951.42 ± 1.82 b | 1526.34 ± 3.33 c | |||||
| Before Digestion | After Digestion | |||||
|---|---|---|---|---|---|---|
| Seeds (G) | Control Sprouts (CS) | Probiotic-Rich Sprouts (PRS) | Seeds (G) | Control Sprouts (CS) | Probiotic-Rich Sprouts (PRS) | |
| Total flavonoids content (mg QE per g d.w.) | 14.10 ± 0.64 a | 20.88 ± 0.59 b | 52.98 ± 0.77 c | 4.88 ± 1.18 a | 7.75 ± 0.78 b | 15.75 ± 0.62 c |
| ABTS+• (mg TE/1 g) | 5.77 ± 0.83 a | 12.63 ± 0.50 b | 19.78 ± 1.37 c | 8.82 ± 0.42 a | 9.13 ± 0.54 a | 11.05 ± 0.29 b |
| Metal chelating activity (mg EDTA/g d.w.) | 4.08 ± 0.5 a | 11.58 ± 0.34 b | 11.70 ± 0.13 b | 11.40 ± 1.23 a | 102.15 ± 8.10 b | 117.78 ± 4.95 c |
| Reducing power (mg TE/g d.w.) | 11.24 ± 0.75 a | 17.59 ± 0.84 b | 31.15 ± 0.42 c | 2.87 ± 0.05 a | 6.50 ± 0.11 b | 11.24 ± 0.34 c |
| COX-1 inhibitory activity (IU/g d.w.) | 1664.18 ± 0.37 a | 2119.97 ± 4.51 b | 3037.15 ± 2.30 c | 390.20 ± 0.39 a | 682.00 ± 5.24 b | 904.00 ± 4.92 c |
| COX-2 inhibitory activity (IU/g d.w.) | 1215.42 ± 0.50 a | 1562.75 ± 0.50 b | 2431.24 ± 0.17 c | 128.00 ± 0.50 a | 213.62 ± 0.25 b | 470.00 ± 0.25 c |
| LOX inhibitory activity (IU/g d.w.) | 442.78 ± 5.91 a | 740.80 ± 10.55 b | 4721.56 ± 9.34 c | 693.94 ± 12.91 a | 773.69 ± 8.35 b | 1250.00 ± 5.52 c |
| Total Flavonoids Content (mg QE per g d.w.) | Total Phenols Compounds (µg/g DM) | |||
|---|---|---|---|---|
| Before Digestion | After Digestion | |||
| ABTS+• (mg TE/1 g) | Before digestion | R = 0.872155 p < 0.00 | R = 0.915499 p < 0.00 | R = 0.947758 p < 0.00 |
| After digestion | R = 0.770579 p < 0.00 | R = 0,721835 p < 0.00 | R = 0.784862 p < 0.00 | |
| Metal chelating activity (mg EDTA/g d.w.) | Before digestion | R = 0.819616 p < 0.00 | R = 0.713033 p < 0.00 | R = 0.814480 p < 0.00 |
| After digestion | R = 0.853147 p < 0.00 | R = 0,882262 p < 0.00 | R = 0.946100 p < 0.00 | |
| Reducing power (mg TE/g d.w.) | Before digestion | R = 0.905269 p < 0.00 | R = 0.945328 p < 0.00 | R = 0.949425 p < 0.00 |
| After digestion | R = 0.888112 p < 0.00 | R = 0.938502 p < 0.00 | R = 0.946100 p < 0.00 | |
| COX-1 inhibitory activity (IU/g d.w.) | Before digestion | R = 0.882663 p < 0.00 | R = 0.924302 p < 0.00 | R = 0.947758 p < 0.00 |
| After digestion | R = 0.892807 p < 0.00 | R = 0.909894 p < 0.00 | R = 0.951101 p < 0.00 | |
| COX-2 inhibitory activity (IU/g d.w.) | Before digestion | R = 0.950583 p < 0.00 | R = 0.873902 p < 0.00 | R = 0.956183 p < 0.00 |
| After digestion | R = 0.888213 p < 0.00 | R = 0.923267 p < 0.00 | R = 0.961347 p < 0.00 | |
| LOX inhibitory activity (IU/g d.w.) | Before digestion | R = 0.937063 p < 0.00 | R = 0.864687 p < 0.00 | R = 0.946100 p < 0.00 |
| After digestion | R = 0.931700 p < 0.00 | R = 0.832752 p < 0.00 | R = 0.947758 p < 0.00 | |
| Total dietary fiber (%) | R = 0.904644 p < 0.00 | R = 0,888112 p < 0.00 | R = 0.956183 p < 0.00 | |
| Insoluble dietary fiber (%) | R = −0.791564 p < 0.00 | R = −0.838378 p < 0.00 | R = −0.836660 p < 0.00 | |
| Soluble dietary fiber (%) | R = 0.904644 p < 0.00 | R = 0.888112 p < 0.00 | R = 0.956183 p < 0.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molska, M.; Reguła, J.; Kapusta, I.; Świeca, M. Analysis of Phenolic Compounds in Buckwheat (Fagopyrum esculentum Moench) Sprouts Modified with Probiotic Yeast. Molecules 2022, 27, 7773. https://doi.org/10.3390/molecules27227773
Molska M, Reguła J, Kapusta I, Świeca M. Analysis of Phenolic Compounds in Buckwheat (Fagopyrum esculentum Moench) Sprouts Modified with Probiotic Yeast. Molecules. 2022; 27(22):7773. https://doi.org/10.3390/molecules27227773
Chicago/Turabian StyleMolska, Marta, Julita Reguła, Ireneusz Kapusta, and Michał Świeca. 2022. "Analysis of Phenolic Compounds in Buckwheat (Fagopyrum esculentum Moench) Sprouts Modified with Probiotic Yeast" Molecules 27, no. 22: 7773. https://doi.org/10.3390/molecules27227773
APA StyleMolska, M., Reguła, J., Kapusta, I., & Świeca, M. (2022). Analysis of Phenolic Compounds in Buckwheat (Fagopyrum esculentum Moench) Sprouts Modified with Probiotic Yeast. Molecules, 27(22), 7773. https://doi.org/10.3390/molecules27227773









