How Well Do We Handle the Sample Preparation, FT-ICR Mass Spectrometry Analysis, and Data Treatment of Atmospheric Waters?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site and Cloud Sampling
2.2. Sample Treatment and Schema of the Experiment
2.3. ESI FT-ICR MS Analysis
2.4. Preliminary Treatment with DataAnalysis
2.5. Formula Assignment with Composer, DataAnalysis, and MFAssignR
2.5.1. Composer
2.5.2. DataAnalysis
2.5.3. MFAssignR
3. Results and Discussion
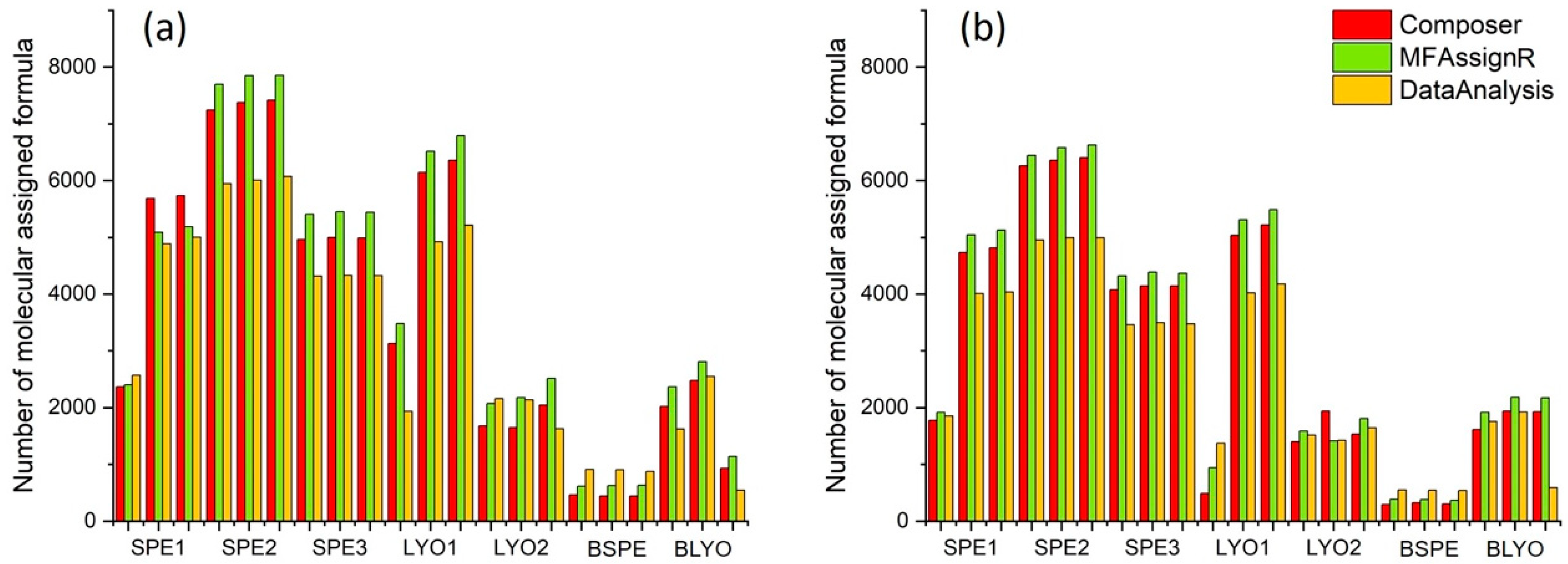
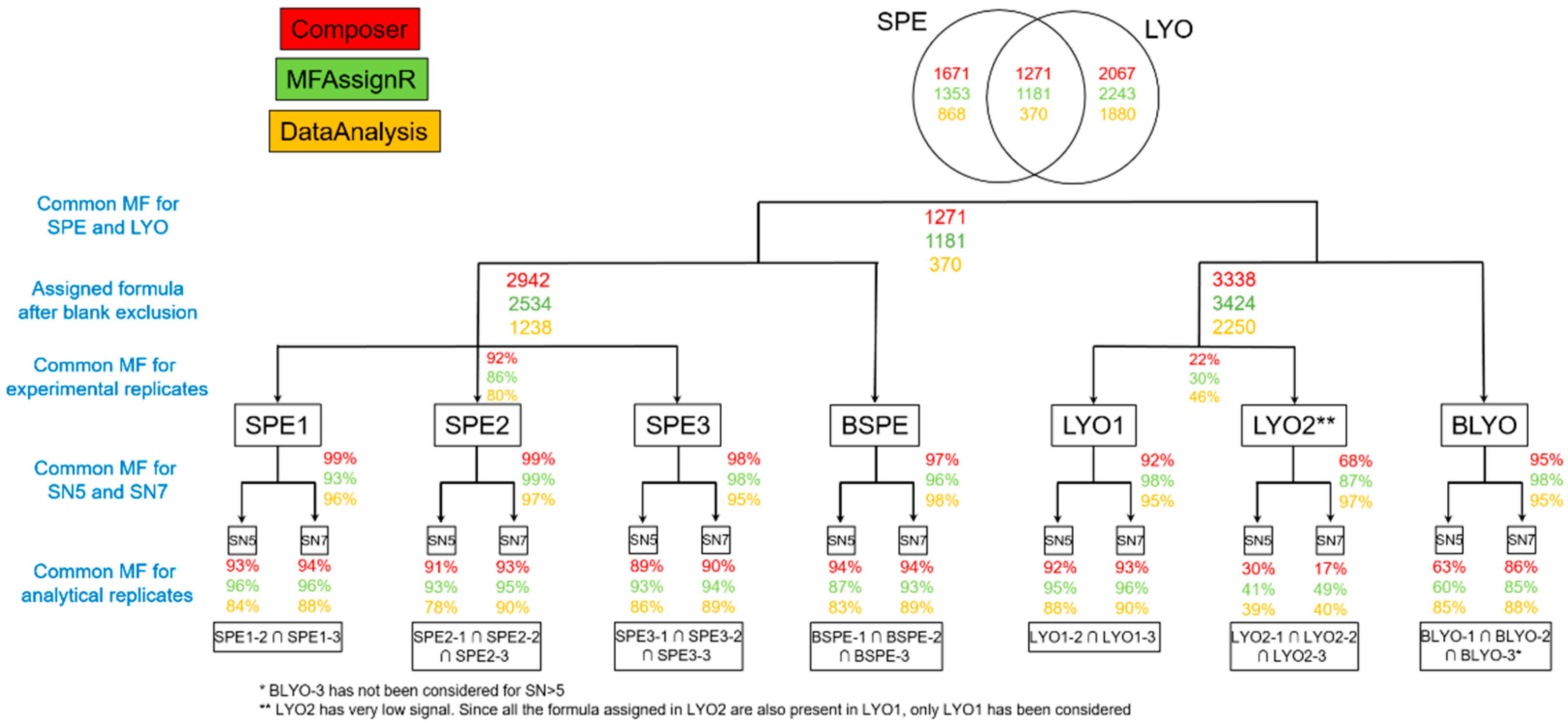
3.1. Analytical Replicates
3.2. S/N Comparison—SPE
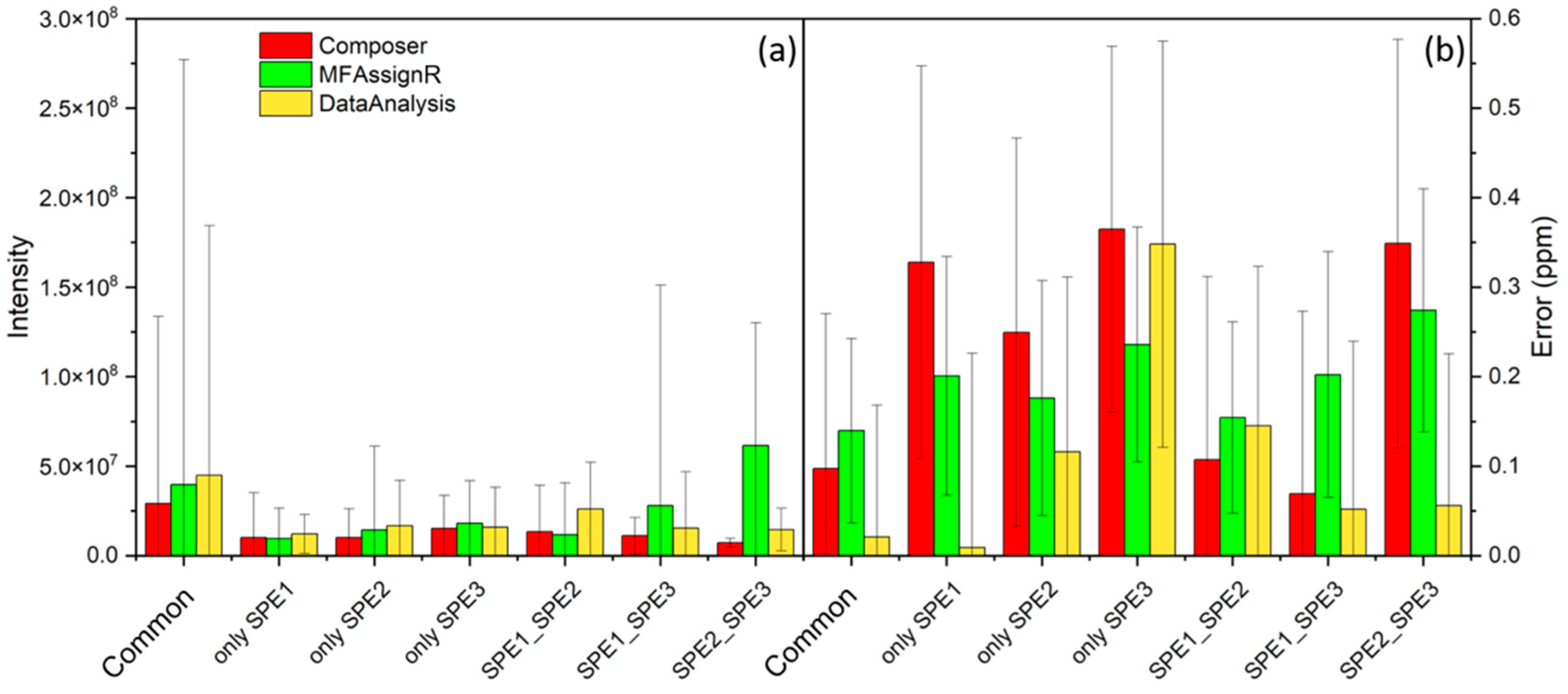
3.3. Experimental Replicates—SPE
3.4. S/N Comparison and Experimental Replicates—LYO
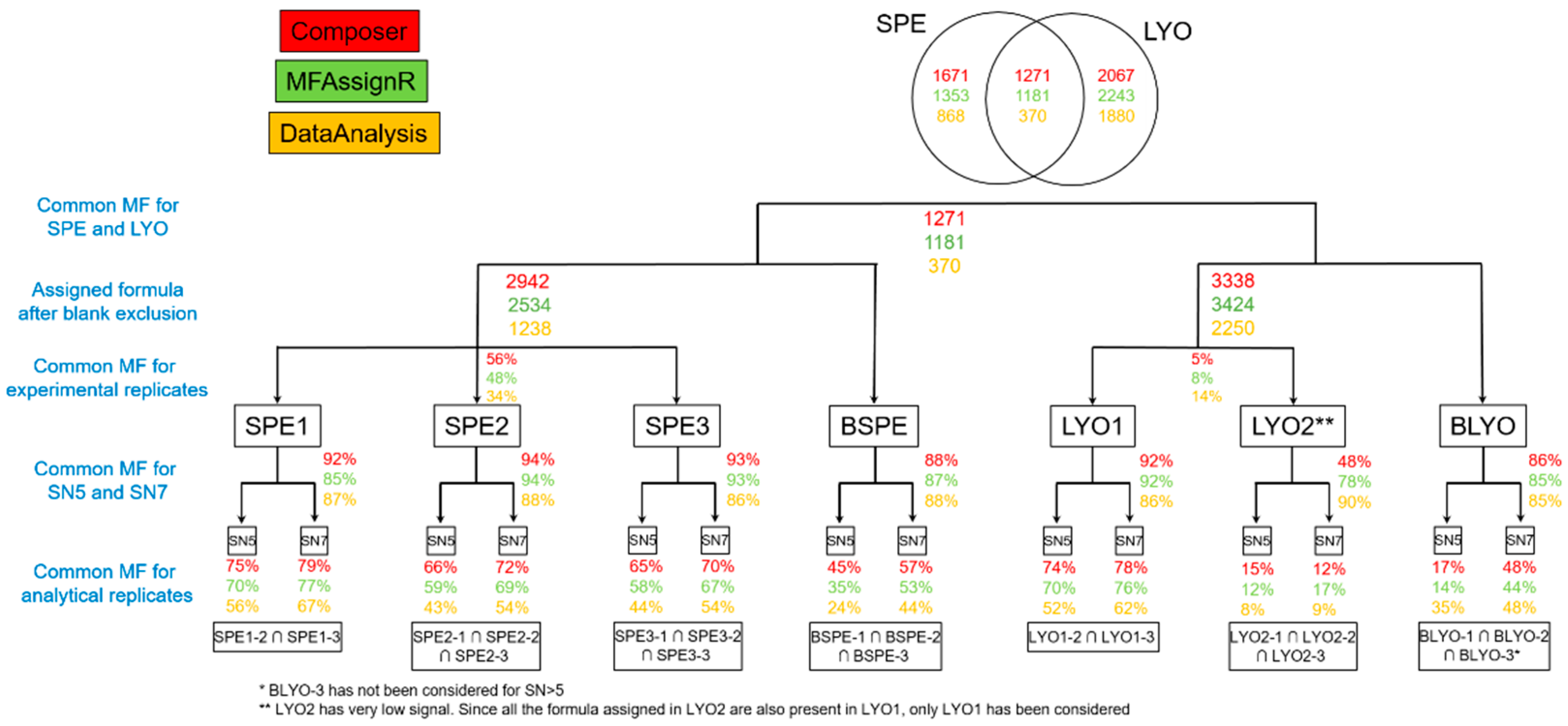
3.5. Blank Exclusion
3.6. Comparison SPE vs. LYO
3.7. Software Program Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pöschl, U. Atmospheric aerosols: Composition, transformation, climate and health effects. Angew. Chem. Int. Ed. 2005, 44, 7520–7540. [Google Scholar] [CrossRef] [PubMed]
- Pye, H.O.T.; Nenes, A.; Alexander, B.; Ault, A.P.; Barth, M.C.; Clegg, S.L.; Collett, J.L., Jr.; Fahey, K.M.; Hennigan, C.J.; Herrmann, H.; et al. The acidity of atmospheric particles and clouds. Atmos. Chem. Phys. 2020, 20, 4809–4888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons: New York, NY, USA, 2016. [Google Scholar]
- Pio, C.A.; Legrand, M.; Oliveira, T.; Afonso, J.; Santos, C.; Caseiro, A.; Fialho, P.; Barata, F.; Puxbaum, H.; Sanchez-Ochoa, A.; et al. Climatology of aerosol composition (organic versus inorganic) at nonurban sites on a west-east transect across Europe. J. Geophys. Res. Atmospheres 2007, 112, D23S02. [Google Scholar] [CrossRef] [Green Version]
- Nozière, B.; Kalberer, M.; Claeys, M.; Allan, J.; D’Anna, B.; Decesari, S.; Finessi, E.; Glasius, M.; Grgić, I.; Hamilton, J.F.; et al. The molecular identification of organic compounds in the atmosphere: State of the art and challenges. Chem. Rev. 2015, 115, 3919–3983. [Google Scholar] [CrossRef]
- Morgan, W.T.; Allan, J.D.; Bower, K.N.; Highwood, E.J.; Liu, D.; McMeeking, G.R.; Northway, M.J.; Williams, P.I.; Krejci, R.; Coe, H. Airborne measurements of the spatial distribution of aerosol chemical composition across Europe and evolution of the organic fraction. Atmos. Chem. Phys. 2010, 10, 4065–4083. [Google Scholar] [CrossRef] [Green Version]
- Lanz, V.A.; Prévôt, A.S.H.; Alfarra, M.R.; Weimer, S.; Mohr, C.; DeCarlo, P.F.; Gianini, M.F.D.; Hueglin, C.; Schneider, J.; Favez, O.; et al. Characterization of Aerosol Chemical Composition with Aerosol Mass Spectrometry in Central Europe: An Overview. Atmos. Chem. Phys. 2010, 10, 10453–10471. [Google Scholar] [CrossRef] [Green Version]
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J.; et al. Organic aerosol and global climate modelling: A review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef] [Green Version]
- Ervens, B. Modeling the processing of aerosol and trace gases in clouds and fogs. Chem. Rev. 2015, 115, 4157–4198. [Google Scholar] [CrossRef]
- Herrmann, H.; Schaefer, T.; Tilgner, A.; Styler, S.A.; Weller, C.; Teich, M.; Otto, T. Tropospheric aqueous-phase chemistry: Kinetics, mechanisms, and its coupling to a changing gas phase. Chem. Rev. 2015, 115, 4259–4334. [Google Scholar] [CrossRef]
- Li, T.; Wang, Z.; Wang, Y.; Wu, C.; Liang, Y.; Xia, M.; Yu, C.; Yun, H.; Wang, W.; Wang, Y.; et al. Chemical characteristics of cloud water and the impacts on aerosol properties at a subtropical mountain site in Hong Kong SAR. Atmos. Chem. Phys. 2020, 20, 391–407. [Google Scholar] [CrossRef]
- Löflund, M.; Kasper-Giebl, A.; Schuster, B.; Giebl, H.; Hitzenberger, R.; Puxbaum, H. Formic, acetic, oxalic, malonic and succinic acid concentrations and their contribution to organic carbon in cloud water. Atmos. Environ. 2002, 36, 1553–1558. [Google Scholar] [CrossRef]
- Herrmann, H. Kinetics of aqueous phase reactions relevant for atmospheric chemistry. Chem. Rev. 2003, 103, 4691–4716. [Google Scholar] [CrossRef] [PubMed]
- Franco, B.; Blumenstock, T.; Cho, C.; Clarisse, L.; Clerbaux, C.; Coheur, P.-F.; De Mazière, M.; De Smedt, I.; Dorn, H.-P.; Emmerichs, T.; et al. Ubiquitous atmospheric production of organic acids mediated by cloud droplets. Nature 2021, 593, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Altieri, K.E.; Seitzinger, S.P.; Carlton, A.G.; Turpin, B.J.; Klein, G.C.; Marshall, A.G. Oligomers formed through in-cloud methylglyoxal reactions: Chemical composition, properties, and mechanisms investigated by ultra-high resolution FT-ICR mass spectrometry. Atmos. Environ. 2008, 42, 1476–1490. [Google Scholar] [CrossRef]
- De Haan, D.O.; Tapavicza, E.; Riva, M.; Cui, T.; Surratt, J.D.; Smith, A.C.; Jordan, M.-C.; Nilakantan, S.; Almodovar, M.; Stewart, T.N.; et al. Nitrogen-containing, light-absorbing oligomers produced in aerosol particles exposed to methylglyoxal, photolysis, and cloud cycling. Environ. Sci. Technol. 2018, 52, 4061–4071. [Google Scholar] [CrossRef] [PubMed]
- Amato, P.; Besaury, L.; Joly, M.; Penaud, B.; Deguillaume, L.; Delort, A.-M. Metatranscriptomic Exploration of microbial functioning in clouds. Sci. Rep. 2019, 9, 4383. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Deguillaume, L.; Chaumerliac, N.; Vaïtilingom, M.; Wang, M.; Delort, A.-M.; Bridoux, M.C. Effect of endogenous microbiota on the molecular composition of cloud water: A study by fourier-transform ion cyclotron resonance mass spectrometry (FT-ICR MS). Sci. Rep. 2019, 9, 7663. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Passananti, M.; Brigante, M.; Mailhot, G. Photochemistry of the cloud aqueous phase: A review. Molecules 2020, 25, 423. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Voyard, G.; Deguillaume, L.; Mailhot, G.; Brigante, M. Improving the Characterization of dissolved organic carbon in cloud water: Amino acids and their impact on the oxidant capacity. Sci. Rep. 2016, 6, 37420. [Google Scholar] [CrossRef] [Green Version]
- Renard, P.; Brissy, M.; Rossi, F.; Leremboure, M.; Jaber, S.; Baray, J.-L.; Bianco, A.; Delort, A.-M.; Deguillaume, L. Free amino acid quantification in cloud water at the Puy de Dôme Station (France). Atmos. Chem. Phys. 2022, 22, 2467–2486. [Google Scholar] [CrossRef]
- Keene, W.C.; Mosher, B.W.; Jacob, D.J.; Munger, J.W.; Talbot, R.W.; Artz, R.S.; Maben, J.R.; Daube, B.C.; Galloway, J.N. Carboxylic acids in clouds at a high-elevation forested site in central Virginia. J. Geophys. Res. 1995, 100, 9345. [Google Scholar] [CrossRef]
- Van Pinxteren, D.; Fomba, K.W.; Mertes, S.; Müller, K.; Spindler, G.; Schneider, J.; Lee, T.; Collett, J.L.; Herrmann, H. Cloud water composition during HCCT-2010: Scavenging efficiencies, solute concentrations, and droplet size dependence of inorganic ions and dissolved organic carbon. Atmos. Chem. Phys. 2016, 16, 3185–3205. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Fu, P.; Yue, S.; Li, L.; Xie, Q.; Zhu, C.; Wei, L.; Ren, H.; Li, P.; Li, W.; et al. Excitation-Emission matrix fluorescence, molecular characterization and compound-specific stable carbon isotopic composition of dissolved organic matter in cloud water over Mt. Tai. Atmos. Environ. 2019, 213, 608–619. [Google Scholar] [CrossRef]
- Triesch, N.; van Pinxteren, M.; Engel, A.; Herrmann, H. Concerted measurements of free amino acids at the Cabo Verde Islands: High enrichments in submicron sea spray aerosol particles and cloud droplets. Atmos. Chem. Phys. 2021, 21, 163–181. [Google Scholar] [CrossRef]
- Bianco, A.; Deguillaume, L.; Vaïtilingom, M.; Nicol, E.; Baray, J.-L.; Chaumerliac, N.; Bridoux, M.C. Molecular characterization of cloud water samples collected at the Puy de Dôme (France) by Fourier transform ion cyclotron resonance mass spectrometry. Environ. Sci. Technol. 2018, 52, 10275–10285. [Google Scholar] [CrossRef]
- Zhao, Y.; Hallar, A.G.; Mazzoleni, L.R. Atmospheric organic matter in clouds: Exact masses and molecular formula identification using ultrahigh-resolution FT-ICR mass spectrometry. Atmos. Chem. Phys. 2013, 13, 12343–12362. [Google Scholar] [CrossRef] [Green Version]
- Cook, R.D.; Lin, Y.-H.; Peng, Z.; Boone, E.; Chu, R.K.; Dukett, J.E.; Gunsch, M.J.; Zhang, W.; Tolic, N.; Laskin, A. Biogenic, urban, and wildfire influences on the molecular composition of dissolved organic compounds in cloud water. Atmos. Chem. Phys. 2017, 17, 15167–15180. [Google Scholar] [CrossRef] [Green Version]
- Renard, P.; Bianco, A.; Jänis, J.; Kekäläinen, T.; Bridoux, M.; Deguillaume, L. Puy de Dôme Station (France): A stoichiometric approach to compound classification in clouds. J. Geophys. Res. Atmos. 2022, 127, e2022JD036635. [Google Scholar] [CrossRef]
- Sun, W.; Fu, Y.; Zhang, G.; Yang, Y.; Jiang, F.; Lian, X.; Jiang, B.; Liao, Y.; Bi, X.; Chen, D.; et al. Measurement report: Molecular characteristics of cloud water in southern China and insights into aqueous-phase processes from fourier transform ion cyclotron resonance mass spectrometry. Atmos. Chem. Phys. 2021, 21, 16631–16644. [Google Scholar] [CrossRef]
- Dominutti, P.A.; Renard, P.; Vaïtilingom, M.; Bianco, A.; Baray, J.-L.; Borbon, A.; Bourianne, T.; Burnet, F.; Colomb, A.; Delort, A.-M.; et al. Insights into tropical cloud chemistry in Réunion (Indian Ocean): Results from the BIO-MAÏDO campaign. Atmos. Chem. Phys. 2022, 22, 505–533. [Google Scholar] [CrossRef]
- Bianco, A.; Passananti, M.; Perroux, H.; Voyard, G.; Mouchel-Vallon, C.; Chaumerliac, N.; Mailhot, G.; Deguillaume, L.; Brigante, M. A better understanding of hydroxyl radical photochemical sources in cloud waters collected at the Puy de Dôme station—Experimental versus modelled formation rates. Atmos. Chem. Phys. 2015, 15, 9191–9202. [Google Scholar] [CrossRef] [Green Version]
- Aleksic, N.; Roy, K.; Sistla, G.; Dukett, J.; Houck, N.; Casson, P. Analysis of Cloud and precipitation chemistry at Whiteface Mountain, NY. Atmos. Environ. 2009, 43, 2709–2716. [Google Scholar] [CrossRef]
- Herckes, P.; Valsaraj, K.T.; Collett, J.L. A review of observations of organic matter in fogs and clouds: Origin, processing and fate. Atmos. Res. 2013, 132–133, 434–449. [Google Scholar] [CrossRef]
- Altieri, K.E.; Hastings, M.G.; Peters, A.J.; Sigman, D.M. Molecular characterization of water soluble organic nitrogen in marine rainwater by ultra-high resolution electrospray ionization mass spectrometry. Atmos. Chem. Phys. 2012, 12, 3557–3571. [Google Scholar] [CrossRef] [Green Version]
- Altieri, K.E.; Turpin, B.J.; Seitzinger, S.P. Composition of dissolved organic nitrogen in continental precipitation investigated by ultra-high resolution FT-ICR mass spectrometry. Environ. Sci. Technol. 2009, 43, 6950–6955. [Google Scholar] [CrossRef]
- Kourtchev, I.; Fuller, S.J.; Giorio, C.; Healy, R.M.; Wilson, E.; O’Connor, I.; Wenger, J.C.; McLeod, M.; Aalto, J.; Ruuskanen, T.M.; et al. Molecular composition of biogenic secondary organic aerosols using ultrahigh-resolution mass spectrometry: Comparing laboratory and field studies. Atmos. Chem. Phys. 2014, 14, 2155–2167. [Google Scholar] [CrossRef] [Green Version]
- Kourtchev, I.; Fuller, S.; Aalto, J.; Ruuskanen, T.M.; McLeod, M.W.; Maenhaut, W.; Jones, R.; Kulmala, M.; Kalberer, M. Molecular composition of boreal forest aerosol from Hyytiälä, Finland, using ultrahigh resolution mass spectrometry. Environ. Sci. Technol. 2013, 47, 4069–4079. [Google Scholar] [CrossRef]
- An, Y.; Xu, J.; Feng, L.; Zhang, X.; Liu, Y.; Kang, S.; Jiang, B.; Liao, Y. Molecular characterization of organic aerosol in the Himalayas: Insight from ultra-high-resolution mass spectrometry. Atmos. Chem. Phys. 2019, 19, 1115–1128. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Feng, Y.; Chen, J.; Xie, Q.; Chen, S.; Sheng, M.; Zhong, S.; Wei, W.; Su, S.; Fu, P. Acidification impacts on the molecular composition of dissolved organic matter revealed by FT-ICR MS. Sci. Total Environ. 2022, 805, 150284. [Google Scholar] [CrossRef]
- Qi, Y.; Ma, C.; Chen, S.; Ge, J.; Hu, Q.; Li, S.-L.; Volmer, D.A.; Fu, P. Online liquid chromatography and FT-ICR MS enable advanced separation and profiling of organosulfates in dissolved organic matter. ACS EST Water 2021, 1, 1975–1982. [Google Scholar] [CrossRef]
- Xie, Q.; Su, S.; Chen, S.; Zhang, Q.; Yue, S.; Zhao, W.; Du, H.; Ren, H.; Wei, L.; Cao, D.; et al. Molecular characterization of size-segregated organic aerosols in the urban boundary layer in wintertime Beijing by FT-ICR MS. Faraday Discuss. 2021, 226, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Schum, S.K.; Brown, L.E.; Mazzoleni, L.R. MFAssignR: Molecular formula assignment software for ultrahigh resolution mass spectrometry analysis of environmental complex mixtures. Environ. Res. 2020, 191, 110114. [Google Scholar] [CrossRef] [PubMed]
- Soule, M.C.K.; Longnecker, K.; Giovannoni, S.J.; Kujawinski, E.B. Impact of instrument and experiment parameters on reproducibility of ultrahigh resolution ESI FT-ICR mass spectra of natural organic matter. Org. Geochem. 2010, 41, 725–733. [Google Scholar] [CrossRef]
- He, C.; Zhang, Y.; Li, Y.; Zhuo, X.; Li, Y.; Zhang, C.; Shi, Q. In-house standard method for molecular characterization of dissolved organic matter by FT-ICR mass spectrometry. ACS Omega 2020, 5, 11730–11736. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, J.A.; D’Andrilli, J.; Agar, J.N.; Barrow, M.P.; Berg, S.M.; Catalán, N.; Chen, H.; Chu, R.K.; Cole, R.B.; Dittmar, T.; et al. An international laboratory comparison of dissolved organic matter composition by high resolution mass spectrometry: Are We getting the same answer? Limnol. Oceanogr. Methods 2020, 18, 235–258. [Google Scholar] [CrossRef]
- Baray, J.-L.; Deguillaume, L.; Colomb, A.; Sellegri, K.; Freney, E.; Rose, C.; Van Baelen, J.; Pichon, J.-M.; Picard, D.; Fréville, P.; et al. Cézeaux-Aulnat-Opme-Puy De Dôme: A multi-site for the long-term survey of the tropospheric composition and climate change. Atmos. Meas. Tech. 2020, 13, 3413–3445. [Google Scholar] [CrossRef]
- Renard, P.; Bianco, A.; Baray, J.-L.; Bridoux, M.; Delort, A.-M.; Deguillaume, L. Classification of clouds sampled at the Puy de Dôme Station (France) based on chemical measurements and air mass history matrices. Atmosphere 2020, 11, 732. [Google Scholar] [CrossRef]
- Bianco, A.; Riva, M.; Baray, J.-L.; Ribeiro, M.; Chaumerliac, N.; George, C.; Bridoux, M.; Deguillaume, L. Chemical characterization of cloudwater collected at Puy de Dôme by FT-ICR MS reveals the presence of SOA components. ACS Earth Space Chem. 2019, 3, 2076–2087. [Google Scholar] [CrossRef]
- Palacio Lozano, D.C.; Gavard, R.; Arenas-Diaz, J.P.; Thomas, M.J.; Stranz, D.D.; Mejía-Ospino, E.; Guzman, A.; Spencer, S.E.F.; Rossell, D.; Barrow, M.P. Pushing the analytical limits: New insights into complex mixtures using mass spectra segments of constant ultrahigh resolving power. Chem. Sci. 2019, 10, 6966–6978. [Google Scholar] [CrossRef] [Green Version]
- Mazzoleni, L.R.; Saranjampour, P.; Dalbec, M.M.; Samburova, V.; Hallar, A.G.; Zielinska, B.; Lowenthal, D.H.; Kohl, S. Identification of water-soluble organic carbon in non-urban aerosols using ultrahigh-resolution FT-ICR mass spectrometry: Organic anions. Environ. Chem. 2012, 9, 285. [Google Scholar] [CrossRef]
- Dzepina, K.; Mazzoleni, C.; Fialho, P.; China, S.; Zhang, B.; Owen, R.C.; Helmig, D.; Hueber, J.; Kumar, S.; Perlinger, J.A.; et al. Molecular characterization of free tropospheric aerosol collected at the Pico Mountain observatory: A case study with a long-range transported biomass burning plume. Atmos. Chem. Phys. 2015, 15, 5047–5068. [Google Scholar] [CrossRef] [Green Version]
- Schum, S.K.; Zhang, B.; Džepina, K.; Fialho, P.; Mazzoleni, C.; Mazzoleni, L.R. Molecular and physical characteristics of aerosol at a remote free troposphere site: Implications for atmospheric aging. Atmos. Chem. Phys. 2018, 18, 14017–14036. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Xie, Q.; Wang, J.-J.; He, D.; Bao, H.; Fu, Q.-L.; Su, S.; Sheng, M.; Li, S.-L.; Volmer, D.A.; et al. Deciphering dissolved organic matter by Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS): From bulk to fractions and individuals. Carbon Res. 2022, 1, 3. [Google Scholar] [CrossRef]
- Leyva, D.; Tose, L.V.; Porter, J.; Wolff, J.; Jaffé, R.; Fernandez-Lima, F. Understanding the structural complexity of dissolved organic matter: Isomeric diversity. Faraday Discuss. 2019, 218, 431–440. [Google Scholar] [CrossRef]
- Giannopoulos, K.; Benettoni, P.; Holbrook, T.R.; Reemtsma, T.; Wagner, S.; Lechtenfeld, O.J. Direct analysis of fulvic acids adsorbed onto capped gold nanoparticles by laser desorption ionization fourier-transform ion cyclotron resonance mass spectrometry. Environ. Sci. Nano 2021, 8, 2336–2346. [Google Scholar] [CrossRef]
- Koch, B.P.; Kattner, G.; Witt, M.; Passow, U. Molecular insights into the microbial formation of marine dissolved organic matter: Recalcitrant or labile? Biogeosciences 2014, 11, 4173–4190. [Google Scholar] [CrossRef] [Green Version]
- Koch, B.P.; Witt, M.; Engbrodt, R.; Dittmar, T.; Kattner, G. Molecular formulae of marine and terrigenous dissolved organic matter detected by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Geochim. Cosmochim. Acta 2005, 69, 3299–3308. [Google Scholar] [CrossRef]
- Koch, B.P.; Dittmar, T. From mass to structure: An aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun. Mass Spectrom. 2006, 20, 926–932. [Google Scholar] [CrossRef] [Green Version]
- De Vijlder, T.; Valkenborg, D.; Lemière, F.; Romijn, E.P.; Laukens, K.; Cuyckens, F. A Tutorial in small molecule identification via electrospray ionization-mass spectrometry: The practical art of structural elucidation. Mass Spectrom. Rev. 2018, 37, 607–629. [Google Scholar] [CrossRef]
- Zielinski, A.T.; Kourtchev, I.; Bortolini, C.; Fuller, S.J.; Giorio, C.; Popoola, O.A.M.; Bogialli, S.; Tapparo, A.; Jones, R.L.; Kalberer, M. A new processing scheme for ultra-high resolution direct infusion mass spectrometry data. Atmos. Environ. 2018, 178, 129–139. [Google Scholar] [CrossRef]
- Patriarca, C.; Hawkes, J.A. High molecular weight spectral interferences in mass spectra of dissolved organic matter. J. Am. Soc. Mass Spectrom. 2021, 32, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Abdul Jameel, A.G.; Alquaity, A.B.S.; Campuzano, F.; Emwas, A.-H.; Saxena, S.; Sarathy, S.M.; Roberts, W.L. Surrogate formulation and molecular characterization of sulfur species in vacuum residues using APPI and ESI FT-ICR mass spectrometry. Fuel 2021, 293, 120471. [Google Scholar] [CrossRef]

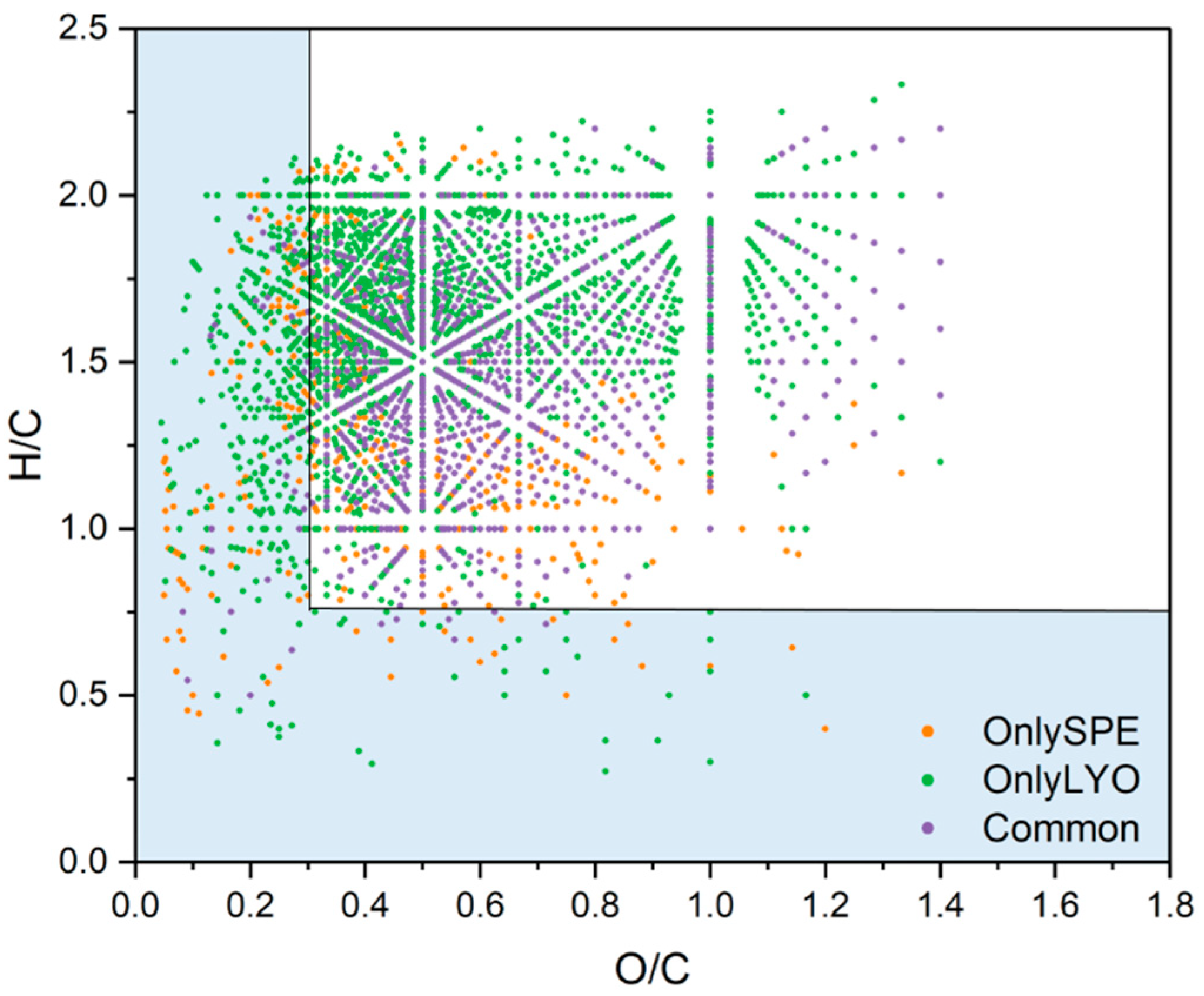
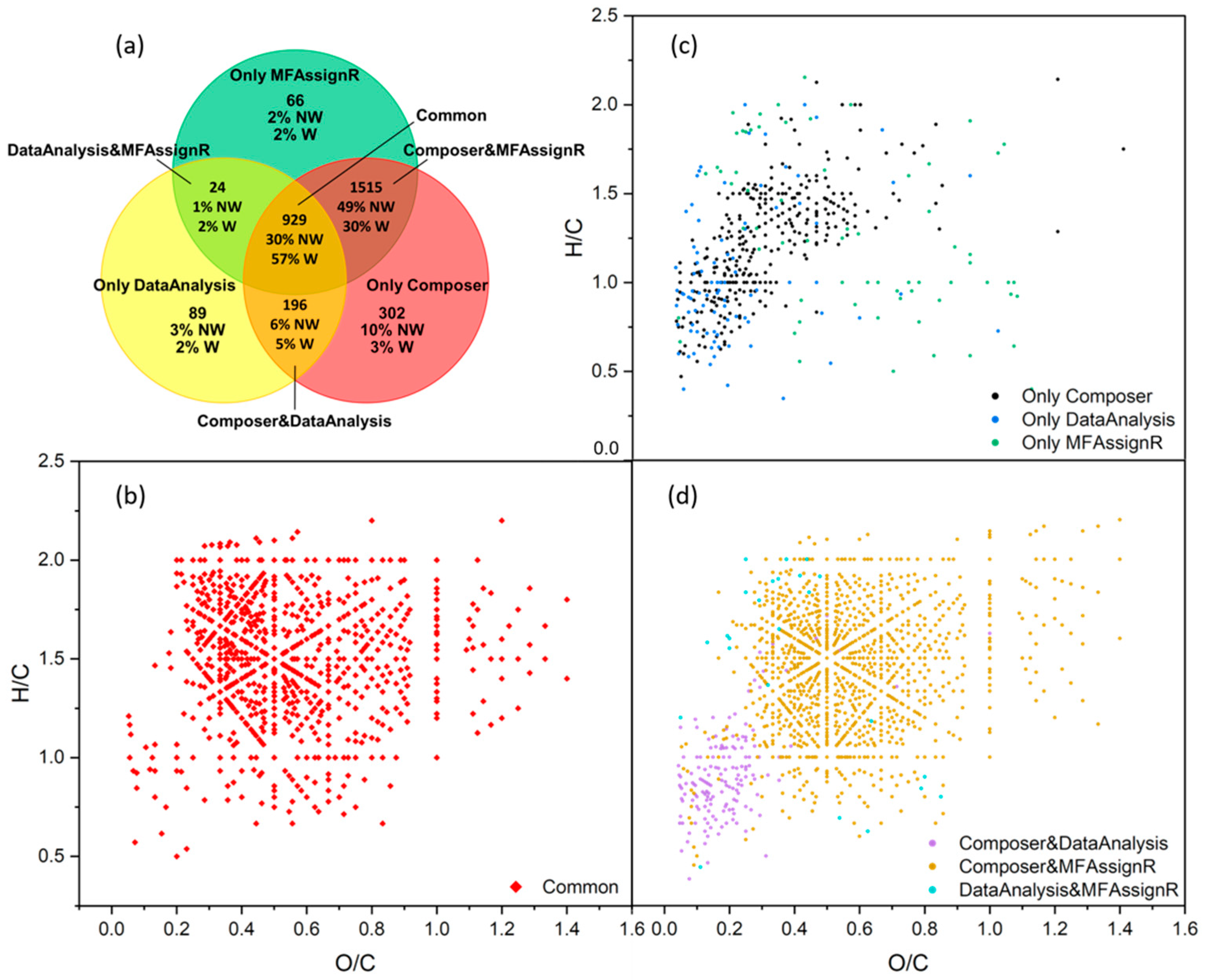

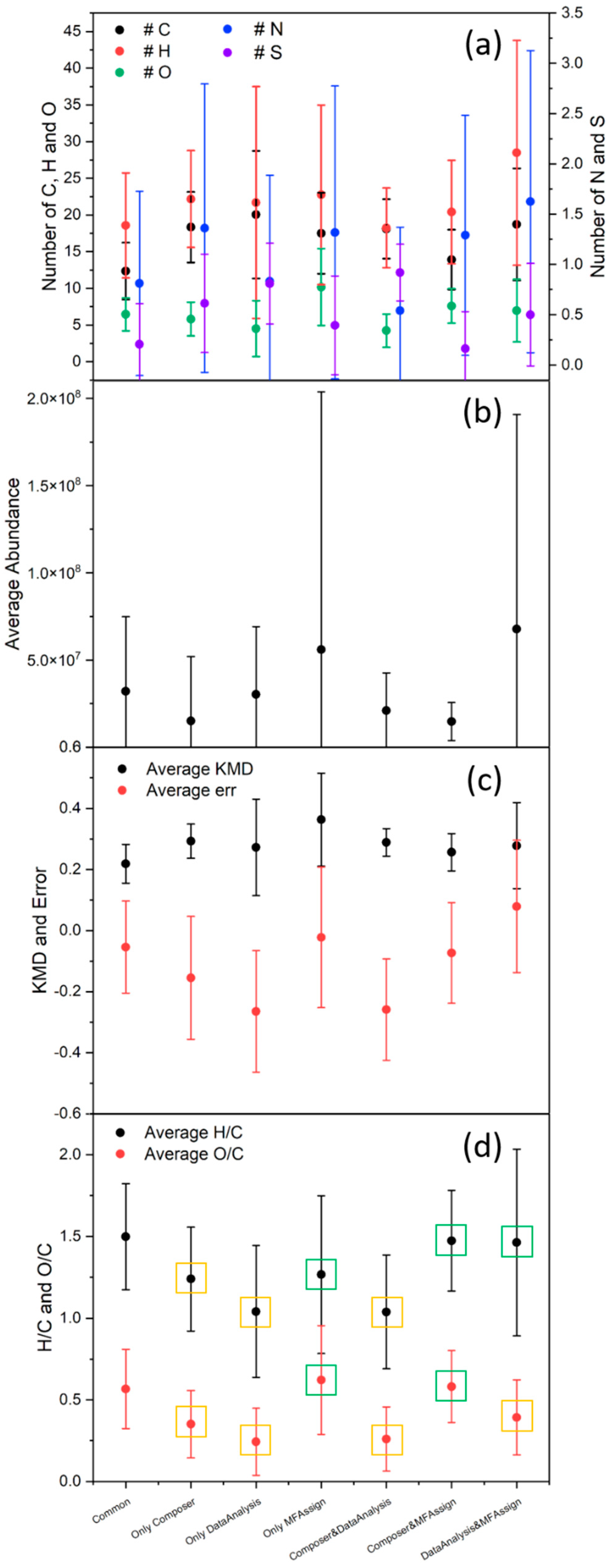
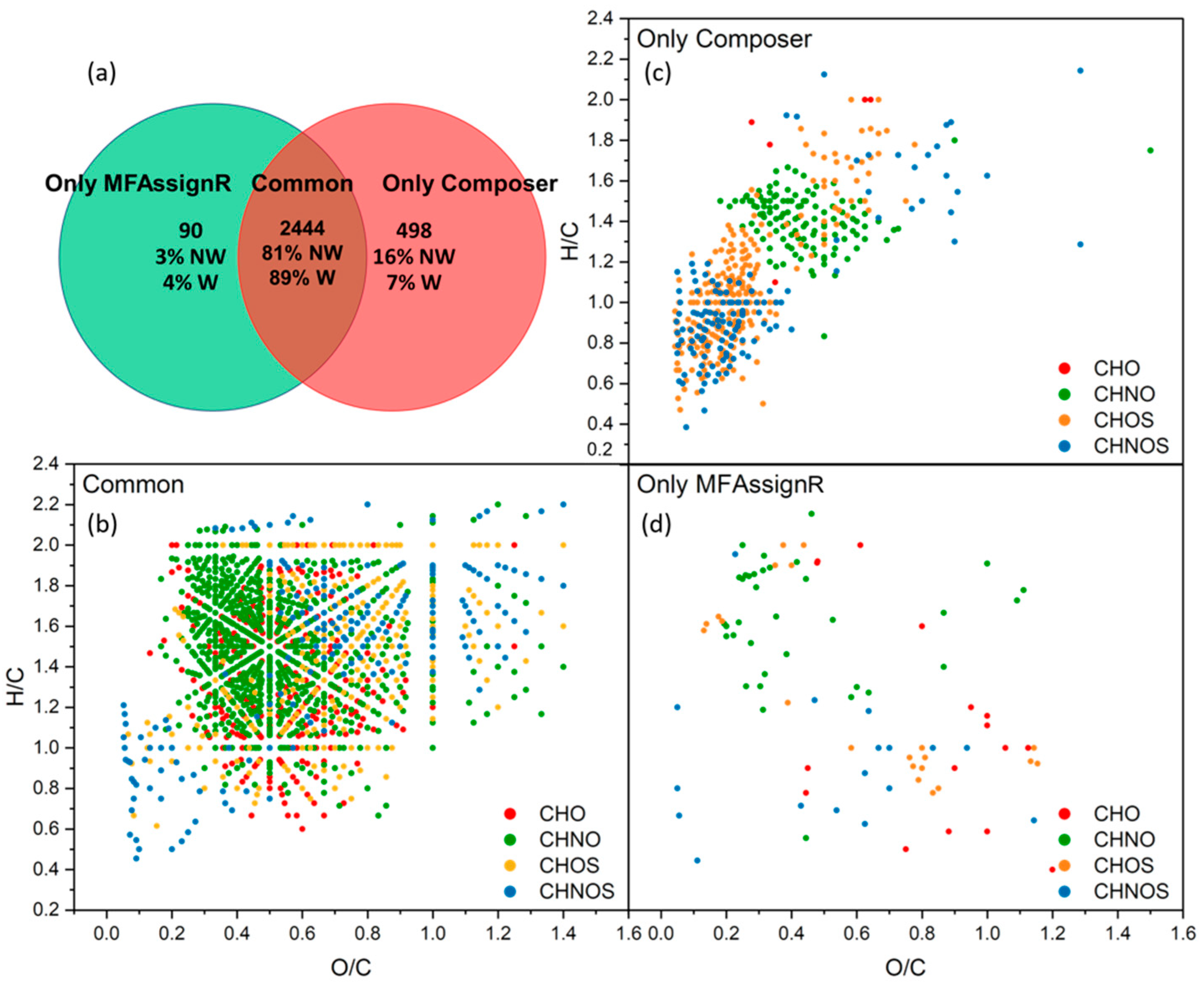
| Software Program | Fraction | Percentage Non-Weighted | Percentage Weighted |
|---|---|---|---|
| Composer | SPE | 26.6 | 23.2 |
| LYO | 32.9 | 30.3 | |
| Common | 40.5 | 46.4 | |
| MFAssignR | SPE | 22.7 | 24.4 |
| LYO | 37.7 | 29.7 | |
| Common | 39.6 | 45.9 | |
| DataAnalysis | SPE | 24.9 | 24.0 |
| LYO | 53.9 | 45.1 | |
| Common | 21.2 | 30.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pailler, L.; Renard, P.; Nicol, E.; Deguillaume, L.; Bianco, A. How Well Do We Handle the Sample Preparation, FT-ICR Mass Spectrometry Analysis, and Data Treatment of Atmospheric Waters? Molecules 2022, 27, 7796. https://doi.org/10.3390/molecules27227796
Pailler L, Renard P, Nicol E, Deguillaume L, Bianco A. How Well Do We Handle the Sample Preparation, FT-ICR Mass Spectrometry Analysis, and Data Treatment of Atmospheric Waters? Molecules. 2022; 27(22):7796. https://doi.org/10.3390/molecules27227796
Chicago/Turabian StylePailler, Lucas, Pascal Renard, Edith Nicol, Laurent Deguillaume, and Angelica Bianco. 2022. "How Well Do We Handle the Sample Preparation, FT-ICR Mass Spectrometry Analysis, and Data Treatment of Atmospheric Waters?" Molecules 27, no. 22: 7796. https://doi.org/10.3390/molecules27227796
APA StylePailler, L., Renard, P., Nicol, E., Deguillaume, L., & Bianco, A. (2022). How Well Do We Handle the Sample Preparation, FT-ICR Mass Spectrometry Analysis, and Data Treatment of Atmospheric Waters? Molecules, 27(22), 7796. https://doi.org/10.3390/molecules27227796






