Matrix-Specific Effects on Caffeine and Chlorogenic Acid Complexation in a Novel Extract of Whole Coffea arabica Coffee Cherry by NMR Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. 1H NMR Spectra of CEWCCE
2.2. 1H NMR Studies of Solutions of Caffeine and Chlorogenic Acids
3. Materials and Method
3.1. Materials
3.2. Chemicals
3.3. NMR Spectroscopy
3.4. Sample Preparation for NMR Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bastian, F.; Hutabarat, O.S.; Dirpan, A.; Nainu, F.; Harapan, H.; Emran, T.B.; Simal-Gandara, J. From plantation to cup: Changes in bioactive compounds during coffee processing. Foods 2021, 10, 2827. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Pourshahidi, L.K.; Navarini, L.; Petracco, M.; Strain, J.J. A comprehensive overview of the risks and benefits of coffee consumption. Comp. Rev. Food Sci. Food Saf. 2016, 15, 671–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yang, H.; Li, S.; Li, W.-D.; Wang, Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: A cohort study in the UK Biobank. PLOS Med. 2021, 18, e1003830. [Google Scholar] [CrossRef] [PubMed]
- Abalo, R. Coffee and caffeine consumption for human health. Nutrients 2021, 13, 2918. [Google Scholar] [CrossRef]
- Eskelinen, M.H.; Kivipelto, M. Caffeine as a protective factor in dementia and Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Prediger, R.D.S. Effects of caffeine in Parkinson’s disease: From neuroprotection to the management of motor and non-motor symptoms. J. Alzheimer’s Dis. 2010, 20, S205–S220. [Google Scholar] [CrossRef] [Green Version]
- Nehlig, A.; Daval, J.-L.; Debry, G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 1992, 17, 139–170. [Google Scholar] [CrossRef]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef]
- Winston, A.P.; Hardwick, E.; Jaberi, N. Neuropsychiatric effects of caffeine. Adv. Psychiatr. Treat. 2005, 11, 432–439. [Google Scholar] [CrossRef]
- Spilling the Beans: How Much Caffeine is Too Much? Available online: https://www.fda.gov/consumers/consumer-updates/spilling-beans-how-much-caffeine-too-much (accessed on 11 October 2022).
- Liang, N.; Kitts, D.D. Role of Chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Dellalibera, O.; Lemaire, B.; Lafay, S. Svetol®, green coffee extract, induces weight loss and increases the lean to fat mass ratio in volunteers with overweight problem. Phytotherapie 2006, 4, 194–197. [Google Scholar] [CrossRef]
- Robinson, J.L.; Yanes, J.A.; Reid, M.A.; Murphy, J.E.; Busler, J.N.; Mumford, P.W.; Young, K.C.; Pietrzkowski, Z.J.; Nemzer, B.V.; Hunter, J.M.; et al. Neurophysiological effects of whole coffee cherry extract in older adults with subjective cognitive impairment: A randomized, double-blind, placebo-controlled, cross-over pilot study. Antioxidants 2021, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.F.; Loh, J.; Brown, P.H.; Macaulay, C.E.; Lyle, B.J. US 2012/0269913 A1. 2012. Available online: https://patentimages.storage.googleapis.com/fc/86/3e/d069a2491579f1/US20120269913A1.pdf (accessed on 11 November 2022).
- Nemzer, B.; Kalita, D.; Abshiru, N. Quantification of major bioactive constituents, antioxidant activity, and enzyme inhibitory effects of whole coffee cherries (Coffea arabica) and their extracts. Molecules 2021, 26, 4306. [Google Scholar] [CrossRef] [PubMed]
- Fritzsche, H.; Petri, I.; Schütz, H.; Weller, K.; Sedmera, P.; Lang, H. On the interaction of caffeine with nucleic acids. III. 1H NMR studies of caffeine--5’-adenosine monophosphate and caffeine-poly(riboadenylate) interactions. Biophys. Chem. 1980, 11, 109. [Google Scholar] [CrossRef]
- Ishizu, T.; Tsutsumi, H.; Sato, T. Mechanism of creaming down Based on chemical characterization of a complex of caffeine and tea catechins. Chem. Pharm. Bull. 2016, 64, 676. [Google Scholar] [CrossRef] [Green Version]
- Fouad, E.A.; El-Badry, M.; Alanazi, F.K.; Arafah, M.M.; Al-Ashban, R.; Alsarra, I.A. Preparation and investigation of acetyl salicylic acid-caffeine complex for rectal administration. Pharm. Dev. Technol. 2010, 15, 249. [Google Scholar] [CrossRef]
- Gorter, K. Beritage Zur Kenntnis des Kaffes. Ann. Chem. 1907, 359, 217. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.; Lilley, T.H.; Bailey, N.A.; Falshaw, C.P.; Haslam, H.; Magnolato, D.; Begley, M.J. Polyphenol-caffeine complexation. J. Chem. Soc. Chem. Commun. 1986, 105–106. [Google Scholar] [CrossRef]
- Horman, I.; Viani, R. The nature and conformation of the caffeine-chloroginate complex of coffee. J. Food Sci. 1972, 37, 925. [Google Scholar] [CrossRef]
- Mosli, S.; Waldhauser, M.; Baumann, T.W. Compartmentation of caffeine and related alkaloids depends exclusively on the physical chemistry of their vacuolar complex formation with the chlorogenic acids. Phytochemistry 1996, 42, 985–996. [Google Scholar]
- Mullen, W.; Nemzer, B.; Stalmach, A.; Ali, S.; Combet, E. Polyphenolic and hydroxycinnamate contents of whole coffee fruits from China, India, and Mexico. J. Agric. Food. Chem. 2013, 61, 5298–5309. [Google Scholar] [CrossRef] [Green Version]
- Nemzer, B.; Abshiru, N.; Al-Taher, F. Identification of phytochemical compounds in coffea arabica whole coffee cherries and their extracts by LC-MS/MS. J. Agric. Food Chem. 2021, 69, 3430–3438. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Surucu, B.; Kuhnert, N. Characterization by LC-MS(n) of four new classes of chlorogenic acids in green coffee beans: Dimethoxycinnamoylquinic acids, diferuloylquinic acids, caffeoyl-dimethoxycinnamoylquinic acids, and feruloyl-dimethoxycinnamoylquinic acids. J. Agric. Food Chem. 2006, 54, 1957–1969. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, N.; Papamokos, G.; Dreyer, J.; Carloni, P.; Navarini, L. NMR studies of hetero-association of caffeine with di-O-Caffeoylquinic acid isomers in aqueous solution. Food Biophys. 2014, 10, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amelio, N.; Fontanive, L.; Uggeri, F.; Liverani-Suggi, F.; Navarini, L. NMR investigation of the caffeine-chloroginate complex in aqueous solution and in coffee brews. Food Biophys. 2009, 4, 321–330. [Google Scholar] [CrossRef]
- Sitkowski, J.; Stefaniak, L.; Nicol, L.; Martin, L.; Martin, G.J.; Webb, G.A. Complete assignment of the 1H, 13C and 15N NMR spectra of caffeine. Spectrochim. Act 1995, 51, 839–841. [Google Scholar] [CrossRef]
- Wei, F.; Furihata, K.; Hu, F.; Miyakawa, T.; Tanokura, M. Complex mixture analysis of organic compounds in green coffee bean extract by two-dimensional NMR spectroscopy. Magn. Reson. Chem. 2010, 48, 857–865. [Google Scholar] [CrossRef]
- Martin, R.; Lilley, T.H.; Falshaw, C.P.; Haslam, E.; Begley, M.J.; Magnolato, D. The caffeine- potassium chlorogenate molecular complex. Phytochemistry 1987, 26, 273–279. [Google Scholar] [CrossRef]
- Okuom, M.O.; Wilson, M.V.; Jackon, A.; Holmes, A.E. Intermolecular interactions between eosin Y and caffeine using 1H NMR spectroscopy. Int. J. Spectrosc. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Kan, L.S.; Borer, P.N.; Cheng, D.M.; TS’O, P.O.P. 1H and 13C-NMR studies on caffeine and its interaction with nucleic acids. Biopolymers 1980, 19, 1641–1654. [Google Scholar] [CrossRef]
- Belay, A.; Kim, H.K.K.; Hwang, Y.H. Binding of caffeine with caffeic acid and chlorogenic acid using flurescence quenching, UV/vis and FTIR spectroscopic techniques. Luminescence 2016, 31, 565–572. [Google Scholar] [CrossRef]
- Choi, K.; Myoung, S.; Seo, Y.; Ahn, S. Quantitative NMR as a versatile tool for the reference material preparation. Magnetochemistry 2021, 7, 15. [Google Scholar] [CrossRef]
- Pauli, G.F.; Chen, S.N.; Simmler, C.; Lankin, D.C.; Gödecke, T.; Jaki, B.U.; Friesen, J.B.; McAlpine, J.B.; Napolitano, J.G. Importance of purity evaluation and the potential of quantitative 1H NMR as a purity assay. J. Med. Chem. 2014, 57, 9220–9231. [Google Scholar] [CrossRef]





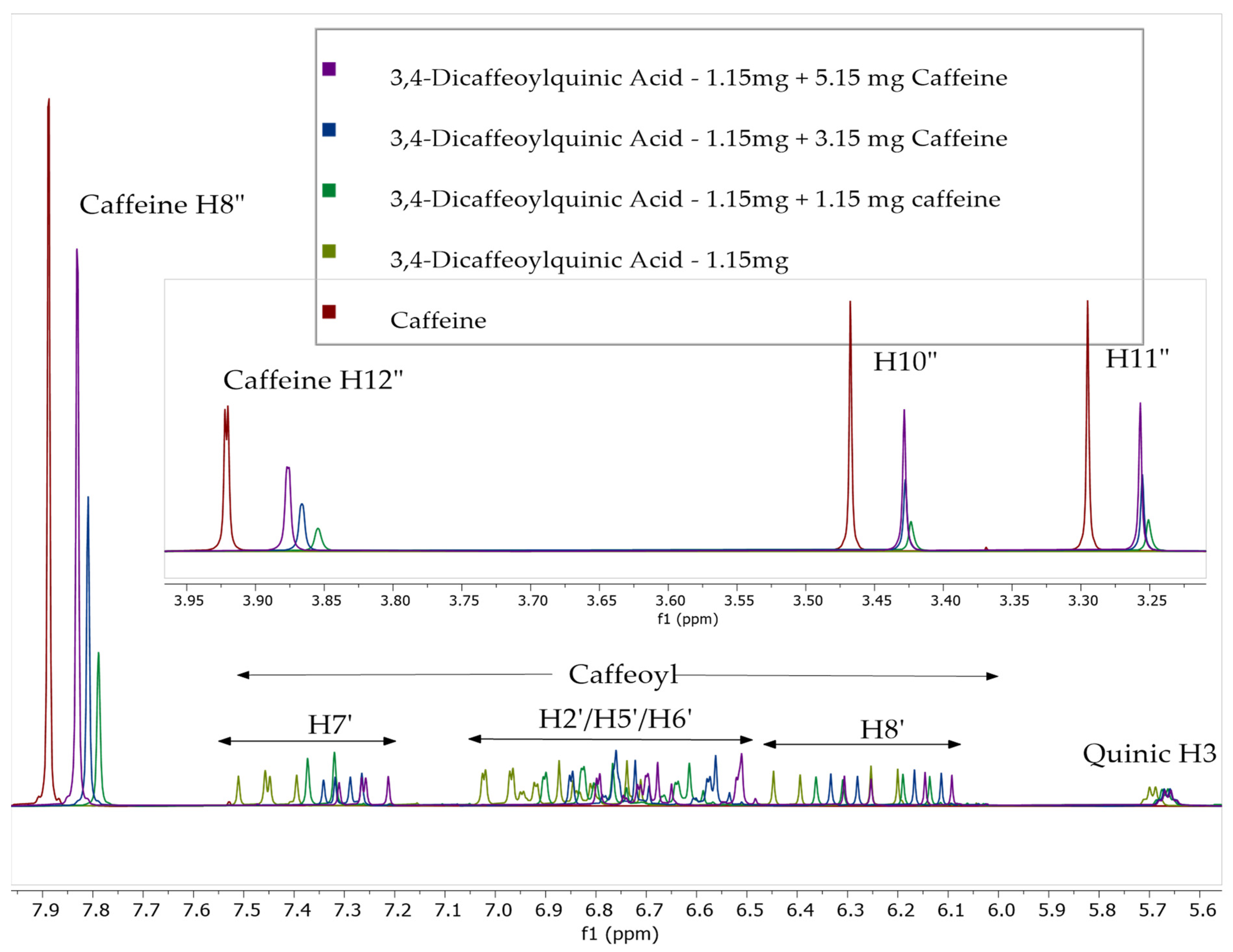




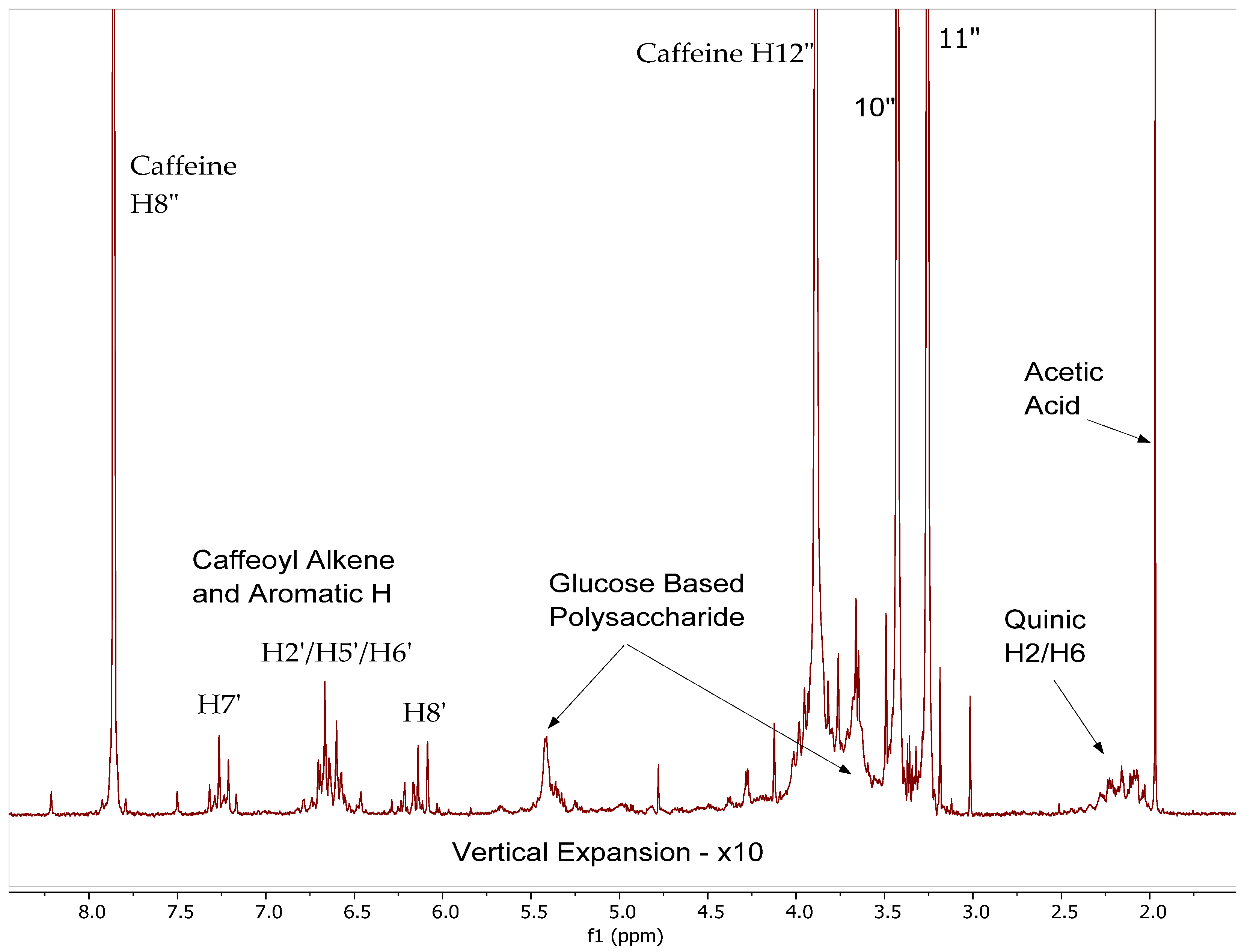

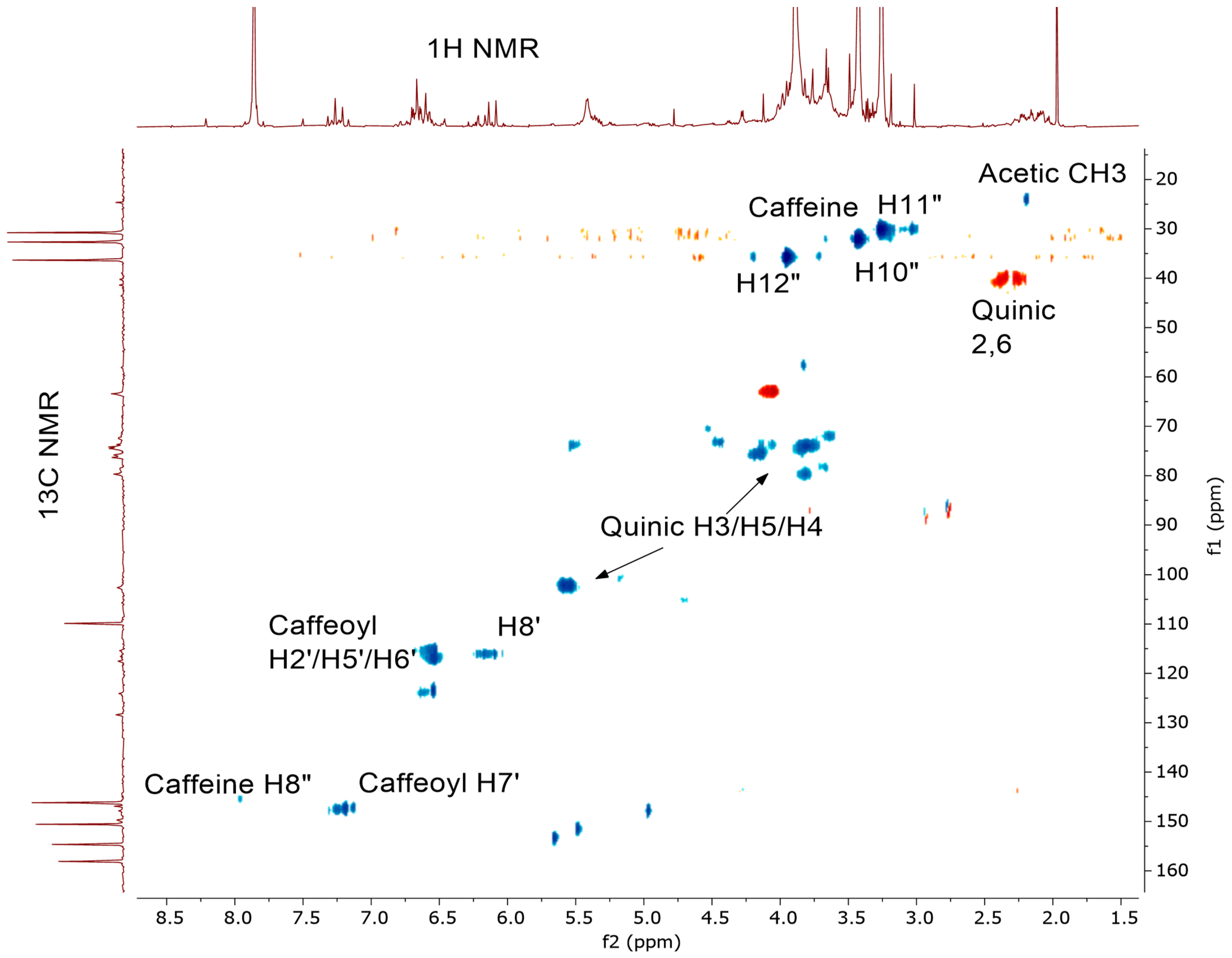

| Sample | Chemical Shift, ppm (Carbon Number and Type) | |||
|---|---|---|---|---|
| H8 (CH) | H12” (CH3) | H10” (CH3) | H11” (CH3) | |
| 1. GCBE (Caffeine, 99%) | 7.875 | 3.900 | 3.441 | 3.270 |
| 2. CEWCCE (70% Caffeine, 6% CA) | 7.859 | 3.887 | 3.426 | 3.256 |
| Chemical shift difference between 1 and 2 | 0.016 | 0.013 | 0.015 | 0.014 |
| 3. Mixture of Caffeine and CQA (natural extract) | 7.838 | 3.831 | 3.329 | 3.163 |
| Chemical shift difference between 1 and 3 | 0.037 | 0.069 | 0.112 | 0.107 |
| 4. Mixture of Caffeine and 5CQA (sigma) | 7.865 | 3.850 | 3.340 | 3.173 |
| Chemical shift difference between 1 and 4 | 0.010 | 0.050 | 0.101 | 0.097 |
| CEWCCE | Composition (%) |
|---|---|
| Caffeine | 73.25 |
| Chlorogenic acid | 7.63 |
| Glucose | 15.08 |
| Malic acid | 0.22 |
| Acetic acid | 0.47 |
| Lactic acid | 0.04 |
| Ethanol | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemzer, B.; Edwards, J.; Kalita, D. Matrix-Specific Effects on Caffeine and Chlorogenic Acid Complexation in a Novel Extract of Whole Coffea arabica Coffee Cherry by NMR Spectroscopy. Molecules 2022, 27, 7803. https://doi.org/10.3390/molecules27227803
Nemzer B, Edwards J, Kalita D. Matrix-Specific Effects on Caffeine and Chlorogenic Acid Complexation in a Novel Extract of Whole Coffea arabica Coffee Cherry by NMR Spectroscopy. Molecules. 2022; 27(22):7803. https://doi.org/10.3390/molecules27227803
Chicago/Turabian StyleNemzer, Boris, John Edwards, and Diganta Kalita. 2022. "Matrix-Specific Effects on Caffeine and Chlorogenic Acid Complexation in a Novel Extract of Whole Coffea arabica Coffee Cherry by NMR Spectroscopy" Molecules 27, no. 22: 7803. https://doi.org/10.3390/molecules27227803






