Protective Effect of Flavonoids against Methylglyoxal-Induced Oxidative Stress in PC-12 Neuroblastoma Cells and Its Structure–Activity Relationships
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of Flavonoids to PC-12 Cells
2.2. The Protective Activities and SAR Analysis of Different Flavonoids With Respect to the Damage Induced by MG
2.3. Molecular Docking Study
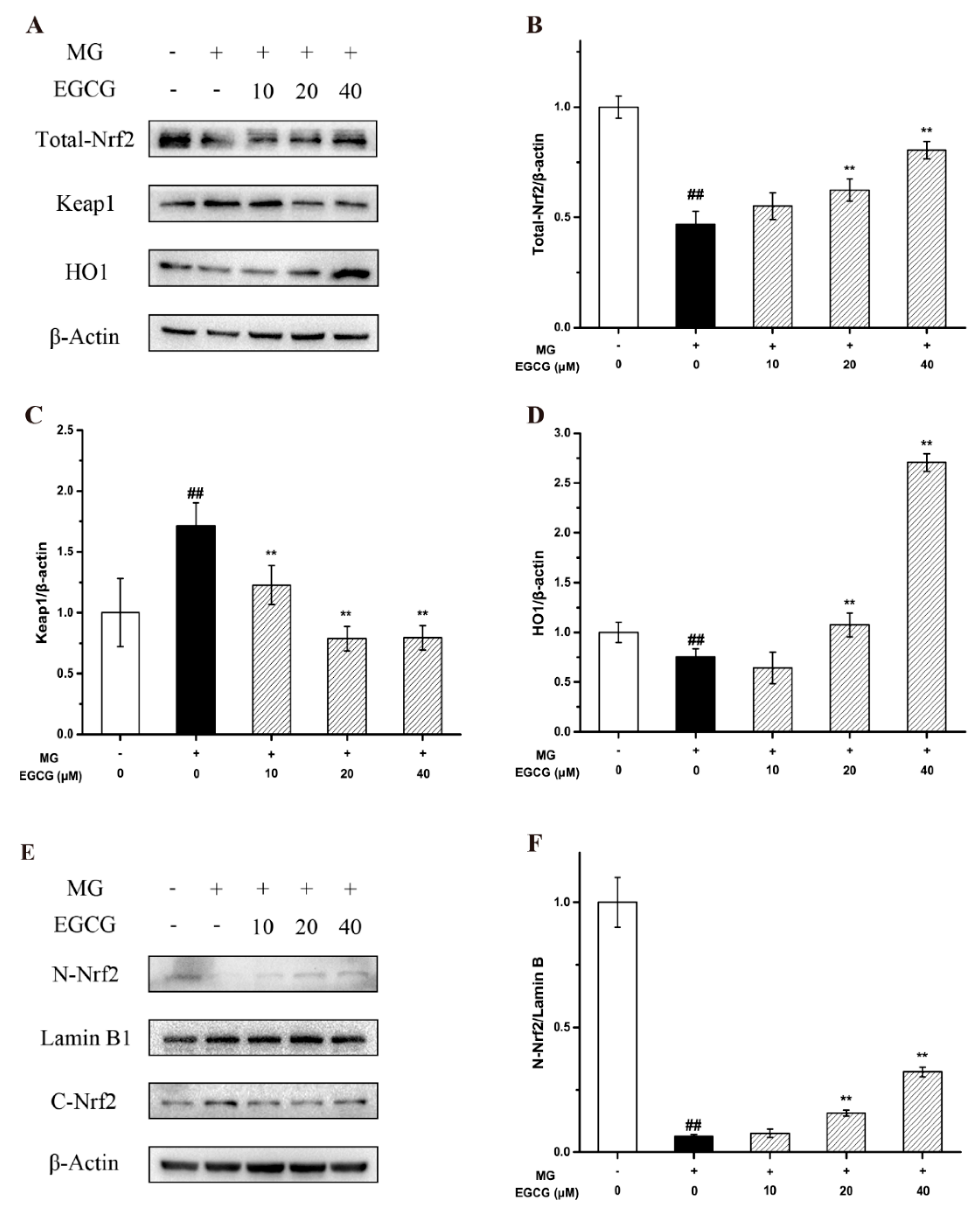
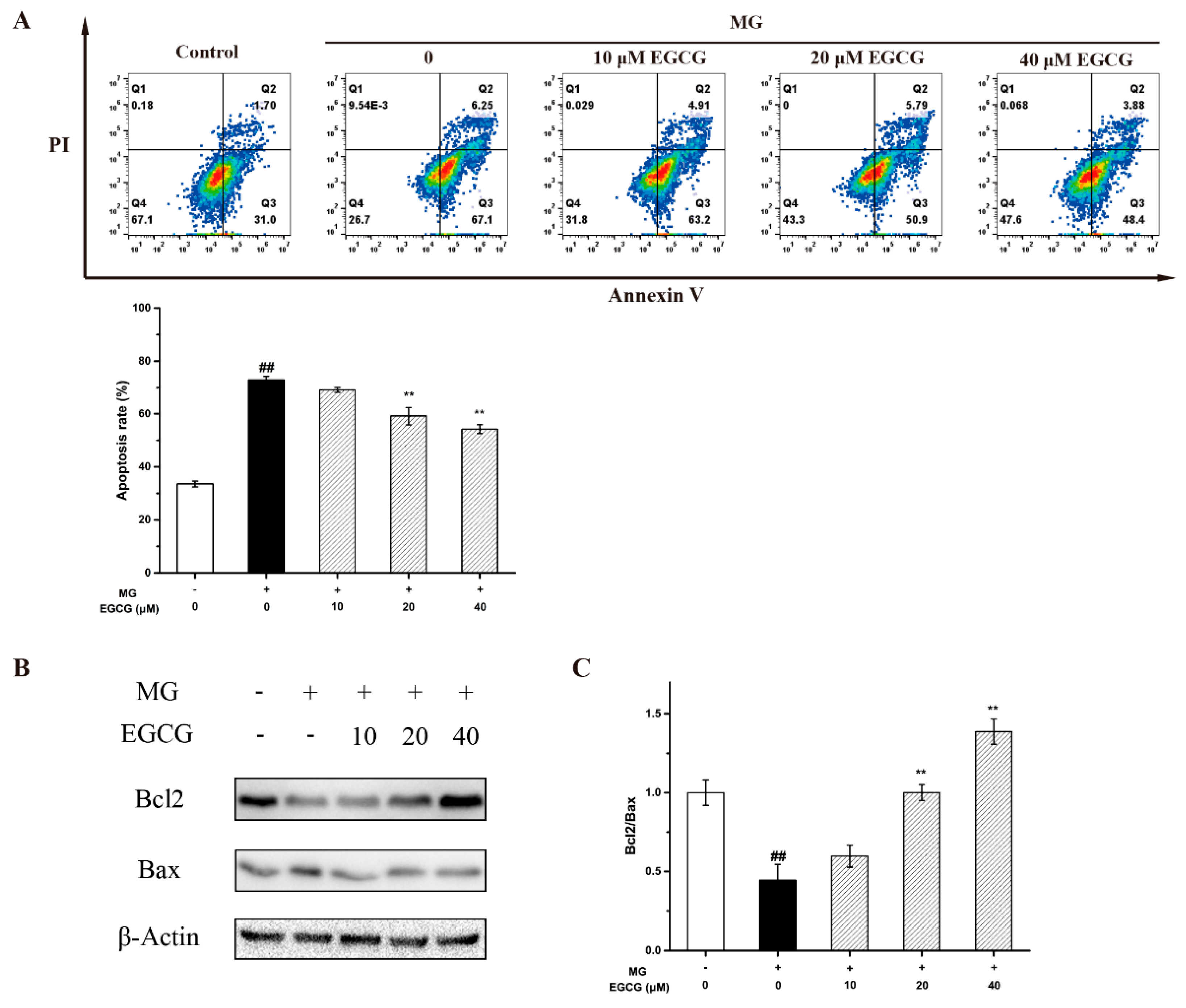
2.4. The Mechanism of EGCG on MG-Induced Oxidative Stress and Apoptosis in PC-12 Neuroblastoma Cells
3. Materials and Methods
3.1. Reagents and Antibodies
3.2. Cell Culture and Treatment
3.3. Analysis of Cell Viability
3.4. Western Blot Analysis
3.5. Molecular Docking
3.6. Flow Cytometry
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Northam, E.A.; Rankins, D.; Lin, A.; Wellard, R.M.; Pell, G.S.; Finch, S.J.; Werther, G.A.; Cameron, F.J. Central Nervous System Function in Youth with Type 1 Diabetes 12 Years After Disease Onset. Diabetes Care 2009, 32, 445–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhad, A.; Chopra, K. Curcumin attenuates diabetic encephalopathy in rats: Behavioral and biochemical evidences. Eur. J. Pharmacol. 2007, 576, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Peters, S.; Woodward, M.; Arango, S.M.; Huxley, R.R. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared with Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Chan, S.L.; de Souza-Pinto, N.C.; Slevin, J.R.; Wersto, R.P.; Zhan, M.; Mustafa, K.; de Cabo, R.; Mattson, M.P. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromol. Med. 2006, 8, 389–413. [Google Scholar] [CrossRef] [Green Version]
- Kierdorf, K.; Wang, Y.; Neumann, H. Immune-mediated CNS damage. Results Probl. Cell Differ. 2009, 51, 173–196. [Google Scholar]
- Sugimoto, K.; Nishizawa, Y.; Horiuchi, S.; Yagihashi, S. Localization in human diabetic peripheral nerve of Nɛ-carboxymethyllysine-protein adducts, an advanced glycation endproduct. Diabetologia 1997, 40, 1380–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arriba, S.; Stuchbury, G.; Yarin, J.; Burnell, J.; Münch, G. Methylglyoxal impairs glucose metabolism and leads to energy depletion in neuronal cells--protection by carbonyl scavengers. Neurobiol. Aging 2007, 28, 1044–1050. [Google Scholar] [CrossRef]
- Maessen, D.E.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 2015, 128, 839–861. [Google Scholar] [CrossRef]
- Palsamy, P.; Bidasee, K.R.; Ayaki, M.; Augusteyn, R.C.; Chan, J.Y.; Shinohara, T. Methylglyoxal induces endoplasmic reticulum stress and DNA demethylation in the Keap1 promoter of human lens epithelial cells and age-related cataracts. Free Radic. Biol. Med. 2014, 72, 134–148. [Google Scholar] [CrossRef] [Green Version]
- Ota, K.; Nakamura, J.; Li, W.; Kozakae, M.; Watarai, A.; Nakamura, N.; Yasuda, Y.; Nakashima, E.; Naruse, K.; Watabe, K.; et al. Metformin prevents methylglyoxal-induced apoptosis of mouse Schwann cells. Biochem. Biophys. Res. Commun. 2007, 357, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344, 109–116. [Google Scholar] [CrossRef]
- Liu, P.; Yin, Z.; Chen, M.; Huang, C.; Wu, Z.; Huang, J.; Ou, S.; Zheng, J. Cytotoxicity of adducts formed between quercetin and methylglyoxal in PC-12 cells. Food Chem. 2021, 352, 129424. [Google Scholar] [CrossRef] [PubMed]
- Mmk, A.; Smh, B.; Ams, C.; Ay, A. Ameliorate impacts of scopoletin against vancomycin-induced intoxication in rat model through modulation of Keap1-Nrf2/HO-1 and IκBα-P65 NF-κB/P38 MAPK signaling pathways: Molecular study, molecular docking evidence and network pharmacology analysis. Int. Immunopharmacol. 2021, 102, 108382. [Google Scholar]
- Su, J.; Yen, J.; Li, S.; Weng, C.; Lin, M.; Ho, C.; Wu, M. 3′,4′-Didemethylnobiletin induces phase II detoxification gene expression and modulates PI3K/Akt signaling in PC12 cells. Free. Radic. Biol. Med. 2012, 52, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, S. Oxidative metabolism deficiencies in brains of patients with Alzheimer’s disease. Acta Neurol. Scand. 2015, 94, 18–24. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef]
- Ponte, L.; Pavan, I.; Mancini, M.; Silva, L.D.; Morelli, A.; Severino, M.; Bezerra, R.; Simabuco, F. The Hallmarks of Flavonoids in Cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef]
- Xie, C.L.; Park, K.H.; Kang, S.S.; Cho, K.M.; Lee, D.H. Isoflavone-enriched soybean leaves attenuate ovariectomy-induced osteoporosis in rats by anti-inflammatory activity. J. Sci. Food Agric. 2020, 101, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Deng, Y.; Wang, J.; Zhou, M.; Liao, L.; Wang, C.; Peng, C.; Li, Y. Hydroxysafflor yellow A, a natural compound from Carthamus tinctorius L with good effect of alleviating atherosclerosis. Phytomedicine 2021, 91, 153694. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Structure-activity relationships of flavonoids in the cellular antioxidant activity assay. J. Agric. Food Chem. 2008, 56, 8404–8411. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.X.; Bai, R.F.; Zhao, Y.Q.; Li, J.; Wei, Z.M. Protective Effect of Grape Seed Procyanidins against H2O2 -Induced Oxidative Stress in PC-12 Neuroblastoma Cells: Structure-Activity Relationships. J. Food Sci. 2018, 83, 2622–2628. [Google Scholar] [CrossRef]
- Hwang, S.L.; Yen, G.C. Effect of Hesperetin against Oxidative Stress via ER- and TrkA-Mediated Actions in PC12 Cells. J. Agric. Food Chem. 2011, 59, 5779–5785. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liang, L.; Pan, H.; Hu, K.; Xiao, P. Dihydromyricetin ameliorates the oxidative stress response induced by methylglyoxal via the AMPK/GLUT4 signaling pathway in PC12 cells. Brain Res. Bull. 2014, 109, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Hu, R.D.; Lu, X.Y.; Ding, X.Y.; Zhang, S.J. Polyphenols from the flower of Hibiscus syriacus Linn ameliorate neuroinflammation in LPS-treated SH-SY5Y cell. Biomed. Pharmacother. 2020, 130, 110517. [Google Scholar] [CrossRef] [PubMed]
- Jnoff, E.; Albrecht, C.; Barker, J.J.; Barker, O.; Beaumont, E.; Bromidge, S.; Brookfield, F.; Brooks, M.; Bubert, C.; Ceska, T. Inside Cover: Binding Mode and Structure-Activity Relationships around Direct Inhibitors of the Nrf2-Keap1 Complex. ChemMedChem 2014, 9, 674. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Song, J.H.; Shin, M.; Hwang, G.S.; Oh, S.T.; Hwang, J.J.; Kang, K.S. Chebulinic acid attenuates glutamate-induced HT22 cell death by inhibiting oxidative stress, calcium influx and MAPKs phosphorylation. Bioorganic Med. Chem. Lett. 2018, 28, 249–253. [Google Scholar] [CrossRef]
- Xu, Y.; Hua, Z.; Zhu, Q. The Impact of Microbiota-Gut-Brain Axis on Diabetic Cognition Impairment. Front. Aging Neurosci. 2017, 9, 106. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Shi, J.J.; Fang, J.; Wang, Q.; Chen, Y.B.; Zhang, S.J. Quercetin ameliorates diabetic encephalopathy through SIRT1/ER stress pathway in db/db mice. Aging 2020, 12, 7015–7029. [Google Scholar] [CrossRef]
- Shyma, R.L.; Mini, S. Neuroprotective effect of Morin via TrkB/Akt pathway against diabetes mediated oxidative stress and apoptosis in neuronal cells. Toxicol. Mech. Methods 2022, 32, 695–704. [Google Scholar] [CrossRef]
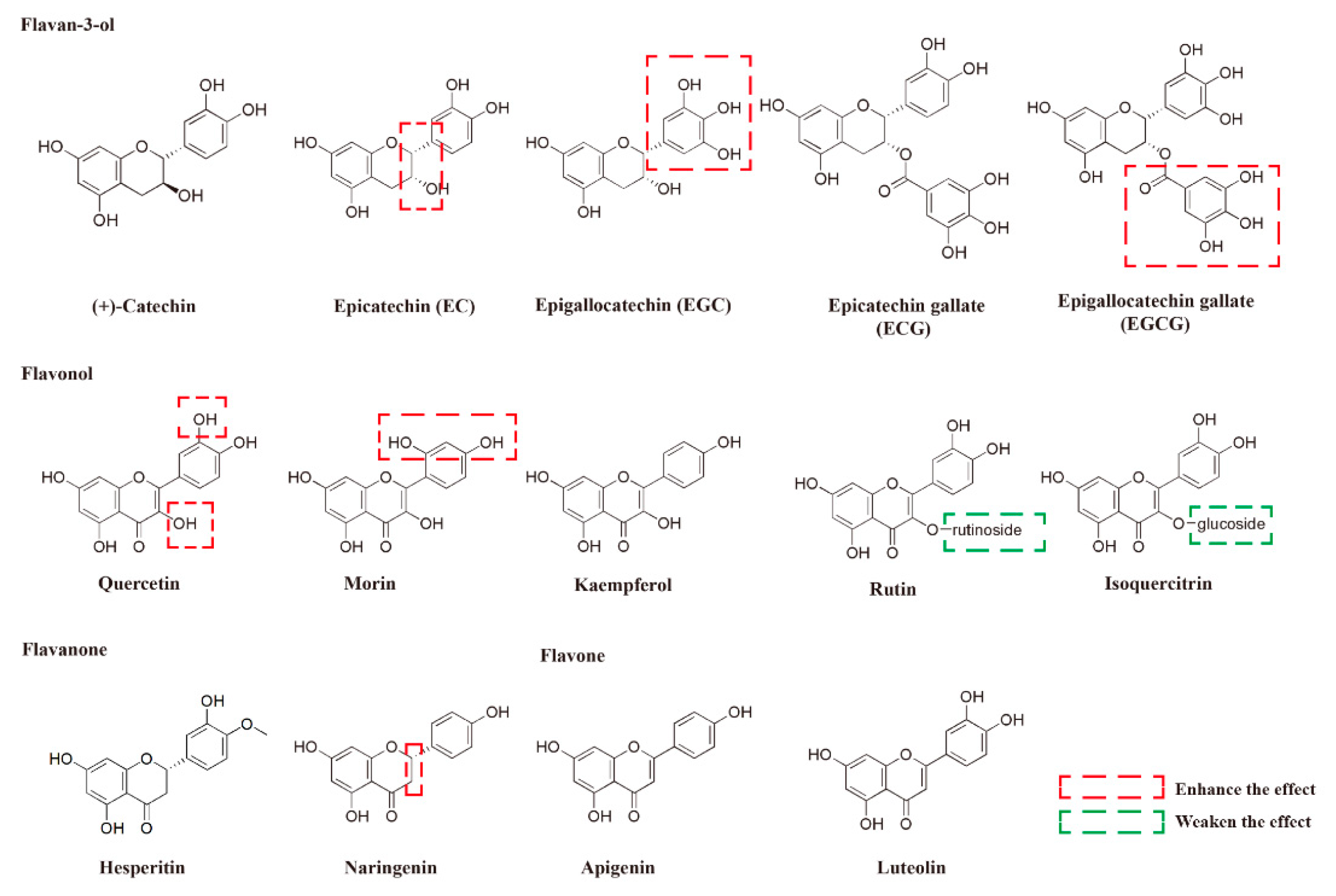
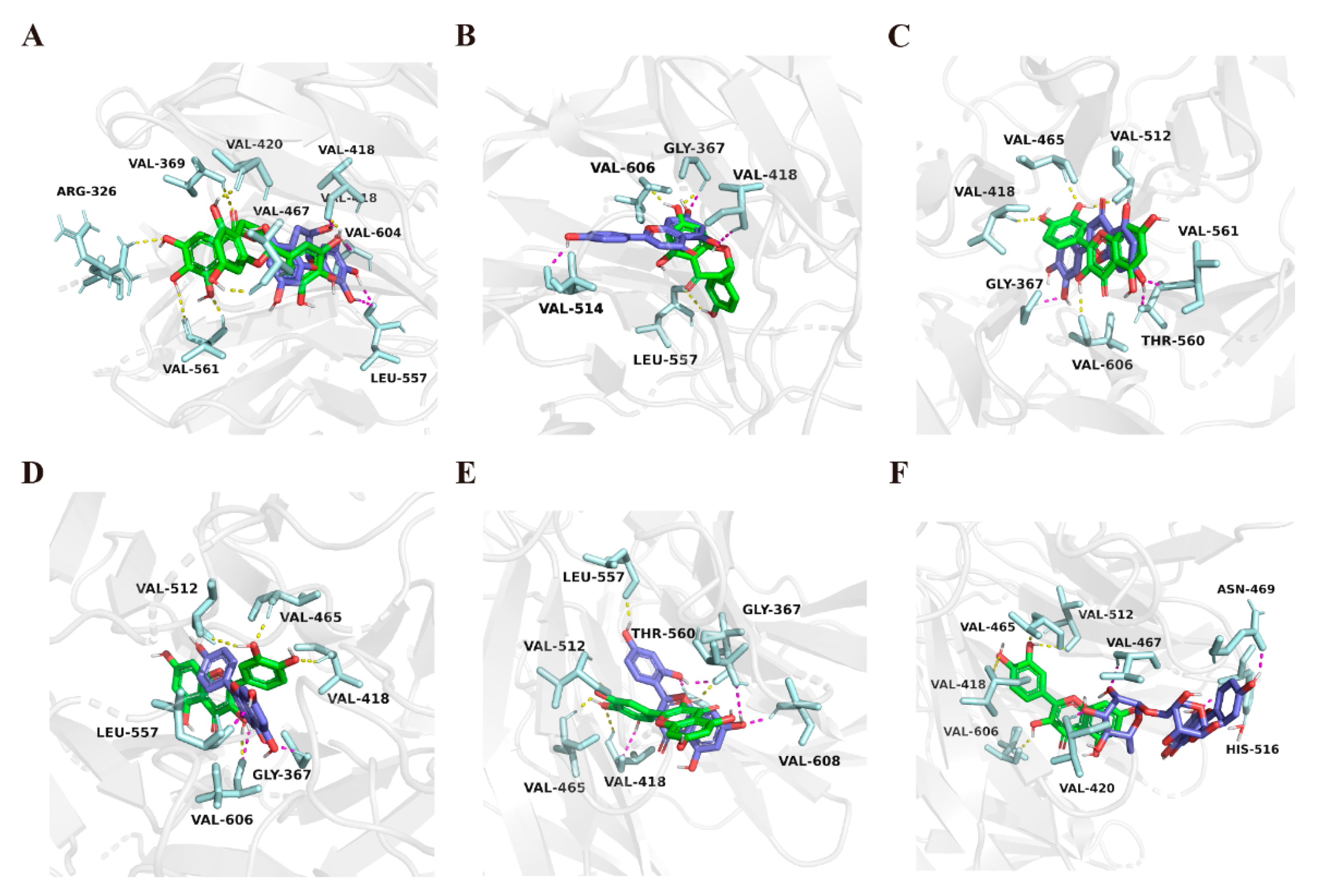
| Compound | EC50 (μM) | Binding Energy (kJ/mol) | Number of H-Bonds | |
|---|---|---|---|---|
| Flavan-3-ol | ||||
| (+)-Catechin | >100 | −24.811 | 3 | |
| EC | 52.34 ± 3.99 | −35.313 | 3 | |
| EGC | 43.06 ± 1.18 | −35.439 | 4 | |
| ECG | 34.52 ± 2.69 | −39.790 | 5 | |
| EGCG | 11.98 ± 0.49 | −40.041 | 8 | |
| Flavanone | ||||
| Naringenin | 13.35 ± 1.92 | −35.271 | 3 | |
| Hesperitin | 48.14 ± 2.31 | −34.058 | 3 | |
| Flavonol | ||||
| Kaempferol | >100 | −32.719 | 3 | |
| Quercetin | 28.83 ± 2.76 | −34.351 | 4 | |
| Morin | 14.83 ± 1.70 | −34.895 | 6 | |
| Isoquercitrin | >100 | −22.175 | 3 | |
| Rutin | >100 | −29.079 | 4 | |
| Flavone | ||||
| Luteolin | >100 | −33.932 | 3 | |
| Apigenin | >100 | −33.639 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Li, X.; He, X.; Xing, Y.; Jiang, B.; Xiu, Z.; Bao, Y.; Dong, Y. Protective Effect of Flavonoids against Methylglyoxal-Induced Oxidative Stress in PC-12 Neuroblastoma Cells and Its Structure–Activity Relationships. Molecules 2022, 27, 7804. https://doi.org/10.3390/molecules27227804
Zhang D, Li X, He X, Xing Y, Jiang B, Xiu Z, Bao Y, Dong Y. Protective Effect of Flavonoids against Methylglyoxal-Induced Oxidative Stress in PC-12 Neuroblastoma Cells and Its Structure–Activity Relationships. Molecules. 2022; 27(22):7804. https://doi.org/10.3390/molecules27227804
Chicago/Turabian StyleZhang, Danyang, Xia Li, Xiaoshi He, Yan Xing, Bo Jiang, Zhilong Xiu, Yongming Bao, and Yuesheng Dong. 2022. "Protective Effect of Flavonoids against Methylglyoxal-Induced Oxidative Stress in PC-12 Neuroblastoma Cells and Its Structure–Activity Relationships" Molecules 27, no. 22: 7804. https://doi.org/10.3390/molecules27227804
APA StyleZhang, D., Li, X., He, X., Xing, Y., Jiang, B., Xiu, Z., Bao, Y., & Dong, Y. (2022). Protective Effect of Flavonoids against Methylglyoxal-Induced Oxidative Stress in PC-12 Neuroblastoma Cells and Its Structure–Activity Relationships. Molecules, 27(22), 7804. https://doi.org/10.3390/molecules27227804






