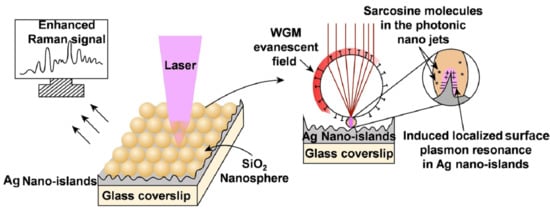
Silica Nanospheres Coated Silver Islands as an Effective Opto-Plasmonic SERS Active Platform for Rapid and Sensitive Detection of Prostate Cancer Biomarkers
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Instrumentation and Methods
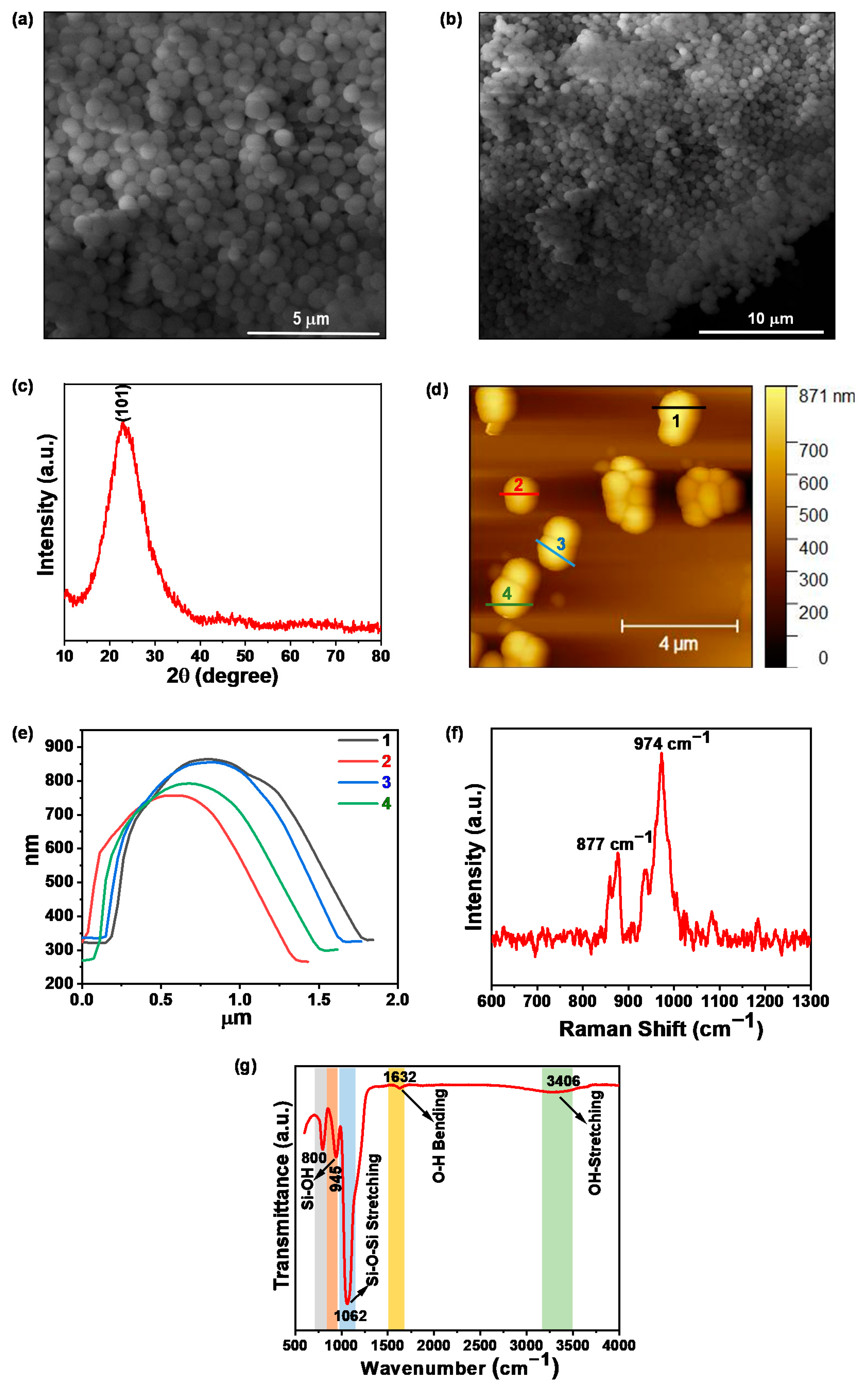
2.2.1. Synthesis of SiO2 Nanospheres
2.2.2. Fabrication of SERS Substrate
2.2.3. SERS Measurements
3. Results and Discussion
3.1. Characterization of As-Synthesized SiO2 Nanoparticles
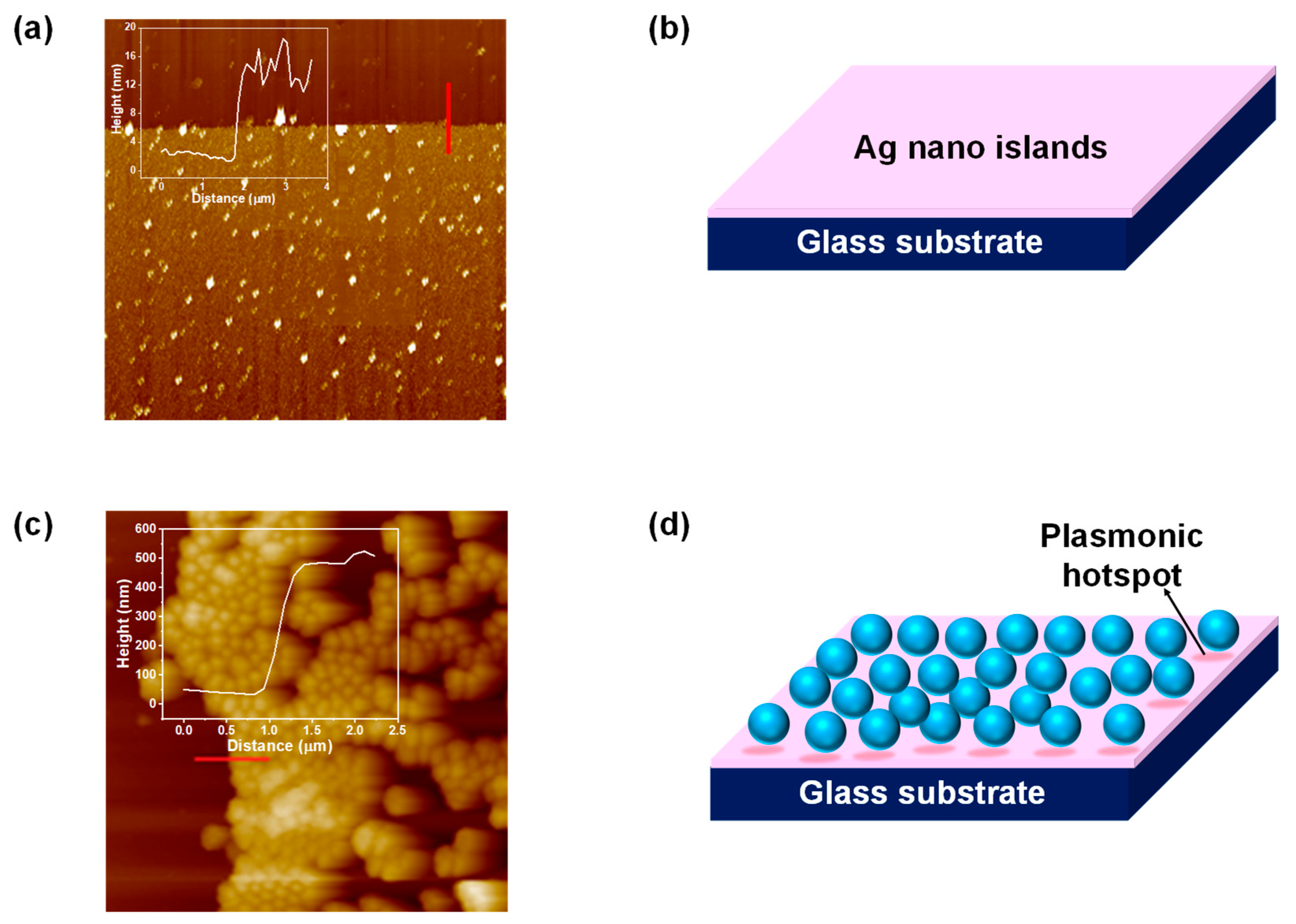
3.2. AFM analysis of Deposited Silver Nano-Islands
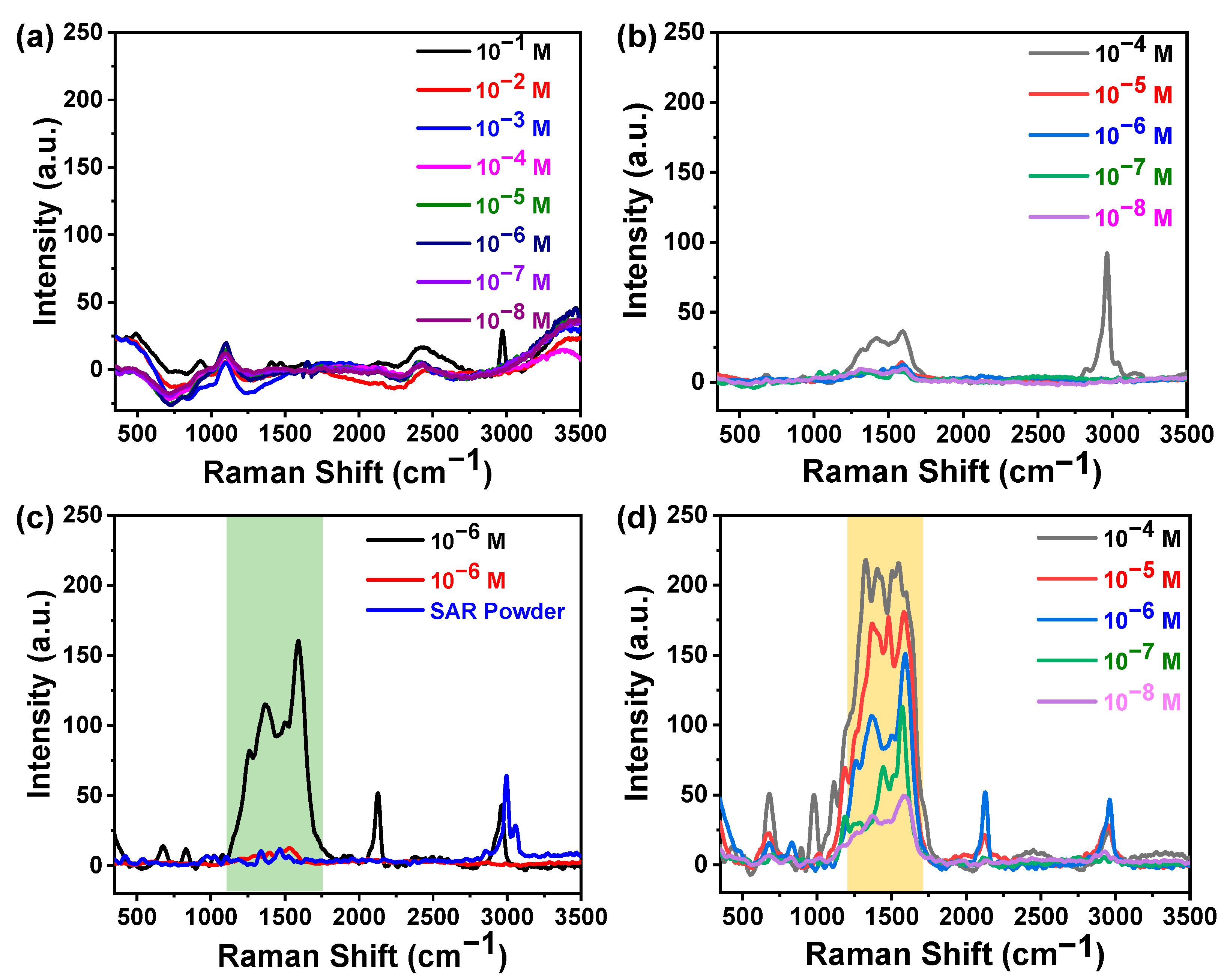
3.3. Calibration Curve
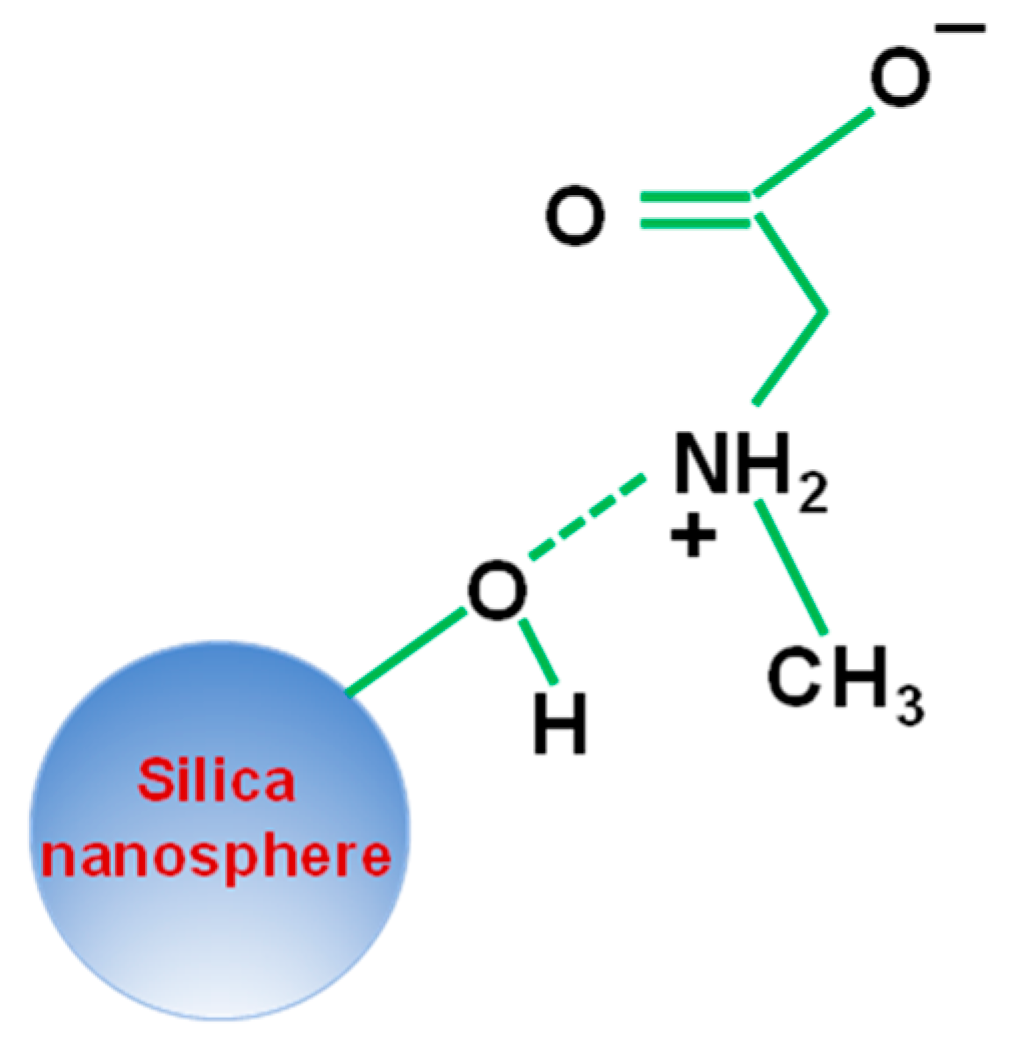
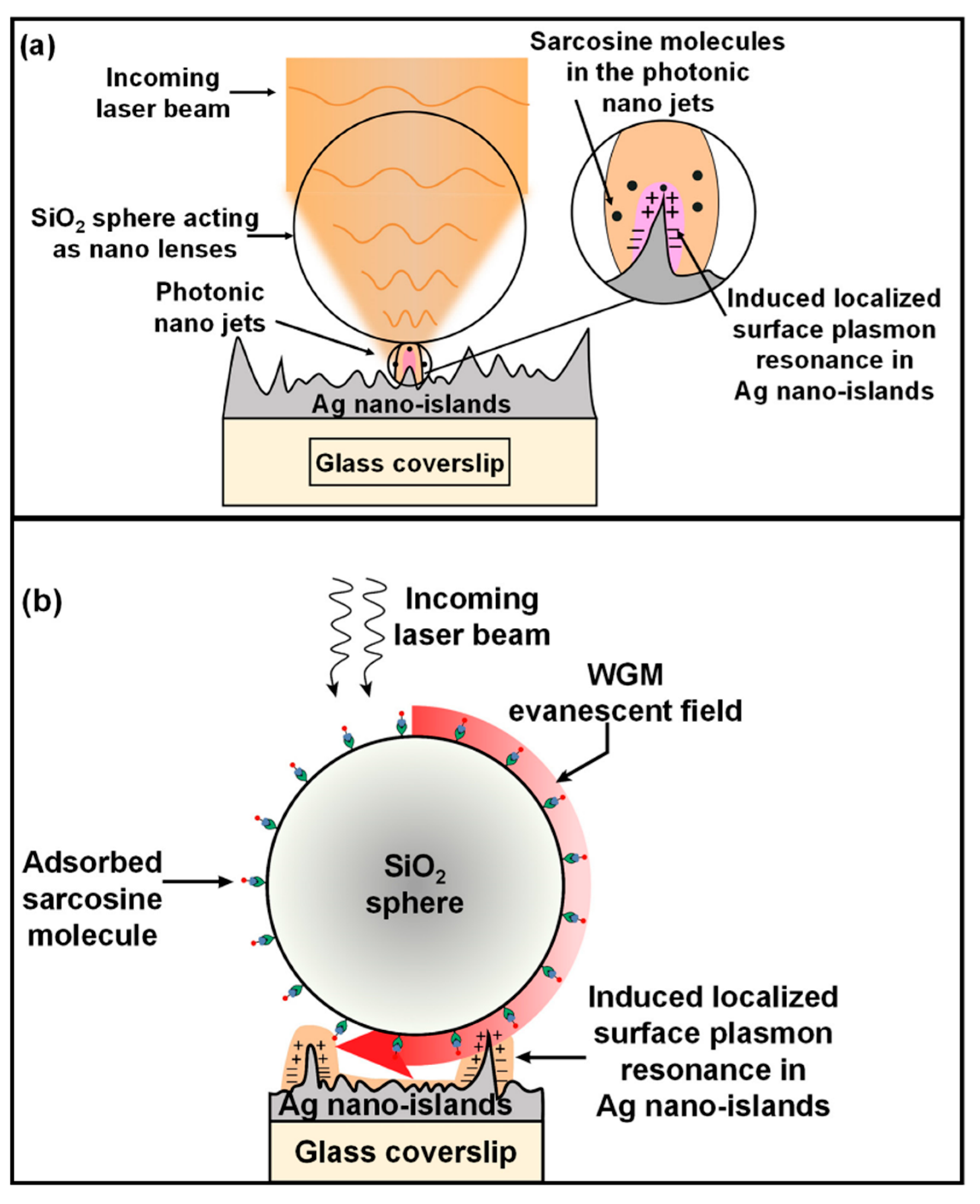
3.4. Understanding the Role of the SiO2@Ag Substrate in the Enhancement of the SERS Signal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yang, Y.; Zhu, J.; Zhao, J.; Weng, G.J.; Li, J.J.; Zhao, J.W. Growth of Spherical Gold Satellites on the Surface of Au@Ag@SiO2 Core-Shell Nanostructures Used for an Ultrasensitive SERS Immunoassay of Alpha-Fetoprotein. ACS Appl. Mater. Interfaces 2019, 11, 3617–3626. [Google Scholar] [CrossRef] [PubMed]
- Parameswari, A.; Mohamed Asath, R.; Premkumar, R.; Milton Franklin Benial, A. SERS and Quantum Chemical Studies on N-Methylglycine Molecule on Silver Nanoparticles. J. Mol. Struct. 2017, 1138, 102–109. [Google Scholar] [CrossRef]
- Cernei, N.; Heger, Z.; Gumulec, J.; Zitka, O.; Masarik, M.; Babula, P.; Eckschlager, T.; Stiborova, M.; Kizek, R.; Adam, V. Sarcosine as a Potential Prostate Cancer Biomarker-a Review. Int. J. Mol. Sci. 2013, 14, 13893–13908. [Google Scholar] [CrossRef] [Green Version]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic Profiles Delineate Potential Role for Sarcosine in Prostate Cancer Progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef] [Green Version]
- Cavaliere, B.; MacChione, B.; Monteleone, M.; Naccarato, A.; Sindona, G.; Tagarelli, A. Sarcosine as a Marker in Prostate Cancer Progression: A Rapid and Simple Method for Its Quantification in Human Urine by Solid-Phase Microextraction-Gas Chromatography-Triple Quadrupole Mass Spectrometry. Anal. Bioanal. Chem. 2011, 400, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.H.; Stabler, S.P.; Lindenbaum, J. Serum betaine, N,N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism 1993, 42, 1448–1460. [Google Scholar] [CrossRef]
- Madu, C.O.; Lu, Y. Novel Diagnostic Biomarkers for Prostate Cancer. J. Cancer 2010, 1, 150–177. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephan, C.; Ralla, B.; Jung, K. Prostate-Specific Antigen and Other Serum and Urine Markers in Prostate Cancer. Biochim. Biophys. Acta-Rev. Cancer 2014, 1846, 99–112. [Google Scholar] [CrossRef]
- Velonas, V.M.; Woo, H.H.; dos Remedios, C.G.; Assinder, S.J. Current Status of Biomarkers for Prostate Cancer. Int. J. Mol. Sci. 2013, 14, 11034–11060. [Google Scholar] [CrossRef]
- Schalken, J.; Dijkstra, S.; Baskin-Bey, E.; Van Oort, I. Potential Utility of Cancer-Specific Biomarkers for Assessing Response to Hormonal Treatments in Metastatic Prostate Cancer. Ther. Adv. Urol. 2014, 6, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zrimsek, A.B.; Chiang, N.; Mattei, M.; Zaleski, S.; McAnally, M.O.; Chapman, C.T.; Henry, A.I.; Schatz, G.C.; Van Duyne, R.P. Single-Molecule Chemistry with Surface- and Tip-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 7583–7613. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.T.X.; Blanch, E.W.; Jones, O.A.H. Surface Enhanced Raman Spectroscopy in Environmental Analysis, Monitoring and Assessment. Sci. Total Environ. 2020, 720, 137601. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ge, M.; Li, P.; Xie, Q.; Yang, L. Development of Surface-Enhanced Raman Spectroscopy Application for Determination of Illicit Drugs: Towards a Practical Sensor. Talanta 2019, 191, 1–10. [Google Scholar] [CrossRef]
- Campion, A.; Kambhampati, P.; Campion, A.; Harris, C. Surface-Enhanced Raman Scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Lin, J.; Shang, Y.; Li, X.; Yu, J.; Wang, X.; Guo, L. Ultrasensitive SERS Detection by Defect Engineering on Single Cu2O Superstructure Particle. Adv. Mater. 2017, 29, 1604797. [Google Scholar] [CrossRef]
- Rieder, K.H.; Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering Downloaded From. Angew. Chem. Int. Ed. Engl 1994, 50, 1828. [Google Scholar]
- Yan, W.; Yang, L.; Chen, J.; Wu, Y.; Wang, P.; Li, Z. In Situ Two-Step Photoreduced SERS Materials for On-Chip Single-Molecule Spectroscopy with High Reproducibility. Adv. Mater. 2017, 29, 1702893. [Google Scholar] [CrossRef]
- Abraham, S.; König, M.; Srivastava, S.K.; Kumar, V.; Walkenfort, B.; Srivastava, A. Carbon Nanostructure (0-3 Dimensional) Supported Isolated Gold Nanoparticles as an Effective SERS Substrate. Sens. Actuators B Chem. 2018, 273, 455–465. [Google Scholar] [CrossRef]
- Graham, D.; Thompson, D.G.; Smith, W.E.; Faulds, K. Control of Enhanced Raman Scattering Using a DNA-Based Assembly Process of Dye-Coded Nanoparticles. Nat. Nanotechnol. 2008, 3, 548–551. [Google Scholar] [CrossRef]
- Wu, D.Y.; Li, J.F.; Ren, B.; Tian, Z.Q. Electrochemical Surface-Enhanced Raman Spectroscopy of Nanostructures. Chem. Soc. Rev. 2008, 37, 1025–1041. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, M.; Wang, Z.; Xu, B.; Zhang, Y.; Wang, S.; He, W.; Wang, C.; Zhou, G.; Chen, Y.; et al. Engineered Optoplasmonic Core-Satellite Microspheres for SERS Determination of Methamphetamine Derivative and Its Precursors. Sens. Actuators B Chem. 2022, 358, 131437. [Google Scholar] [CrossRef]
- Mishra, A.; Tripathy, S.K.; Yun, S. Il Bio-Synthesis of Gold and Silver Nanoparticles from Candida Guilliermondii and Their Antimicrobial Effect against Pathogenic Bacteria. J. Nanosci. Nanotechnol. 2011, 11, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Olea-Mejía, O.; Fernández-Mondragón, M.; Rodríguez-De La Concha, G.; Camacho-López, M. SERS-Active Ag, Au and Ag-Au Alloy Nanoparticles Obtained by Laser Ablation in Liquids for Sensing Methylene Blue. Appl. Surf. Sci. 2015, 348, 66–70. [Google Scholar] [CrossRef]
- Padovani, S.; D’Acapito, F.; Cattaruzza, E.; De Lorenzi, A.; Gonella, F.; Mattei, G.; Maurizio, C.; Mazzoldi, P.; Montagna, M.; Ronchin, S.; et al. Metal Nanocluster Formation in Silica Films Prepared by Rf-Sputtering: An Experimental Study. Eur. Phys. J. B 2002, 25, 11–17. [Google Scholar] [CrossRef]
- Kapaklis, V.; Poulopoulos, P.; Karoutsos, V.; Manouras, T.; Politis, C. Growth of Thin Ag Films Produced by Radio Frequency Magnetron Sputtering. Thin Solid Film. 2006, 510, 138–142. [Google Scholar] [CrossRef]
- Zuo, J. Deposition of Ag Nanostructures on TiO2 Thin Films by RF Magnetron Sputtering. Appl. Surf. Sci. 2010, 256, 7096–7101. [Google Scholar] [CrossRef]
- Ausman, L.K.; Schatz, G.C. Whispering-Gallery Mode Resonators: Surface Enhanced Raman Scattering without Plasmons. J. Chem. Phys. 2008, 129, 054704. [Google Scholar] [CrossRef]
- Cao, H.; Wiersig, J. Dielectric Microcavities: Model Systems for Wave Chaos and Non-Hermitian Physics. Rev. Mod. Phys. 2015, 87, 61–111. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.K.; Kitahama, Y.; Huang, G.G.; Han, X.; Ozaki, Y. Surface-Enhanced Raman Scattering: Realization of Localized Surface Plasmon Resonance Using Unique Substrates and Methods. Anal. Bioanal. Chem. 2009, 394, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Ciobota, V.; Srivastava, S.; Srivastava, S.K.; Singh, R.K.; Dellith, J.; Malhotra, B.D.; Schmitt, M.; Popp, J.; Srivastava, A. Mesoporous Silica Particle Embedded Functional Graphene Oxide as an Efficient Platform for Urea Biosensing. Anal. Methods 2014, 6, 6711–6720. [Google Scholar] [CrossRef]
- Srivastava, R.R.; Mishra, H.; Singh, V.K.; Vikram, K.; Srivastava, R.K.; Srivastava, S.K.; Srivastava, A. PH Dependent Luminescence Switching of Tin Disulfide Quantum Dots. J. Lumin. 2019, 213, 401–408. [Google Scholar] [CrossRef]
- Stolen, R.H.; Walrafen, G.E. Water and Its Relation to Broken Bond Defects in Fused Silica. J. Chem. Phys. 1976, 64, 2623–2631. [Google Scholar] [CrossRef]
- Verma, R.; Singh, J.; Samdarshi, S.K.; Srivastava, A. Autonomous Self-Optimizing Defects by Refining Energy Levels through Hydrogenation in CeO2−x Polymorphism: A Walking Mobility of Oxygen Vacancy with Enhanced Adsorption Capabilities and Photocatalytic Stability. New J. Chem. 2022, 46, 5869–5880. [Google Scholar] [CrossRef]
- Tu, J.; Yuan, Y.; Zhan, P.; Jiao, H.; Wang, X.; Zhu, H.; Jiao, S. Straightforward Approach toward SiO2 Nanospheres and Their Superior Lithium Storage Performance. J. Phys. Chem. C 2014, 118, 7357–7362. [Google Scholar] [CrossRef]
- Panwar, K.; Jassal, M.; Agrawal, A.K. In Situ Synthesis of Ag-SiO2 Janus Particles with Epoxy Functionality for Textile Applications. Particuology 2015, 19, 107–112. [Google Scholar] [CrossRef]
- Balakrishnan, V.; Ab Wab, H.A.; Abdul Razak, K.; Shamsuddin, S. In Vitro Evaluation of Cytotoxicity of Colloidal Amorphous Silica Nanoparticles Designed for Drug Delivery on Human Cell Lines. J. Nanomater. 2013, 2013, 729306. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Singh, D.K.; Gupta, V.; Asthana, B.P.; Mishra, P.C.; Singh, R.K. Study of Hydration of Sarcosine, Formation of Its Zwitterion and Their Different Oligomers in Aqueous Media: A Raman Spectroscopic and Theoretical Study. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2013, 116, 74–83. [Google Scholar] [CrossRef]
- Chen, N.; Rong, M.; Shao, X.; Zhang, H.; Liu, S.; Dong, B.; Xue, W.; Wang, T.; Li, T.; Pan, J. Surface-Enhanced Raman Spectroscopy of Serum Accurately Detects Prostate Cancer in Patients with Prostate-Specific Antigen Levels of 4–10 Ng/ML. Int. J. Nanomed. 2017, 12, 5399–5407. [Google Scholar] [CrossRef] [Green Version]
- Kapustin, E.A.; Minkov, V.S.; Boldyreva, E.V. Sarcosine and Betaine Crystals upon Cooling: Structural Motifs Unstable at High Pressure Become Stable at Low Temperatures. Phys. Chem. Chem. Phys. 2015, 17, 3534–3543. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Kim, D.Y. 3D Hotspot Matrix of Au Nanoparticles on Au Island Film with a Spacer Layer of Dithiol Molecules for Highly Sensitive Surface-Enhanced Raman Spectroscopy. Sci. Rep. 2021, 11, 22399. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Gao, R.; Zhang, F.; Zhu, A.; Chen, L.; Wang, Y. Facile SERS-Active Chip (PS@Ag/SiO2/Ag) for the Determination of HCC Biomarker. Sens. Actuators B Chem. 2018, 272, 34–42. [Google Scholar] [CrossRef]
- Gartia, M.R.; Seo, S.; Kim, J.; Chang, T.W.; Bahl, G.; Lu, M.; Liu, G.L.; Eden, J.G. Injection-Seeded Optoplasmonic Amplifier in the Visible. Sci. Rep. 2014, 4, 6168. [Google Scholar] [CrossRef] [Green Version]
- Yi, K.J.; Wang, H.; Lu, Y.F.; Yang, Z.Y. Enhanced Raman Scattering by Self-Assembled Silica Spherical Microparticles. J. Appl. Phys. 2007, 101, 063528. [Google Scholar] [CrossRef] [Green Version]
- Alessandri, I.; Bontempi, N.; Depero, L.E. Colloidal Lenses as Universal Raman Scattering Enhancers. RSC Adv. 2014, 4, 38152–38158. [Google Scholar] [CrossRef]
- White, I.M.; Hanumegowda, N.M.; Oveys, H.; Fan, X. Tuning Whispering Gallery Modes in Optical Microspheres with Chemical Etching. Opt. Express 2005, 13, 10754. [Google Scholar] [CrossRef]
- Huang, S.H.; Jiang, X.; Peng, B.; Janisch, C.; Cocking, A.; Özdemir, Ş.K.; Liu, Z.; Yang, L. Surface-Enhanced Raman Scattering on Dielectric Microspheres with Whispering Gallery Mode Resonance. Photonics Res. 2018, 6, 346. [Google Scholar] [CrossRef]
- Das, S.; Hossain, M.J.; Leung, S.F.; Lenox, A.; Jung, Y.; Davis, K.; He, J.H.; Roy, T. A Leaf-Inspired Photon Management Scheme Using Optically Tuned Bilayer Nanoparticles for Ultra-Thin and Highly Efficient Photovoltaic Devices. Nano Energy 2019, 58, 47–56. [Google Scholar] [CrossRef]
- Inoue, M. Enhanced Raman Scattering By Dielectric Spheres. Pure Appl. Chem. 1987, 59, 1253–1262. [Google Scholar] [CrossRef]
- Asnaashari, M.; Esmaeilzadeh Kenari, R.; Farahmandfar, R.; Taghdisi, S.M.; Abnous, K. Fluorescence Quenching Biosensor for Acrylamide Detection in Food Products Based on Double-Stranded DNA and Gold Nanoparticles. Sens. Actuators B Chem. 2018, 265, 339–345. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, S.; Liu, L.; Kuang, H.; Xu, C. An Indirect Competitive Enzyme-Linked Immunosorbent Assay for Acrylamide Detection Based on a Monoclonal Antibody. Food Agric. Immunol. 2016, 27, 796–805. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Mao, L.G.; Wang, Y.L.; Shi, X.B.; Liu, Y.; Yang, Y.; He, Z. Electrochemical Sensor Based on Imprinted Sol-Gel Polymer on Au NPs-MWCNTs-CS Modified Electrode for the Determination of Acrylamide. Food Anal. Methods 2016, 9, 114–121. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X. Research on Determination of Acrylamide in Fried Food by Flow Injection Chemiluminescence. In 2017 2nd International Conference on Education, Sports, Arts and Management Engineering (ICESAME 2017); Atlantis Press: Paris, France, 2017; Volume 123, pp. 724–727. [Google Scholar] [CrossRef] [Green Version]
- Asnaashari, M.; Kenari, R.E.; Farahmandfar, R.; Abnous, K.; Taghdisi, S.M. An Electrochemical Biosensor Based on Hemoglobin-Oligonucleotides-Modified Electrode for Detection of Acrylamide in Potato Fries. Food Chem. 2019, 271, 54–61. [Google Scholar] [CrossRef]

| S. No. | Technique | Material | Detection Range | Limit of Detection | Reference |
|---|---|---|---|---|---|
| 1. | Fluorescence | Au NPs | 100 nM–50 mM | 10 nM | [53] |
| 2. | icELISA | Acrylamide-4-MBA | 124.79 nM–1590 nM | 124.79 nM | [54] |
| 3. | MIP-DPV | Au NPs-MWCNTs | 703.4 nM–70.34 μM | 393.9 nM | [55] |
| 4. | FIC | Luminol-Hydrogen Peroxide | 1.0 μM–1.0 mM | 39.5 nM | [56] |
| 5. | SWV | Au NPs | 2.0 μM–50 mM | 158 nM | [57] |
| 6. | SERS | Silica coated Ag nano-islands | 10 nM–100 μM | 1.76 nM | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, A.; Sarkar, S.; Pandey, S.K.; Srivastava, A. Silica Nanospheres Coated Silver Islands as an Effective Opto-Plasmonic SERS Active Platform for Rapid and Sensitive Detection of Prostate Cancer Biomarkers. Molecules 2022, 27, 7821. https://doi.org/10.3390/molecules27227821
Pandey A, Sarkar S, Pandey SK, Srivastava A. Silica Nanospheres Coated Silver Islands as an Effective Opto-Plasmonic SERS Active Platform for Rapid and Sensitive Detection of Prostate Cancer Biomarkers. Molecules. 2022; 27(22):7821. https://doi.org/10.3390/molecules27227821
Chicago/Turabian StylePandey, Anamika, Subhankar Sarkar, Sumit Kumar Pandey, and Anchal Srivastava. 2022. "Silica Nanospheres Coated Silver Islands as an Effective Opto-Plasmonic SERS Active Platform for Rapid and Sensitive Detection of Prostate Cancer Biomarkers" Molecules 27, no. 22: 7821. https://doi.org/10.3390/molecules27227821
APA StylePandey, A., Sarkar, S., Pandey, S. K., & Srivastava, A. (2022). Silica Nanospheres Coated Silver Islands as an Effective Opto-Plasmonic SERS Active Platform for Rapid and Sensitive Detection of Prostate Cancer Biomarkers. Molecules, 27(22), 7821. https://doi.org/10.3390/molecules27227821