Development and Validation of an LC-MS/MS Method for Simultaneous Determination of Short Peptide-Based Drugs in Human Blood Plasma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Validation
2.1.1. Selectivity
2.1.2. Accuracy and Precision
2.1.3. Matrix Effect
2.1.4. Lower Limit of Quantification (LLOQ)
2.1.5. Stability
2.1.6. Carryover
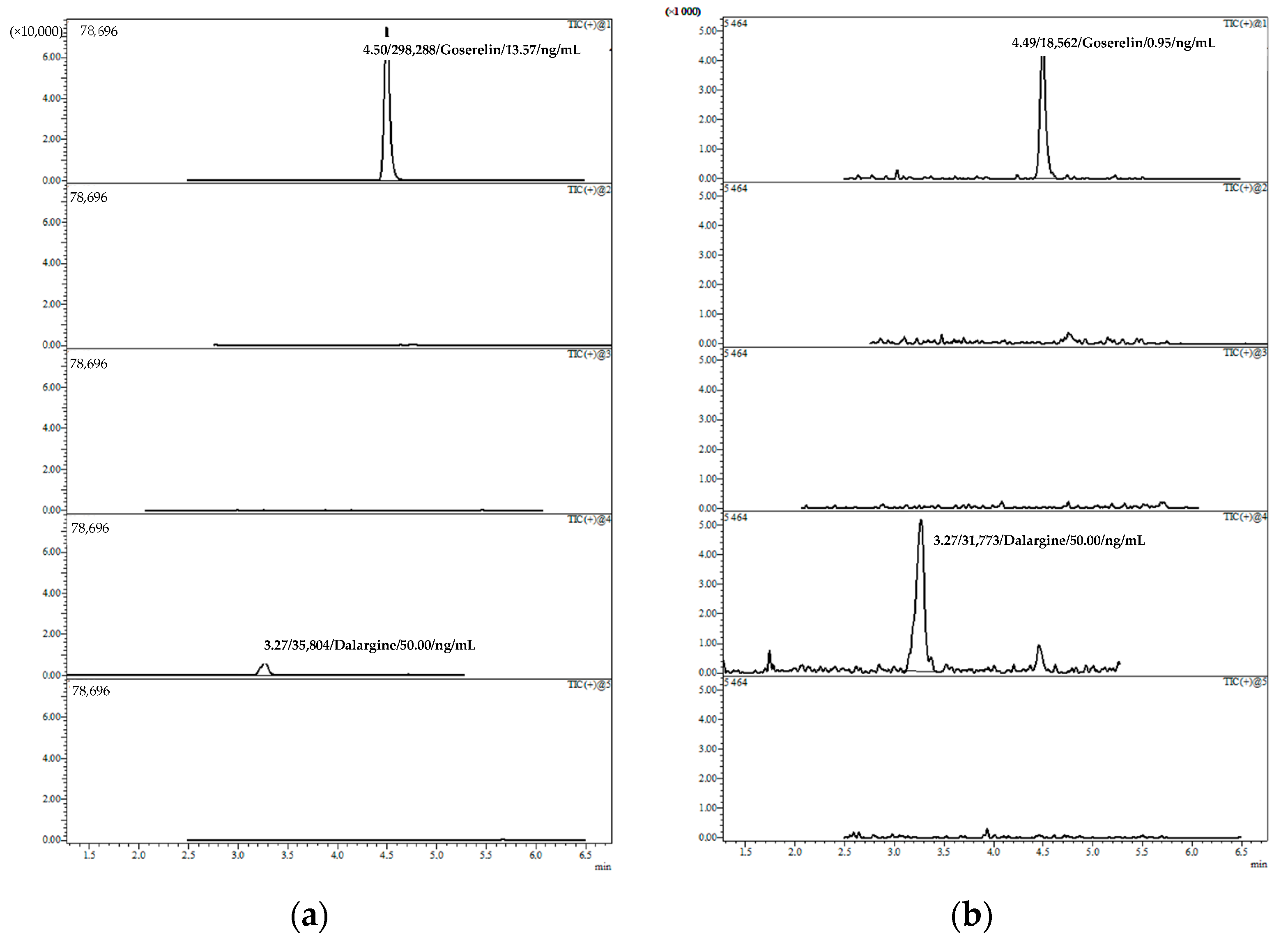
2.2. Practical Application of the Developed Methodology
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Stock and Working Standard Solutions
3.3. Calibration and Quality Control Samples
3.4. Sample Preparation
3.5. Analytical Instrumentation
3.6. LC-MS/MS Operation Conditions
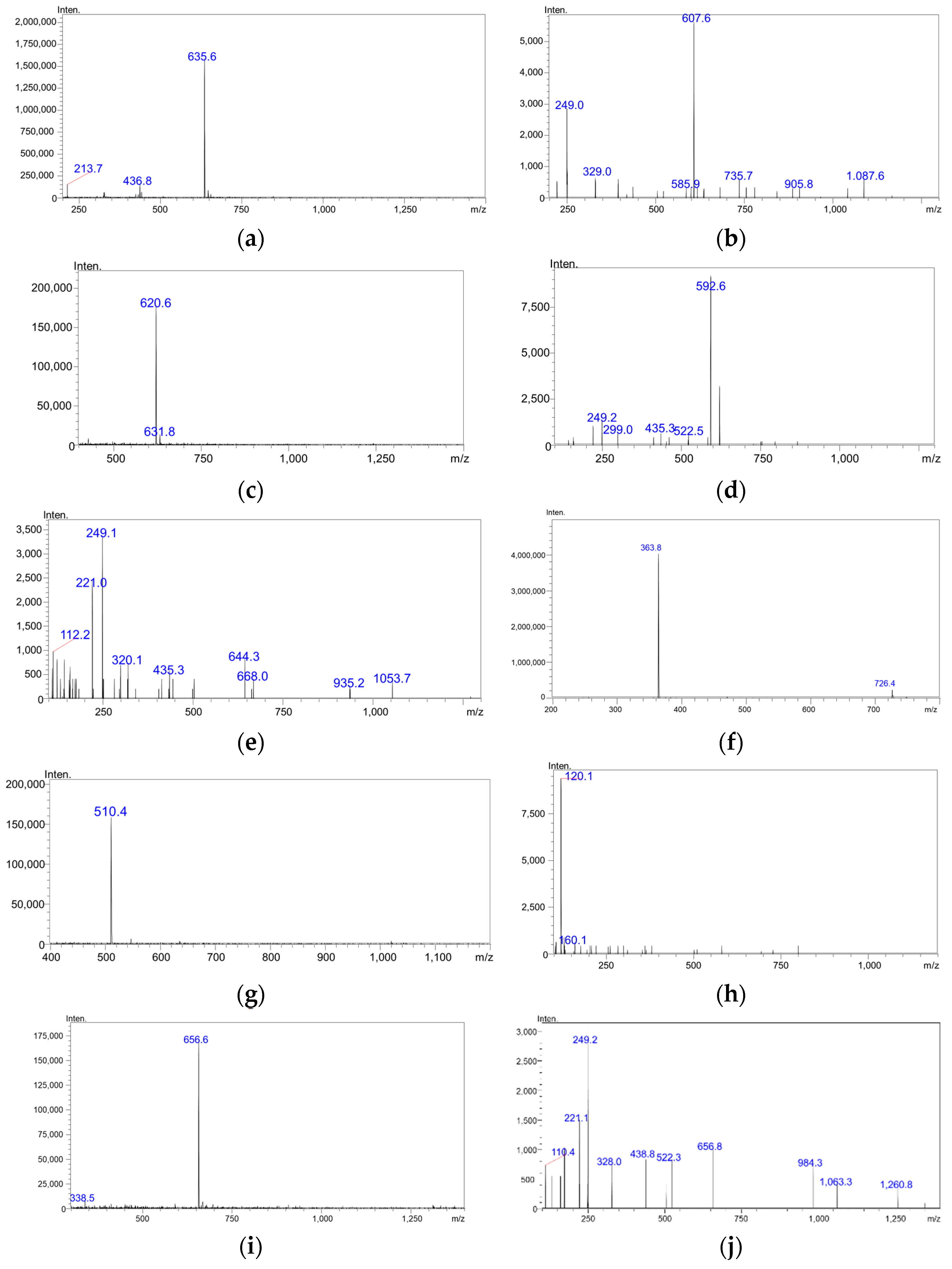
3.7. Development and Optimization of the MS/MS Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohlsson, B. Gonadotropin-releasing hormone and its role in the enteric nervous system. Front. Endocrinol. 2017, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, B. Gonadotropin-releasing hormone and its physiological and pathophysiological roles in relation to the structure and function of the gastrointestinal tract. Eur. Surg. Res. 2016, 57, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Chrisp, P.; Goa, L.K. Goserelin. A review of its pharmacodynamic and pharmacokinetic properties, and clinical use in sex hormone-related conditions. Drugs 1991, 41, 254–288. [Google Scholar] [CrossRef]
- Connolly, R.M.; Carducci, M.A.; Antonarakis, E.S. Use of androgen deprivation therapy in prostate cancer: Indications and prevalence. Asian J. 2012, 14, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, J.-F. Oligopeptide. In Encyclopedia of Astrobiology; Gargaud, M., Amils, R., Quintanilla, J.C., Cleaves, H.J., Irvine, W.M., Pinti, D.L., Viso, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; p. 1174. [Google Scholar]
- Kumar, P.; Sharma, A. Gonadotropin-releasing hormone analogs: Understanding advantages and limitations. J. Hum. Reprod. Sci. 2014, 7, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Perrett, R.M.; McArdle, C.A. Molecular mechanisms of gonadotropin-releasing hormone signaling: Integrating cyclic nucleotides into the network. Front. Endocrinol. 2013, 4, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zompra, A.A.; Magafa, V.; Lamari, F.N.; Nikolopoulou, A.; Nock, B.; Maina, T.; Spyroulias, G.A.; Karamanos, N.K.; Cordopatis, P. GnRH analogues containing conformationally restricted amino acids in positions 3 and 6: Differential impact on pituitary binding affinity and direct antiproliferative effect on breast cancer cells. Chem. Biol. Drug Des. 2005, 66, 57–64. [Google Scholar] [CrossRef]
- Wilson, A.C.; Meethal, V.M.; Bowen, R.L.; Atwood, C.S. Leuprolide acetate: A drug of diverse clinical applications. Expert Opin. Investig. Drugs 2007, 16, 1851–1863. [Google Scholar] [CrossRef]
- Eigler, T.; Ben-Shlomo, A. Somatostatin system: Molecular mechanisms regulating anterior pituitary hormones. J. Mol. Endocrinol. 2014, 53, R1–R19. [Google Scholar] [CrossRef] [Green Version]
- Dalm, V.A.; Hofland, L.J.; Lamberts, S.W. Future clinical prospects in somatostatin/cortistatin/somatostatin receptor field. Mol. Cell. Endocrinol. 2008, 286, 262–277. [Google Scholar] [CrossRef]
- Seery, T.E.; Choudhry, A.; Eapen, A.; Cheng, Y. Pancreatic Neuroendocrine Tumors Therapy. J. Pancreas 2018, 3, 358–365. [Google Scholar]
- Abramson, M.A.; Jazag, A.; Zee, J.A.; Whang, E.E. The molecular biology of pancreatic cancer. Gastrointest. Cancer Res. 2007, 1, S7–S12. [Google Scholar]
- Kogot, J.M.; Sarkes, D.A.; Val-Addo, I.; Pellegrino, P.M.; Stratis-Cullum, D.N. Increased affinity and solubility of peptides used for direct peptide ELISA on polystyrene surfaces through fusion with a polystyrene-binding peptide tag. BioTechniques 2012, 52, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.E.; Hammarlund, E.; Slifka, M.K. Optimization of peptide-based ELISA for serological diagnostics: A retrospective study of human monkeypox infection. Vector Borne Zoonotic Dis. 2012, 12, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Trudeau, K.; Vu, K.D.; Shareck, F.; Lacroix, M. Capillary electrophoresis separation of protein composition of γ-irradiated food pathogens Listeria monocytogenes and Staphylococcus aureus. PLoS ONE 2012, 7, e32488. [Google Scholar] [CrossRef] [Green Version]
- Bergman, T. Capillary Electrophoresis in Structural Characterization of Polypeptides. In Proceedings of the Ninth International Conference on Methods in Protein Sequence Analysis, Otsu, Japan, 20–24 September 1992; pp. 21–28. [Google Scholar]
- Albright, J.C.; Beussman, D.J. Capillary zone electrophoresis for the analysis of peptides: Fostering students’ problem-solving and discovery learning in an undergraduate laboratory experiment. J. Chem. Educ. 2017, 94, 361–366. [Google Scholar] [CrossRef]
- Szökö, E. Protein and peptide analysis by capillary zone electrophoresis and micellar electrokinetic chromatography. Electrophoresis 1997, 18, 74–81. [Google Scholar] [CrossRef]
- Messana, I.; Rossetti, D.V.; Cassiano, L.; Misiti, F.; Giardina, B.; Castagnola, M. Peptide analysis by capillary (zone) electrophoresis. J. Chromatogr. B 1997, 699, 149–171. [Google Scholar] [CrossRef]
- Gartenmann, K.; Kochhar, S. Short-chain peptide analysis by high-performance liquid chromatography coupled to electrospray ionization mass spectrometer after derivatization with 9-fluorenylmethyl chloroformate. J. Agric. Food Chem. 1999, 47, 5068–5071. [Google Scholar] [CrossRef]
- Syka, E.P.; Coon, J.J.; Schroeder, M.J.; Shabanowitz, J.; Hunt, D.F. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. USA 2004, 101, 9528–9533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, S.A.; Annan, R.S. Overview of peptide and protein analysis by mass spectrometry. Curr. Protoc. Protein Sci. 2001, 16, 1–27. [Google Scholar] [PubMed]
- Gillet, M.A.; Carr, S.A. Quantitative analysis of peptides and proteins in biomedicine by targeted mass spectrometry. Nat. Methods 2013, 10, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, M.E.; Krogsrud, R.L. Principles of immunochemical techniques used in clinical laboratories. Lab. Med. 2006, 37, 490–497. [Google Scholar] [CrossRef]
- Gazzaz, S.S.; Rasco, B.A.; Dong, F.M. Application of immunochemical assays to food analysis. Crit. Rev. Food Sci. Nutr. 1992, 32, 197–229. [Google Scholar] [CrossRef]
- Jung, F.; Gee, S.J.; Harrison, R.O.; Goodrow, M.H.; Karu, A.E.; Braun, A.L.; Li, Q.X.; Hammock, B.D. Use of immunochemical techniques for the analysis of pesticides. Pestic. Sci. 1989, 26, 303–317. [Google Scholar] [CrossRef]
- Lesnik, B. Immunoassay Techniques in Environmental Analyses. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 2653–2672. [Google Scholar]
- D’Hondt, M.; Gevaert, B.; Stalmans, S.; Van Dorpe, S.; Wynendaele, E.; Peremans, K.; Burvenich, C.; De Spiegeleer, B. Reversed-phase fused-core HPLC modeling of peptides. J. Pharm. Anal. 2013, 3, 93–101. [Google Scholar] [CrossRef]
- Böttger, R.; Hoffmann, R.; Knappe, D. Differential stability of therapeutic peptides with different proteolytic cleavage sites in blood, plasma and serum. PLoS ONE 2017, 12, e0178943. [Google Scholar] [CrossRef] [Green Version]
- Kuhtreiber, W.M.; Washer, S.L.L.; Hsu, E.; Zhao, M.; Reinhold, P.; Burger, D.; Zheng, H.; Faustman, D.L. Low levels of C-peptide have clinical significance for established Type 1 diabetes. Diabet. Med. 2015, 32, 1346–1353. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Lee, T.H.; Suh, J.H.; Eom, H.Y.; Min, J.W.; Yeom, H. Development and validation of a liquid chromatography–tandem mass spectrometry method for the determination of goserelin in rabbit plasma. J. Chromatogr. B 2010, 878, 2235–2242. [Google Scholar] [CrossRef]
- Zhang, S.; Han, J.; Leng, G.; Di, X.; Sha, C.; Zhang, X.; Li, Y.; Liu, W. An LC-MS/MS method for the simultaneous determination of goserelin and testosterone in rat plasma for pharmacokinetic and pharmacodynamic studies. J. Chromatogr. B 2014, 965, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, O.A.; Zhang, T.; Jenkins, R.; Karnes, H.T. Determination of octreotide and assessment of matrix effects in human plasma using ultra high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2011, 879, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, J.; Wang, Y.; Du, X.; Zhang, Y.; Fawcett, J.P.; Gu, J. Determination of long-acting release octreotide, an octapeptide analogue of somatostation, in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3982–3986. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Sun, J.; Sha, C.; Zhang, J.; Gai, Y.; Li, Y.; Liu, W. Quantitation of slow release triptorelin in beagle dog plasma by liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 66, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Capron, A.; Destree, J.; Maiter, D.; Wallemacq, P. Validation of a rapid liquid chromatography–tandem mass spectrometric assay for the determination of octreotide plasma concentrations. Clin. Biochem. 2013, 47, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services; Food and Drug Administration; Center for Drug Evolution and Research (CDER). Guidance for Industry: Bioanalytical Method Validation; U.S. Government Printing Office: Washington, DC, USA, 2018.
- European Medicines Agency. Guideline on Bioanalytical Method Validation; Committee for Medicinal Products for Human Use: London, UK, 2011.
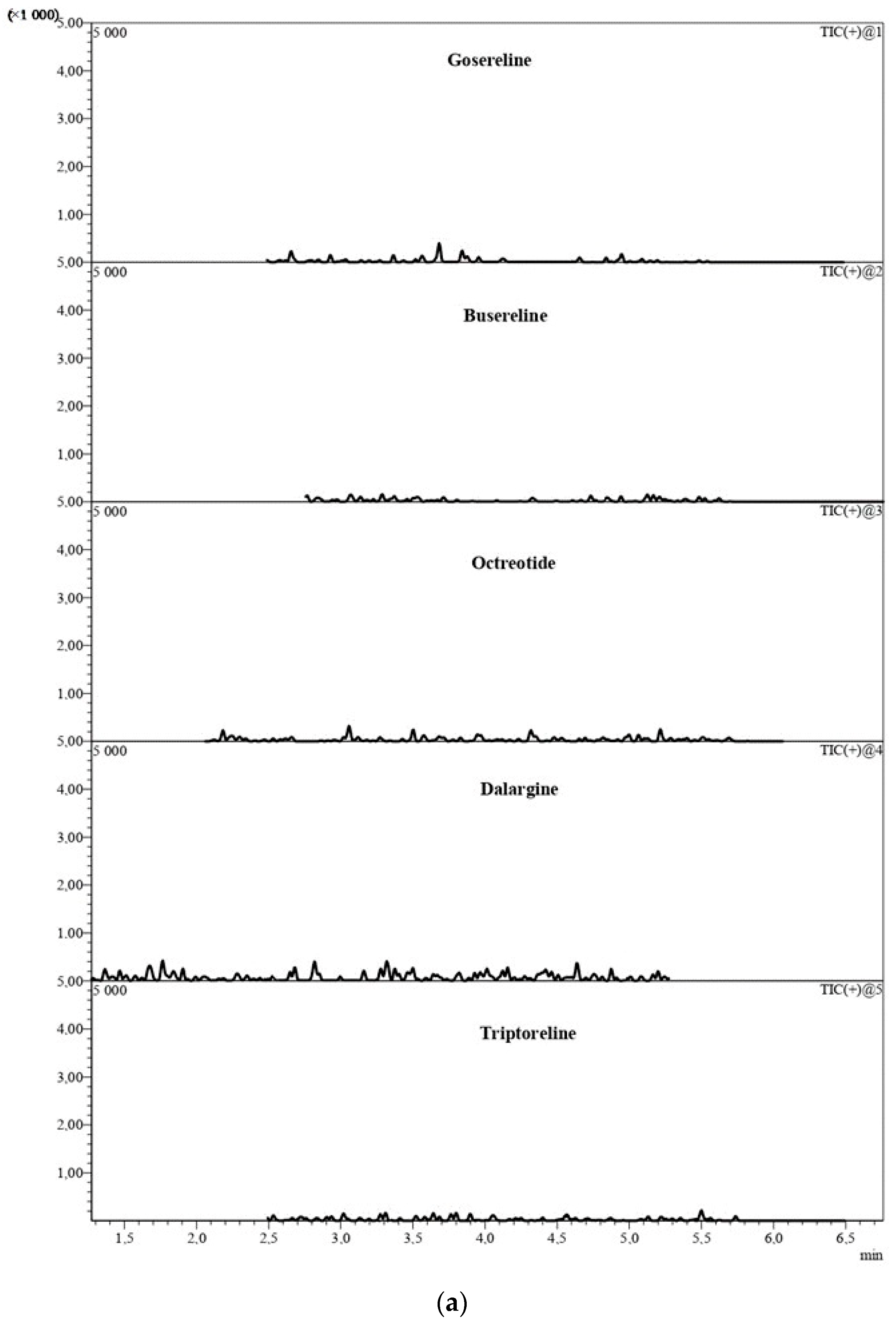
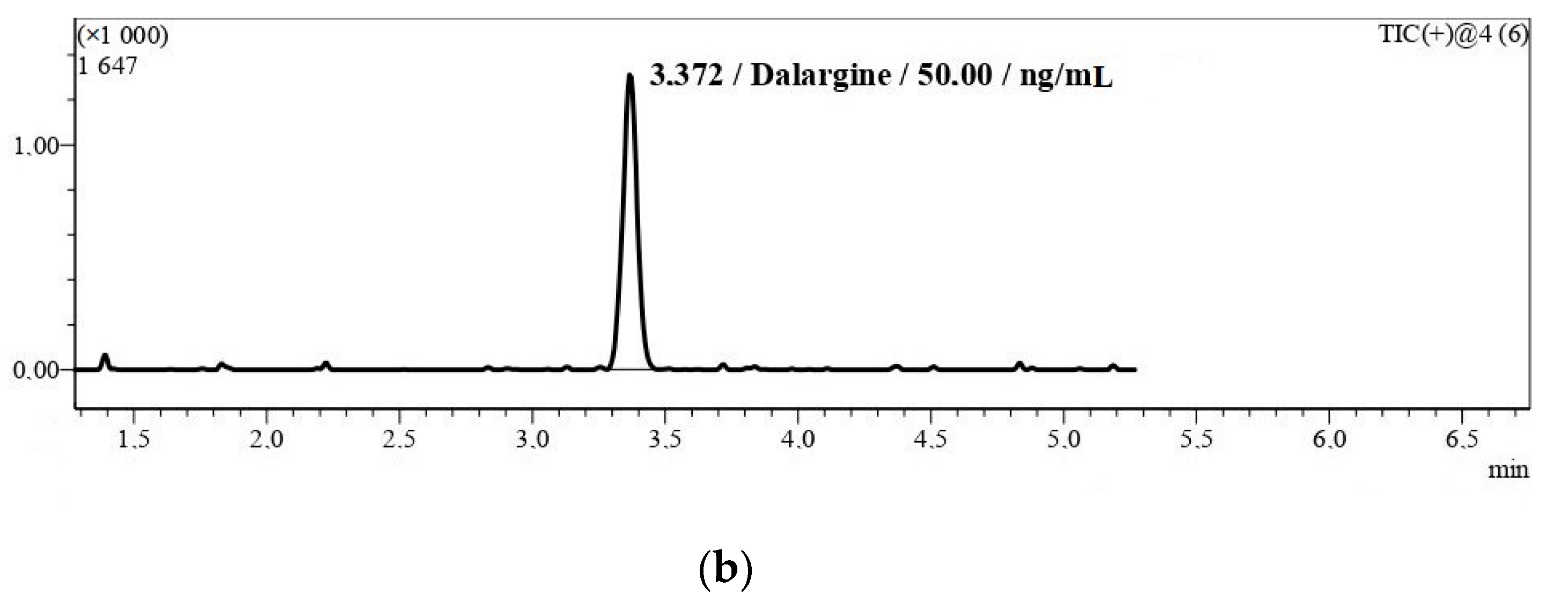

| Analyte | Regression Analysis | Nominal Concentration (ng/mL) | Measured Concentration (ng/mL) | Maximum E (%) |
|---|---|---|---|---|
| Goserelin | y = 1.9602x + 0.0007 (R = 0.998) | 0.50 | 0.46–0.58 | 16.0 |
| 1.00 | 0.96–1.14 | 14.0 | ||
| 2.50 | 2.34–2.72 | 8.8 | ||
| 5.00 | 4.64–5.23 | 7.2 | ||
| 10.00 | 9.37–10.47 | 6.3 | ||
| 20.00 | 17.49–21.04 | 12.5 | ||
| Buserelin | y = 8.2778x + 0.0006 (R = 0.999) | 0.50 | 0.48–0.53 | 6.00 |
| 1.00 | 0.91–1.03 | 9.00 | ||
| 2.50 | 2.13–2.17 | 14.8 | ||
| 5.00 | 5.11–5.34 | 6.8 | ||
| 10.00 | 10.84–11.22 | 12.20 | ||
| 20.00 | 19.75–20.12 | 1.25 | ||
| Triptorelin | y = 0.8506x + 0.0014 (R = 0.998) | 0.50 | 0.48–0.53 | 6.00 |
| 1.00 | 0.91–1.03 | 9.00 | ||
| 2.50 | 2.13–2.17 | 14.8 | ||
| 5.00 | 5.11–5.34 | 6.8 | ||
| 10.00 | 10.84–11.22 | 12.20 | ||
| 20.00 | 19.75–20.12 | 1.25 | ||
| Octreotide | y = 1.6853x + 0.0003 (R = 0.998) | 0.50 | 0.49–0.53 | 6.0 |
| 1.00 | 1.00–1.12 | 12.0 | ||
| 2.50 | 2.46–2.70 | 8.00 | ||
| 5.00 | 4.89–5.14 | 2.80 | ||
| 10.00 | 10.17–10.39 | 3.90 | ||
| 20.00 | 17.82–19.16 | 10.90 |
| Intra-Day (n = 5) | Inter-Day (n = 10) | ||||||
|---|---|---|---|---|---|---|---|
| Analyte | Nominal Concentration/ng/mL | Average Measured Concentration/ng/mL | RSD/% | E/% | Average Measured Concentration/ng/mL | RSD/% | E/% |
| Goserelin | 0.50 | 0.46 | 8.71 | 7.60 | 0.51 | 8.40 | 1.00 |
| 1.00 | 1.04 | 11.10 | 3.80 | 1.02 | 6.18 | 2.10 | |
| 10.00 | 9.73 | 7.26 | 2.70 | 10.31 | 6.10 | 3.07 | |
| 20.00 | 19.49 | 7.63 | 2.54 | 20.21 | 7.03 | 1.07 | |
| Buserelin | 0.50 | 0.51 | 8.89 | 2.40 | 0.48 | 4.67 | 4.60 |
| 1.00 | 0.97 | 6.60 | 2.80 | 0.96 | 7.27 | 4.50 | |
| 10.00 | 10.44 | 4.75 | 4.36 | 9.99 | 3.99 | 0.06 | |
| 20.00 | 20.44 | 5.80 | 2.18 | 20.07 | 5.31 | 0.33 | |
| Triptorelin | 0.50 | 0.50 | 6.15 | 0.80 | 0.49 | 7.88 | 1.40 |
| 1.00 | 1.00 | 7.67 | 0.40 | 1.01 | 8.04 | 0.90 | |
| 10.00 | 10.14 | 8.28 | 1.36 | 9.81 | 6.99 | 1.92 | |
| 20.00 | 19.93 | 10.14 | 0.34 | 19.79 | 8.02 | 1.03 | |
| Octreotide | 0.50 | 0.49 | 7.79 | 1.20 | 0.49 | 5.29 | 2.20 |
| 1.00 | 0.98 | 8.86 | 2.40 | 1.03 | 5.84 | 2.50 | |
| 10.00 | 10.17 | 10.17 | 1.74 | 10.25 | 6.58 | 2.48 | |
| 20.00 | 19.98 | 4.11 | 0.09 | 19.75 | 2.28 | 1.27 | |
| Substance | Matrix Effect (ME)/% | IS-Normalized Matrix Effect (MEnorm)/% | Recovery (RE)/% | |||
|---|---|---|---|---|---|---|
| 1 ng/mL | 20 ng/mL | 1 ng/mL | 20 ng/mL | 1 ng/mL | 20 ng/mL | |
| Goserelin | 91 | 89 | 107 | 101 | 77 | 87 |
| Buserelin | 87 | 81 | 102 | 92 | 82 | 77 |
| Triptorelin | 73 | 79 | 85 | 90 | 53 | 65 |
| Octreotide | 66 | 79 | 77 | 89 | 69 | 65 |
| 50 ng/mL | ||||||
| IS | 85 | 88 | - | - | 80 | 88 |
| Patient No. | Received Drug, Active Substance, Dose | Duration of the Received Therapy | Day of the Sample Collection | Measured Concentration/ng/mL |
|---|---|---|---|---|
| 1 | Zoladex®, goserelin acetate, 10.8 mg | 6 months | 29th/84 | 13.57 |
| 2 | Zoladex®, goserelin acetate, 3.6 mg | 9 days | 8th/28 | 0.95 |
| 3 | Zoladex®, goserelin acetate, 10.8 mg | 2 months | 59th/84 | 0.55 |
| 4 | Zoladex®, goserelin acetate, 10.8 mg | 3 days | 3rd/84 | - |
| 5 | Triptorelin-long®, triptorelin acetate, 11.25 mg | 3 months | 83rd/84 | - |
| 6 | Zoladex®, goserelin acetate, 10.8 mg | 3 months | 74th/84 | - |
| 7 | Zoladex®, goserelin acetate, 10.8 mg | 8 months | 10th/84 | 7.81 |
| 8 | Zoladex®, goserelin acetate, 3.6 mg | 2 years | 16th/28 | 16.23 |
| 9 | Buserelin-depo®, buserelin acetate, 3.75 mg | 2 years | 27th/28 | - |
| Time/Minutes | Solvent B, % |
|---|---|
| 0–0.3 | 5 |
| 0.3–4.6 | 5–35 |
| 4.6–5.1 | 35–95 |
| 5.1–6.5 | 95 |
| 6.5–8.2 | 95–5 |
| 8.2–10 | 5 |
| Analyte | RT/Min | Precursor Ion/m/z | Product Ion/m/z | Collision Energy/V |
|---|---|---|---|---|
| Goserelin | 4.61 | 635.50 | 607.45 | −20.0 |
| Buserelin | 4.88 | 620.45 | 248.90 | −37.0 |
| 592.35 | −19.0 | |||
| Triptorelin | 4.59 | 510.40 | 120.05 | −34.0 |
| Dalargin | 3.37 | 363.85 | 120.10 | −43.0 |
| 135.95 | −22.0 | |||
| 492.30 | −13.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher, E.N.; Melnikov, E.S.; Gegeckori, V.; Potoldykova, N.V.; Enikeev, D.V.; Pavlenko, K.A.; Agatonovic-Kustrin, S.; Morton, D.W.; Ramenskaya, G.V. Development and Validation of an LC-MS/MS Method for Simultaneous Determination of Short Peptide-Based Drugs in Human Blood Plasma. Molecules 2022, 27, 7831. https://doi.org/10.3390/molecules27227831
Fisher EN, Melnikov ES, Gegeckori V, Potoldykova NV, Enikeev DV, Pavlenko KA, Agatonovic-Kustrin S, Morton DW, Ramenskaya GV. Development and Validation of an LC-MS/MS Method for Simultaneous Determination of Short Peptide-Based Drugs in Human Blood Plasma. Molecules. 2022; 27(22):7831. https://doi.org/10.3390/molecules27227831
Chicago/Turabian StyleFisher, Elizaveta N., Evgeny S. Melnikov, Vladimir Gegeckori, Natalya V. Potoldykova, Dmitry V. Enikeev, Kirill A. Pavlenko, Snezana Agatonovic-Kustrin, David W. Morton, and Galina V. Ramenskaya. 2022. "Development and Validation of an LC-MS/MS Method for Simultaneous Determination of Short Peptide-Based Drugs in Human Blood Plasma" Molecules 27, no. 22: 7831. https://doi.org/10.3390/molecules27227831
APA StyleFisher, E. N., Melnikov, E. S., Gegeckori, V., Potoldykova, N. V., Enikeev, D. V., Pavlenko, K. A., Agatonovic-Kustrin, S., Morton, D. W., & Ramenskaya, G. V. (2022). Development and Validation of an LC-MS/MS Method for Simultaneous Determination of Short Peptide-Based Drugs in Human Blood Plasma. Molecules, 27(22), 7831. https://doi.org/10.3390/molecules27227831








