Essential Oils from the Leaves, Stem, and Roots of Blumea lanceolaria (Roxb.) Druce in Vietnam: Determination of Chemical Composition, and In Vitro, In Vivo, and In Silico Studies on Anti-Inflammatory Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the BEOs
2.2. Effect of the BEOs on Macrophage Viability
2.3. Inhibition of NO Production by BEOs
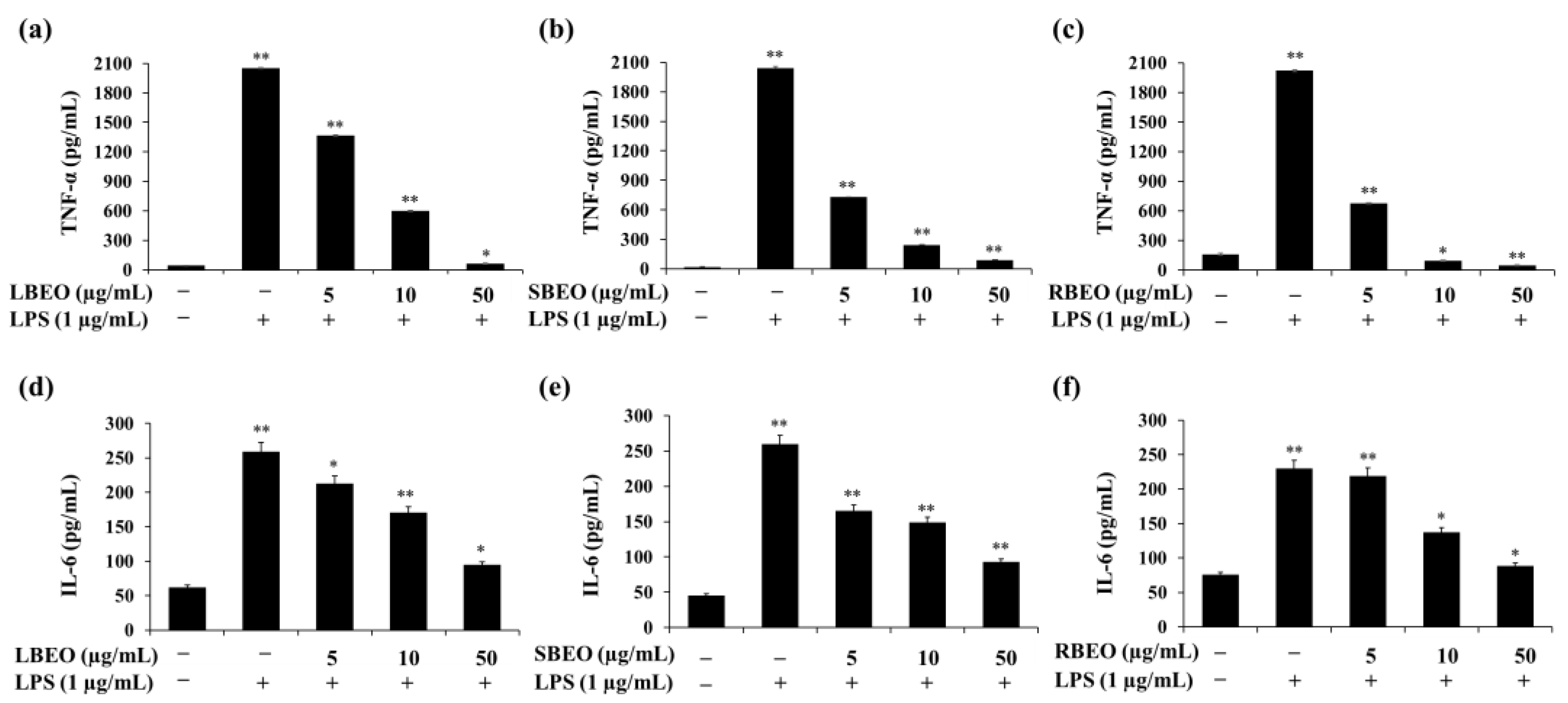
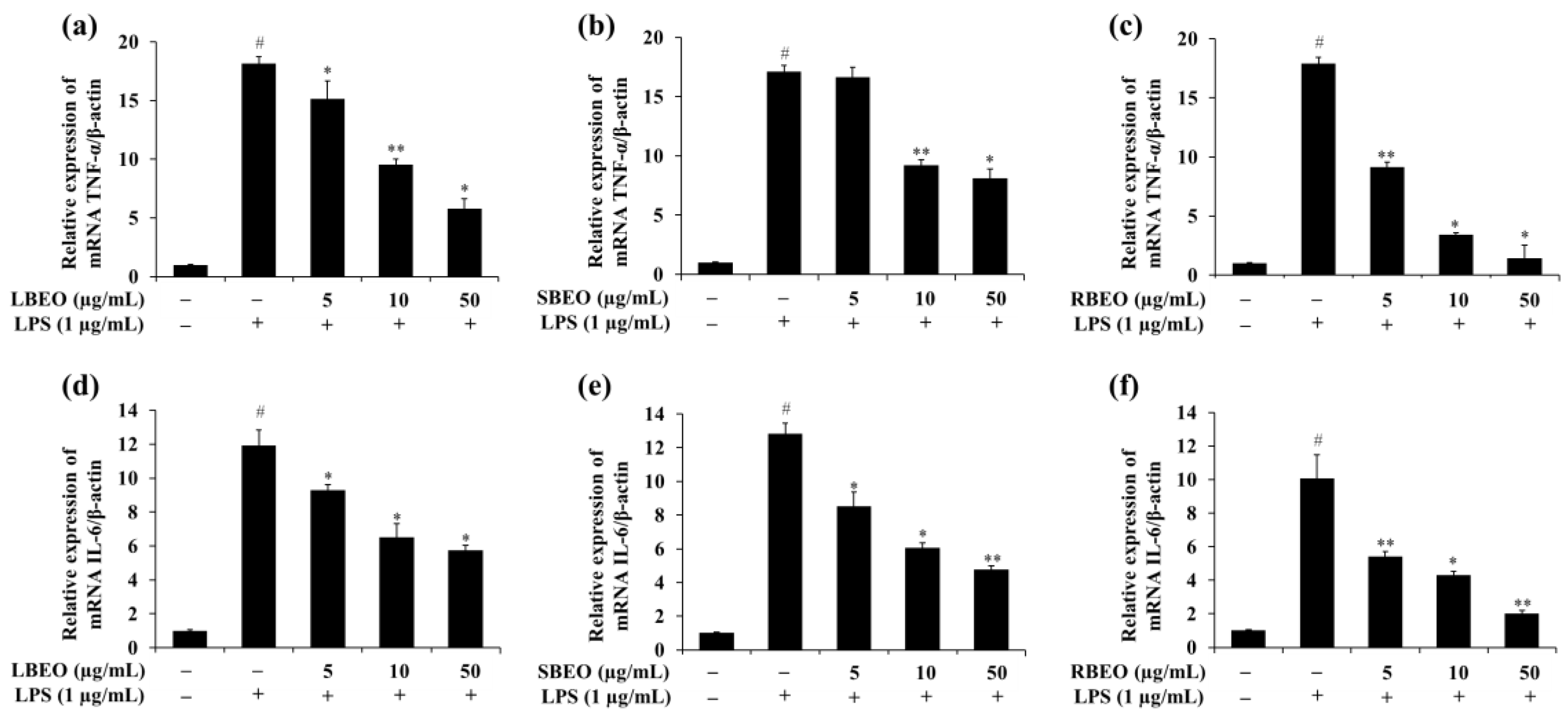
2.4. Effect of the BEOs on Transcription and Translation of TNF-α and IL-6
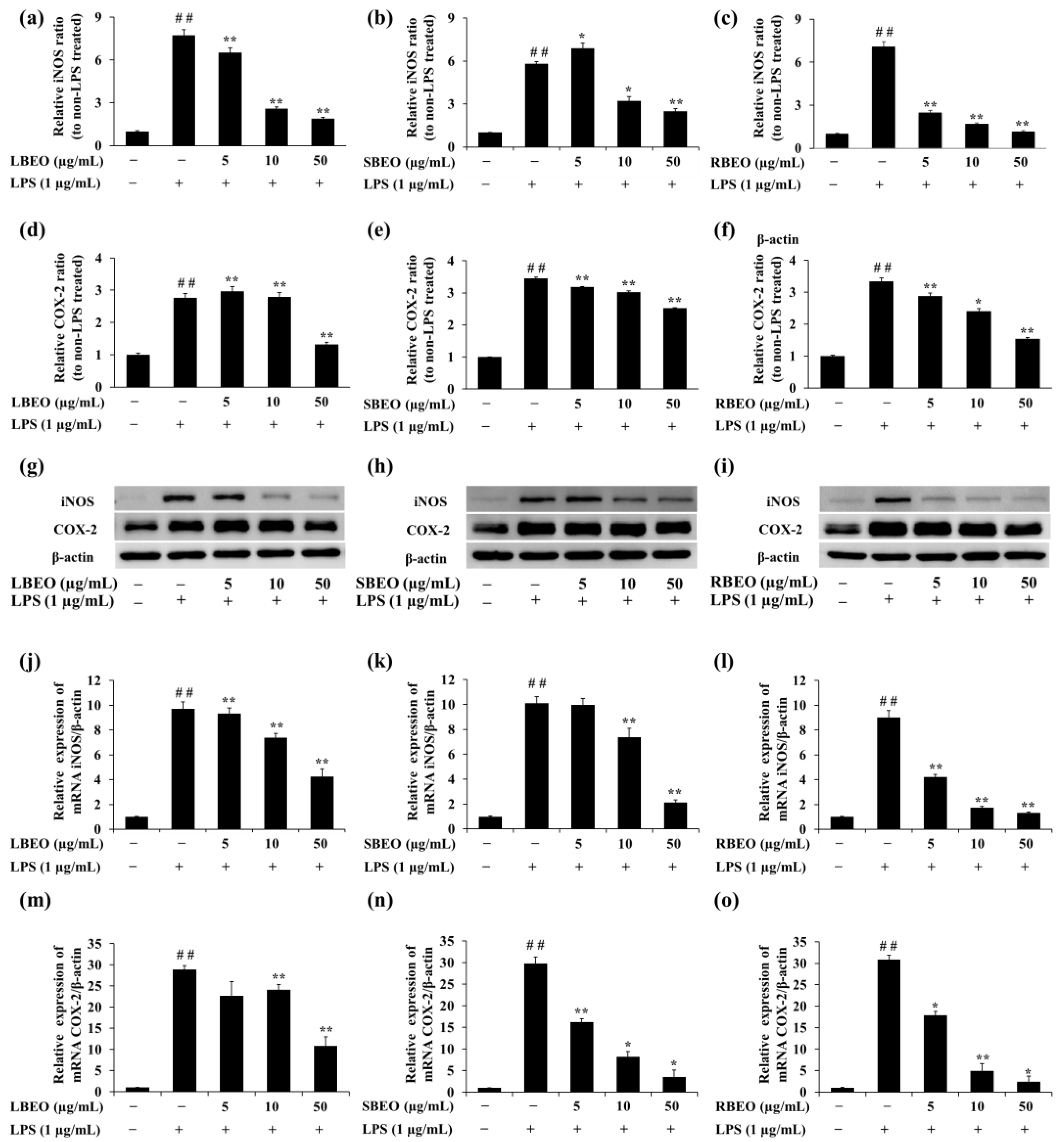
2.5. Effect of the BEOs on the Transcription and Translation of iNOS and COX-2
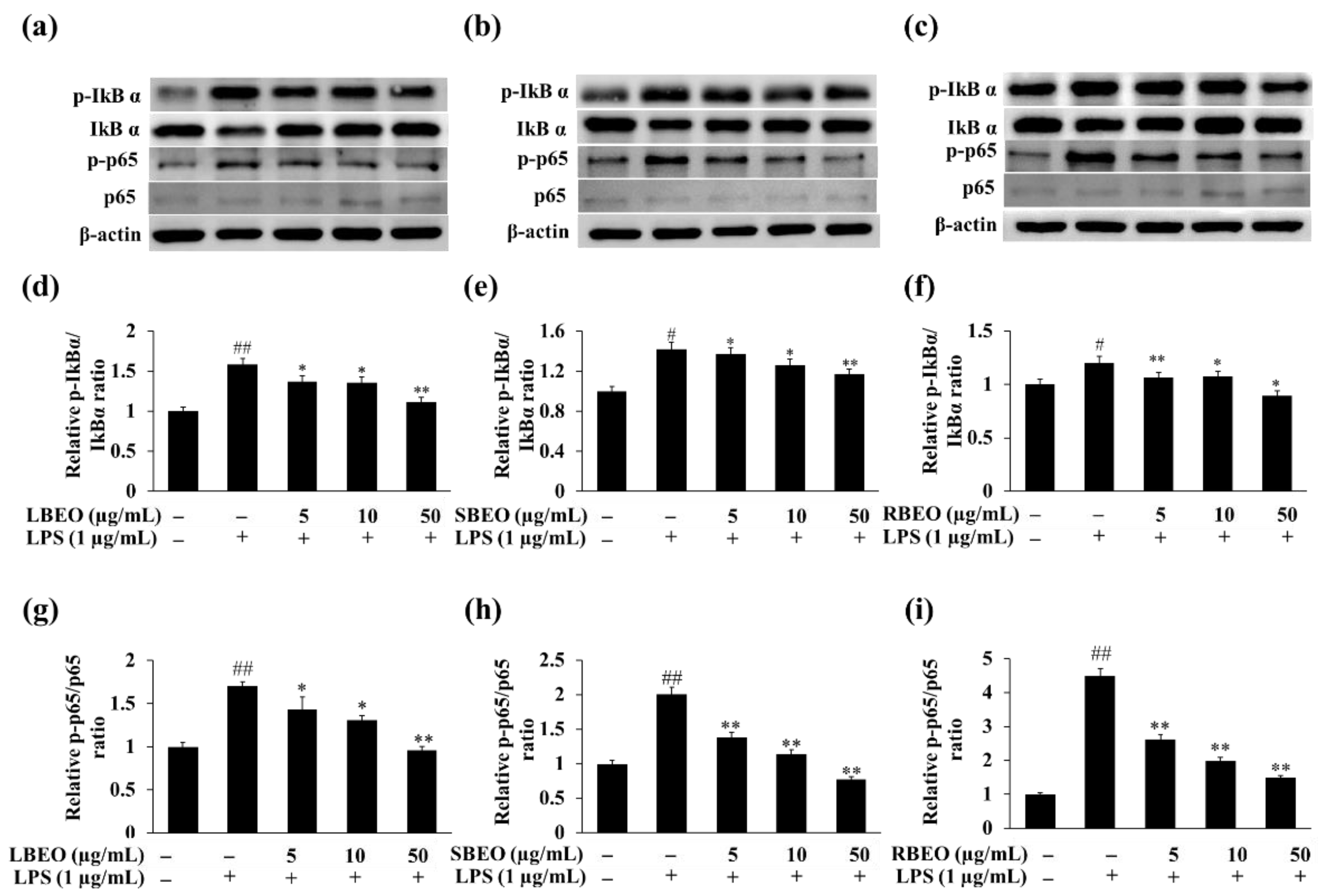
2.6. Inhibitory Effect of BEOs on the NF-κB Pathway in RAW 264.7 Macrophages
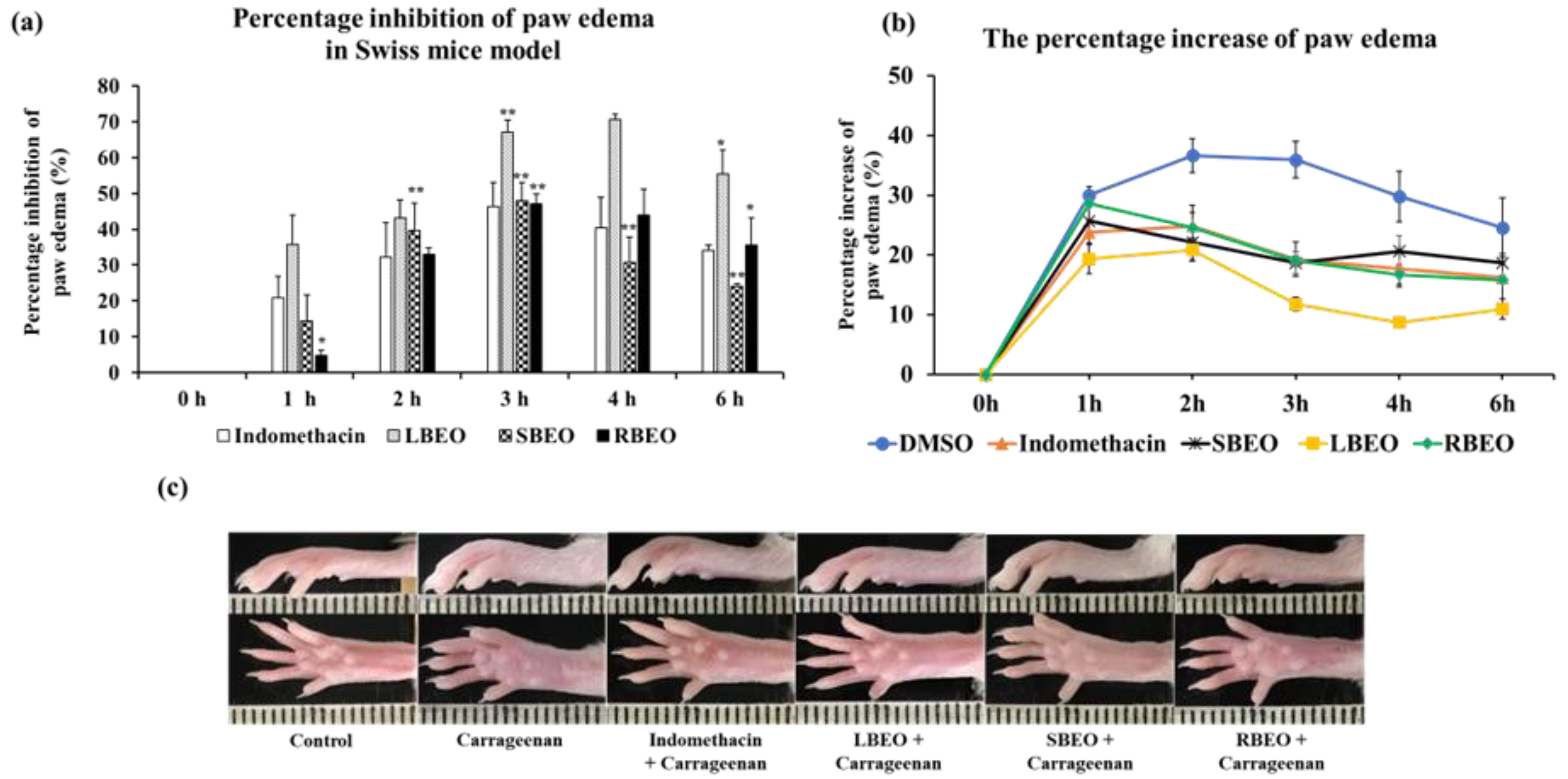
2.7. In Vivo Anti-Inflammatory Assay
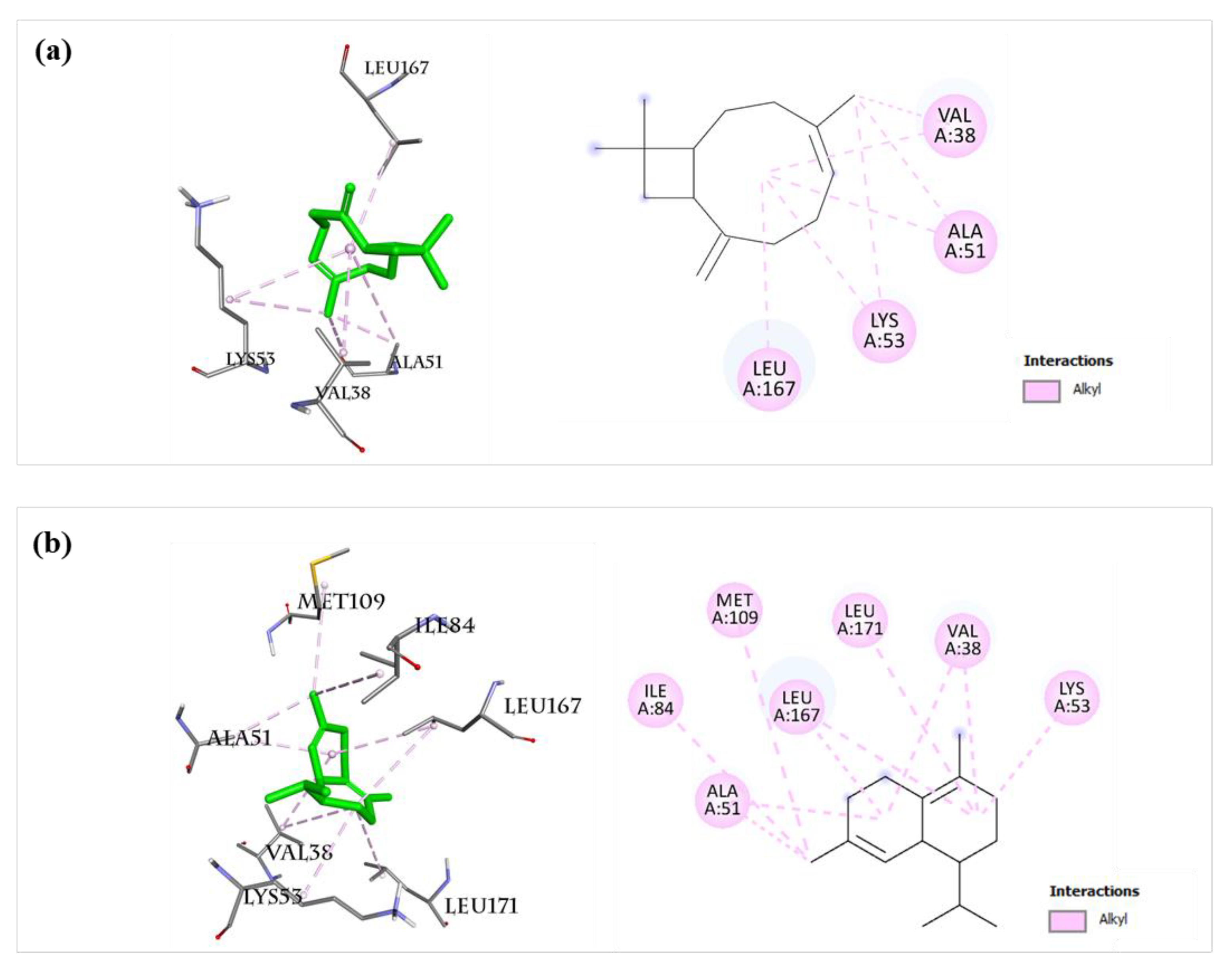
2.8. Molecular Docking of 12 Main Compounds with Anti-Inflammatory Protein Targets
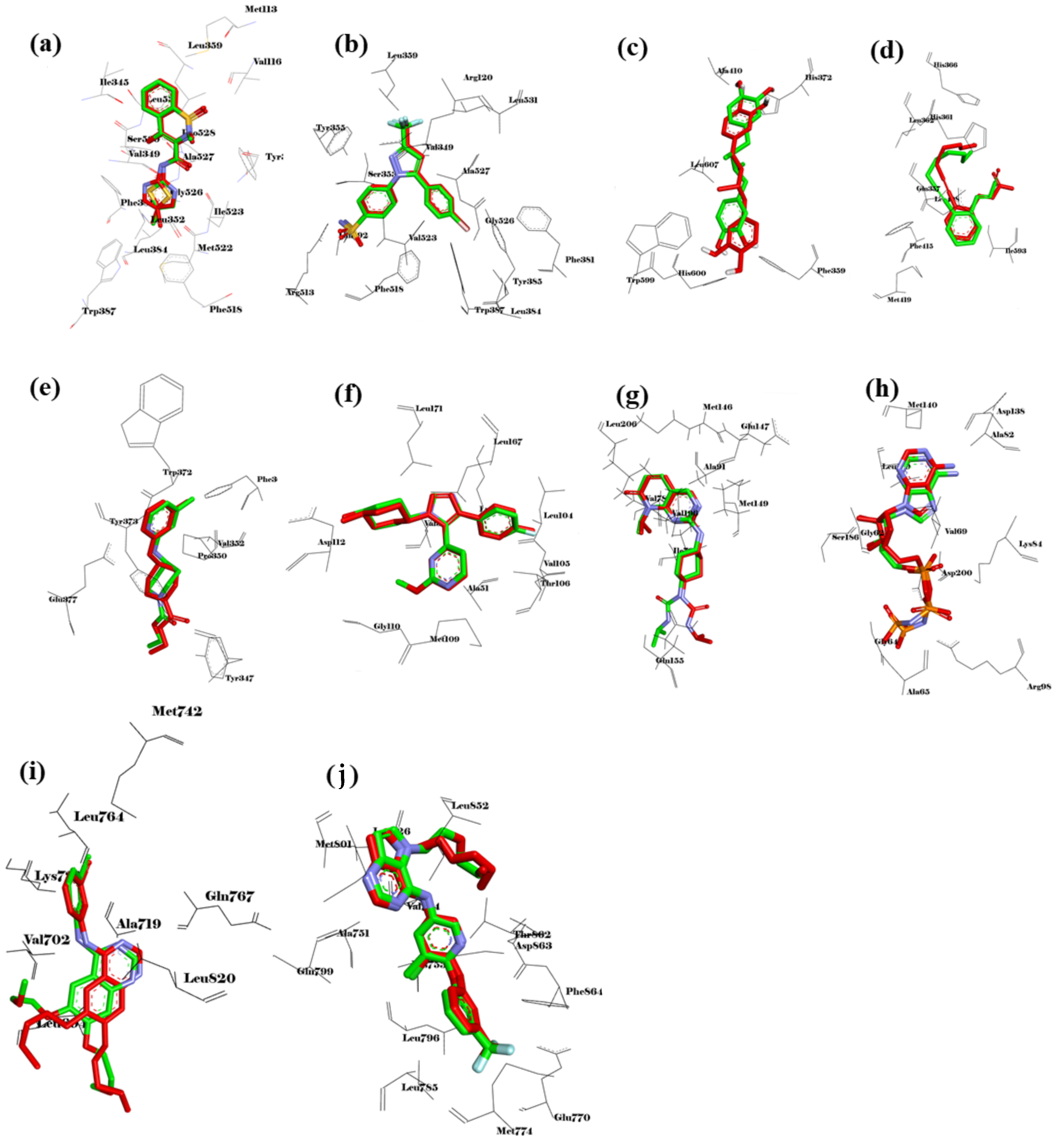
2.8.1. Validation of the Molecular Docking Method
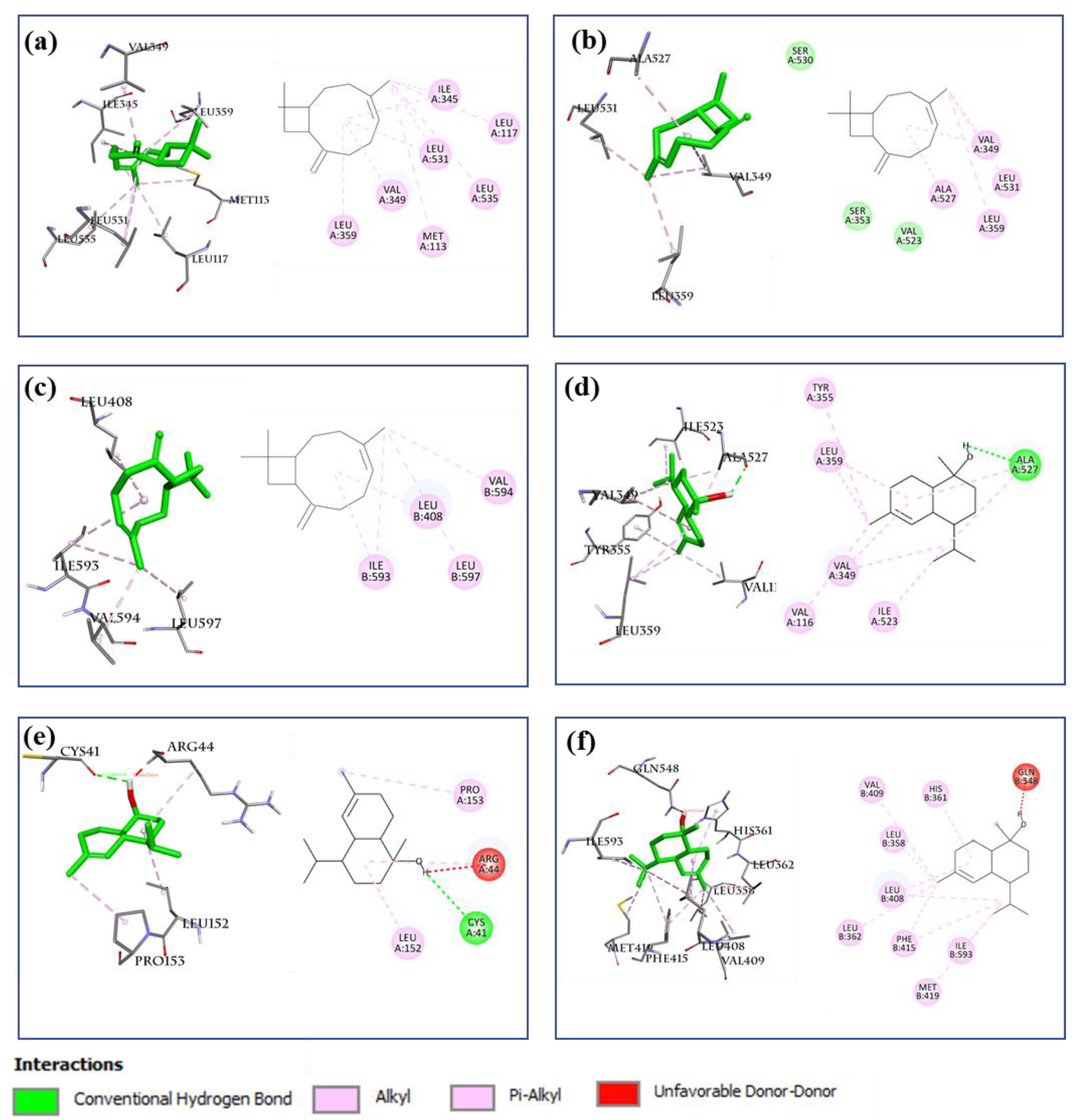
2.8.2. AA Metabolic Pathway
2.8.3. NF-κB Signaling Pathway
2.8.4. MAPK Signaling Pathway
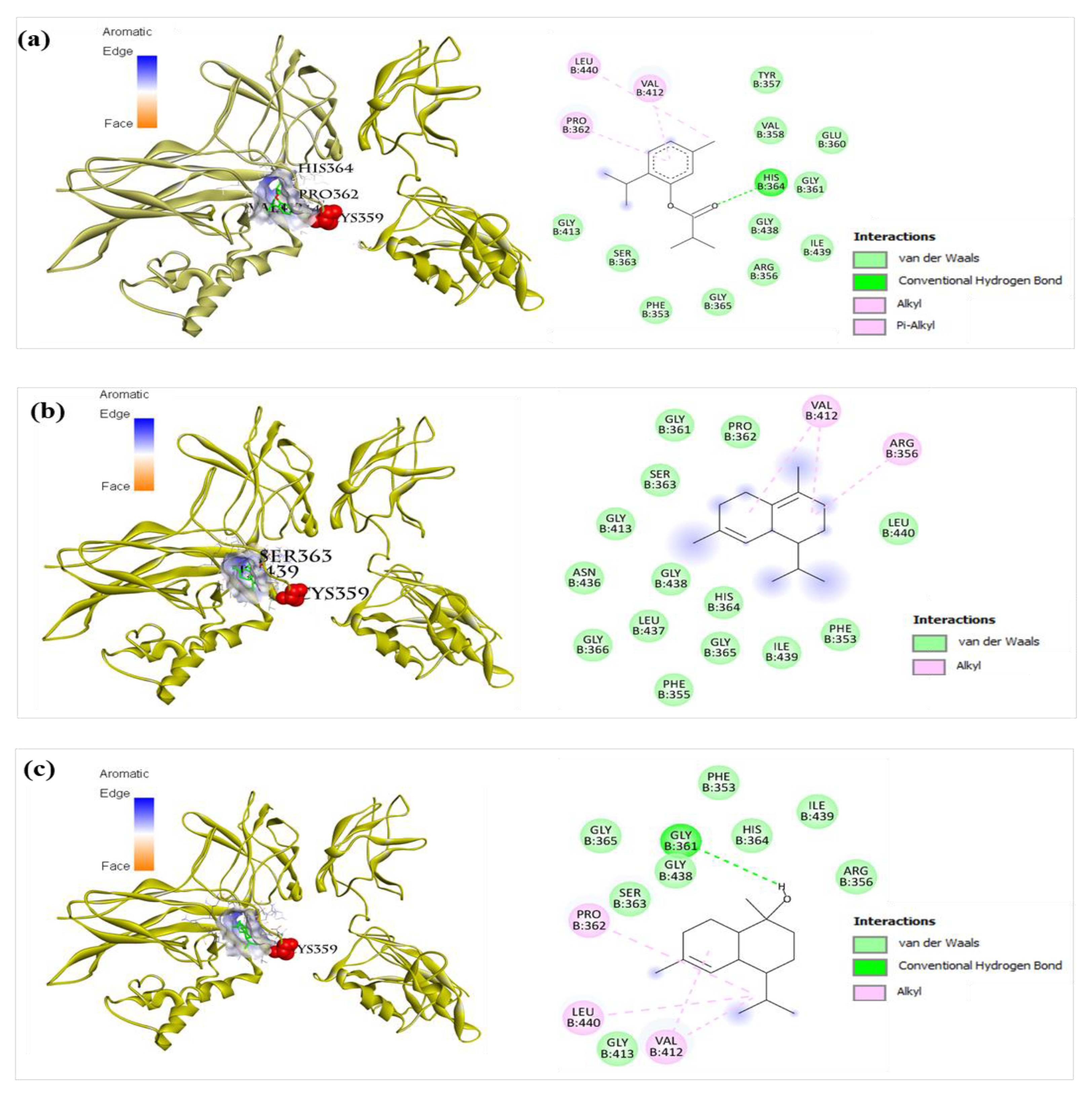
2.8.5. Inflammatory Interleukins (IL-6 and IL-23R)
3. Materials and Methods
3.1. Experimental Animals and Ethics Statement
3.2. Plant Material
3.3. Preparation of EOs
3.4. GC/MS Analyses
3.5. Chemicals and Reagents
3.6. Cell Culture
3.7. Cell Viability Assay
3.8. Measuring NO Production
3.9. ELISA
3.10. Western Blotting
3.11. qRT-PCR
3.12. Anti-Inflammation Assay Using Carrageenan-Induced Edema Model
3.13. Molecular Docking
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Scrivo, R.; Vasile, M. Inflammation as “common soil” of the multifactorial diseases. Autoimmun. Rev. 2011, 10, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Gu, Y.; Wang, C.; Zhang, J.; Zhang, J.; Wang, G.; Wang, F. A Systematic Review of the Anti-Inflammatory and Immunomodulatory Properties of 16 Essential Oils of Herbs. Evid. Based Complement. Alternat. Med. 2020, 2020, 8878927. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- McCarberg, B.; Gibofsky, A. Need to Develop New Nonsteroidal Anti-Inflammatory Drug Formulations. Clin. Ther. 2012, 34, 1954–1963. [Google Scholar] [CrossRef]
- Davies, N.M.; Reynolds, J.K.; Undeberg, M.R.; Gates, B.J.; Ohgami, Y.; Vega-Villa, K.R. Minimizing risks of NSAIDs: Cardiovascular, gastrointestinal and renal. Expert Rev. Neurother. 2006, 6, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.G.; Jick, H. Risk of upper gastrointestinal bleeding and perforation associated with Individual non-steroidal anti-inflammatory drugs. Lancet 1994, 343, 769–772. [Google Scholar] [CrossRef]
- Mahesh, G.; Kumar, K.A.; Reddanna, P. Overview on the Discovery and Development of Anti-Inflammatory Drugs: Should the Focus Be on Synthesis or Degradation of PGE2? J. Inflamm. Res. 2021, 14, 253–263. [Google Scholar] [CrossRef]
- Kim, K.-N.; Ko, S.C.; Ye, B.R.; Kim, M.S.; Kim, J.; Ko, E.Y.; Cho, S.-H.; Kim, D.; Heo, S.-J.; Jung, W.-K. 5-Bromo-2-hydroxy-4-methyl-benzaldehyde inhibited LPS-induced production of pro-inflammatory mediators through the inactivation of ERK, p38, and NF-κB pathways in RAW 264.7 macrophages. Chem. -Biol. Interact. 2016, 258, 108–114. [Google Scholar] [CrossRef]
- Saleh, H.A.; Yousef, M.H.; Abdelnaser, A. The anti-inflammatory properties of phytochemicals and their effects on epigenetic mechanisms involved in TLR4/NF-κB-mediated inflammation. Front. Immunol. 2021, 12, 606069. [Google Scholar] [CrossRef]
- Fujihara, M.; Muroi, M.; Tanamoto, K.-I.; Suzuki, T.; Azuma, H.; Ikeda, H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: Roles of the receptor complex. Pharmacol. Ther. 2003, 100, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef] [PubMed]
- Hongzhi, D.; Xiaoying, H.; Yujie, G.; Le, C.; Yuhuan, M.; Dahui, L.; Luqi, H. Classic mechanisms and experimental models for the anti-inflammatory effect of traditional Chinese medicine. Anim. Model. Exp. Med. 2022, 5, 108–119. [Google Scholar] [CrossRef]
- Mahajan, A.; Hardyal, S.; Tung, B. Effect of concurrent use of cimetidine and anti-inflammatory agents in experimental models of peptic ulcer and inflammation. Indian J. Pharmacol. 1984, 16, 132. [Google Scholar]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. Methods Mol. Biol. 2003, 225, 115–121. [Google Scholar]
- Tsuji, R.; Hoshino, K.; Noro, Y.; Tsuji, N.M.; Kurokawa, T.; Masuda, T.; Akira, S.; Nowak, B. Suppression of allergic reaction by λ-carrageenan: Toll-like receptor 4/MyD88-dependent and-independent modulation of immunity. Clin. Exp. Allergy 2003, 33, 249–258. [Google Scholar] [CrossRef]
- Loi, D.T. Những Cây Thuốc và vị Thuốc Việt Nam [Book in Vietnamese]; Medica Publishing House: Hanoi, Vietnam, 1999; pp. 689–690. [Google Scholar]
- Joshi, R.K. GC-MS Analysis of Volatile Organic Constituents of Traditionally Used Medicinal Plants from the Western Ghatsof India: Blumea lanceolaria (Roxb.) Druce., Heliotropium indicum L. and Triumfetta rhomboidea Jacq. J. Mex. Chem. Soc. 2020, 64, 74–82. [Google Scholar] [CrossRef]
- Lalmuanthanga, C.; Roy, D.C.; Roy, R.K.; Sarma, Y.; Borah, P.; Tamuli, S.; Hmarthansanga, L.; Inaotombi, L.; Ali, D.M.A. Antioxidant activity of methanolic extract of Blumea lanceolaria. Int. J. Chem. Stud. 2019, 7, 3546–3548. [Google Scholar]
- Vineet, K.M.; Ajit, K.P.; Nachimuthu, S.K.; Bhim, P.S.; Mishra, V.K.; Passari, A.; Vanlalhmangaihi, K.; Kumar, N.S.; Singh, B.P. Antimicrobial and antioxidant activities of Blumea lanceolaria (Roxb.). J. Med. Plants Res. 2015, 9, 84–90. [Google Scholar] [CrossRef][Green Version]
- Nguyêñ Xuân Duñg, D.T.L.; Dô Tât, H.ù.n.g.; Piet, A. Leclercq Chemical Composition of the Oil of Blumea lanceolaria (Roxb.) Druce from Vietnam. J. Essent. Oil Res. 1991, 3, 285–286. [Google Scholar] [CrossRef]
- FitzGerald, G.A.; Patrono, C. The Coxibs, Selective Inhibitors of Cyclooxygenase-2. N. Engl. J. Med. 2001, 345, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2020, 133, 110985. [Google Scholar] [CrossRef]
- Li, G.; Fang, Y.; Ma, Y.; Dawa, Y.; Wang, Q.; Gan, J.; Dang, J. Screening and Isolation of Potential Anti-Inflammatory Compounds from Saxifraga atrata via Affinity Ultrafiltration-HPLC and Multi-Target Molecular Docking Analyses. Nutrients 2022, 14, 2405. [Google Scholar] [CrossRef]
- Alamzeb, M.; Setzer, W.N.; Ali, S.; Khan, B.; Rashid, M.-U.; Salman, S.M.; Omer, M.; Ali, J. Spectral, Anti-Inflammatory, Anti-Pyretic, Leishmanicidal, and Molecular Docking Studies, Against Selected Protein Targets, of a New Bisbenzylisoquinoline Alkaloid. Front. Chem. 2021, 9, 711190. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Jaiswal, V.; Akhtar, S.; Jayashree, B.S.; Dhar, K.L. Isolation of isoflavones from Iris kashmiriana Baker as potential anti proliferative agents targeting NF-kappaB. Phytochemistry 2017, 136, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Piccagli, L.; Thangavel, S.; Alam, A. Virtual screening against nuclear factor kappaB (NF-kappaB) of a focus library: Identification of bioactive furocoumarin derivatives inhibiting NF-kappaB dependent biological functions involved in cystic fibrosis. Bioorg. Med. Chem. 2010, 18, 8341–8349. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Loh, X.Y.; Wijaya, H.; Wang, J.; Lin, Q.; Lam, Y.; Wong, W.-S.F.; Mok, Y.K. Specificity and inhibitory mechanism of andrographolide and its analogues as antiasthma agents on NF-kappaB p50. J. Nat. Prod. 2015, 78, 208–217. [Google Scholar] [CrossRef]
- Elkamhawy, A.; Hassan, A.H.; Paik, S.; Lee, Y.S.; Lee, H.-H.; Shin, J.-S.; Lee, K.-T.; Roh, E.J. EGFR inhibitors from cancer to inflammation: Discovery of 4-fluoro-N-(4-(3-(trifluoromethyl)phenoxy)pyrimidin-5-yl)benzamide as a novel anti-inflammatory EGFR inhibitor. Bioorganic Chem. 2019, 86, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Yang, Y.; Tan, Y.; Lu, C.; Pan, Y.; Chen, L.; Lu, G. ERBB2-induced inflammation in lung carcinogenesis. Mol. Biol. Rep. 2012, 39, 7911–7917. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8 (Suppl. S2), S3. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Gepdiremen, A.; Mshvildadze, V.; Süleyman, H.; Elias, R. Acute and chronic antiinflammatory effects of Hedera colchica in rats. J. Ethnopharmacol. 2004, 94, 191–195. [Google Scholar] [CrossRef]
- Lee, W.-S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and TNF-α production by AP-1 and NF-κB inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.-H. Anti-inflammatory effect of the water fraction from hawthorn fruit on LPS-stimulated RAW 264.7 cells. Nutr. Res. Pr. 2011, 5, 101–106. [Google Scholar] [CrossRef]
- Liao, J.; Xie, X.; Wang, W.; Gao, Y.; Cai, Y.; Peng, J.; Li, T.; Yi, Q.; He, C.; Wang, L. Anti-inflammatory activity of essential oil from leaves of Blumea balsamifera (L.) DC through inhibiting TLR4/NF-kB signaling pathways and NLRP3 inflammasome activation in LPS-induced RAW264. 7 macrophage cells. J. Essent. Oil Bear. Plants 2021, 24, 160–176. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hermanson, D.J.; Banerjee, S.; Ghebreselasie, K.; Clayton, G.M.; Garavito, R.M.; Marnett, L.J. Oxicams Bind in a Novel Mode to the Cyclooxygenase Active Site via a Two-water-mediated H-bonding Network. J. Biol. Chem. 2014, 289, 6799–6808. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N.C.; Gerstmeier, J.; Schexnaydre, E.E.; Börner, F.; Garscha, U.; Neau, D.B.; Werz, O.; Newcomer, M.E. Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products. Nat. Chem. Biol. 2020, 16, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Chon, J.K.; Kim, S.; Shin, W. Conformational flexibility in mammalian 15S-lipoxygenase: Reinterpretation of the crystallographic data. Proteins 2008, 70, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.S.; Kim, S.E.; Heo, J.Y.; Lee, M.E.; Kim, H.M.; Paik, S.-G.; Lee, H.; Lee, J.-O. Crystal Structure of the TLR1-TLR2 Heterodimer Induced by Binding of a Tri-Acylated Lipopeptide. Cell 2007, 130, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.E.; Huang, D.B.; Chen, Y.Q.; Ghosh, G. Crystal structure of p50/p65 heterodimer of transcription factor NF-kappaB bound to DNA. Nature 1998, 391, 410–413. [Google Scholar] [CrossRef]
- Shibata, H.; Yoshioka, Y.; Ohkawa, A.; Minowa, K.; Mukai, Y.; Abe, Y.; Taniai, M.; Nomura, T.; Kayamuro, H.; Nabeshi, H.; et al. Creation and X-ray structure analysis of the tumor necrosis factor receptor-1-selective mutant of a tumor necrosis factor-alpha antagonist. J. Biol. Chem. 2008, 283, 998–1007. [Google Scholar] [CrossRef]
- Garcin, E.D.; Arvai, A.S.; Rosenfeld, R.J.; Kroeger, M.D.; Crane, B.R.; Andersson, G.; Andrews, G.; Hamley, P.; Mallinder, P.R.; Nicholls, D.J.; et al. Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat. Chem. Biol. 2008, 4, 700–707. [Google Scholar] [CrossRef]
- Watterson, D.M.; Grum-Tokars, V.L.; Roy, S.M.; Schavocky, J.P.; Bradaric, B.; Bachstetter, A.; Xing, B.; Dimayuga, E.; Saeed, F.; Zhang, H.; et al. Development of Novel In Vivo Chemical Probes to Address CNS Protein Kinase Involvement in Synaptic Dysfunction. PLoS ONE 2013, 8, e66226. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Park, C.M.; Iqbal, S.; Hernandez, P.; Park, H.; LoGrasso, P.V.; Feng, Y. Pyridopyrimidinone Derivatives as Potent and Selective c-Jun N-Terminal Kinase (JNK) Inhibitors. ACS Med. Chem. Lett. 2015, 6, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Glatz, G.; Gógl, G.; Alexa, A.; Reményi, A. Structural Mechanism for the Specific Assembly and Activation of the Extracellular Signal Regulated Kinase 5 (ERK5) Module. J. Biol. Chem. 2013, 288, 8596–8609. [Google Scholar] [CrossRef] [PubMed]
- Stamos, J.; Sliwkowski, M.X.; Eigenbrot, C. Structure of the Epidermal Growth Factor Receptor Kinase Domain Alone and in Complex with a 4-Anilinoquinazoline Inhibitor. J. Biol. Chem. 2002, 277, 46265–46272. [Google Scholar] [CrossRef]
- Aertgeerts, K.; Skene, R.; Yano, J.; Sang, B.-C.; Zou, H.; Snell, G.; Jennings, A.; Iwamoto, K.; Habuka, N.; Hirokawa, A.; et al. Structural Analysis of the Mechanism of Inhibition and Allosteric Activation of the Kinase Domain of HER2 Protein. J. Biol. Chem. 2011, 286, 18756–18765. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, M.J.; Chow, D.C.; Brevnova, E.E.; Garcia, K.C. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science 2003, 300, 2101–2104. [Google Scholar] [CrossRef]
- Lupardus, P.J.; Garcia, K.C. The structure of interleukin-23 reveals the molecular basis of p40 subunit sharing with interleukin-12. J. Mol. Biol. 2008, 382, 931–941. [Google Scholar] [CrossRef]
- BIOVIA, D.S. Discovery Studio Visualizer; v21.1.0.20298; Dassault Systèmes: San Diego, CA, USA, 2021; Available online: https://discover.3ds.com/discovery-studio-visualizer-download (accessed on 12 May 2022)v21.1.0.20298.
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Suzek, T.O.; Zhang, J.; Wang, J.; Bryant, S.H. PubChem: A public information system for analyzing bioactivities of small molecules. Nucleic Acids Res. 2009, 37, W623–W633. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System; Version 2.4.0; Schrodinger, L.L.C.: New York, NY, USA, 2020.
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]



| No. | RT (min) | RI | Chemical Composition | Relative Content (%) | ||
|---|---|---|---|---|---|---|
| Leaf (LBEO) | Stem (SBEO) | Root (RBEO) | ||||
| 1 | 10.22 | 930 | α-Thujene | - | - | 0.14 |
| 2 | 10.50 | 939 | α-Pinene | - | 2.28 | 33.36 |
| 3 | 13.35 | 1029 | o-Cymene | 38.29 | 3.35 | 0.92 |
| 4 | 13.51 | 1033 | Limonene | - | - | 0.14 |
| 5 | 15.82 | 1101 | Linalool | - | 0.12 | - |
| 6 | 18.23 | 1169 | Albene | - | - | 0.72 |
| 7 | 19.22 | 1198 | α-Terpineol | - | - | 0.14 |
| 8 | 20.38 | 1231 | Nerol | - | - | 0.57 |
| 9 | 20.66 | 1239 | Thymol methyl ether | 0.24 | 0.36 | 21.70 |
| 10 | 20.75 | 1242 | Carvacrol methyl ether | 58.28 | 89.40 | - |
| 11 | 22.50 | 1293 | Thymol | - | - | 4.25 |
| 12 | 24.94 | 1366 | α-Longipinene | - | - | 0.14 |
| 13 | 25.49 | 1383 | Cyclosativene | - | - | 0.24 |
| 14 | 25.70 | 1389 | α-Copaene | - | 0.37 | 0.65 |
| 15 | 26.47 | 1413 | Petasitene | - | - | 0.12 |
| 16 | 27.22 | 1437 | β-Caryophyllene | 0.26 | 0.82 | 0.53 |
| 17 | 27.91 | 1459 | Epi-β-Santalene | - | - | 0.11 |
| 18 | 27.95 | 1460 | (Z)-β-Farnesene | - | - | 0.19 |
| 19 | 28.30 | 1471 | α-Humulene | - | 0.17 | 0.24 |
| 20 | 28.93 | 1492 | Thymol isobutyrate | - | - | 29.23 |
| 21 | 29.12 | 1498 | Germacrene D | - | 0.16 | 0.16 |
| 22 | 29.22 | 1501 | β-Selinene | - | - | 0.68 |
| 23 | 30.28 | 1537 | δ-Cadinene | - | 0.18 | 0.26 |
| 24 | 31.54 | 1579 | Geranyl n-butanoate | - | - | 0.47 |
| 25 | 31.98 | 1593 | Scapanol | - | 0.16 | 0.15 |
| 26 | 32.08 | 1597 | Prenopsan-8-ol | - | - | 0.21 |
| 27 | 33.84 | 1659 | Tau-Cadinol | - | 0.29 | 0.30 |
| 28 | 34.21 | 1672 | α-Cadinol | - | 0.27 | - |
| 29 | 38.16 | 1818 | Hexadecanal | - | 0.25 | - |
| 30 | 39.76 | 1881 | Hexadecanol | 2.05 | 0.26 | - |
| Total | 99.12 | 98.44 | 95.62 | |||
| Monoterpenes | 96.81 | 95.51 | 61.94 | |||
| Sesquiterpenes | 0.26 | 2.13 | 4.45 | |||
| Other | 2.05 | 0.51 | 29.23 | |||
| Compounds | Calculated Affinity Energy (kcal/mol) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Name | COX-1 | COX-2 | 5-LOX | ALOX15 | TLR | NF- κB p50p65 | TNF-α | iNOS | p38MAPK | JNK | ERK5 | EGFR | ERBB2 | IL-6 | IL-23R |
| 2 | α-Pinene | −6.1 | −6.2 | −4.9 | −6.4 | −3.5 | −4.9 | −4.9 | −5.3 | −5.9 | −5.5 | −5.3 | −5.5 | −6.4 | −4.7 | −4.3 |
| 3 | o-Cymene | −6.4 | −6.3 | −5.0 | −6.2 | −4.0 | −5.9 | −4.7 | −5.8 | −6.3 | −5.7 | −5.7 | −5.6 | −6.3 | −4.7 | −4.3 |
| 9 | Thymol methyl ether | −6.4 | −6.7 | −5.5 | −6.4 | −4.0 | −5.9 | −5.4 | −5.5 | −6.1 | −5.8 | −5.8 | −5.6 | −6.6 | −5.0 | −4.5 |
| 10 | Carvacrol methyl ether | −6.2 | −6.9 | −5.8 | −6.7 | −4.1 | −6.2 | −5.3 | −6.2 | −6.5 | −5.8 | −6.0 | −6.0 | −6.9 | −4.9 | −4.4 |
| 11 | Thymol | −6.3 | −6.5 | −5.7 | −6.3 | −4.0 | −6.1 | −5.3 | −6.2 | −6.6 | −5.6 | −6.0 | −5.9 | −6.9 | −4.9 | −4.4 |
| 14 | α-Copaene | −7.5 | −7.7 | −6.4 | −7.6 | −4.4 | −5.8 | −6.3 | −6.1 | −7.4 | −7.2 | −7.3 | −6.9 | −7.2 | −5.4 | −5.5 |
| 16 | β- Caryophyllene | −9.0 | −8.1 | −6.2 | −8.2 | −5.2 | −6.7 | −6.6 | −7.1 | −8.2 | −8.0 | −7.2 | −7.4 | −7.7 | −6.1 | −5.7 |
| 19 | α-Humulene | −7.2 | −7.7 | −6.1 | −7.4 | −5.0 | −5.8 | −6.7 | −6.1 | −7.8 | −7.1 | −6.9 | −7.2 | −7.3 | −5.6 | −5.7 |
| 20 | Thymol isobutyrate | −7.6 | −7.7 | −6.1 | −7.6 | −4.7 | −7.0 | −6.1 | −6.4 | −7.1 | −6.5 | −6.7 | −6.5 | −8.0 | −5.3 | −5.2 |
| 23 | δ-Cadinene | −7.4 | −7.8 | −6.1 | −7.7 | −4.7 | −7.6 | −6.5 | −6.5 | −8.5 | −7.4 | −7.5 | −7.0 | −8.3 | −5.9 | −5.8 |
| 27 | Tau-Cadinol | −7.0 | −7.8 | −6.4 | −9.1 | −5.0 | −7.1 | −6.6 | −6.3 | −7.4 | −7.3 | −7.0 | −7.5 | −8.2 | −6.2 | −6.1 |
| 30 | 1-Hexadecanol | −5.9 | −5.7 | −4.4 | −4.7 | −3.1 | −4.6 | −4.6 | −5.5 | −5.7 | −5.0 | −4.8 | −4.7 | −6.4 | −4.1 | −3.6 |
| Reference ligands | Docking scores | −9.4 | −11.0 | −7.1 | −7.3 | NA | NA | NA | −8.3 | −7.8 | −7.8 | −8.0 | −7.4 | −11.4 | NA | NA |
| Name | Meloxi-cam | Celeco-xib | 30Z | RS7 | NA | NA | NA | AT2 | SB0 | PDB ID:519 | ANP | AQ4 | 03Q | NA | NA | |
| No. | Name | Sequence (5′-3′) | Length (nm) | Size (bp) | Reference |
|---|---|---|---|---|---|
| 1 | β-actin-F | ATCACTATTGGCAACGAGCG | 20 | 191 | Lee, 2016 [41] |
| 2 | β-actin-R | TCAGCAATGCCTGGGTACAT | 20 | ||
| 3 | iNOS-F | TTCCGAAGTTTCTGGCAGCAGC | 25 | 491 | Li, 2011 [42] |
| 4 | iNOS-R | TGTCAGAGCCTCGTGGCTTTGG | 25 | ||
| 5 | COX2-F | GGAGTCTGGAACATTGTGAAC | 21 | 156 | Tian, 2021 [43] |
| 6 | COX2-R | GTAGTAGGAGAGGTTGGAGAAG | 22 | ||
| 7 | TNF-α-F | ATGAGCACAGAAAGCATGATC | 21 | 276 | Kim, 2016 [9] |
| 8 | TNF-α-R | TACAGGCTTGTCACTCGAATT | 21 | ||
| 9 | IL-6-F | GAGGATACCACTCCCAACAGACC | 23 | 141 | Lee, 2016 [41] |
| 10 | IL-6-R | AAGTGCATCATCGTTGTTCATACA | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, T.T.H.; Nguyen, T.U.; Nguyen, T.T.H.; Ho, T.Y.; Pham, T.L.H.; Le, T.S.; Nguyen, T.H.V.; Nguyen, P.-H.; Nguyen, Q.H.; Nguyen, V.S. Essential Oils from the Leaves, Stem, and Roots of Blumea lanceolaria (Roxb.) Druce in Vietnam: Determination of Chemical Composition, and In Vitro, In Vivo, and In Silico Studies on Anti-Inflammatory Activity. Molecules 2022, 27, 7839. https://doi.org/10.3390/molecules27227839
Do TTH, Nguyen TU, Nguyen TTH, Ho TY, Pham TLH, Le TS, Nguyen THV, Nguyen P-H, Nguyen QH, Nguyen VS. Essential Oils from the Leaves, Stem, and Roots of Blumea lanceolaria (Roxb.) Druce in Vietnam: Determination of Chemical Composition, and In Vitro, In Vivo, and In Silico Studies on Anti-Inflammatory Activity. Molecules. 2022; 27(22):7839. https://doi.org/10.3390/molecules27227839
Chicago/Turabian StyleDo, Thi Thanh Huyen, Thi Uyen Nguyen, Thi Thu Huyen Nguyen, Thi Yen Ho, Thi Luong Hang Pham, Tho Son Le, Thi Hong Van Nguyen, Phi-Hung Nguyen, Quang Huy Nguyen, and Van Sang Nguyen. 2022. "Essential Oils from the Leaves, Stem, and Roots of Blumea lanceolaria (Roxb.) Druce in Vietnam: Determination of Chemical Composition, and In Vitro, In Vivo, and In Silico Studies on Anti-Inflammatory Activity" Molecules 27, no. 22: 7839. https://doi.org/10.3390/molecules27227839
APA StyleDo, T. T. H., Nguyen, T. U., Nguyen, T. T. H., Ho, T. Y., Pham, T. L. H., Le, T. S., Nguyen, T. H. V., Nguyen, P.-H., Nguyen, Q. H., & Nguyen, V. S. (2022). Essential Oils from the Leaves, Stem, and Roots of Blumea lanceolaria (Roxb.) Druce in Vietnam: Determination of Chemical Composition, and In Vitro, In Vivo, and In Silico Studies on Anti-Inflammatory Activity. Molecules, 27(22), 7839. https://doi.org/10.3390/molecules27227839






