Status Quo on Graphene Electrode Catalysts for Improved Oxygen Reduction and Evolution Reactions in Li-Air Batteries
Abstract
1. Introduction

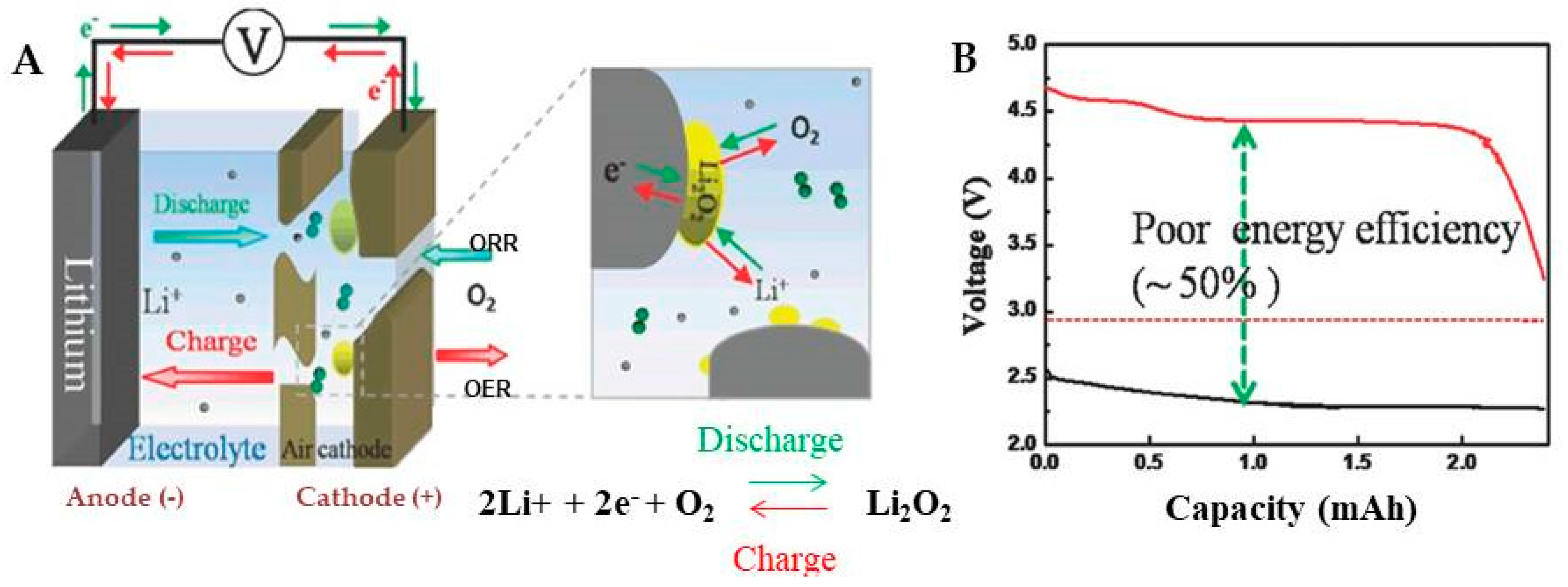
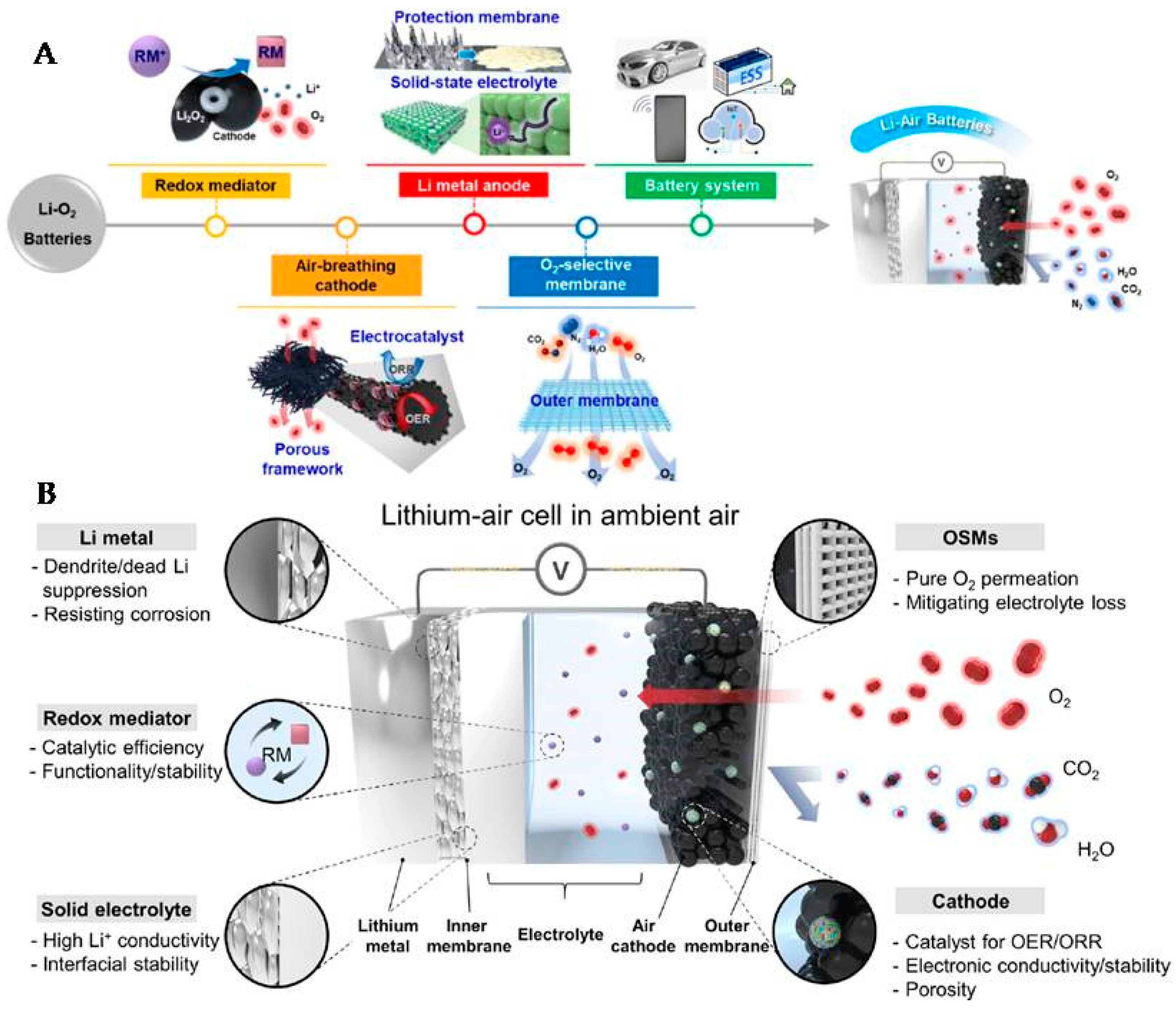
2. Li-O2 Batteries
2.1. Anode
2.2. Cathode
2.3. Electrolyte
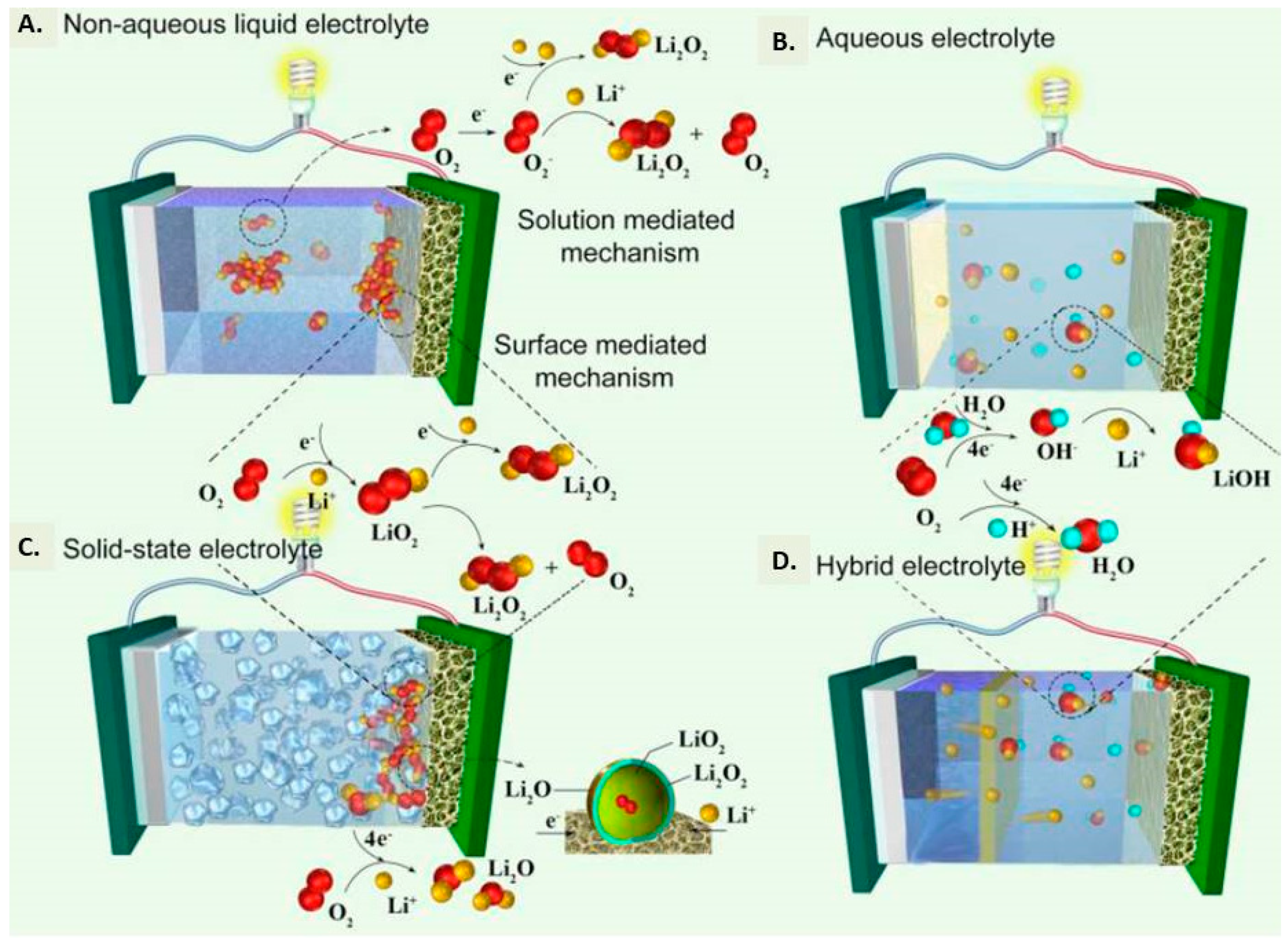
2.3.1. Non-Aqueous/Aprotic/Organic Li-O2 Batteries
2.3.2. Aqueous Electrolyte Li-O2 Battery
2.3.3. Solid-State Li-O2 Battery
2.3.4. Hybrid (Aprotic/Aqueous/Solid) Li-O2 Battery
2.4. Separator
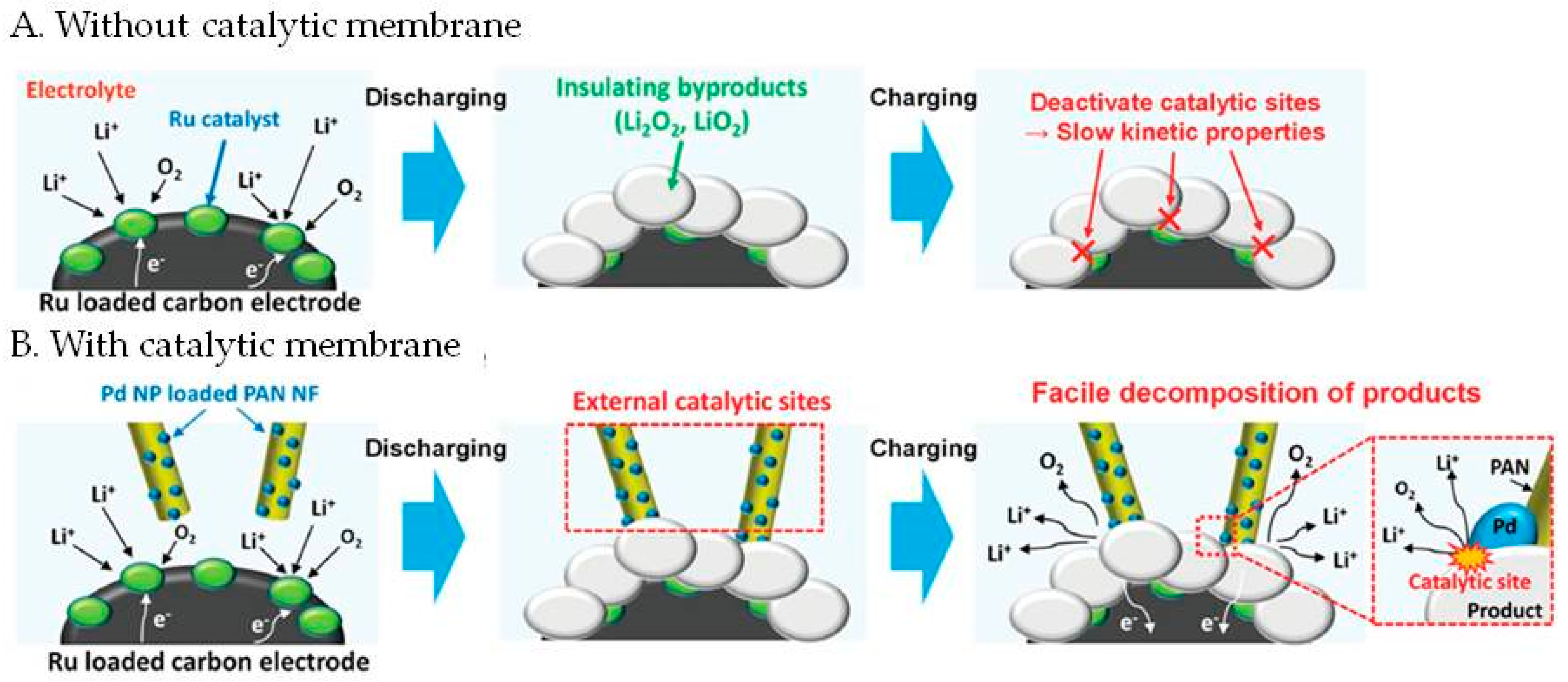
2.5. Electrode Membrane
3. Graphene and its Composite Catalysts as Electrodes
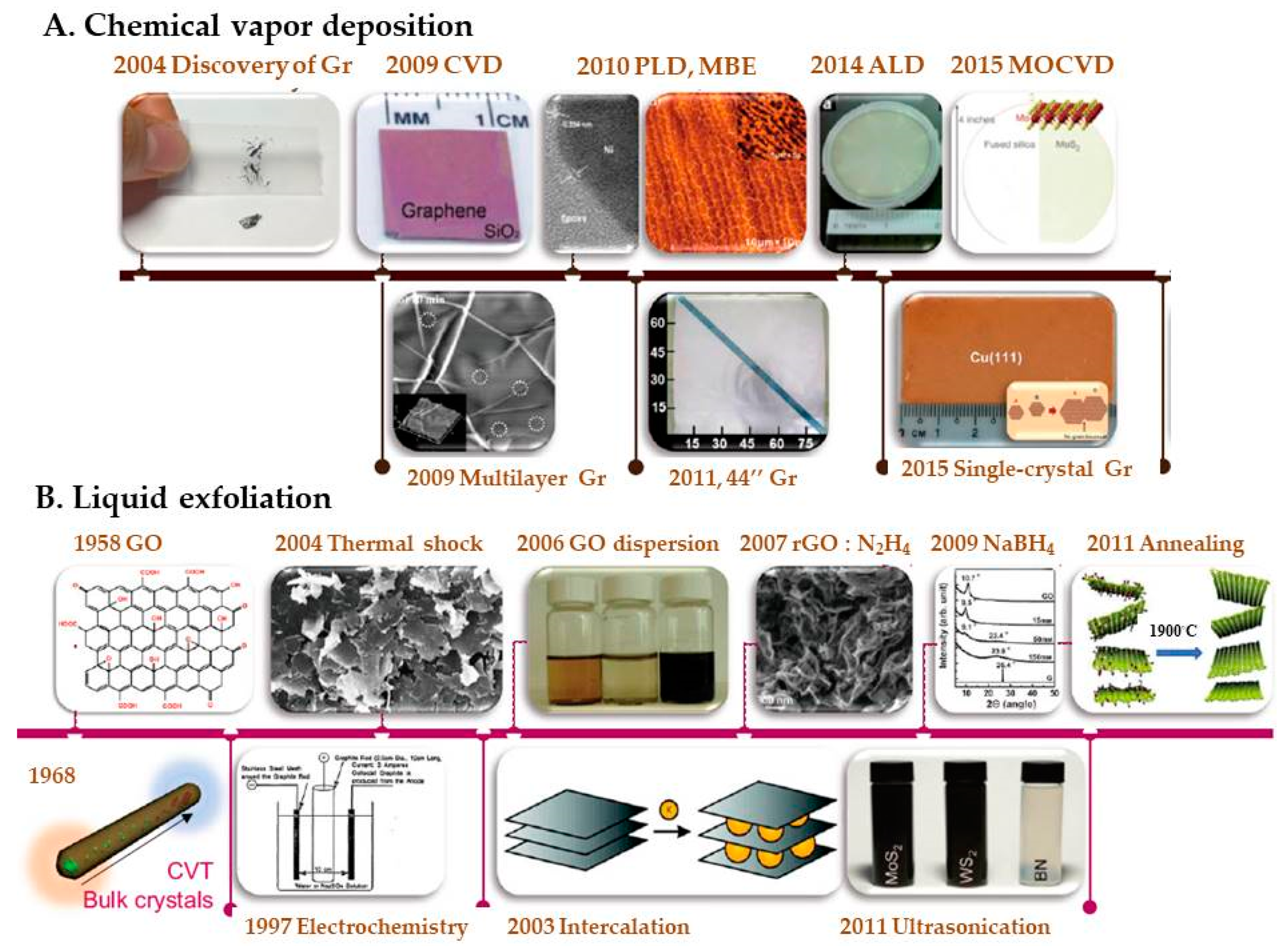
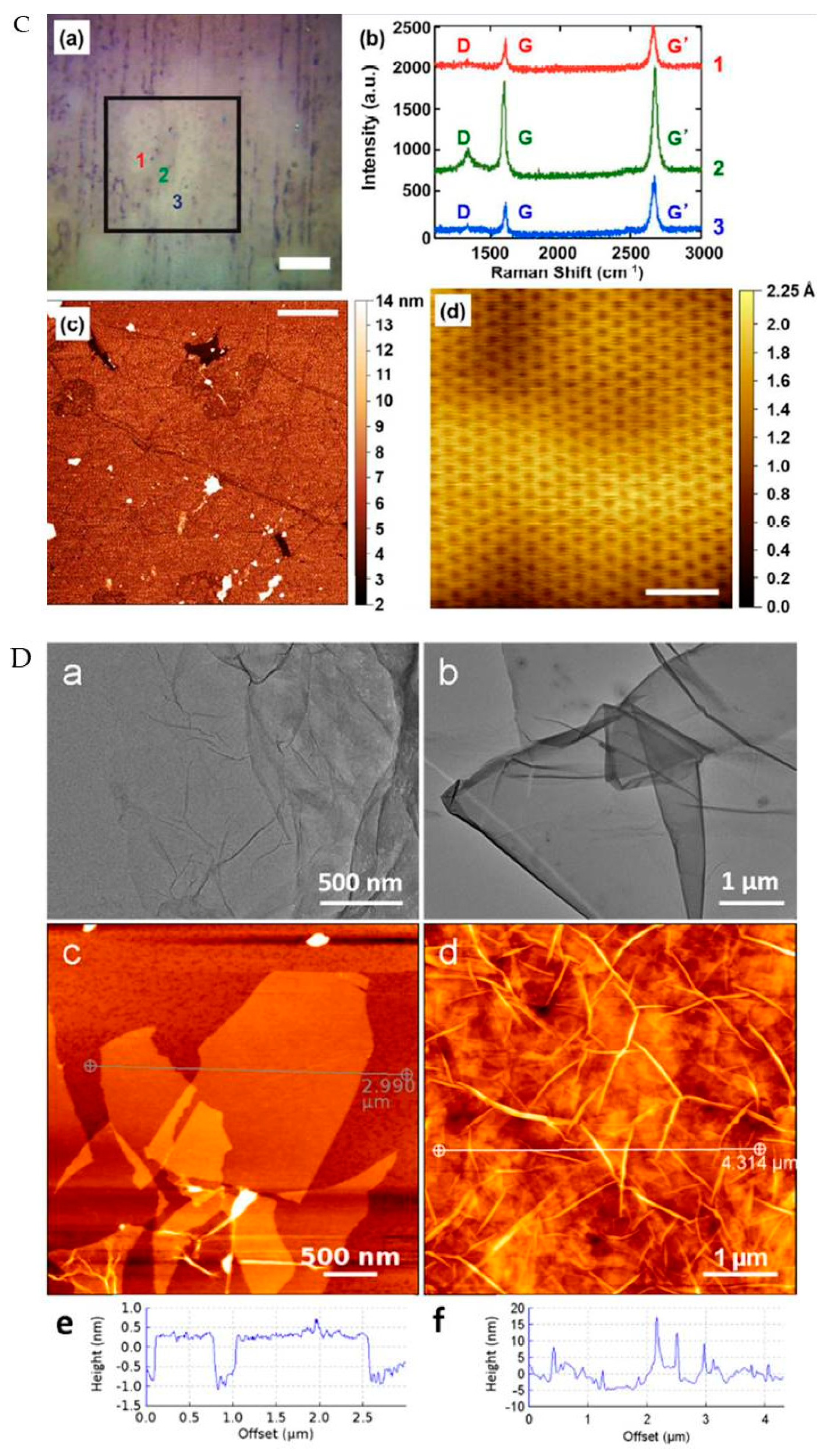
3.1. Synthesis and Characterization of Graphene Materials
3.2. Graphene and Non-Metal Doped Graphene
3.2.1. Graphene
3.2.2. Porous Graphene
3.2.3. Non-Metal Doped Graphene
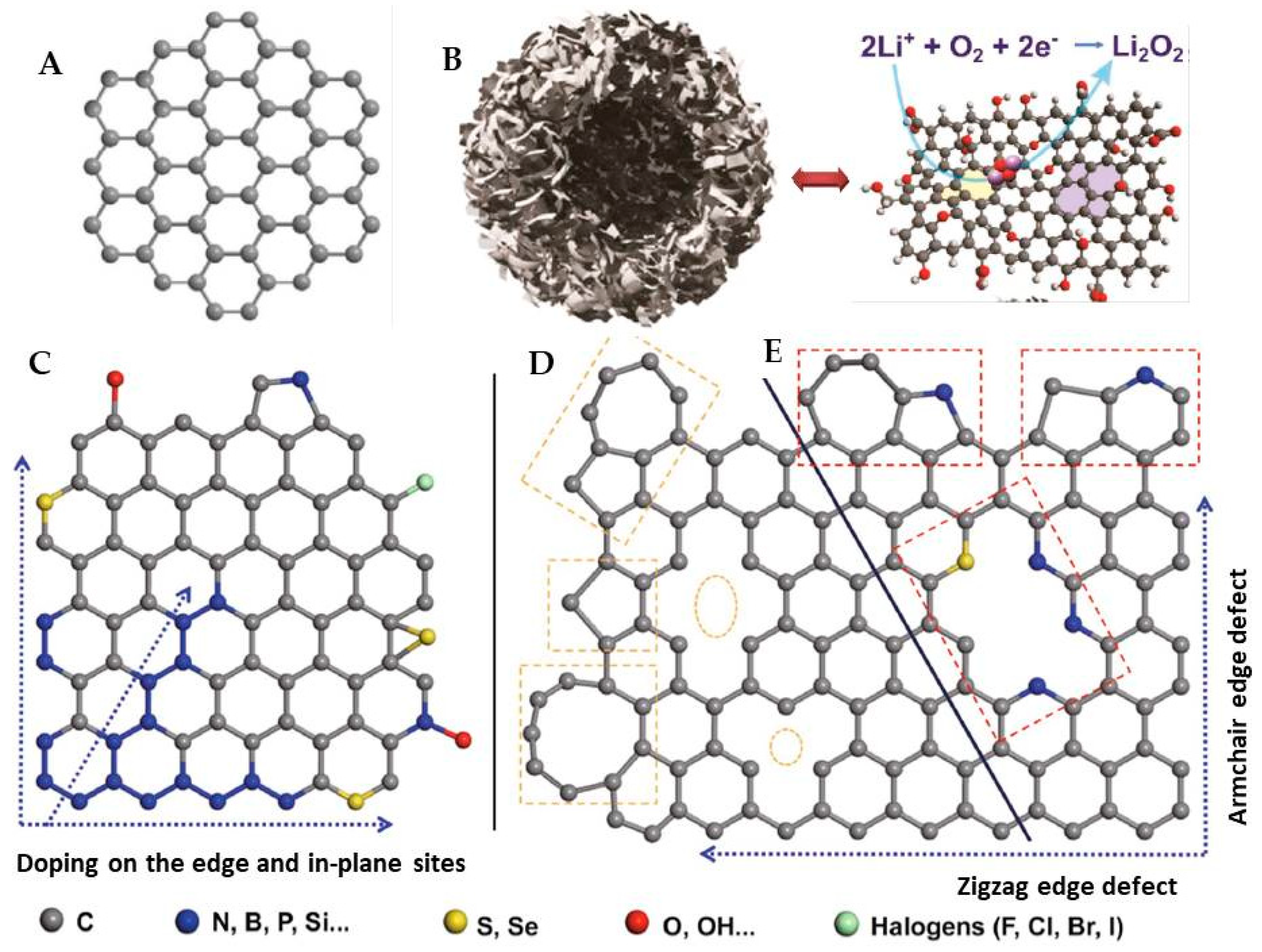
3.3. Graphene-Metals
3.4. Graphene-Metal Oxides
| Graphene Supported Metal Catalysts for ORR and OER | ||||||
|---|---|---|---|---|---|---|
| S. No. | Catalyst | Electrolyte | Discharge Voltage (V) | Discharge Capacity (mA h g−1) | Current Density | Ref. No. |
| 1 | Ru-rGO | LiCF3SO3 -TEGDME | 3.7 | 5000 | 500 mA g−1 | [74] |
| 2 | Porous Graphene—Ru | LiClO4—DMSO | 2.79 | 17,700 | 200 mA g−1 | [126] |
| 3 | N doped Co @ Graphene | LiCF3SO3 in TEGDME | 2.8 | 3.65 mA h cm−2 | 0.1 mA cm−2 | [128] |
| 4 | MnNiFe/Laser Induced Graphene | LiCF3SO3/G4 | 2.9 | 26.3 mA h cm−2 | 0.08 mA cm−2 | [129] |
| Graphene supported metal oxide catalysts for ORR and OER | ||||||
| 5 | RuO2 decorated graphene nanoribbons | LiCF3SO3 in TEGDME | 1.652 | 5397 mA h g−1 | 100 mA g−1 | [152] |
| 6 | CuCr2O4@rGO nanocomposites | LiCF3SO3 in TEGDME | 0.99 | 1000 mA h g−1 | 100 mA g−1 | [154] |
| 7 | MnO2@rGO | LiCF3SO3 in TEGDME | 0.15 | 5139 mA h g−1 | 100 mA g−1 | [155] |
| 8 | 3DNiCo2O4 dodecahedron nanosheets decorated@ 2D graphene nanosheets. | LiCF3SO3 in TEGDME | 2.79 | 7201 mA h g−1 | 100 mA g−1 | [156] |
| 9 | ZrO2@NiCo2O4/GNS | LiCF3SO3 in TEGDME | 2.95 | 9034 mA h g−1 | 50 mA g−1 | [157] |
3.5. Other Carbon Nanomaterials (CNTs, CNFs, CDs)
- Graphene has the highest surface sensitivity, optical transparency, chemical stability, and flexibility of any substance in the universe. It is also the strongest and thinnest material known to man. These qualities are good enough to make a material for electrodes [29].
- Several inorganic and organic materials can be doped or functionalized to create composite materials that give the catalyst a necessary property because of the high surface area and surface chemistry of graphene [30].
- Lithium ions can embed and de-embed more quickly in multilayer graphene materials because the space between layers is much wider than it is in graphite.
- Graphite is typically the most adaptable anode material. However, because of its high surface area, thermal and electrical conductivity, Young’s modulus, flexibility, and strength for use in flexible electrodes, etc., graphene might provide higher performance. Although it can hold more Li+, it is inactive while performing its function.
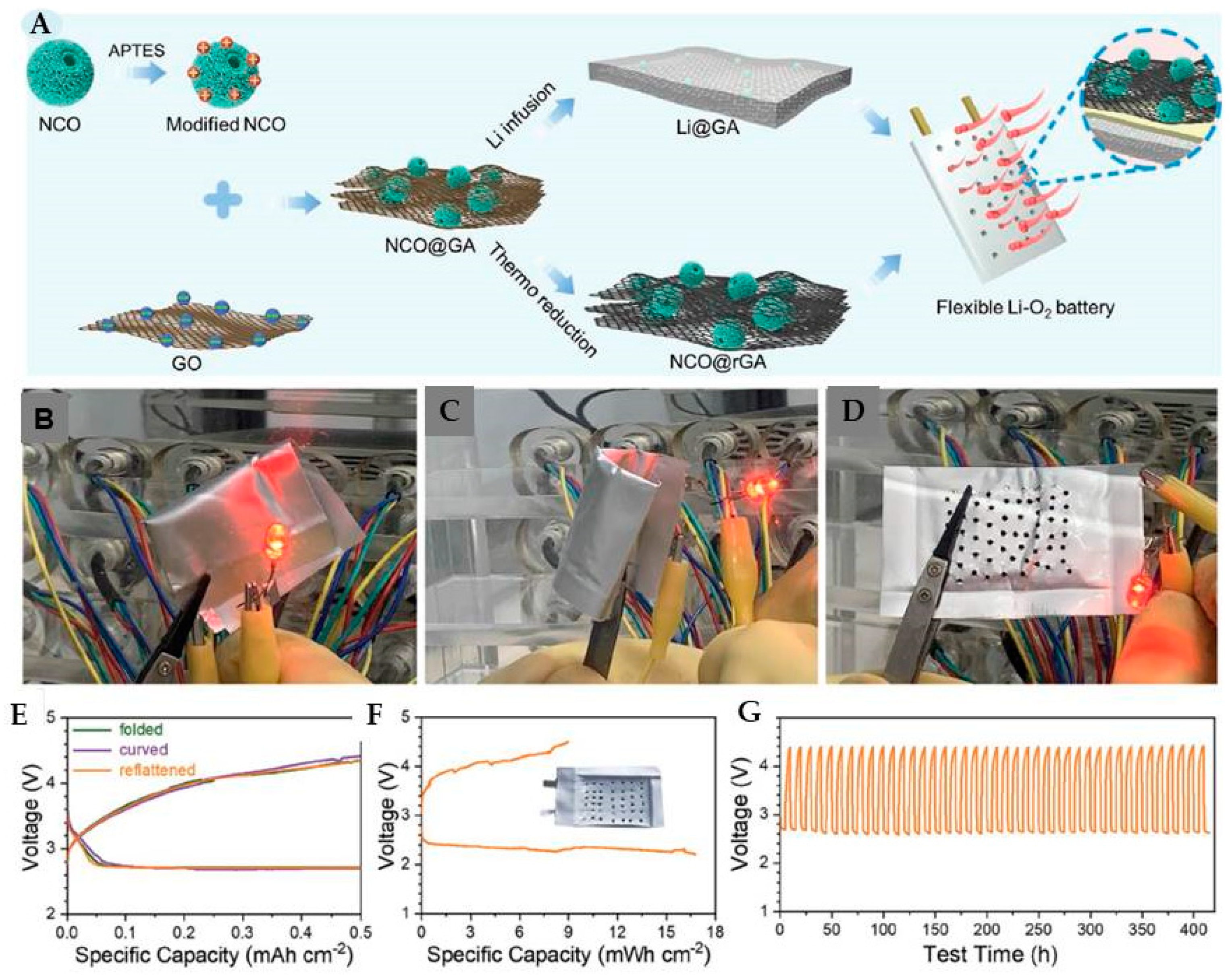
- Graphene has the flexibility and chemical inertness to operate as both a cathode and anode, providing outstanding performance in a variety of folded, curved, and reflattened geometries to inhibit the Li dendrites. Anode (Li) and cathode (NiCo2O4 and O2) are shielded with GO and rGO to prevent their deterioration or volume expansion to provide reliable performance with improved ORR and OER [38].
- The doped graphene can lower the overpotential and act as a cathode protective membrane and improve battery stability and longevity [72].
- Economical carbon materials such as graphene and CDs can be used in place of costlier noble metals and their oxides [87].
- The cost of the graphene electrodes can be minimized by using leftover graphite from old batteries and renewable sources in its preparation.
- Versatile morphology and dimensions with porosity and tunable ethylene and oxygenated carbons. These functional groups play crucial roles in the graphene chemical properties for many reactions by functionalizing a variety of molecules on top of it, both covalently and non-covalently [87].
- Graphene to rGO and GO band gaps are adjustable.
- Graphene can replace common catalysts such as Pt/C, since it possesses changeable energy levels and catalytic sites with dopants [102].
- Due to its exceptional hydrophobicity, chemical resistance, and high conductivity, graphene can prevent the growth of lithium dendrites at the anode and passivate the lithium anode to prevent interactions with moisture and oxygen [91].
- The graphene’s morphology, which ranges from a sheet-like structure to a porous one, is crucial in boosting the effectiveness of power densities [98].
- The lavish physical and chemical inertness of graphene was able to withstand the severe reaction circumstances and various electrode media, and can help to lower the overpotential.
- Owing to the aforementioned advantages, graphene electrode Li-O2 batteries are expected to have high charge speeds, stability, and a long life.
- The single layer graphene is expensive and requires a skilled technician to synthesize it in the lab.
- There is a chance of the reassembling the graphene monolayers into multilayers, which causes a decrease in the specific surface area for a catalytic reaction.
- Low-quality graphene may be prone to oxidation in a rich O2 environment. It may not provide the expected catalytic results and provide reversible capacity.
- The oxygen functional groups on GO may interact with the electrolytes and may cause overpotential.
- Physically adsorbed metals and metal oxides with graphene may cause the sintering/aggregation of the nanoparticles and may lower cell performance.
4. Challenges and Strategies
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grand View Research. Battery Market Size, Share & Trends Analysis Report by Product (Lead Acid, Li-ion, Nickle Metal Hydride, Ni-cd), By Application (Automotive, Industrial, Portable), By Region, And Segment Forecasts, 2020–2027; Grand View Research: San Francisco, CA, USA, 2020; Available online: https://www.grandviewresearch.com/industry-analysis/battery-market (accessed on 4 October 2022).
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Hosaka, T.; Kubota, K.; Hameed, A.A.; Komaba, S. Research Development on K-Ion Batteries. Chem. Rev. 2020, 120, 6358–6466. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Sun, Y.; Liu, Y.; Jiang, K.; Cao, A. Stabilization of High-Energy Cathode Materials of Metal-Ion Batteries: Control Strategies and Synthesis Protocols. Energy Fuels 2021, 35, 7511–7527. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, Z.; Zhang, Z.; Du, A.; Dong, S.; Li, Z.; Li, G.; Cui, G. Current Design Strategies for Rechargeable Magnesium-Based Batteries. ACS Nano 2021, 15, 15594–15624. [Google Scholar] [CrossRef]
- Gummow, R.J.; Vamvounis, G.; Kannan, M.B.; He, Y. Calcium-Ion Batteries: Current State-of-the-Art and Future Perspectives. Adv. Mater. 2018, 30, 1801702. [Google Scholar] [CrossRef]
- Tu, J.; Song, W.; Lei, H.; Yu, Z.; Chen, L.; Wang, M.; Jiao, S. Nonaqueous Rechargeable Aluminum Batteries: Progresses, Challenges, and Perspectives. Chem. Rev. 2021, 121, 4903–4961. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Kumar, D. Metal-ion batteries for electric vehicles: Current state of the technology, issues and future perspectives. Nanoscale Adv. 2021, 3, 3384–3394. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.A.; Ma, L.; Pastel, G.; Xu, K. The mystery and promise of multivalent metal-ion batteries. Curr. Opin. Electrochem. 2021, 29, 100819. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, L.Z.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.Q.; Yu, D.; Liu, Y.; Titirici, M.M.; Chueh, Y.L.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.; Xia, Q.; Hu, Z.; Zhu, Y.; Yan, S.; Ge, C.; Zhang, Q.; Wang, X.; Shang, X.; et al. Extra storage capacity in transition metal oxide lithium-ion batteries revealed by in situ magnetometry. Nat. Mater. 2021, 21, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.S.; Lu, Y. 2019 Nobel Prize for the Li-Ion Batteries and New Opportunities and Challenges in Na-Ion Batteries. ACS Energy Lett. 2019, 4, 2689–2690. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A review on the key issues of the lithium ion battery degradation among the whole life cycle. Etransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Shan, X.; Zhong, Y.; Zhang, L.; Zhang, Y.; Xia, X.; Wang, X.; Tu, J. A Brief Review on Solid Electrolyte Interphase Composition Characterization Technology for Lithium Metal Batteries: Challenges and Perspectives. J. Phys. Chem. C 2021, 125, 19060–19080. [Google Scholar] [CrossRef]
- Ye, F.; Liao, K.; Ran, R.; Shao, Z. Recent Advances in Filler Engineering of Polymer Electrolytes for Solid-State Li-Ion Batteries: A Review. Energy Fuels 2020, 34, 9189–9207. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Recent Advances in Lithium-Carbon Dioxide Batteries. Small Struct. 2020, 1, 2000027. [Google Scholar] [CrossRef]
- Pan, H.; Cheng, Z.; He, P.; Zhou, H. A Review of Solid-State Lithium-Sulfur Battery: Ion Transport and Polysulfide Chemistry. Energy Fuels 2020, 34, 11942–11961. [Google Scholar] [CrossRef]
- Chang, Z.; Xu, J.; Zhang, X. Recent Progress in Electrocatalyst for Li-O2 Batteries. Adv. Energy Mater. 2017, 7, 1700875. [Google Scholar] [CrossRef]
- Srinivaas, M.; Wu, C.-Y.; Duh, J.-G.; Hu, Y.-C.; Wu, J.M. Multi-walled carbon-nanotube-decorated tungsten ditelluride nanostars as anode material for lithium-ion batteries. Nanotechnology 2020, 31, 035406. [Google Scholar] [CrossRef]
- Srinivaas, M.; Wu, C.-Y.; Duh, J.-G.; Hu, Y.-C.; Wu, J.M. Highly Rich 1T Metallic Phase of Few-Layered WS2 Nanoflowers for Enhanced Storage of Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 10363–10370. [Google Scholar] [CrossRef]
- Research and Markets. Lithium-Air Battery Market—Growth, Trends, COVID-19 Impact, And Forecasts (2022–2027). Lithium-Air Battery Market—Growth, Trends, COVID-19 Impact, And Forecasts (2022–2027), Mordor Intelligence, 2022–2027; Research and Markets: Dublin, Ireland, 2022; Available online: https://www.researchandmarkets.com/reports/4897099/lithium-air-battery-market-growth-trends (accessed on 4 October 2022).
- Liu, T.; Vivek, J.P.; Zhao, E.W.; Lei, J.; Garcia-Araez, N.; Grey, C.P. Current Challenges and Routes Forward for Nonaqueous Lithium-Air Batteries. Chem. Rev. 2020, 120, 6558–6625. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, J.; Feng, Q.; Yan, J.; Tang, Y.; Wang, H.J. Significantly enhanced oxygen reduction activity of Cu/CuNxCy co-decorated ketjenblack catalyst for Al–air batteries . Energy Chem. 2018, 27, 419–425. [Google Scholar]
- Torabi, F.; Ahmadi, P. Part I Basic Fundamentals. In Battery Technologies, Simulation of Battery Systems, Fundamentals and Applications, 1st ed.; Torabi, F., Ahmadi, P., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Xu, X.; Shao, Z. Chapter 3 Design of Bifunctional Oxygen Catalysts in Rechargeable Zinc-Air Batteries. In Zinc-Air Batteries: Introduction, Design Principles and Emerging Technologies; Xu, X., Shao, Z., Eds.; Wiley-VCH GmbH: Weinheim, Germany, 2022; pp. 69–110. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Gollavelli, G.; Ling, Y.C. Chapter 21 Ultrathin graphene structure, fabrication and characterization for clinical diagnosis applications. In Smart Nanodevices for Point-of-Care Applications; Kanchi, S., Chokkareddy, R., Rezakazemi, M., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 263–280. [Google Scholar]
- Yanxia, W.; Shengxi, W.; Kyriakos, K. A review of graphene synthesis by indirect and direct deposition methods. J. Mater. Res. 2020, 35, 76–89. [Google Scholar]
- Park, M.S.; Ma, S.B.; Lee, D.J.; Im, D.; Doo, S.G.; Yamamoto, O. A Highly Reversible Lithium Metal Anode. Sci. Rep. 2014, 4, 3815. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.; Banerjee, S.; Kim, J.; Dutta, P. A Review of Lithium-Air Battery Modeling Studies. Energies 2017, 10, 1748. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Millholland, C.D. Looking at Anode Materials in Lithium-Ion Batteries—Advancing Materials; Thermo Fisher Scientific: Waltham, MA, USA, 2018; Available online: https://www.thermofisher.com/blog/materials/looking-at-anode-materials-in-lithium-ion-batteries/ (accessed on 4 October 2022).
- Sui, D.; Si, L.; Li, C.; Yang, Y.; Zhang, Y.; Yan, W. A Comprehensive Review of Graphene-Based Anode Materials for Lithium-ion Capacitors. Chemistry 2021, 3, 1215–1246. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, L.; Gu, Y.; Hu, J.; Chen, Y.; Qi, P.; Zhao, X.; Peng, Y.; Deng, Z.; Liu, Z. High-Performance Li-O2 Batteries Based on All-Graphene Backbone. Adv. Funct. Mater. 2020, 30, 2007218. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, C.; Liu, S.; Yang, J.; Fan, X.; Huang, H. 3D Porous N-Doped Graphene Frameworks Made of Interconnected Nanocages for Ultrahigh-Rate and Long-Life Li-O2 Batteries. Adv. Funct. Mater. 2015, 25, 6913. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Yin, R.; Qian, L.; Huang, X.; Liu, H.; Zhang, J.; Wang, J.; Ding, T.; Guo, Z. Urchin-like NiO-NiCo2O4 heterostructure microsphere catalysts for enhanced rechargeable non-aqueous Li-O2 batteries. Nanoscale 2019, 11, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.H.; Yoon, T.H.; Song, S.H.; Jeon, S.; Park, Y.J.; Kim, I.D.; Ryu, W.H.; Yoon, T.H.; Song, S.H.; Jeon, S.; et al. Bifunctional Composite Catalysts Using Co3O4 Nanofibers Immobilized on Nonoxidized Graphene Nanoflakes for High-Capacity and Long-Cycle Li-O2 Batteries. Nano Lett. 2013, 13, 4190–4197. [Google Scholar] [CrossRef]
- Li, F.; Tang, D.M.; Zhang, T.; Liao, K.; He, P.; Golberg, D.; Yamada, A.; Zhou, H. Superior performance of a Li-O2 battery with metallic RuO2 hollow spheres as the carbon-free cathode. Adv. Energy Mater. 2015, 5, 1500294. [Google Scholar] [CrossRef]
- Guo, X.; Liu, P.; Han, J.; Ito, Y.; Hirata, A.; Fujita, T.; Chen, M. 3D Nanoporous Nitrogen-Doped Graphene with Encapsulated RuO2 Nanoparticles for Li-O2 Batteries. Adv. Mater. 2015, 27, 6137. [Google Scholar] [CrossRef]
- Hu, A.; Long, J.; Shu, C.; Liang, R.; Li, J. Three-Dimensional Interconnected Network Architecture with Homogeneously Dispersed Carbon Nanotubes and Layered MoS2 as a Highly Efficient Cathode Catalyst for Lithium-Oxygen Battery. ACS Appl. Mater. Interfaces 2018, 10, 34077–34086. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Dong, S.; Ge, X.; Zhang, P.; Miao, X.; Zhang, Z.; Wang, C.; Yin, L. One-Step Route Synthesized Co2 P/Ru/N-Doped Carbon Nanotube Hybrids as Bifunctional Electrocatalysts for High-Performance Li-O2 Batteries. Small 2019, 15, 1900001. [Google Scholar] [CrossRef]
- Imanishi, N.; Yamamoto, O. Perspectives and challenges of rechargeable lithium-air batteries. Mater. Today Adv. 2019, 4, 100031. [Google Scholar] [CrossRef]
- Suryatna, A.; Raya, I.; Thangavelu, L.; Alhachami, F.R.; Kadhim, M.M.; Altimari, U.S.; Mahmoud, Z.H.; Mustafa, Y.F.; Kianfar, E. A Review of High-Energy Density Lithium-Air Battery Technology: Investigating the Effect of Oxides and Nanocatalysts. J. Chem. 2022, 1, 2762647. [Google Scholar] [CrossRef]
- Abraham, K.M.; Jiang, Z. A polymer electrolyte based rechargeable lithium oxygen battery. J. Electrochem. Soc. 1996, 143, 1–5. [Google Scholar] [CrossRef]
- Ogasawara, T.; Debart, A.; Holzapfei, M.; Novak, P.; Bruce, P.G. Rechargeable Li2O2 electrode for lithium batteries. J. Am. Chem. Soc. 2006, 128, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Read, J. Characterization of the lithium-oxygen organic electrolyte battery. J. Electrochem. Soc. 2002, 149, A1190–A1195. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Review article Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef]
- Lai, J.; Xing, Y.; Chen, N.; Li, L.; Wu, F.; Chen, R. A comprehensive insight into the electrolytes for rechargeable lithium-air batteries. Angew. Chem. Int. Ed. 2019, 59, 2974–2997. [Google Scholar] [CrossRef] [PubMed]
- Balaish, M.; Kraytsberg, A.; Ein-Eli, Y. A critical review on lithium-air battery electrolytes. Phys. Chem. Chem. Phys. 2014, 16, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Ichida, S.; Mori, D.; Taminato, S.; Zhang, T.; Takeda, Y.; Yamamoto, O.; Imanishi, N. A Rechargeable Aqueous Lithium-Air Battery with an Acetic Acid Catholyte Operated at High Pressure. J. Energy Power Technol. 2022, 4, 009. [Google Scholar] [CrossRef]
- Yamamoto, O.; Imanishi, N. Aqueous Lithium-Air Batteries. Green Energy Technol. 2015, 5, 559–585. [Google Scholar]
- Stevens, P.; Toussaint, G.L.; Caillon, G.; Viaud, P.; Vinatier, P.; Cantau, C.; Fichet, O.; Sarrazin, C.; Mallouki, M. Development of an aqueous, rechargeable Lithium-Air battery operating with untreated air. ECS Trans. 2010, 28, 1–12. [Google Scholar] [CrossRef]
- Andrei., P.; Zheng, J.P.; Hendrickson, M.; Plichta, E.J. Modeling of Li-Air Batteries with Dual Electrolyte. J. Electrochem. Soc. 2012, 159, A770. [Google Scholar] [CrossRef]
- Crowther, O.; Salomon, M. Aqueous Lithium-Air Systems. In Lithium Batteries: Advanced Technologies and Applications, 1st ed.; Scrosati, B., Abraham, K.M., Schalkwijk, W.V., Hassoun, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 191–216. [Google Scholar]
- Wang, H.F.; Wang, X.X.; Li, F.; Xu, J.J. Fundamental Understanding and Construction of Solid-State Li−Air Batteries. Small Sci. 2022, 2, 2200005. [Google Scholar] [CrossRef]
- Chen, W.P.; Duan, H.; Shi, J.L.; Qian, Y.; Wan, J.; Zhang, X.D.; Sheng, H.; Guan, B.; Wen, R.; Yin, Y.X.; et al. Bridging Interparticle Li+ Conduction in a Soft Ceramic Oxide Electrolyte. J. Am. Chem. Soc. 2021, 143, 5717–5726. [Google Scholar] [CrossRef]
- Walle, K.Z.; Babulal, L.M.; Wu, S.H.; Chien, W.C.; Jose, R.; Lue, S.J.; Chang, J.K.; Yang, C.C. Electrochemical Characteristics of a Polymer/Garnet Trilayer Composite Electrolyte for Solid-State Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 2507–2520. [Google Scholar] [CrossRef]
- He, P.; Zhang, T.; Jiang, J.; Zhou, H. Lithium−Air Batteries with Hybrid Electrolytes, J. Phys. Chem. Lett. 2016, 7, 1267–1280. [Google Scholar] [CrossRef]
- Manthiram, A.; Li, L. Hybrid and Aqueous Lithium-Air Batteries. Adv. Energy Mater. 2014, 5, 1401302. [Google Scholar] [CrossRef]
- Kellera, M.; Varzia, A.; Passerinia, S. Hybrid electrolytes for lithium metal batteries. J. Power Sources 2018, 392, 206–225. [Google Scholar] [CrossRef]
- Xiao, J.; Xu, W.; Wang, D.; Zhang, J.G. Hybrid Air-Electrode for Li/Air Batteries. J. Electrochem. Soc. 2010, 157, A294. [Google Scholar] [CrossRef][Green Version]
- Geng, L.; Wang, X.; Han, K.; Hu, P.; Zhou, L.; Zhao, Y.; Luo, W.; Mai, L. Eutectic Electrolytes in Advanced Metal-Ion Batteries. ACS Energy Lett. 2022, 7, 247–260. [Google Scholar] [CrossRef]
- Li, C.L.; Huang, G.; Yu, Y.; Xiong, Q.; Yan, J.M.; Zhang, X. A Low-Volatile and Durable Deep Eutectic Electrolyte for High-Performance Lithium-Oxygen Battery. J. Am. Chem. Soc. 2022, 144, 5827–5833. [Google Scholar] [CrossRef]
- Lithium Anode Protective Layers for Li-Air Batteries. Available online: https://tlo.mit.edu/technologies/lithium-anode-protective-layers-li-air-batteries (accessed on 4 October 2022).
- Chang, H.-H.; Ho, T.-H.; Su, Y.-S. Graphene-Enhanced Battery Components in Rechargeable Lithium-Ion and Lithium Metal Batteries. C 2021, 7, 65. [Google Scholar] [CrossRef]
- Weber, C.J.; Geiger, S.; Falusi, S.; Roth, M. Material review of Li ion battery separators. AIP Conf. Proc. 1597, 2014, 66–81. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Choi, K.H.; Yu, H.K.; Kim, J.H.; Lee, J.S.; Lee, S.Y. Inverse Opal-Inspired, Nanoscaffold Battery Separators: A New Membrane Opportunity for High-Performance Energy Storage Systems. Nano Lett. 2014, 14, 4438–4448. [Google Scholar] [CrossRef]
- Kim, J.H.; Gu, M.; Lee, D.H.; Kim, J.H.; Oh, Y.S.; Min, S.H.; Kim, B.S.; Lee, S.Y. Functionalized Nanocellulose-Integrated Heterolayered Nanomats toward Smart Battery Separators. Nano Lett. 2016, 16, 5533–5541. [Google Scholar] [CrossRef]
- Ryu, W.H.; Gittleson, F.S.; Schwab, M.; Goh, T.; Taylor, A.D. A Mesoporous Catalytic Membrane Architecture for Lithium-Oxygen Battery Systems. Nano Lett. 2015, 15, 434–441. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, P.; Cheng, Z.; Mao, J.; Jiang, L.; Ni, C.; Park, S.; Deng, K.; Hu, Y.; Fu, K.K. Flexible, Mechanically Robust, Solid-State Electrolyte Membrane with Conducting Oxide-Enhanced 3D Nanofiber Networks for Lithium Batteries. Nano Lett. 2021, 21, 7070–7078. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Mack, N.H.; Gao, W.; Ma, S.; Zhong, R.; Han, J.; Baldwin, J.K.; Zelena, P. Nitrogen-Doped Graphene-Rich Catalysts Derived from Heteroatom Polymers for Oxygen Reduction in Nonaqueous Lithium-O2 Battery Cathodes. ACS Nano 2012, 6, 9764–9776. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.G.; Jeong, Y.S.; Park, J.B.; Sun, Y.K.; Scrosati, B.; Lee, Y.J. Ruthenium-Based Electrocatalysts Supported on Reduced Graphene Oxide for Lithium-Air Batteries. ACS Nano 2013, 7, 3532–3539. [Google Scholar] [CrossRef]
- Choi, S.H.; Yun, S.; Won, Y.S.; Oh, C.S.; Kim, S.M.; Kim, K.K.; Lee, Y.H. Large-scale synthesis of graphene and other 2D materials towards industrialization. Nat. Commun. 2022, 13, 1484. [Google Scholar] [CrossRef]
- Koepke, J.C.; Wood, J.D.; Estrada, D.; Ong, Z.Y.; He, K.T.; Pop, E.; Lyding, J.W. Atomic-Scale Evidence for Potential Barriers and Strong Carrier Scattering at Graphene Grain Boundaries: A Scanning Tunneling Microscopy Study. ACS Nano 2013, 7, 75–86. [Google Scholar] [CrossRef]
- Liu, S.W.; Zhang, X.; Wei, G.; Su, Z. Reduced Graphene Oxide-Based Double Network Polymeric Hydrogels for Pressure and Temperature. Sensors 2018, 18, 3162. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Zanoni, R.; Branchi, M.; Marcucci, C.; Zamparelli, C.; Altimari, P.; Navarra, M.A.; Pagnanelli, F. Upcycling Real Waste Mixed Lithium-Ion Batteries by Simultaneous Production of rGO and Lithium-Manganese-Rich Cathode Material. ACS Sustain. Chem. Eng. 2021, 9, 13303–13311. [Google Scholar] [CrossRef]
- Gollavelli, G.; Chang, C.C.; Ling, Y.C. Facile synthesis of smart magnetic graphene for safe drinking water: Heavy metal removal and disinfection control. ACS Sustain. Chem. Eng. 2013, 1, 462–472. [Google Scholar] [CrossRef]
- Gollavelli, G.; Ling, Y.C. Multi-functional graphene as an in vitro and in vivo imaging probe. Biomaterials 2012, 33, 2532–2545. [Google Scholar] [CrossRef]
- Gollavelli, G.G.; Ling, Y.C. Magnetic and fluorescent graphene for dual modal imaging and single light induced photothermal and photodynamic therapy of cancer cells. Biomaterials 2014, 35, 4499–4507. [Google Scholar] [CrossRef]
- Sinha, M.; Gollavelli, G.; Ling, Y.C. Exploring the photothermal hot spots of graphene in the first and second biological window to inactivate cancer cells and pathogens. RSC Adv. 2016, 6, 63859–63866. [Google Scholar] [CrossRef]
- Gollavelli, G.; Ghule, A.V.; Ling, Y.-C. Multimodal Imaging and Phototherapy of Cancer and Bacterial Infection by Graphene and Related Nanocomposites. Molecules 2022, 27, 558. [Google Scholar] [CrossRef]
- Yuan, H.; Hou, Y.; Wen, Z.; Guo, X.; Chenb, J.; Hea, Z. Porous carbon nanosheets co-doped with nitrogen and sulfur for oxygen reduction reaction in microbial fuel cells. ACS Appl. Mater. Interfaces 2015, 7, 18672–18678. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, H. To draw an air electrode of a Li-air battery by pencil. Energy Environ. Sci. 2011, 4, 1704. [Google Scholar] [CrossRef]
- Yoo, E.; Zhou, H. Li-Air Rechargeable Battery Based on Metal-free Graphene Nanosheet Catalysts. ACS Nano 2011, 5, 3020–3026. [Google Scholar] [CrossRef]
- Sun, B.; Wang, B.; Su, D.; Xiao, L.; Ahn, H.; Wang, G. Graphene nanosheets as cathode catalysts for lithium-air batteries with an enhanced electrochemical performance. Carbon 2012, 50, 727–733. [Google Scholar] [CrossRef]
- Storm, M.M.; Overgaard, M.; Younesi, R.; Reeler, N.E.A.; Vosch, T.; Nielsen, U.G.; Edstro¨m, K.; Norby, P. Reduced graphene oxide for Li-air batteries: The effect of oxidation time and reduction conditions for graphene oxide. Carbon 2015, 85, 233–244. [Google Scholar] [CrossRef]
- Al-Ogaili, A.; Pakseresht, S.; Cetinkaya, T.; Akbulut, H. Reduction of graphene oxide using Salvia Officinalis plant extract and its utilization for Li-O2 batteries. Diam. Relat. Mater. 2022, 126, 109118. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, P.; Ma, J.; Wei, L.; Zhao, L.; Cheng, J.; Su, Y.; Gu, Y.; Lian, Y.; Peng, Y.; et al. Wax-Transferred Hydrophobic CVD Graphene Enables Water-Resistant and Dendrite-Free Lithium Anode Toward Long Cycle Li-Air Battery. Adv. Sci. 2021, 8, 2100488. [Google Scholar] [CrossRef] [PubMed]
- Bulbulaa, S.T.; Lua, Y.; Dong, Y.; Yang, X.Y. Hierarchically porous graphene for batteries and supercapacitors. New J. Chem. 2018, 42, 5634–5655. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, M.; Kim, D.W.; Suk, J.; Park, O.O.; Kang, Y. Flexible binder-free graphene paper cathodes for high-performance Li-O2 batteries. Carbon 2015, 93, 625–635. [Google Scholar] [CrossRef]
- Zhong, X.; Papandrea, B.; Xu, Y.; Lin, Z.; Zhang, H.; Liu, Y.; Huang, Y.; Duan, X. Three-dimensional graphene membrane cathode for high energy density rechargeable lithium-air batteries in ambient conditions. Nano Res. 2017, 10, 472–482. [Google Scholar] [CrossRef]
- Xiao, J.; Mei, D.; Li, X.; Xu, W.; Wang, D.; Graff, G.L.; Bennett, W.D.; Nie, Z.; Saraf, L.V.; Aksay, I.A.; et al. Hierarchically Porous Graphene as a Lithium Air Battery Electrode. Nano Lett. 2011, 11, 5071–5078. [Google Scholar] [CrossRef]
- Lin, Y.; Moitoso, B.; Martinez-Martinez, C.; Walsh, E.D.; Lacey, S.D.; Kim, J.W.; Dai, L.; Hu, L.; Connell, J.W. Ultrahigh-Capacity Lithium−Oxygen Batteries Enabled by Dry-Pressed Holey Graphene Air Cathodes. Nano Lett. 2017, 17, 3252–3260. [Google Scholar] [CrossRef]
- Lim, K.H.; Kim, S.; Kweon, H.; Moon, S.; Lee, C.H.; Kim, H. Preparation of graphene hollow spheres from vacuum residue of ultra-heavy oil as an effective oxygen electrode for Li-O2 batteries. J. Mater. Chem. A 2018, 6, 4040–4047. [Google Scholar] [CrossRef]
- Li, W.; Han, C.; Zhang, K.; Chou, S.; Dou, S. Strategies for boosting carbon electrocatalysts for the oxygen reduction reaction in non-aqueous metal-air battery systems. J. Mater. Chem. A 2021, 9, 6671–6693. [Google Scholar] [CrossRef]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural Defects in Graphene. ACS Nano 2011, 5, 26–41. [Google Scholar] [CrossRef]
- Yoon, T.; Kim, J.H.; Choi, J.H.; Jung, D.Y.; Park, I.J.; Choi, S.Y.; Cho, N.S.; Lee, J.I.; Kwon, Y.D.; Cho, S.; et al. Healing Graphene Defects using Selective Electrochemical Deposition: Toward Flexible and Stretchable Devices. ACS Nano 2016, 10, 1539–1545. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; He, F.; Chen, Y.; Zhu, J.; Wang, D.; Mu, S.; Yang, H.Y. Defect and Doping Co-Engineered Non-Metal Nanocarbon ORR Electrocatalyst. Nano-Micro Lett. 2021, 13, 65. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Ren, X.; Zhu, J.; Du, F.; Liu, J.; Zhang, W. B-Doped Graphene as Catalyst to Improve Charge Rate of Lithium−Air Battery. J. Phys. Chem. C 2014, 118, 22412–22418. [Google Scholar] [CrossRef]
- Hou, B.; Lei, X.; Zhong, S.; Sun, B.; Ouyang, C. Dissociation of (Li2O2)0,+ on graphene and boron-doped graphene: Insights from first-principles calculations. Phys. Chem. Chem. Phys. 2020, 22, 14216–14224. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Yao, S.; Hao, C.; Pan, C.; Xiang, X.; Tian, Q.; Shen, P.K.; Shao, Z.; Jiang, S.P. Boosting Electrocatalytic Activity of Single Atom Catalysts Supported on Nitrogen-Doped Carbon through N Coordination Environment Engineering. Small 2022, 18, 2105329. [Google Scholar] [CrossRef]
- Liu, L.-H.; Li, N.; Han, M.; Han, J.-R.; Liang, H.-Y. Scalable synthesis of nanoporous high entropy alloys for electrocatalytic oxygen evolution. Rare Metals. 2022, 41, 125–131. [Google Scholar] [CrossRef]
- Wu, F.; Xing, Y.; Li, L.; Qian, J.; Qu, W.; Wen, J.; Miller, D.J.; Ye, Y.; Chen, R.; Amine, K.; et al. Facile Synthesis of Boron-Doped RGO as Cathode Material for High Energy Li-O2 Batteries. ACS Appl. Mater. Interfaces 2016, 8, 23635–23645. [Google Scholar] [CrossRef]
- Shui, J.; Lin, Y.; Connell, J.W.; Xu, J.; Fan, X.; Dai, L. Nitrogen-Doped Holey Graphene for High-Performance Rechargeable Li-O2 Batteries. ACS Energy Lett. 2016, 1, 260–265. [Google Scholar] [CrossRef]
- Wu, A.; Shen, S.; Yan, X.; Xia, G.; Zhang, Y.; Zhu, F.; Zhang, J. CxNy particles@N-doped porous graphene. Nanoscale 2018, 10, 12763–12770. [Google Scholar] [CrossRef]
- Ding, S.; Yu, X.; Ma, Z.F.; Yuan, X. A review of rechargeable aprotic lithium-oxygen batteries based on theoretical and computational investigations. J. Mater. Chem. A 2021, 9, 8160–8194. [Google Scholar] [CrossRef]
- Zheng, T.; Ren, Y.; Han, X.; Zhang, J. Design principles of nitrogen-doped graphene nanoribbons as highly effective bifunctional catalysts for Li-O2 batteries. Phys. Chem. Chem. Phys. 2022, 24, 22589–22598. [Google Scholar] [CrossRef]
- Benti, N.E.; Tiruye, G.A.; Mekonnen, Y.S. Boron and pyridinic nitrogen-doped graphene as potential catalysts for rechargeable non-aqueous sodium-air batteries. RSC Adv. 2020, 10, 21387. [Google Scholar] [CrossRef]
- Gong, Y.; Ding, W.; Li, Z.; Su, R.; Zhang, X.; Wang, J.; Zhou, J.; Wang, Z.; Gao, Y.; Li, S.; et al. An inverse spinel cobalt-iron oxide and N-doped graphene composite as an efficient and durable bifuctional catalyst for Li-O2 batteries. ACS Catal. 2018, 8, 4082–4090. [Google Scholar] [CrossRef]
- Chang, Z.; Yu, F.; Liu, Z.; Peng, S.; Guan, M.; Shen, X.; Zhao, S.; Liu, N.; Wu, Y.; Chen, Y. Co−Ni Alloy Encapsulated by N-doped Graphene as a Cathode Catalyst for Rechargeable Hybrid Li−Air Batteries. ACS Appl. Mater. Interfaces 2020, 12, 4366–4372. [Google Scholar] [CrossRef]
- Cui, H.; Zhou, Z.; Jia, D. Heteroatom-doped graphene as electrocatalysts for air cathodes. Mater. Horiz. 2017, 4, 7–19. [Google Scholar] [CrossRef]
- Shao, Y.; Jiang, Z.; Zhang, Q.; Guan, J. Progress in nonmetal-doped graphene electrocatalysts for oxygen reduction reaction. ChemSusChem 2019, 12, 2133–2146. [Google Scholar] [CrossRef]
- Yin, H.; Li, D.; Chi, Z.; Zhang, Q.; Liu, X.; Ding, L.; Li, S.; Liu, J.; Guo, Z.; Wang, L. Iridium coated Co nanoparticles embedded into highly porous N-doped carbon nanocubes grafted with carbon nanotubes as a catalytic cathode for high-performance Li-O2 batteries. J. Mater. Chem. A 2021, 9, 17865–17875. [Google Scholar] [CrossRef]
- Kavalsky, L.; Mukherjee, S.; Singh, C.V. Phosphorene as a Catalyst for Highly Efficient Nonaqueous Li-Air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 499–510. [Google Scholar] [CrossRef]
- Jiang, Z.L.; Sun, H.; Shi, W.K.; Cheng, J.Y.; Hu, J.Y.; Guo, H.L.; Gao, M.Y.; Zhou, H.; Sun, S.G. P-Doped Hive-like Carbon Derived from Pinecone Biomass as Efficient Catalyst for Li-O2 Battery. ACS Sustain. Chem. Eng. 2019, 7, 14161–14169. [Google Scholar] [CrossRef]
- Yin, Y.C.; Deng, R.X.; Yang, D.R.; Sun, Y.B.; Liand, Z.Q.; Xia, X.H. Synthesis of Pure Thiophene-Sulfur-Doped Graphene for an Oxygen Reduction Reaction with High Performance. J. Phys. Chem. Lett. 2022, 13, 4350–4356. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Li, X.; Geng, D.; Banis, M.N.; Tang, Y.; Wang, D.; Li, R.; Sham, T.K.; Sun, X. Discharge product morphology and increased charge performance of lithium-oxygen batteries with graphene nanosheet electrodes: The effect of sulphur doping. J. Mater. Chem. 2012, 22, 20170–20174. [Google Scholar] [CrossRef]
- Kim, J.H.; Kannan, A.G.; Woo, H.S.; Jin, D.G.; Kim, W.; Ryu, K.; Kim, D.W. A bi-functional metal-free catalyst composed of dual-doped graphene and mesoporous carbon for rechargeable lithium-oxygen batteries. J. Mater. Chem. A 2015, 3, 18456–18465. [Google Scholar] [CrossRef]
- Kong, F.; Yue, Y.; Li, Q. Ren. Sulfur-Doped Graphdiyne as a High-Capacity Anode Material for Lithium-Ion Batteries. Nanomaterials 2021, 11, 1161. [Google Scholar]
- Nam, G.; Jang, H.; Sung, J.; Chae, S.; Soule, L.; Zhao, B.; Cho, J.; Liu, M. Evaluation of the Volumetric Activity of the Air Electrode in a Zinc-Air Battery Using a Nitrogen and Sulfur Co-doped Metal-free Electrocatalyst. ACS Appl. Mater. Interfaces 2020, 12, 57064–57070. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef]
- Sun, B.P.; Huang, X.; Chen, S.; Munroe, P.; Wang, G. Porous Graphene Nanoarchitectures: An Efficient Catalyst for Low Charge-Overpotential, Long Life, and High Capacity Lithiu—Oxygen Batteries. Nano Lett. 2014, 14, 3145–3152. [Google Scholar] [CrossRef]
- Liao, K.; Zhang, T.; Wang, Y.; Li, F.; Jian, Z.; Yu, H.; Zhou, H. Nanoporous Ru as a Carbon- and Binder-Free Cathode for Li-O2 Batteries. ChemSusChem 2015, 8, 1429–1434. [Google Scholar] [CrossRef]
- Tan, G.; Chong, L.; Amine, R.; Lu, J.; Liu, C.; Yuan, Y.; Wen, J.; He, K.; Bi, X.; Guo, Y.; et al. Toward Highly Efficient Electrocatalyst for Li-O2 Batteries Using Biphasic N Doping Cobalt@Graphene Multiple-Capsule Heterostructures. Nano Lett. 2017, 17, 2959–2966. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, J.; Fan, M.; Ajayan, P.M.; Tour, J.M. Li-Breathing Air Batteries Catalyzed by MnNiFe/Laser-Induced Graphene Catalysts. Adv. Mater. Interfaces 2019, 6, 1901035. [Google Scholar] [CrossRef]
- He, C.; Ma, Z.; Wu, Q.; Cai, Y.; Huang, Y.; Liu, K.; Fan, Y.; Wang, H.; Li, Q.; Qi, J.; et al. Promoting the ORR catalysis of Pt-Fe intermetallic catalysts by increasing atomic utilization and electronic regulation. Electrochim. Acta 2020, 330, 135119. [Google Scholar] [CrossRef]
- Once, A.; Cetinkaya, T.; Akbulut, H. Enhancement of the electrochemical performance of free-standing graphene electrodes with manganese dioxide and ruthenium nanocatalysts for lithium-oxygen batteries. Int. J. Hydro. Energy 2021, 46, 17173–17186. [Google Scholar] [CrossRef]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Topalov, A.A.; Kostka, A.; Schüth, F.; Mayrhofer, K.J.J. Degradation mechanisms of Pt/C fuel cell catalysts under simulated start-stop conditions. ACS Catal. 2012, 2, 832–843. [Google Scholar] [CrossRef]
- Proch, S.; Wirth, M.; White, H.S.; Anderson, S.L. Strong effects of cluster size and air exposure on oxygen reduction and carbon oxidation electrocatalysis by size-selected Ptn (n ≤ 11) on glassy carbon electrodes. J. Am. Chem. Soc. 2013, 135, 3073–3086. [Google Scholar] [CrossRef] [PubMed]
- Bing, Y.; Liu, H.; Zhang, L.; Ghosh, D.; Zhang, J. Nanostructured Pt-alloy electrocatalysts for PEM fuel cell oxygen reduction reaction. Chem. Soc. Rev. 2010, 39, 2184–2202. [Google Scholar] [CrossRef]
- Liu, R.; von Malotki, C.; Arnold, L.; Koshino, N.; Higashimura, H.; Baumgarten, M.; Müllen, K. Triangular trinuclear metal-N4 complexes with high electrocatalytic activity for oxygen reduction. J. Am. Chem. Soc. 2011, 133, 10372–10375. [Google Scholar] [CrossRef]
- Zhou, C.; Kumar, S.; Doyle, C.D.; Tour, J.M. Functionalized single wall carbon nanotubes treated with pyrrole for electrochemical supercapacitor membranes. Chem. Mater. 2005, 17, 1997–2002. [Google Scholar] [CrossRef]
- Lee, J.Y.; An, K.H.; Heo, J.K.; Lee, Y.H. Fabrication of supercapacitor electrodes using fluorinated single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 8812–8815. [Google Scholar] [CrossRef]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable thin film supercapacitors using single-walled carbon nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from “Supercapacitor” to “Battery” behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539. [Google Scholar] [CrossRef]
- Cottineau, T.; Toupin, M.; Delahaye, T.; Brousse, T.; Bélanger, D. Nanostructured transition metal oxides for aqueous hybrid electrochemical supercapacitors. Appl. Phys. A 2006, 82, 599–606. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, P.; Wang, B.; Chen, Y.; Tian, S.; Wu, Y.; Holze, R. Electrochemical performance of MnO2 nanorods in neutral aqueous electrolytes as a cathode for asymmetric supercapacitors. J. Phys. Chem. C 2009, 113, 14020–14027. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Sun, W.; Ning, G.; Wei, T.; Zhang, Q.; Zhang, R.; Zhi, L.; Wei, F. Advanced asymmetric supercapacitors based on Ni(OH)2/graphene and porous graphene electrodes with high energy density. Adv. Funct. Mater. 2012, 22, 2632–2641. [Google Scholar] [CrossRef]
- Fusalba, F.; Gouérec, P.; Villers, D.; Bélanger, D. Electrochemical characterization of polyaniline in nonaqueous electrolyte and its evaluation as electrode material for electrochemical supercapacitors. J. Electrochem. Soc. 2001, 148, A1. [Google Scholar] [CrossRef]
- Laforgue, A.; Simon, P.; Sarrazin, C.; Fauvarque, J.-F. Polythiophene-based supercapacitors. J. Power Sources 1999, 80, 142–148. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Wei, T.; Zhi, L.; Ning, G.; Li, T.; Wei, F. Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv. Funct. Mater. 2011, 21, 2366–2375. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.; Wu, X.; Han, Q.; Wang, X. Graphene Oxide-MnO2 nanocomposites for supercapacitors. ACS Nano 2010, 4, 2822–2830. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chang, Y.-X.; Li, Y.-R.; Zhang, S.-L.; Xu, S.-L. Well-dispersed NiCoS2 nanoparticles rGO composite with a large specific surface area as an oxygen evolution electrocatalyst. Rare Metals. 2021, 40, 3156–3165. [Google Scholar] [CrossRef]
- Li, J.; Liu, G.; Liu, B.; Min, Z.; Qian, D.; Jiang, J.; Li, J. Fe-doped CoSe2 nanoparticles encapsulated in N-doped bamboo-like carbon nanotubes as an efficient electrocatalyst for oxygen evolution reaction. Electrochem. Acta. 2018, 265, 577–585. [Google Scholar] [CrossRef]
- Wang, S.; Sha, Y.; Zhu, Y.; Xu, X.; Shao, Z. Modified template synthesis and electrochemical performance of a Co3O4/mesoporous cathode for lithium-oxygen batteries. J. Mater. Chem. A 2015, 3, 16132–16141. [Google Scholar] [CrossRef]
- Li, C.; Zhao, D.-H.; Long, H.-L.; Li, M. Recent advances in carbonized non-noble metal-organic frameworks for electrochemical catalyst of oxygen reduction reaction. Rare Metals. 2021, 40, 2657–2689. [Google Scholar] [CrossRef]
- Xua, P.; Chen, C.; Zhu, J.; Xie, J.; Zhaoc, P.; Wang, M. RuO2-particle-decorated graphene-nanoribbon cathodes for long-cycle Li-O2 batteries. J. Electroanal. Chem. 2019, 842, 98–106. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, J.; Tour, J.M. Laser-induced graphene synthesis of Co3O4 in graphene for oxygen electrocatalysis and metal-air batteries. Carbon 2018, 139, 880–887. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Li, X.; Wang, C.; Zeng, Y.; Yue, G.; Chen, Q. CuCr2O4 @rGO Nanocomposites as High-Performance Cathode Catalyst for Rechargeable Lithium-Oxygen Batteries. Nano-Micro Lett. 2018, 10, 22. [Google Scholar] [CrossRef]
- Zhu, L.; Scheiba, F.; Trouillet, V.; Georgian, M.; Fu, Q.; Sarapulpva, A.; Sigel, F.; Hua, W.; Ehrenberg, H. MnO2 and Reduced Graphene Oxide as Bifunctional Electro-catalysts for Li-O2 Batteries. ACS Appl. Energy Mater. 2019, 2, 7121–7131. [Google Scholar] [CrossRef]
- Palani, R.; Karuppiah, C.; Yang, C.C.; Piraman, S. Metal-organic frameworks derived spinel NiCo2O4/graphene nanosheets composite as a bi-functional cathode for high energy density Li-O2 battery applications. Int. J. Hydro. Energy 2021, 46, 14288–14300. [Google Scholar] [CrossRef]
- Palani, R.; Wu, Y.S.; Wu, S.H.; Jose, R.; Yang, C.C. Metal-organic framework-derived ZrO2/NiCo2O4/graphene mesoporous cake-like structure as enhanced bifunctional electrocatalytic cathodes for long life Li-O2 batteries. Electrochim. Acta 2022, 412, 140147. [Google Scholar] [CrossRef]
- Chen, K.; Yang, D.Y.; Huang, G.; Zhang, X.B. Lithium-air batteries: Air-electrochemistry and anode stabilization. Acc. Chem. Res. 2021, 54, 632–641. [Google Scholar] [CrossRef]
- Pan, J.; Tian, X.L.; Zaman, S.; Dong, Z.; Liu, H.; Park, H.S.; Xia, B.Y. Recent progress on transition metal oxides as bifunctional catalysts for lithium-air and zinc-air batteries. Batter. Supercaps. 2019, 2, 336–347. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhang, Y.; Wang, B.; Peng, H. The recent progress of nitrogen-doped carbon nanomaterials for electrochemical batteries. J. Mater. Chem. A 2018, 6, 12932–12944. [Google Scholar] [CrossRef]
- Jung, J.W.; Cho, S.H.; Nam, J.S.; Kim, I.D. Current and future cathode materials for non-aqueous Li-air (O2) battery technology-A focused review. Energy Storage Mater. 2020, 24, 512–528. [Google Scholar] [CrossRef]
- Chawla, N. Recent advances in air-battery chemistries. Mater. Today Chem. 2019, 12, 324–331. [Google Scholar] [CrossRef]
- Guo, F.; Kang, T.; Liu, Z.; Tong, B.; Guo, L.; Wang, Y.; Liu, C.; Chen, X.; Zhao, Y.; Shen, Y.; et al. Advanced lithium metal-carbon nanotube composite anode for high-performance lithium-oxygen batteries. Nano Lett. 2019, 19, 6377–6384. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Li, X.; Liu, J.; Geng, D.; Yang, J.; Li, R.; Sun, X. Nitrogen-doped carbon nanotubes as cathode for lithium-air batteries. Electrochem. Commun. 2011, 13, 668–672. [Google Scholar] [CrossRef]
- Kwak, W.J.; Lau, K.C.; Shin, C.D.; Amine, K.; Curtiss, L.A.; Sun, Y.K. A Mo2C/carbon nanotube composite cathode for lithium-oxygen batteries with high energy efficiency and long cycle life. ACS Nano 2015, 9, 4129–4137. [Google Scholar] [CrossRef]
- Pham, H.T.T.; Kim, Y.; Kim, Y.J.; Lee, J.W.; Park, M.S. Robust Design of Dual-Phasic Carbon Cathode for Lithium-Oxygen Batteries. Adv. Funct. Mater. 2019, 29, 1902915. [Google Scholar] [CrossRef]
- Nie, H.; Xu, C.; Zhou, W.; Wu, B.; Li, X.; Liu, T.; Zhang, H. Free-standing thin webs of activated carbon nanofibers by electrospinning for rechargeable Li-O2 batteries. ACS Appl. Mater. Interfaces 2016, 8, 1937–1942. [Google Scholar] [CrossRef]
- Sun, B.; Chen, S.; Liu, H.; Wang, G. Mesoporous carbon nanocube architecture for high-performance lithium-oxygen batteries. Adv. Funct. Mater. 2015, 25, 4436–4444. [Google Scholar] [CrossRef]
- Zhu, X.; Shang, Y.; Lu, Y.; Liu, C.; Li, Z.; Liu, Q. A free-standing biomass-derived RuO2/N-doped porous carbon cathode towards highly performance lithium-oxygen batteries. J. Power Sources 2020, 471, 228444. [Google Scholar] [CrossRef]
- Song, T.B.; Huang, Z.H.; Niu, X.Q.; Liu, J.; Wei, J.S.; Chen, X.B.; Xiong, H.M. Applications of Carbon Dots in Next-generation Lithium-Ion Batteries. Chem. Nano. Mat. 2020, 6, 1421–1436. [Google Scholar] [CrossRef]
- Kumar, Y.R.; Deshmukh, K.; Sadasivuni, K.K.; Pasha, S.K. Graphene quantum dot-based materials for sensing, bio-imaging and energy storage applications: A review. RSC Adv. 2020, 10, 23861–23898. [Google Scholar] [CrossRef]
- Guo, R.; Li, L.; Wang, B.; Xiang, Y.; Zou, G.; Zhu, Y.; Hou, H.; Ji, X. Functionalized carbon dots for advanced batteries. Energy Storage Mater. 2021, 37, 8–39. [Google Scholar] [CrossRef]
- Zulfajri, M.; Gedda, G.; Chang, C.J.; Chang, Y.P.; Huang, G.G. Cranberry beans derived carbon dots as a potential fluorescence sensor for selective detection of Fe3+ ions in aqueous solution. ACS Omega 2019, 4, 15382–15392. [Google Scholar] [CrossRef] [PubMed]
- Gudimella, K.K.; Appidi, T.; Wu, H.F.; Battula, V.; Jogdand, A.; Rengan, A.K.; Gedda, G. Sand bath assisted green synthesis of carbon dots from citrus fruit peels for free radical scavenging and cell imaging. Colloids Surf. B Biointerfaces 2021, 197, 111362. [Google Scholar] [CrossRef]
- Gudimella, K.; Gedda, G.; Kumar, P.S.; Babu, B.K.; Yamajala, B.; Rao, B.V.; Singh, P.P.; Kumar, D.; Sharma, A. Novel synthesis of fluorescent carbon dots from bio-based Carica Papaya Leaves: Optical and structural properties with antioxidant and anti-inflammatory activities. Environ. Res. 2022, 204, 111854. [Google Scholar] [CrossRef] [PubMed]
- Gedda, G.; Tiruveedhi, V.B.G.; Ganesh, G.; Suribabu, J. Recent advancements of carbon dots in analytical techniques. In Carbon Dots in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 13–147. [Google Scholar]
- Gedda, G.; Bhupathi, A.; Tiruveedhi, V.B.G. Naturally Derived Carbon Dots as Bioimaging Agents. In Biomechanics and Functional Tissue Engineering; IntechOpen: London, UK, 2021. [Google Scholar]
- Pandey, R.R.; Chusuei, C.C. Carbon nanotubes, graphene, and carbon dots as electrochemical biosensing composites. Molecules 2021, 26, 6674. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.; Riahifar, R.; Li, Y. A review on the superb contribution of carbon and graphene quantum dots to electrochemical capacitors’ performance: Synthesis and application. FlatChem 2020, 22, 100171. [Google Scholar] [CrossRef]
- Gao, R.; Li, Z.; Zhang, X.; Zhang, J.; Hu, Z.; Liu, X. Carbon-dotted defective CoO with oxygen vacancies: A synergetic design of bifunctional cathode catalyst for Li-O2 batteries. ACS Catal. 2016, 6, 400–406. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, X.; Ji, X.; Liu, W.; Wan, W.; Wang, Y.; Pan, X.; Lu, Z. Graphene quantum dots as a highly efficient electrocatalyst for lithium-oxygen batteries. J. Mater. Chem. B 2020, 8, 22356–22368. [Google Scholar] [CrossRef]
- Lin, X.; Gao, K.; Han, Y.; Cui, R.; Yu, W.; Zhang, Z. Laser-assisted synthesis of FePc/N-doped carbon dots on Co3O4 flakes as an efficient cathode for lithium-oxygen batteries. J. Nanoparticle Res. 2022, 24, 52. [Google Scholar] [CrossRef]
- Duan, X.; Zhu, W.; Ruan, Z.; Xie, M.; Chen, J.; Ren, X. Recycling of Lithium Batteries—A Review. Energies 2022, 15, 1611. [Google Scholar] [CrossRef]
- Ian Morse. A Dead Battery Dilemma. Science 2021, 372, 780–783.
- Kang, J.H.; Lee, J.; Jung, J.W.; Park, J.; Jang, T.; Kim, H.S.; Nam, J.S.; Lim, H.; Yoon, K.R.; Ryu, W.H.; et al. Lithium-Air Batteries: Air-Breathing Challenges and Perspective. ACS Nano 2020, 14, 14549–14578. [Google Scholar] [CrossRef]
- Tatara, R.; Feng, S.; Crabb, E.; France-Lanord, A.; Tułodziecki, M.; Lopez, J.; Stephens, R.M.; Grossman, J.C.; Shao-Horn, Y. Solvent and anion dependent Li+-O2− coupling strength and implications on the thermodynamics and kinetics of Li-O2 batteries. J. Phys. Chem. C 2020, 124, 4953–4967. [Google Scholar]
- Xu, S.; Liang, X.; Liu, X.; Bai, W.; Liu, Y.; Cai, Z.; Zhang, Q.; Zhao, C.; Wang, K.; Chen, J. Surface engineering donor and acceptor sites with enhanced charge transport for low-overpotential lithium-oxygen batteries. Energy Storage Mater. 2020, 25, 52–61. [Google Scholar] [CrossRef]
- Kwak, W.; Lim, H.S.; Gao, P.; Feng, R.; Chae, S.; Zhong, L.; Read, J.; Engelhard, M.H.; Xu, W.; Zhang, J.G. Effects of fluorinated diluents in localized high-concentration electrolytes for lithium-oxygen batteries. Adv. Funct. Mater. 2021, 31, 2002927. [Google Scholar] [CrossRef]
- Cao, D.; Tan, C.; Chen, Y. Oxidative decomposition mechanisms of lithium carbonate on carbon substrates in lithium battery chemistries. Nature Commun. 2022, 13, 4908. [Google Scholar] [CrossRef]
- Ko, Y.; Park, H.; Lee, K.; Kim, S.J.; Park, H.; Bae, Y.; Kim, J.; Park, S.Y.; Kwon, J.E.; Kang, K. Anchored mediator enabling shuttle-free redox mediation in lithium-oxygen batteries. Angew. Chem. Int. Ed. 2020, 59, 5376–5380. [Google Scholar] [CrossRef]


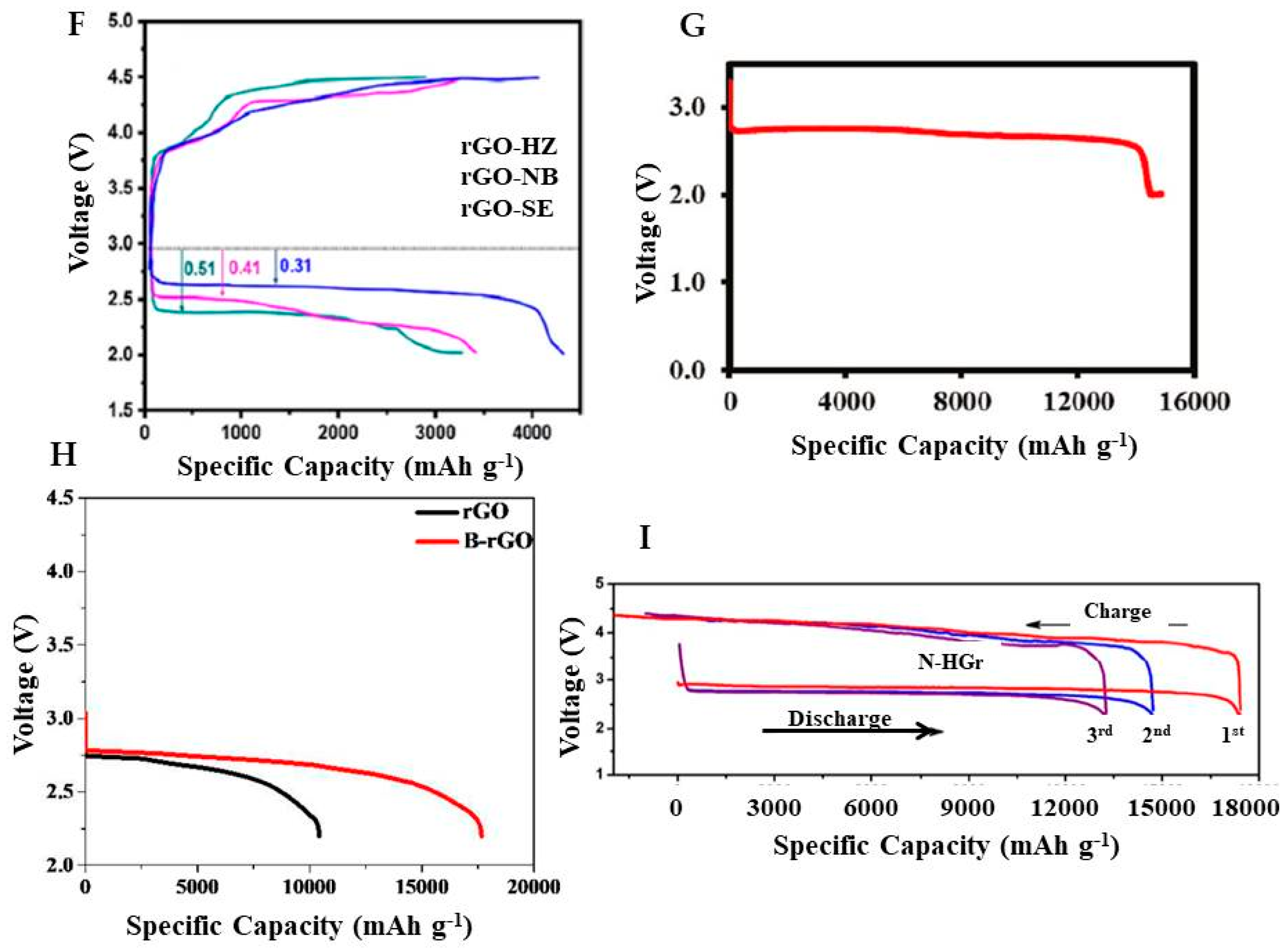
| S. No. | Batteries | Anode | Cathode | Electrolyte | Separator | Expected Performance | Ref. No. |
|---|---|---|---|---|---|---|---|
| 1. | Li+ | Graphite/CNTs/Metals/Alloys | LiCoO2, LiFePO4, LiMn2O4, LiNiCoAlO2 etc. | LiPF6/organic carbonates/polymers | Pos, IOs IOs | 387 W h kg−1 | [17] |
| 2. | Li-CO2 | Li—Metal | Carbon materials Nobel metals Transition metals | LiTFSI in TEGDME, LiTFSI in DMSO LiCF3SO3/TEGDME, LiCF3SO3/TEGDME | (Ru(acac)3) and M-GF | 1876 W h kg−1 | [18] |
| 3. | Li-S | Li—Metal Si/Carbon | Carbon materials (OMCs, CMK-3, CNTs, Graphene, S@BP2000, S/Se@pPAN etc.) and Graphene/S. | NASICON, LISICON, Garnet, Perovskite, Antiperovskite, LiPON, etc. Polymers, Argyrodite, Li2S-SiS2, Li2S-P2S5, etc. | LiTFSI in DOL:DME, Cyclic/short chain/glycol ethers etc. | 2600 W h kg−1 | [19] |
| 4. | Li-Air | Li-Metal | Graphene CNTs Porous carbon Metals, Metal Oxides, Alloys etc. | Li salts of amides, ethers, Ionic liquids, PCs, RMs. Acid, base, H2O etc. | POs LISCON | 3505 W h kg−1 | [20] |
| S. No. | Catalyst | Electrolyte | Discharge Voltage (V) | Discharge Capacity (mA h g−1) | Current Density | Ref. No. |
|---|---|---|---|---|---|---|
| 1 | Multilayer graphene from pencil | Organic/LISCON | 2.6 | 560 | 0.25 mA g−1 | [86] |
| 2 | Graphene (rGO) | LiClO4/propylene carbonate (PPC) | 2.75 | 2332 | 50 mA g−1 | [88] |
| 3 | Graphene (rGO) | LiClO4 salt mixed in TEGDME | 2.65 | 4320 | 150 mA g−1 | [90] |
| 4 | GO/rGO nanoplates | LiNO3 in dimethylacetamide (DMAc) | 2.70 | 6910 | 200 mA g−1 | [93] |
| 5 | 3D Porous graphene membrane | ORG/LiNO3, DMA | 2.71 | 5700 | (2.8 Ag−1) 2800 mA g−1 | [94] |
| 6 | 3D Porous graphene | LITFSI inTEGDME/Triglyme | 2.7 | 15,000 | 0.1 mA cm−2 | [95] |
| 7 | Holey graphene | LiTFSI in TEGDME | 2.7 | 6500 | 0.2 mA cm−2 | [96] |
| 8 | 3D Porous graphene spheres | LiNO3 in N,N-dimethylacetamide (DMA) | 2.8 | 18,578 | 50 mA g−1 | [97] |
| 9 | B-doped porous graphene | LiTFSI in TEGDME | 2.75 | 18,000 | 100 mA g−1 | [107] |
| 10 | N-doped holey graphene | LiCF3SO3 in TEGDME | 2.93 | 17,000 | 800 mA g−1 | [108] |
| 11 | S-doped graphene | LiPF6 in TEGDME | 2.60 | 4300 | 75 mA g−1 | [121] |
| 12 | N- and S-doped graphene | LiTFSI in TEGDME | 2.75 | 11,500 | 100 mA g−1 | [122] |
| S. No. | Catalyst | Electrolyte | Discharge Voltage (V) | Specific Capacity/Discharge Capacity (mA h g−1) | Current Density (mA g−1) | Ref. No. |
|---|---|---|---|---|---|---|
| 1 | N-CNT | LiPF6 dissolved in propylene carbonate/ethylene carbonate | ~2.52 | 866 | 75 | [159] |
| 2 | Mo2C/CNTs | LiCFSO3 in TEGDME | 2.65 | 1000 | 200 | [160] |
| 3 | CNTs/MoS2 Sheets | LiTFSI in TEGDME | 0.29 | 6904 | 200 | [161] |
| 4 | Co2P/Ru/CNT | LiTFSI in TEGDME | 0.7 | 12,800 | 100 | [162] |
| 5 | MOF-C/CNT | LiTFSI in TEGDME | 2.70 | 10,050 | 50 | [163] |
| 6 | 1D activated carbon nanofibers | LiTFSI in TEGDME | 2.75 | 6099 | 200 | [164] |
| 7 | 3D mesoporous carbon nanocubes | LiClO/LiNO3 in DMSO | 2.75 | 26,100 | 200 | [165] |
| 8 | N-HMACs-RuO2 | TEGDME-LiCF3SO3 | 2.0 | 13,400 | 200 | [42] |
| 9 | Carbon-Dotted Defective CoO | LITFSI in TEGDME | 4.5 | 7000 | 100 | [175] |
| 10 | GQDs | LiTFSI in TEGDME | 2.4 | 68,900 | 1400 | [176] |
| 11 | FePc/N-CDs@Co3O4 | KOH | 2.73 V | 28,619 | 100 | [177] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gollavelli, G.; Gedda, G.; Mohan, R.; Ling, Y.-C. Status Quo on Graphene Electrode Catalysts for Improved Oxygen Reduction and Evolution Reactions in Li-Air Batteries. Molecules 2022, 27, 7851. https://doi.org/10.3390/molecules27227851
Gollavelli G, Gedda G, Mohan R, Ling Y-C. Status Quo on Graphene Electrode Catalysts for Improved Oxygen Reduction and Evolution Reactions in Li-Air Batteries. Molecules. 2022; 27(22):7851. https://doi.org/10.3390/molecules27227851
Chicago/Turabian StyleGollavelli, Ganesh, Gangaraju Gedda, Raja Mohan, and Yong-Chien Ling. 2022. "Status Quo on Graphene Electrode Catalysts for Improved Oxygen Reduction and Evolution Reactions in Li-Air Batteries" Molecules 27, no. 22: 7851. https://doi.org/10.3390/molecules27227851
APA StyleGollavelli, G., Gedda, G., Mohan, R., & Ling, Y.-C. (2022). Status Quo on Graphene Electrode Catalysts for Improved Oxygen Reduction and Evolution Reactions in Li-Air Batteries. Molecules, 27(22), 7851. https://doi.org/10.3390/molecules27227851








