Pickering Emulsions as Vehicles for Bioactive Compounds from Essential Oils
Abstract
:1. Introduction
2. Pickering Emulsions
3. Essential Oils
| Essential Oil | Scientific Name | Bioactive Compounds | Functions | Ref(s). |
|---|---|---|---|---|
| Lemon grass | Cymbopogon citratus, Cymbopogon flexuosus | Geranial, neral, myrcene, geraniol, verbenol | Antibacterial agent, insecticidal agent | [15,19] |
| Eucalyptus | Eucalyptus globules | 1,8-cineole, citronellal, citronellol, limonene, α-pinene, α-terpinene | Insecticidal agent | [15] |
| Rosemary | Rosmarinus officinalis | 1,8-cineole, camphor, β-caryophyllene, α-terpineol, verbenone | Antibacterial agent, antioxidant, insecticidal agent | [15,19,23] |
| Clove | Syzigium aromaticum | Eugenol, β-caryophyllene, eugenyl acetate, α-humulene | Antibacterial agent, antioxidant | [14,19,24] |
| Thyme | Thymus vulgaris | γ-Terpinene, thymol, p-cymene, carvacrol, linalool | Antibacterial agent, antioxidant, insecticidal agent | [15,19,25] |
| White peppermint | Mentha piperita | Menthol, menthone, menthyl acetate, 1,8-cineole | Antibacterial agent, insecticidal agent | [14,15,19] |
| Basil | Ocimum basilicum | Linalool, 1,8-cineole, geraniol, eugenol | Antibacterial agent, antioxidant, insecticidal agent | [15,19,26] |
| Oregano | Origanum vulgar | Thymol, carvacrol, γ-terpinene, linalool, p-cymene | Antibacterial agent, antioxidant | [19,23] |
| Tea tree | Melaleuca alternifolia | Terpenen-4-ol, γ-terpinene, 1,8-cineole, α-terpinene, cymene | Antibacterial agent | [19] |
| Citronella | Cymbopogon nardus | Citronellal, geraniol | Antibacterial agent, insecticidal agent | [15,19] |
| Cinnamon | Cinnamomum zeilanicum | Eugenol, cinnamaldehyde, linalool, geraniol | Antibacterial agent, antioxidant | [19,23] |
| Lavender | Lavandula hybrida, Lavandula angustifolia | Octyl acetate, linalool, camphor | Antibacterial agent, insecticidal agent | [15,19] |
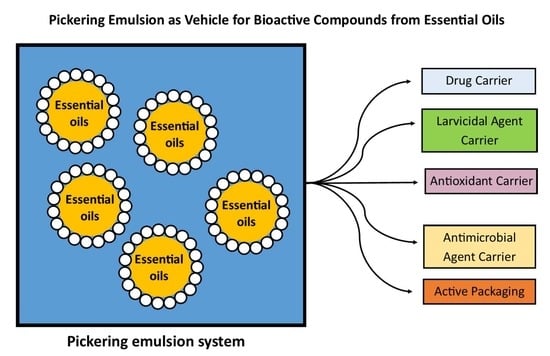
4. Application of Essential Oil Pickering Emulsions
4.1. Essential Oil Pickering Emulsions as Drug Carriers
4.2. Essential Oil Pickering Emulsions (EO-PEs) as Larvicidal Agent Carriers
4.3. Essential Oil Pickering Emulsions as Antioxidant Carriers
4.4. Essential Oil Pickering Emulsions as Antimicrobial Agents
4.4.1. Emulsification of Cedarwood Essential Oil (CEO) by OSA-Modified Starch Pickering Emulsions
4.4.2. Emulsification of Cinnamon Essential Oil (CEO) by Zein–Pectin (ZP) Composite Nanoparticles
4.4.3. Emulsification of Thymol with Zein/Gum Nanoparticle (ZGP)-Stabilized Pickering Emulsions
4.4.4. Emulsification of Cinnamon Essential Oil with Nanocellulose Pickering Emulsions
4.4.5. Emulsification of Rosmarinus officinalis Essential Oils with Chitosan–Benzoic Acid Nanogels
4.4.6. Emulsification of Citronella Oil with Composite Microcapsules
4.4.7. Emulsification of Peppermint Oil with Composite Microcapsules
4.4.8. Emulsification of Cinnamon Oil with Composite Microcapsules
4.5. Essential Oil Pickering Emulsions as Active Packaging
4.5.1. Clove Essential Oil Pickering Emulsions and Gelatin/Agar Bio-Based Films as Active Packaging
4.5.2. Clove Essential Oil Pickering Emulsions and Chitosan Films as Active Packaging
4.5.3. Oregano Essential Oil Pickering Emulsions and Cellulose Nanofibrils as Active Packaging
4.5.4. Oregano Essential Oil Pickering Emulsions and Soluble Soybean Polysaccharide as Active Packaging
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, A.G.; Ferreira, R.R.; Paula, L.C.; Mitra, S.K.; Rosa, D.S. Starch-Based Films Enriched with Nanocellulose-Stabilized Pickering Emulsions Containing Different Essential Oils for Possible Applications in Food Packaging. Food Packag. Shelf Life 2021, 27, 100615. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.A. Emulsions Stabilized with Solid Nanoparticles: Pickering Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-Grade Pickering Emulsions for Encapsulation and Delivery of Bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, S.; Guan, X.; McClements, D.J.; Liu, X.; Liu, F.; Ngai, T. Polysaccharide-Based Pickering Emulsions: Formation, Stabilization and Applications. Food Hydrocoll. 2021, 119, 106812. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as Surfactants—Similarities and Differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Pickering, S.U. Emulsions. J. Chem. Soc. 1907, 91, 2001–2021. [Google Scholar] [CrossRef] [Green Version]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical Characteristics, Applications and Research Trends of Edible Pickering Emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Chen, L.; Ao, F.; Ge, X.; Shen, W. Food-Grade Pickering Emulsions: Preparation, Stabilization and Applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef] [PubMed]
- Linke, C.; Drusch, S. Pickering Emulsions in Foods—Opportunities and Limitations. Crit. Rev. Food Sci. Nutr. 2018, 58, 1971–1985. [Google Scholar] [CrossRef] [PubMed]
- Kierulf, A.; Whaley, J.; Liu, W.; Enayati, M.; Tan, C.; Perez-Herrera, M.; You, Z.; Abbaspourrad, A. Protein Content of Amaranth and Quinoa Starch Plays a Key Role in Their Ability as Pickering Emulsifiers. Food Chem. 2020, 315, 126246. [Google Scholar] [CrossRef]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of Essential Oils: A Review on Their Interaction with Food Components. Front. Microbiol. 2015, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Said-, H.A.H.; Ahl, A.; Hikal, W.; Said-Al, H.A.H.; Said-Al Ahl, H.A.H.; Hikal, W.M.; Tkachenko, K.G. Essential Oils with Potential as Insecticidal Agents: A Review. Int. J. Environ. Plan. Manag. 2017, 3, 23–33. [Google Scholar]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of Plant Volatiles: Nature’s Diversity and Ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef] [Green Version]
- Bowles, E. The Chemistry of Aromatherapeutic Oils; Routledge: London, UK, 2003. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils–a Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Santos, M.I.S.; Marques, C.; Mota, J.; Pedroso, L.; Lima, A. Applications of Essential Oils as Antibacterial Agents in Minimally Processed Fruits and Vegetables—A Review. Microorganisms 2022, 10, 760. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [Green Version]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar]
- Cardoso-Ugarte, G.A.; Sosa-Morales, M.E. Essential Oils from Herbs and Spices as Natural Antioxidants: Diversity of Promising Food Applications in the Past Decade. Food Rev. Int. 2021, 00, 1–31. [Google Scholar] [CrossRef]
- Bao, X.; Wu, J.; Ma, G. Sprayed Pickering Emulsion with High Antibacterial Activity for Wound Healing. Prog. Nat. Sci. Mater. Int. 2020, 30, 669–676. [Google Scholar] [CrossRef]
- Horváth, B.; Pál, S.; Széchenyi, A. Preparation and in Vitro Diffusion Study of Essential Oil Pickering Emulsions Stabilized by Silica Nanoparticles. Flavour Fragr. J. 2018, 33, 385–396. [Google Scholar] [CrossRef]
- Seo, S.M.; Lee, J.W.; Shin, J.; Tak, J.H.; Hyun, J.; Park, I.K. Development of Cellulose Nanocrystal-Stabilized Pickering Emulsions of Massoia and Nutmeg Essential Oils for the Control of Aedes Albopictus. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Shin, J.; Na, K.; Shin, S.; Seo, S.M.; Youn, H.J.; Park, I.K.; Hyun, J. Biological Activity of Thyme White Essential Oil Stabilized by Cellulose Nanocrystals. Biomolecules 2019, 9, 799. [Google Scholar] [CrossRef] [Green Version]
- Harsanto, B.W.; Supriyanto; Kartini, I.; Pranoto, Y. The Ability of Breadfruit Starch Nanoparticle-Stabilized Pickering Emulsion for Encapsulating Cinnamon Essential Oil. Malays. Appl. Biol. 2022, 51, 83–90. [Google Scholar] [CrossRef]
- Comunian, T.A.; Grassmann Roschel, G.; da Silva Anthero, A.G.; de Castro, I.A.; Dupas Hubinger, M. Influence of Heated, Unheated Whey Protein Isolate and Its Combination with Modified Starch on Improvement of Encapsulated Pomegranate Seed Oil Oxidative Stability. Food Chem. 2020, 326, 126995. [Google Scholar] [CrossRef]
- Huang, K.; Liu, R.; Zhang, Y.; Guan, X. Characteristics of Two Cedarwood Essential Oil Emulsions and Their Antioxidant and Antibacterial Activities. Food Chem. 2021, 346, 128970. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Li, F.; Li, D.; Huang, Q. Cinnamon Essential Oil Pickering Emulsion Stabilized by Zein-Pectin Composite Nanoparticles: Characterization, Antimicrobial Effect and Advantages in Storage Application. Int. J. Biol. Macromol. 2020, 148, 1280–1289. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; He, X.; Kong, D.; Zhou, C.; Wu, H.; Yang, Z.; Yang, Z.; Hu, Y. Preparation of Cinnamon Oil-Loaded Antibacterial Composite Microcapsules by In Situ Polymerization of Pickering Emulsion Templates. Macromol. Mater. Eng. 2020, 305, 1–10. [Google Scholar] [CrossRef]
- De Souza, A.G.; Ferreira, R.R.; Aguilar, E.S.F.; Zanata, L.; Rosa, D.d.S. Cinnamon Essential Oil Nanocellulose-Based Pickering Emulsions: Processing Parameters Effect on Their Formation, Stabilization, and Antimicrobial Activity. Polysaccharides 2021, 2, 608–625. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Chen, Z.; Wang, T.; Lu, Z.; Hu, W.; Wang, L. Zein/Gum Arabic Nanoparticle-Stabilized Pickering Emulsion with Thymol as an Antibacterial Delivery System. Carbohydr. Polym. 2018, 200, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Chen, M.; Chen, Z.; Wu, H.; Zhang, P.; Yuan, T.; Yang, Z.; Hu, Y. Fabrication of Sustained-Release and Antibacterial Citronella Oil-Loaded Composite Microcapsules Based on Pickering Emulsion Templates. J. Appl. Polym. Sci. 2018, 135, 1–9. [Google Scholar] [CrossRef]
- Hadian, M.; Rajaei, A.; Mohsenifar, A.; Tabatabaei, M. Encapsulation of Rosmarinus Officinalis Essential Oils in Chitosan-Benzoic Acid Nanogel with Enhanced Antibacterial Activity in Beef Cutlet against Salmonella Typhimurium during Refrigerated Storage. LWT Food Sci. Technol. 2017, 84, 394–401. [Google Scholar] [CrossRef]
- Lai, H.; Liu, Y.; Huang, G.; Chen, Y.; Song, Y.; Ma, Y.Q.; Yue, P. Fabrication and Antibacterial Evaluation of Peppermint Oil-Loaded Composite Microcapsules by Chitosan-Decorated Silica Nanoparticles Stabilized Pickering Emulsion Templating. Int. J. Biol. Macromol. 2021, 183, 2314–2325. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Gelatin/Agar-Based Functional Film Integrated with Pickering Emulsion of Clove Essential Oil Stabilized with Nanocellulose for Active Packaging Applications. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 627, 127220. [Google Scholar] [CrossRef]
- Xu, Y.; Chu, Y.; Feng, X.; Gao, C.; Wu, D.; Cheng, W.; Meng, L.; Zhang, Y.; Tang, X. Effects of Zein Stabilized Clove Essential Oil Pickering Emulsion on the Structure and Properties of Chitosan-Based Edible Films. Int. J. Biol. Macromol. 2020, 156, 111–119. [Google Scholar] [CrossRef]
- Wu, M.; Zhou, Z.; Yang, J.; Zhang, M.; Cai, F.; Lu, P. ZnO Nanoparticles Stabilized Oregano Essential Oil Pickering Emulsion for Functional Cellulose Nanofibrils Packaging Films with Antimicrobial and Antioxidant Activity. Int. J. Biol. Macromol. 2021, 190, 433–440. [Google Scholar] [CrossRef]
- Liu, Q.R.; Wang, W.; Qi, J.; Huang, Q.; Xiao, J. Oregano Essential Oil Loaded Soybean Polysaccharide Films: Effect of Pickering Type Immobilization on Physical and Antimicrobial Properties. Elsevier B.V. 2019, 87. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y. Original Article: Antifungal Activities of Cinnamon Oil against Rhizopus Nigricans, Aspergillus Flavus and Penicillium Expansum in Vitro and in Vivo Fruit Test. Int. J. Food Sci. Technol. 2010, 45, 1837–1842. [Google Scholar] [CrossRef]
- Gende, L.B.; Floris, I.; Fritz, R.; Martin Javier Aras, E.G.U. Antimicrobial Activity of Cinnamon (Cinnamomum Zeylanicum) Essential Oil and Its Main Components against Paenibacillus Larvae from Argentine. Bull. Insectology 2008, 61, 1–4. [Google Scholar]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-Step Method for Encapsulation of Oregano Essential Oil in Chitosan Nanoparticles: Preparation, Characterization and in Vitro Release Study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions Stabilised Solely by Colloidal Particles. Adv. Colloid Interface Sci. 2003, 100–102, 503–546. [Google Scholar] [CrossRef]
- Zhou, F.Z.; Huang, X.N.; Wu, Z.L.; Yin, S.W.; Zhu, J.H.; Tang, C.H.; Yang, X.Q. Fabrication of Zein/Pectin Hybrid Particle-Stabilized Pickering High Internal Phase Emulsions with Robust and Ordered Interface Architecture. J. Agric. Food Chem. 2018, 66, 11113–11123. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, H.; Niu, B.; Jin, W. Physical Stabilities of Taro Starch Nanoparticles Stabilized Pickering Emulsions and the Potential Application of Encapsulated Tea Polyphenols. Int. J. Biol. Macromol. 2018, 118, 2032–2039. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and Antifungal Activities of Thymol: A Brief Review of the Literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Tang, C.H.; Yin, S.W.; Yang, X.Q. Development and Characterization of Novel Antimicrobial Bilayer Films Based on Polylactic Acid (PLA)/Pickering Emulsions. Carbohydr. Polym. 2018, 181, 727–735. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Q. A Novel Method of Preparing Stable Zein Nanoparticle Dispersions for Encapsulation of Peppermint Oil. Food Hydrocoll. 2015, 43, 593–602. [Google Scholar] [CrossRef]
- Lu, X.; Xiao, J.; Huang, Q. Pickering Emulsions Stabilized by Media-Milled Starch Particles. Food Res. Int. 2018, 105, 140–149. [Google Scholar] [CrossRef]
- Saffarionpour, S. Nanocellulose for Stabilization of Pickering Emulsions and Delivery of Nutraceuticals and Its Interfacial Adsorption Mechanism. Food Bioprocess. Technol. 2020, 13, 1292–1328. [Google Scholar] [CrossRef]
- Silva, C.E.P.; Tam, K.C.; Bernardes, J.S.; Loh, W. Double Stabilization Mechanism of O/W Pickering Emulsions Using Cationic Nanofibrillated Cellulose. J. Colloid Interface Sci. 2020, 574, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Blanco, A.; Negro, C. Pickering Emulsions Containing Cellulose Microfibers Produced by Mechanical Treatments as Stabilizer in the Food Industry. Appl. Sci. 2019, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Huang, G.; Ma, Y.; Liu, Y.; Huang, X.; Zheng, Q.; Yue, P.; Yang, M. Cellulose Nanocrystals Based Clove Oil Pickering Emulsion for Enhanced Antibacterial Activity. Int. J. Biol. Macromol. 2021, 170, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Beyki, M.; Zhaveh, S.; Khalili, S.T.; Rahmani-Cherati, T.; Abollahi, A.; Bayat, M.; Tabatabaei, M.; Mohsenifar, A. Encapsulation of Mentha Piperita Essential Oils in Chitosan–Cinnamic Acid Nanogel with Enhanced Antimicrobial Activity against Aspergillus Flavus. Ind. Crops Prod. 2014, 54, 310–319. [Google Scholar] [CrossRef]
- Khalili, S.T.; Mohsenifar, A.; Beyki, M.; Zhaveh, S.; Rahmani-Cherati, T.; Abdollahi, A.; Bayat, M.; Tabatabaei, M. Encapsulation of Thyme Essential Oils in Chitosan-Benzoic Acid Nanogel with Enhanced Antimicrobial Activity against Aspergillus Flavus. LWT Food Sci. Technol. 2015, 60, 502–508. [Google Scholar] [CrossRef]
- Severino, R.; Ferrari, G.; Vu, K.D.; Donsì, F.; Salmieri, S.; Lacroix, M. Antimicrobial Effects of Modified Chitosan Based Coating Containing Nanoemulsion of Essential Oils, Modified Atmosphere Packaging and Gamma Irradiation against Escherichia Coli O157:H7 and Salmonella Typhimurium on Green Beans. Food Control. 2015, 50, 215–222. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Fu, X.; Chen, W.; Yang, J.; Chen, Z.; Wang, Z.; Zhuansun, X.; Feng, J.; Chen, Y. Preparation of Peppermint Oil Nanoemulsions: Investigation of Stability, Antibacterial Mechanism and Apoptosis Effects. Colloids Surf. B Biointerfaces. 2021, 201, 111626. [Google Scholar] [CrossRef]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of Essential Oils via Nanoprecipitation Process: Overview, Progress, Challenges and Prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef]
- Pongsumpun, P.; Iwamoto, S.; Siripatrawan, U. Response Surface Methodology for Optimization of Cinnamon Essential Oil Nanoemulsion with Improved Stability and Antifungal Activity. Ultrason. Sonochemistry 2020, 60, 104604. [Google Scholar] [CrossRef]
- Yin, C.; Huang, C.; Wang, J.; Liu, Y.; Lu, P.; Huang, L. Effect of Chitosan- and Alginate-Based Coatings Enriched with Cinnamon Essential Oil Microcapsules to Improve the Postharvest Quality of Mangoes. Materials 2019, 12, 2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial Bio-Nanocomposites and Their Potential Applications in Food Packaging. Food Control. 2020, 112, 107086. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential Oils as Additives in Biodegradable Films and Coatings for Active Food Packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the Antifungal Activity of Clove Essential Oil Encapsulated by Chitosan Nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef]
- Shen, Y.; Ni, Z.J.; Thakur, K.; Zhang, J.G.; Hu, F.; Wei, Z.J. Preparation and Characterization of Clove Essential Oil Loaded Nanoemulsion and Pickering Emulsion Activated Pullulan-Gelatin Based Edible Film. Int. J. Biol. Macromol. 2021, 181, 528–539. [Google Scholar] [CrossRef]
- Mohajer, S.; Rezaei, M.; Hosseini, S.F. Physico-Chemical and Microstructural Properties of Fish Gelatin/Agar Bio-Based Blend Films. Carbohydr. Polym. 2017, 157, 784–793. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wu, Y.; Fang, R.; Lei, C.; Li, Y.; Li, B.; Pei, Y.; Luo, X.; Liu, S. Application of Nanocellulose as Particle Stabilizer in Food Pickering Emulsion: Scope, Merits and Challenges. Trends Food Sci. Technol. 2021, 110, 573–583. [Google Scholar] [CrossRef]
- Wang, X.Y.; Heuzey, M.C. Pickering Emulsion Gels Based on Insoluble Chitosan/Gelatin Electrostatic Complexes. RSC Adv. 2016, 6, 89776–89784. [Google Scholar] [CrossRef]
- Pérez-Córdoba, L.J.; Norton, I.T.; Batchelor, H.K.; Gkatzionis, K.; Spyropoulos, F.; Sobral, P.J.A. Physico-Chemical, Antimicrobial and Antioxidant Properties of Gelatin-Chitosan Based Films Loaded with Nanoemulsions Encapsulating Active Compounds. Food Hydrocoll. 2018, 79, 544–559. [Google Scholar] [CrossRef]
| Application | Method | Results | Ref(s). | ||||
|---|---|---|---|---|---|---|---|
| Essential Oils | Particle Stabilizer | Particle Characteristics/Properties | Oil/Water Phase | Preparation | Pickering Emulsion Characteristics/Properties | ||
| Drug carriers | Tea tree oil; Lavender oil; Clove oil; Olive oil; Corn oil | Chitosan nanoparticles (CS NPs) | Particle size: 300–600 nm; Zeta potential: +16 mV | Oil phase: tea tree oil with 100 microgram/mL curcumin; Water phase: 4 mg/mL CS NPs suspension; Oil/water ratio: 1/9 | EO containing curcumin mixed with CS NPs suspension at concentrations of 2, 3, and 4 mg/mL; pH 7.4 with 4 mol/L added NaOH; Homogenization: ultrasonication | Zeta potential: +24 mV; Droplet size (at respective concentrations): 2.2, 1.9, and 1.7 µm; Viscosity (at respective concentrations): 1.18, 6.36, and 26.46 Pas; Rheology: shear thinning Stability: Good long-term stability (2 months) | [24] |
| Drug carriers | Anise oil; Tea tree oil; Thyme oil | Silica nanoparticle (native silica nanoparticle)/HS (hydrophilic silica), and modified silica (MET, ET, and Ph)) Tween 80 | Particle size: 19.8–20.8 nm; Zeta potential: −116 to +83.1 mV | - | Homogenization: 13,500 rpm for 2 min (rotor–stator homogenizer) | The most stable emulsions were stabilized with particles modified by the ethyl group. Tea tree EO droplet size: 0.292–6.247 µm Thyme EO droplet size: 0.613–5.435 µm Anise EO droplet size: 1.393–5.362 µm | [25] |
| Encapsulated larvicides | Massoia and nutmeg essential oils | Cellulose nanocrystals (CNCs) | - | Aqueous phase: CNC solution (270 mg/mL CNC for massoia oil, 180 mg/mL CNC for nutmeg oil); Oil phase: massoia and nutmeg essential oils | Homogenization: ultrasonication at a 60% amplitude for 1 min using an ultrasonic processor | Droplet size: 3 to 15 μm | [26] |
| Encapsulated larvicides | White thyme essential oil | Cellulose nanocrystals (CNCs) | - | Aqueous phase: CNC solution (45, 90, 135, and 180 mg/mL EO); Oil phase: thymol white oil | Homogenization: ultrasonication at a 50% amplitude for 30 s | TSI values after 24 h for CNCs at 135 and 180 mg/mL: <12; TSI values for CNCs at 45 and 90 mg/mL: >50; Average shell size: 45 μm (45 mg/mL CNCs), 5 μm (135 and 180 mg/mL CNCs) | [27] |
| Antioxidant encapsulation | Cinnamon essential oil | Breadfruit starch nanoparticles | - | Oil phase: cinnamon oil (0.05, 0.1, 0.5, and 1% (w/w)) and dissolved in MCT oil; Aqueous phase: distilled water; Oil/water ratio: 40%:60% | Dispersed breadfruit starch (3 g) in distilled water with added oil phase; Homogenization: 10,000 rpm for 5 min using a rotor–stator homogenizer (T50 basic, IKA) | Droplet size: 11.66–23.62 µm (before storage) and 20.7–25.64 µm (after 7 days of storage); Viscosity: 629–813 cP (before storage) and 3209–5849 cP (after 7 days of storage); EI: 1 (before and after 7 days of storage) | [28] |
| Antioxidant encapsulation | Pomegranate seed oil (PSO) | Whey protein isolate (WPI) microgel; Modified starch (Capsul®) combined with WPI | - | Oil phase: pomegranate seed oil (PSO); Continuous phase: combination of WPI (8% w/w) and modified starch or WPI microgels (heated WPI); Oil/water ratio: 1:4 | Homogenization: 18,000 rpm/10 min using a rotor–stator homogenizer (Ultra-Turrax IKA T18) Stability test: pH (2.0, 4.0, 6.0, 8.0), NaCl solutions (0.5, 1.0 and 2.0% w/v); Spray-drying stability test: 0, 7, 15, 21, 30 days at 40 °C | Zeta potential (under stress conditions/different pH values and NaCl concentrations): −63 to −37 mV; Zeta potential of reconstituted emulsion over time: −39 to −33 (WPI microgel), −55 to −45 (WPI), −41 to −33 mV (WPI: Capsul); Droplet size: 6.49–9.98 µm (WPI microgel), 1.88–4.62 µm (WPI), 1.68–5.62 µm (WPI Capsul). The average droplet size increased over time | [29] |
| Antibacterial microcapsules | Cedarwood essential oil (CEO) | OSA-modified starch | - | Particle concentration: 0.1, 0.5, 1, 2, and 3%; Oil phase: CEO 5% Aqueous phase: deionized water | Homogenization: 15,000 rpm for 20 min | Best formulation for CEO-PE with OSA-modified starch: 1%; Droplet size: 0.626 µm; Zeta potential: +27.58 mV; Viscosity: 1.0–1.3 mPas | [30] |
| Antibacterial microcapsules | Cinnamon essential oil | Zein–pectin composite nanoparticles | ZCP properties; Particle size: 660.8 nm; Zeta potential: +31.23 mV; Contact angle: 89.2° | Particle concentration: 2, 1.75, 1.5, 1.25, 1, 0.75, 0.5, 0.25, 0.125%; Oil phase: CEO; Aqueous phase: deionized water; Oil/water ratio: 1:1 | Homogenization: 14,000 rpm for 4 min using a rotor–stator homogenizer | 1% ZCPs while maintaining good physical stability. Above concentrations of 0.125%, emulsions stabilized by ZCPs were more stable than CP alone or zein alone | [31] |
| Antibacterial microcapsules | Cinnamon essential oil | Silicon dioxide nanoparticles | Average particle size: 22 nm (diluted particles 148.7 nm); Zeta potential: −24.1 mV | Particle concentration: 1, 2, 3, or 4%; Oil phase: cinnamon oil; Water phase: deionized water; Oil/water ratio: 1:4 | Homogenization: 5000 rpm for 2 min using an FJ200S homogenizer | SiO2 nanoparticle concentration was 4 wt%; superfluous SiO2 nanoparticles were sedimented at the bottom of the CMO Pickering emulsion after standing for 2 days | [32] |
| Antibacterial microcapsules | Cinnamon essential oil | Cellulose nanocrystals (CNCs); Cellulose nanofibers (CNFs) | CNCs: Zeta potential: −33 mV; Length: 2 μm; Diameter 131 nm; CNFs: Zeta potential: −33.2 mV; Length: 4 μm; Diameter 66.3 nm | Aqueous phase: CNC or CNF solution; Oil phase: cinnamon oil; Particle concentration: 0.5 or 1%; EO concentration: 20 or 30% | Homogenization: 10,000 rpm or 12,000 rpm for 3 or 7 min | Zeta potential of CNCs: −29.3 to −28.3 mV; Zeta potential of CNFs: −18.3 to −11.1 mV; Droplet size of CNCs: 25 and 50 μm (without phase separation after 30 days); Droplet size of CNFs: 30 and 100 μm (with phase separation after 30 days); Best preparation: cellulose nanofibers, homogenization speed of 12,000 rpm, and oil concentration of 20% | [33] |
| Antibacterial microcapsules | Thymol | Zein–gum Arabic nanoparticles (ZGP) | - | Aqueous phase: Zein/GA–thymol nanoparticle dispersion (6.25% w/v); Oil phase: soybean oil; Oil fraction: 0.3 | Homogenization: 10,000 rpm for 3 min using a high-speed homogenizer | Droplet size: 27.95–69.98 µm; Most stable emulsion: ZGP at a concentration of 2.5% and oil fraction of 0.1. Stable oil–water interfacial layer was destroyed by a higher concentration of NaCl (>150 mmol/L) | [34] |
| Antibacterial microcapsules | Citronella oil (CTO) | HAP (Hydroxyapatite) nanoparticles; Quaternary ammonium salt of chitosan (HACC); Sodium alginate (SA) | HAP Particle size: 397.4 nm; HAP zeta potential: −12.8 mV | Aqueous phase: HAP (hydroxyapatite) nanoparticles dispersion (1.0%) and sodium alginate (SA); Oil phase: citronella oil; Oil/water ratio: 1:1, 1:2, 1:3, 1:4 | Homogenization: at 10,000 rpm for 2 min | CTO-loaded microcapsules possess high thermal stability; the in vitro release study of CTO shows that the microcapsules have sustained release activity | [35] |
| Antibacterial microcapsules | Rosmarinus officinalis essential oils | Chitosan–benzoic acid | - | Oil phase: REOs; Aqueous phase: chitosan–benzoic acid nanogels | Homogenization: sonication at 70 kHz for 5 min | - | [36] |
| Antibacterial microcapsules | Peppermint oil (PO) | Chitosan-decorated Silica nanoparticles | CSNs; Particle size: 118.12–152.5 nm; Zeta potential (from chitosan concentration 0–5%): −41.8 to +42.5 mV; Contact angle (from chitosan concentration 0–5%): 39.4°–67.4° | Oil phase: peppermint oil; Water phase: CSN suspensions (containing water); Oil/water ratio: 1:9 | Homogenization: 22,000 rpm for 1 min using a high-shear homogenizer and high-pressure homogenizer (30 cycles at 800 bar) | Particle size: PO-PE 0.5%: 6.61 µm; PO-PE 2%: 3.73 µm; PO-PE 2% did not cream during storage | [37] |
| Active Packaging | Clove essential oil | Cellulose nanofiber (CNF) | - | Particle concentration: 0.75%; Oil phase: clove oil; Water phase: CNF aqueous solution | Homogenization: 5000 rpm for 1 h using an Ultra-Turrax blender | Particle size: 0.06–0.12 µm; Zeta potential: −51.8 mV | [38] |
| Active Packaging | Clove essential oil | Zein | - | Aqueous phase: zein suspension; Oil phase: clove oil; Zein concentration: 2%, 3% | Homogenization: 12,000 rpm for 10 min | The particle size of the emulsion decreased from 1.73 μm to 1.40 μm when the concentration of zein increased from 2% to 3%, indicating that higher concentrations of zein were required to reduce the size of the oil droplets and stabilize the emulsion | [39] |
| Active Packaging | Oregano essential oil | ZnO nanoparticles | - | Aqueous phase: ZnO nanoparticle dispersion; Oil phase: Mixture of oregano essential oil and soybean oil (1:1 ratio) | Homogenization: high-shear homogenizer at a speed of 10,000 rpm for 6 min | Droplet size: 15 μm to 60 μm. When the concentration of ZnO nanoparticles was 1.5 wt% and the mass fraction of the oil phase was 20%, the Pickering emulsion with a particle size of 26.85 μm exhibited strong standing stability | [40] |
| Active Packaging | Oregano essential oil | Soluble soybean polysaccharide (SSPS); Acid-soluble soy protein (ASSP) | Particle size of ASSP/SSPS complexes: 162.1 nm | Oil phase: Oregano essential oil mixed with soybean oil; Aqueous phase: ASSP dispersion | Homogenization: Homogenizer at a speed of 6000 rpm for 1 min | Particle size: 0.811 to 1.896 µm; Zeta potential: −4.48 to −3.41 mV | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cahyana, Y.; Putri, Y.S.E.; Solihah, D.S.; Lutfi, F.S.; Alqurashi, R.M.; Marta, H. Pickering Emulsions as Vehicles for Bioactive Compounds from Essential Oils. Molecules 2022, 27, 7872. https://doi.org/10.3390/molecules27227872
Cahyana Y, Putri YSE, Solihah DS, Lutfi FS, Alqurashi RM, Marta H. Pickering Emulsions as Vehicles for Bioactive Compounds from Essential Oils. Molecules. 2022; 27(22):7872. https://doi.org/10.3390/molecules27227872
Chicago/Turabian StyleCahyana, Yana, Yunita Safriliani Eka Putri, Dian Siti Solihah, Farrah Shabira Lutfi, Randah Miqbil Alqurashi, and Herlina Marta. 2022. "Pickering Emulsions as Vehicles for Bioactive Compounds from Essential Oils" Molecules 27, no. 22: 7872. https://doi.org/10.3390/molecules27227872
APA StyleCahyana, Y., Putri, Y. S. E., Solihah, D. S., Lutfi, F. S., Alqurashi, R. M., & Marta, H. (2022). Pickering Emulsions as Vehicles for Bioactive Compounds from Essential Oils. Molecules, 27(22), 7872. https://doi.org/10.3390/molecules27227872