Overview of the Polyphenols in Salicornia: From Recovery to Health-Promoting Effect
Abstract
1. Introduction
- S. europea, the common glasswort, appears as a relatively small plant, having bright green stems characterized by small leaves and fleshy fruits that contain a single seed. It is present in Britain, France, and Ireland [3];
- S. bigelovii, the dwarf saltwort Salicornia, is located in USA and Mexico and can be distinguished from other species by its acute and sharply mucronate leaf and bract tips [4];
- S. ramosissima, also known as purple glasswort, is situated in France and Iberia and has stems up to 30 cm high, highly branched, green or purple depending on their youth [5];
- S. herbacea, the dwarf glasswort, is diffused in Korea and Italy and has fleshy, erect stems and opposite leaves, similar in appearance to flattened scales on the stems [6];
- S. brachiata, also named umari keerai, is located in India [7].
 |  |  |  |  | |
| Botanical name | S. bigelovii | S. brachiata | S. europea | S. ramosissima | S. herbacea |
| Common names | Dwarf saltwort | Umari Keerai | Common glasswort | Purple glasswort | Dwarf glasswort |
| Geographical range | USA, Mexico | India | Britain, France, Ireland | France, Iberia | South Korea |
| References | [4] | [7] | [3] | [5] | [6] |
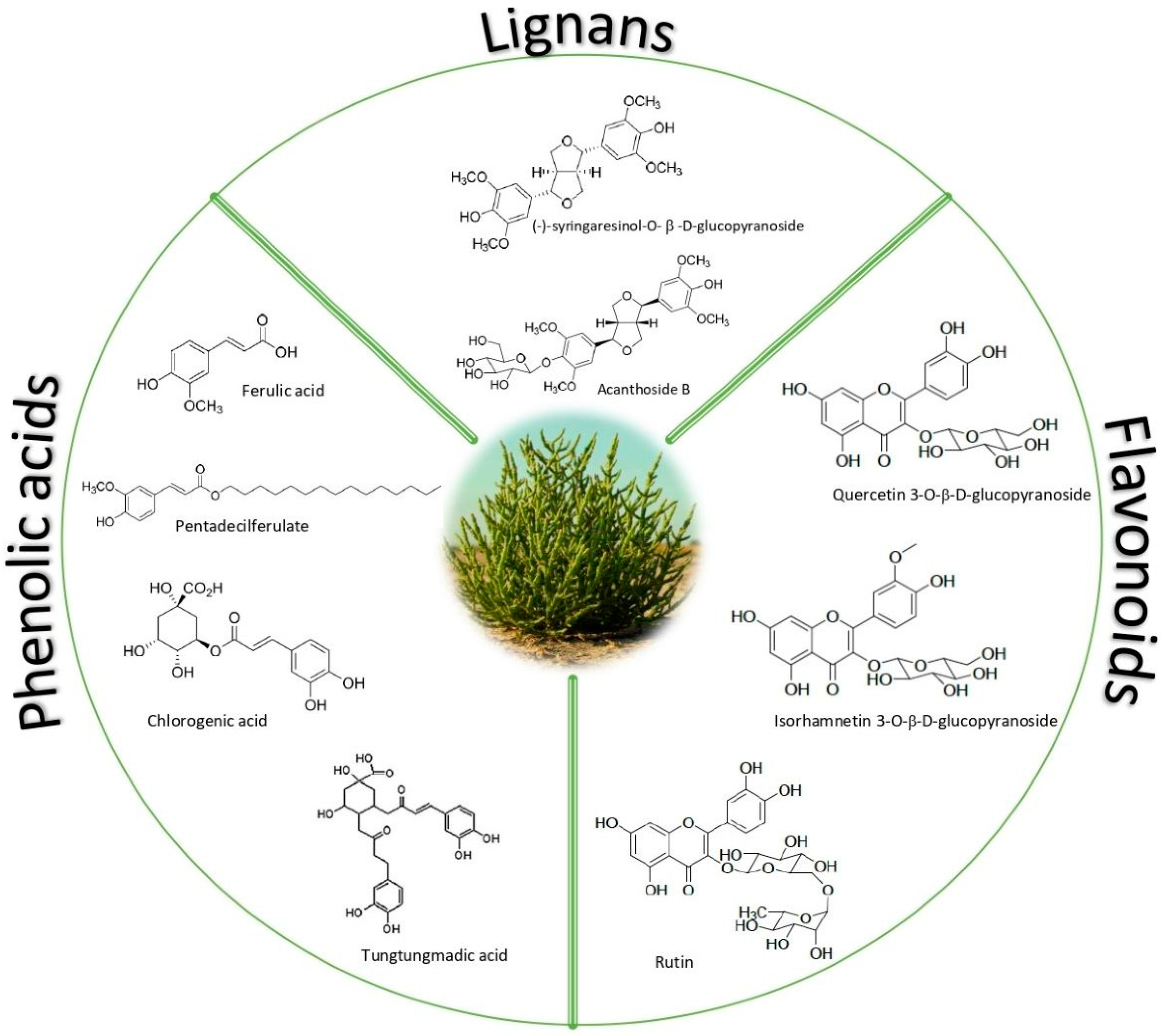
2. Principal Phytochemicals (Especially Polyphenols) Identified in Salicornia
2.1. Phenolic Acids
2.2. Flavonoids
2.3. Lignans
3. Comparison of Extraction Methods of Polyphenols from Salicornia
3.1. Maceration
3.2. Microwave-Assisted Extraction
3.3. Ultrasound-Assisted Extraction
3.4. Supercritical Fluid Extraction
3.5. Enzyme-Assisted Extraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Corbo, F.; Brunetti, G.; Crupi, P.; Bortolotti, S.; Storlino, G.; Piacente, L.; Carocci, A.; Catalano, A.; Milani, G.; Colaianni, G.; et al. Effects of Sweet Cherry Polyphenols on Enhanced Osteoclastogenesis Associated With Childhood Obesity. Front. Immunol. 2019, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Liso, M.; Verna, G.; Curci, F.; Milani, G.; Faienza, M.F.; Franchini, C.; Moschetta, A.; Chieppa, M.; Clodoveo, M.L.; et al. Extra Virgin Olive Oil Extracts Modulate the Inflammatory Ability of Murine Dendritic Cells Based on Their Polyphenols Pattern: Correlation between Chemical Composition and Biological Function. Antioxidants 2021, 10, 1016. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Niu, Y.D.; Huridu, H.; Hao, J.F.; Qi, Z.; Hasi, A. Salicornia europaea L. Na+/H+ antiporter gene improves salt tolerance in transgenic alfalfa (Medicago sativa L.). Genet. Mol Res. 2014, 13, 5350–5360. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, M.; Cao, C.; Ju, Y.; Deng, Y.; Ye, T.; Xia, Z.; Chen, M. Effect and mechanism of Salicornia bigelovii Torr. plant salt on blood pressure in SD rats. Food Funct. 2015, 6, 920–926. [Google Scholar] [CrossRef]
- Isca, V.M.; Seca, A.M.; Pinto, D.C.; Silva, H.; Silva, A.M. Lipophilic profile of the edible halophyte Salicornia ramosissima. Food Chem. 2014, 165, 330–336. [Google Scholar] [CrossRef]
- Cho, H.D.; Lee, J.H.; Jeong, J.H.; Kim, J.Y.; Yee, S.T.; Park, S.K.; Lee, M.K.; Seo, K.I. Production of novel vinegar having antioxidant and anti-fatigue activities from Salicornia herbacea L. J. Sci. Food. Agric. 2015, 96, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.; Singh, N.P.; Mishra, A. Proteome profiling of seed storage proteins reveals the nutritional potential of Salicornia brachiata Roxb.; an extreme halophyte. J. Agric. Food Chem. 2012, 60, 4320–4326. [Google Scholar] [CrossRef] [PubMed]
- Giordano, R.; Aliotta, G.E.; Johannesen, A.S.; Voetmann-Jensen, D.; Laustsen, F.H.; Andersen, L.A.; Rezai, A.; Fredsgaard, M.; Vecchio, S.L.; Arendt-Nielsen, L.; et al. Effects of Salicornia-Based Skin Cream Application on Healthy Humans’ Experimental Model of Pain and Itching. Pharmaceuticals 2022, 15, 150. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Lentini, G.; Catalano, A.; Cavalluzzi, M.M.; Bruno, C.; Muraglia, M.; Franchini, C. Chiral Aryloxyalkylamines: Selective 5-HT1B/1D Activation and Analgesic Activity. ChemMedChem 2010, 5, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Glassworts: From Wild Salt Marsh Species to Sustainable Edible Crops. Agriculture 2019, 9, 14. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Zhao, Y.; Wang, H.; Liu, T.; Xin, Z. Pentadecyl ferulate, a potent antioxidant and antiproliferative agent from the halophyte Salicornia herbacea. Food Chem. 2013, 141, 2066–2074. [Google Scholar] [CrossRef]
- Mallamaci, R.; Budriesi, R.; Clodoveo, M.L.; Biotti, G.; Micucci, M.; Ragusa, A.; Curci, F.; Muraglia, M.; Corbo, F.; Franchini, C. Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation. Molecules 2021, 26, 1072. [Google Scholar] [CrossRef]
- Faienza, M.F.; Corbo, F.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Grano, M.; Wang, D.Q.; D’Amato, G.; Muraglia, M.; Franchini, C.; et al. Novel insights in health-promoting properties of sweet cherries. J. Funct. Foods 2020, 69, 103945. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Corbo, F. Optimization of a Green Extraction of Polyphenols from Sweet Cherry (Prunus avium L.) Pulp. Processes 2022, 10, 1657. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Annunziato, A.; Corbo, F. Innovative extraction technologies for development of functional ingredients based on polyphenols from olive leaves. Foods 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Pérez, S.; Piernik, A.; Chanona-Pérez, J.J.; Grigore, M.N.; Perea-Flores, M.J. An overview of the emerging trends of the Salicornia L. genus as a sustainable crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Li, X.; Sun, J.; Yao, D.; Jiang, H.; Zhou, T.; Liu, Y.; Li, J.; Wang, C.; et al. Extraction, preliminary characterization and antioxidant properties of polysaccharides from the testa of Salicornia herbacea. Carbohydr. Polym. 2017, 176, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zhang, M.; Wang, S.; Cai, J.; Zhou, X.; Zhu, C. Nutritional characterization and changes in quality of Salicornia bigelovii Torr. during storage. LWT-Food Sci. Technol. 2010, 43, 519–524. [Google Scholar] [CrossRef]
- Lima, A.R.; Castaneda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Barreira, L. Influence of cultivation salinity in the nutritional composition, antioxidant capacity and microbial quality of Salicornia ramosissima commercially produced in soilless systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Dong, G.; Shang, Y.; Lyu, Y.; Li, F.; Yu, X. The chemical composition analysis of dwarf saltwort (Salicornia bigelovii Torr.) and its preservative effects on snakehead fish fillets. J. Food Process. Preserv. 2022, 46, e16433. [Google Scholar] [CrossRef]
- Panth, N.; Park, S.H.; Kim, H.J.; Kim, D.H.; Oak, M.H. Protective effect of Salicornia europaea extracts on high salt intake-induced vascular dysfunction and hypertension. Int. J. Mol. Sci. 2016, 17, 1176. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, J.; Fan, P.; Jia, W.; Nie, L.; Jiang, P.; Li, Y. High-throughput deep sequencing reveals that microRNAs play important roles in salt tolerance of euhalophyte Salicornia europaea. BMC Plant Biol. 2015, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jeong, E.M.; Ki, A.Y.; Oh, K.S.; Kwon, J.; Jeong, J.H.; Chung, N.J. Oxidative defense metabolites induced by salinity stress in roots of Salicornia herbacea. J. Plant Physiol. 2016, 206, 133–142. [Google Scholar] [CrossRef]
- Rahmani, R.; Arbi, K.E.; Aydi, S.S.; Hzami, A.; Tlahig, S.; Najar, R.; Debouba, M. Biochemical composition and biological activities of Salicornia europaea L. from southern Tunisia. J. Food Meas. Charact. 2022, 16, 4833–4846. [Google Scholar] [CrossRef]
- Mishra, A.; Patel, M.K.; Jha, B. Non-targeted metabolomics and scavenging activity of reactive oxygen species reveal the potential of Salicornia brachiata as a functional food. J. Funct. Foods 2015, 13, 21–31. [Google Scholar] [CrossRef]
- Kaur, N.; Chaudhary, J.; Jain, A.; Kishore, L. Stigmasterol: A comprehensive review. Int. J. Pharm. Sci. Res. 2011, 2, 2259. [Google Scholar]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The story of beta-sitosterol-a review. Eur. J. Med. Plants 2014, 4, 590. [Google Scholar] [CrossRef]
- Kim, S.; Lee, E.Y.; Hillman, P.F.; Ko, J.; Yang, I.; Nam, S.J. Chemical structure and biological activities of secondary metabolites from Salicornia europaea L. Molecules 2021, 26, 2252. [Google Scholar] [CrossRef]
- Lyu, H.; Ma, X.; Guan, F.; Chen, Y.; Wang, Q.; Feng, X. 30-Noroleanane triterpenoid saponins from Salicornia europaea Linn. and their chemotaxonomic significance. Biochem. Syst. Ecol. 2018, 78, 106–109. [Google Scholar] [CrossRef]
- Patel, S. Salicornia: Evaluating the halophytic extremophile as a food and a pharmaceutical candidate. 3 Biotech 2016, 6, 104. [Google Scholar] [CrossRef]
- Elsebaie, E.M.; Elsanat, S.Y.; Gouda, M.S.; Elnemr, K.M. Oil and fatty acids composition in glasswort (Salicornia fruticosa) seeds. J. App. Chem. 2013, 4, 2278–5736. [Google Scholar]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Zuo, P.Y.; Zha, X.N.; Chen, X.L.; Zhang, R.; He, X.X.; Liu, C.Y. Octacosanol enhances the proliferation and migration of human umbilical vein endothelial cells via activation of the PI3K/Akt and MAPK/Erk pathways. Lipids 2015, 50, 241–251. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Shpigel, M.; Samocha, T.M.; Klim, B.C.; Cohen, S.; Sagi, M. Effects of day length on flowering and yield production of Salicornia and Sarcocornia species. Sci. Hortic. 2011, 130, 510–516. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Gouda, M.S.; Elsebaie, E.M. Glasswort (Salicornia spp) as a source of bioactive compounds and its health benefits: A review. Alex J. Sci. Technol. 2016, 13, 1–7. [Google Scholar]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, J.Y.; Lee, Y.G.; Lee, H.J.; Shim, H.J.; Lee, J.H.; Moon, J.H. Four new dicaffeoylquinic acid derivatives from glasswort (Salicornia herbacea L.) and their antioxidative activity. Molecules 2016, 21, 1097. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012, 35, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.S.; Berk, B.C.; Abe, J.I. Disturbed flow-induced endothelial proatherogenic signaling via regulating post-translational modifications and epigenetic events. Antioxid. Redox Signal. 2016, 25, 435–450. [Google Scholar] [CrossRef]
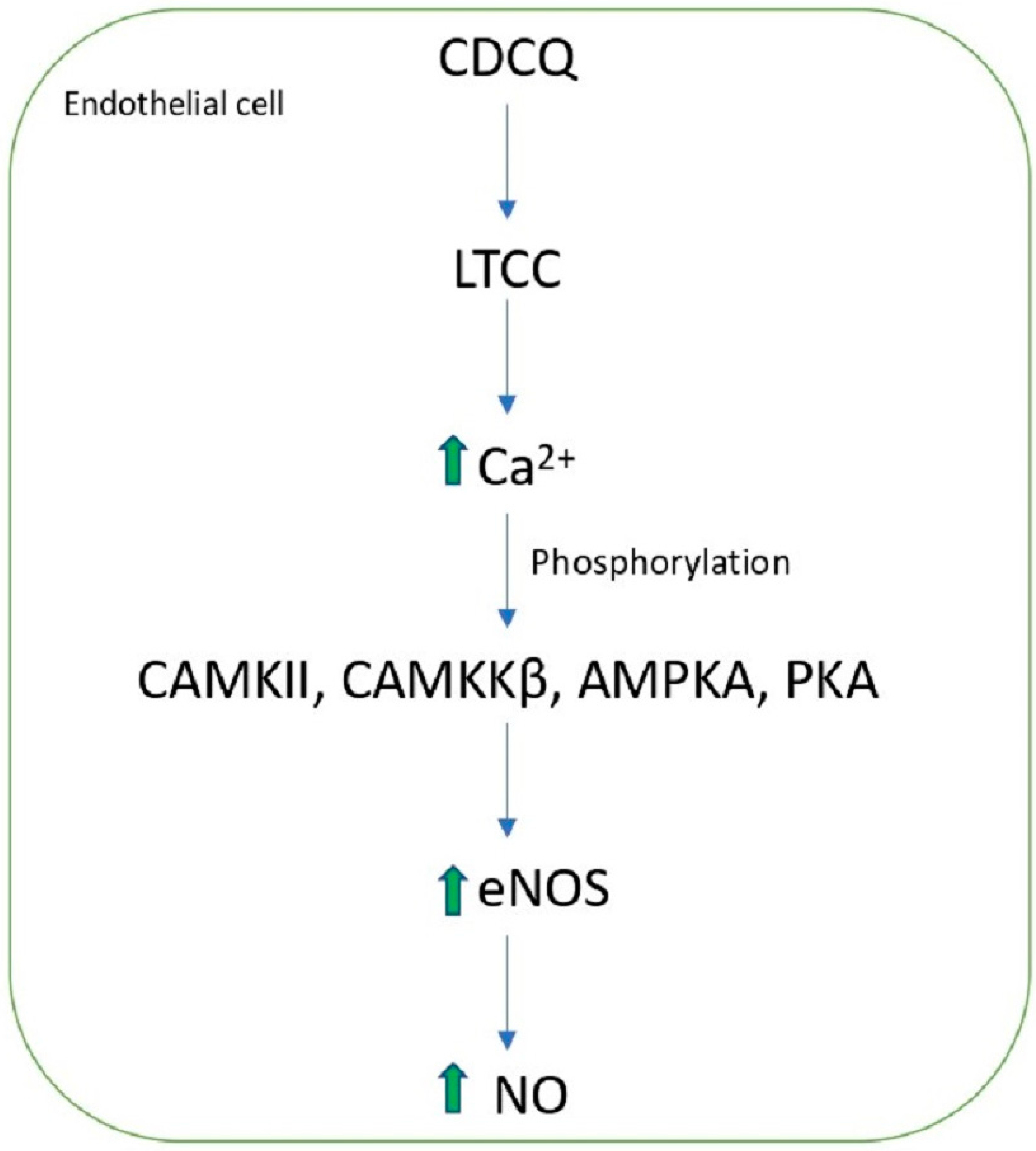
- Lee, G.H.; Lee, S.Y.; Zheng, C.; Pham, H.T.; Kim, C.Y.; Kim, M.Y.; Jeong, H.G. Effect of 3-caffeoyl, 4-dihydrocaffeoylquinic acid from Salicornia herbacea on endothelial nitric oxide synthase activation via calcium signaling pathway. Toxicol. Res. 2022, 38, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Kim, M.J.; Kim, J.H.; Kim, S.H.; Go, H.K.; Kweon, M.H.; Kim, D.H. Desalted Salicornia europaea powder and its active constituent, trans-ferulic acid, exert anti-obesity effects by suppressing adipogenic-related factors. Pharm. Biol. 2018, 56, 183–191. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef]
- Padalino, L.; Costa, C.; Del Nobile, M.A.; Conte, A. Extract of Salicornia europaea in fresh pasta to enhance phenolic compounds and antioxidant activity. Int. J. Food Sci. Technol. 2019, 54, 3051–3057. [Google Scholar] [CrossRef]
- Ferreira, D.; Isca, V.M.; Leal, P.; Seca, A.M.; Silva, H.; de Lourdes Pereira, M.; Pinto, D.C. Salicornia ramosissima: Secondary metabolites and protective effect against acute testicular toxicity. Arab. J. Chem. 2018, 11, 70–80. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- D’Amelia, V.; Aversano, R.; Chiaiese, P.; Carputo, D. The antioxidant properties of plant flavonoids: Their exploitation by molecular plant breeding. Phytochem. Rev. 2018, 17, 611–625. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. 2021 Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Kong, C.S.; Kim, J.A.; Qian, Z.J.; Kim, Y.A.; Im Lee, J.; Kim, S.K.; Seo, Y. Protective effect of isorhamnetin 3-O-β-D-glucopyranoside from Salicornia herbacea against oxidation-induced cell damage. Food Chem. Toxicol. 2009, 47, 1914–1920. [Google Scholar] [CrossRef]
- Cybulska, I.; Zembrzuska, J.; Brudecki, G.; Thomsen, M.H. Optimizing Methods to Characterize Caffeic, Ferulic, and Chlorogenic Acids in Salicornia sinus-persica and Salicornia bigelovii Extracts by Tandem Mass Spectrometry (LC-MS/MS). Bioresources 2021, 16, 5508–5523. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, J.Y.; Ma, Y.K.; Park, K.Y.; Lee, S.H.; Ham, K.S.; Moon, J.H. Dicaffeoylquinic acid derivatives and flavonoid glucosides from glasswort (Salicornia herbacea L.) and their antioxidative activity. Food Chem. 2011, 125, 55–62. [Google Scholar] [CrossRef]
- Masuoka, N.; Matsuda, M.; Kubo, I. Characterisation of the antioxidant activity of flavonoids. Food Chem. 2012, 131, 541–545. [Google Scholar] [CrossRef]
- Noh, E.J.; Lee, J.Y.; Park, S.Y.; Park, J.H.; Cho, J.Y.; Kim, Y.M.; Lee, S.K. Salicornia herbacea Aqueous Extracts Regulate NLRP3 Inflammasome Activation in Macrophages and Trophoblasts. J. Med. Food 2022, 25, 503–512. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, J.Y.; Moon, J.H.; Choi, G.C.; Lee, K.D.; Ham, K.S.; Kim, S.J. Change of phenylpropanoic acid and flavonol contents at different growth stage of glasswort (Salicornia herbacea L.). Food Sci. Biotechnol. 2014, 23, 685–691. [Google Scholar] [CrossRef]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef] [PubMed]
- Won, K.J.; Lee, K.P.; Baek, S.; Cui, L.; Kweon, M.H.; Jung, S.H.; Kim, B. Desalted Salicornia europaea extract attenuated vascular neointima formation by inhibiting the MAPK pathway-mediated migration and proliferation in vascular smooth muscle cells. Biomed. Pharmacother. 2017, 94, 430–438. [Google Scholar] [CrossRef]
- Essaidi, I.; Brahmi, Z.; Snoussi, A.; Koubaier, H.B.H.; Casabianca, H.; Abe, N.; Bouzouita, N. Phytochemical investigation of Tunisian Salicornia herbacea L.; antioxidant, antimicrobial and cytochrome P450 (CYPs) inhibitory activities of its methanol extract. Food Control. 2013, 32, 125–133. [Google Scholar] [CrossRef]
- Pandit, A.; Sachdeva, T.; Bafna, P. Drug-induced hepatotoxicity: A review. J. Appl. Pharm. Sci. 2012, 02, 233–243. [Google Scholar] [CrossRef]
- Greenblatt, D.J.; Zhao, Y.; Hanley, M.J.; Chen, C.; Harmatz, J.S.; Cancalon, P.F.; Gmitter Jr, F.G. Mechanism-based inhibition of human cytochrome P450-3A activity by grapefruit hybrids having low furanocoumarin content. Xenobiotica 2012, 42, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Polat Kose, L.; Gulcin, İ. Evaluation of the antioxidant and antiradical properties of some phyto and mammalian lignans. Molecules 2021, 26, 7099. [Google Scholar] [CrossRef] [PubMed]
- Köse, L.P.; Gulcin, I. Inhibition effects of some lignans on carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase enzymes. Rec. Nat. Prod. 2017, 11, 558–561. [Google Scholar] [CrossRef]
- Zálešák, F.; Bon, D.J.Y.D.; Pospíšil, J. Lignans and Neolignans: Plant secondary metabolites as a reservoir of biologically active substances. Pharmacol. Res. 2019, 146, 104284. [Google Scholar] [CrossRef]
- Wang, X.Y.; Feng, X.; Wang, M.; Chen, Y.; Dong, Y.F.; Zhao, Y.Y.; Sun, H. Studies on the chemical constituents of Salicornia europaea. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2011, 34, 67–69. [Google Scholar]
- Wang, S.; Wu, C.; Li, X.; Zhou, Y.; Zhang, Q.; Ma, F.; Guo, P. Syringaresinol-4-O-β-D-glucoside alters lipid and glucose metabolism in HepG2 cells and C2C12 myotubes. Acta Pharm. Sin. B 2017, 7, 453–460. [Google Scholar] [CrossRef]
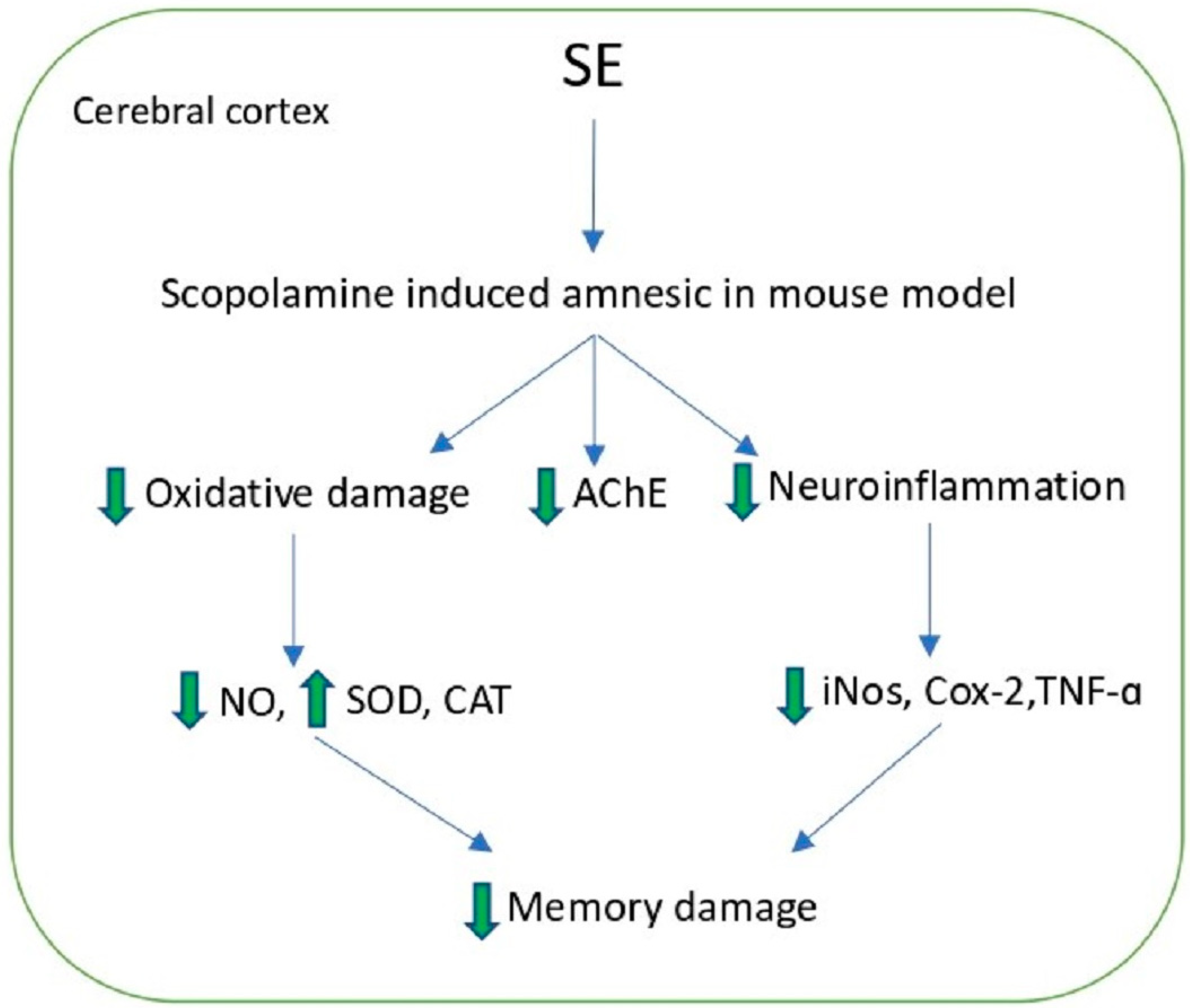
- Karthivashan, G.; Kweon, M.H.; Park, S.Y.; Kim, J.S.; Kim, D.H.; Ganesan, P.; Choi, D.K. Cognitive-enhancing and ameliorative effects of acanthoside B in a scopolamine-induced amnesic mouse model through regulation of oxidative/inflammatory/cholinergic systems and activation of the TrkB/CREB/BDNF pathway. Food Chem. Toxicol. 2019, 129, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Crupi, P.; Muraglia, M.; Corbo, F. Ultrasound Assisted Extraction of Polyphenols from Ripe Carob Pods (Ceratonia siliqua L.): Combined Designs for Screening and Optimizing the Processing Parameters. Foods 2022, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Crupi, P.; Dipalmo, T.; Clodoveo, M.L.; Toci, A.T.; Coletta, A. Seedless table grape residues as a source of polyphenols: Comparison and optimization of non-conventional extraction techniques. Eur. Food Res. Technol. 2018, 244, 1091–1100. [Google Scholar] [CrossRef]
- Jovanović, A.; Petrović, P.; Đorđević, V.; Zdunić, G.; Šavikin, K.; Bugarski, B. Polyphenols extraction from plant sources. Lek. Sirovine 2017, 37, 45–49. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Saini, A.; Panesar, P.S. Beneficiation of food processing by-products through extraction of bioactive compounds using neoteric solvents. LWT 2020, 134, 110263. [Google Scholar] [CrossRef]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A powerful and greener alternative to the latest solid-liquid extraction techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef]
- Silva, A.M.; Lago, J.P.; Pinto, D.; Moreira, M.M.; Grosso, C.; Cruz Fernandes, V.; Rodrigues, F. Salicornia ramosissima bioactive composition and safety: Eco-friendly extractions approach (microwave-assisted extraction vs. conventional maceration). Appl. Sci. 2021, 11, 4744. [Google Scholar] [CrossRef]
- Ko, Y.C.; Choi, H.S.; Kim, S.L.; Yun, B.S.; Lee, D.S. Anti-Inflammatory Effects of (9Z, 11E)-13-Oxooctadeca-9, 11-dienoic Acid (13-KODE) Derived from Salicornia herbacea L. on Lipopolysaccharide-Stimulated Murine Macrophage via NF-kB and MAPK Inhibition and Nrf2/HO-1 Signaling Activation. Antioxidants 2022, 11, 180. [Google Scholar] [CrossRef]
- Pinto, D.; Reis, J.; Silva, A.M.; Salazar, M.; Dall’Acqua, S.; Delerue-Matos, C.; Rodrigues, F. Valorisation of Salicornia ramosissima biowaste by a green approach–An optimizing study using response surface methodology. Sustain. Chem. Pharm. 2021, 24, 100548. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M. Olive tree leaves—A source of valuable active compounds. Processes 2020, 8, 1177. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochemistry 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Cristina, C.; Lucia, P.; Sara, S.; Francesco, S.; Nobile Matteo Alessandro, D.; Amalia, C. Study of the efficacy of two extraction techniques from Crithmum maritimum and Salicornia europaea. J. Food Nutr. Res. 2018, 6, 456–463. [Google Scholar] [CrossRef]
- Magiera, S.; Sobik, A. Ionic liquid-based ultrasound-assisted extraction coupled with liquid chromatography to determine isoflavones in soy foods. J. Food Compos. Anal. 2017, 57, 94–101. [Google Scholar] [CrossRef]
- Sicaire, A.G.; Vian, M.A.; Fine, F.; Carré, P.; Tostain, S.; Chemat, F. Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrason. Sonochemistry 2016, 31, 319–329. [Google Scholar] [CrossRef]
- Souza, M.M.; Silva, B.D.; Costa, C.S.; Badiale-Furlong, E. Free phenolic compounds extraction from Brazilian halophytes, soybean and rice bran by ultrasound-assisted and orbital shaker methods. An. Da Acad. Bras. Ciências 2018, 90, 3363–3372. [Google Scholar] [CrossRef]
- Faria, G.Y.Y.; Souza, M.M.; Oliveira, J.R.M.; Costa, C.S.B.; Collares, M.P.; Prentice, C. Effect of ultrasound-assisted cold plasma pretreatment to obtain sea asparagus extract and its application in Italian salami. Food Res. Int. 2020, 137, 109435. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Andrade, F.; Prazeres, I.; Silva, A.B.; Capelo, J.; Duarte, B.; Bronze, M.R. Impact of drying processes on the nutritional composition, volatile profile, phytochemical content and bioactivity of Salicornia ramosissima J. woods. Antioxidants 2021, 10, 1312. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Optimization of ultrasound-assisted extraction of phenolic compounds from Salicornia herbacea powder. Prev. Nutr. Food Sci. 2009, 14, 129–133. [Google Scholar] [CrossRef][Green Version]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Xynos, N.; Papaefstathiou, G.; Psychis, M.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A.L. Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. J. Supercrit. Fluids 2012, 67, 89–93. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub-and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae–An update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; FitzGerald, R.J.; Vila-Soler, A.; Mendiola, J.; Ibáñez, E.; Brunton, N.P. Comparison of extraction methods for selected carotenoids from macroalgae and the assessment of their seasonal/spatial variation. Innov. Food Sci. Emerg. Technol. 2016, 37, 221–228. [Google Scholar] [CrossRef]
- Folayan, A.J.; Anawe, P.A.L.; Ayeni, A.O. Synthesis and characterization of Salicornia bigelovii and Salicornia brachiata halophytic plants oil extracted by supercritical CO2 modified with ethanol for biodiesel production via enzymatic transesterification reaction using immobilized Candida antarctica lipase catalyst in tert-butyl alcohol (TBA) solvent. Cogent Eng. 2019, 6, 1625847. [Google Scholar]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Enzyme-assisted extraction of bioactive non-extractable polyphenols from sweet cherry (Prunus avium L.) pomace. Food Chem. 2021, 339, 128086. [Google Scholar] [CrossRef]
- Akyüz, A.; Ersus, S. Optimization of enzyme assisted extraction of protein from the sugar beet (Beta vulgaris L.) leaves for alternative plant protein concentrate production. Food Chem. 2021, 335, 127673. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, E.O.; Lee, S.K.; Woo, M.H.; Choi, S.W. Antioxidant activities of the ethanol extract of hamcho (Salicornia herbacea L.) cake prepared by enzymatic treatment. Food Sci. Biotechnol. 2007, 16, 90–98. [Google Scholar]
- Karthivashan, G.; Park, S.Y.; Kweon, M.H.; Kim, J.; Haque, M.; Cho, D.Y.; Choi, D.K. Ameliorative potential of desalted Salicornia europaea L. extract in multifaceted Alzheimer’s-like scopolamine-induced amnesic mice model. Sci. Rep. 2018, 8, 7174. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Muraglia, M.; Crupi, P.; Hbaieb, R.H.; De Santis, S.; Desantis, A.; Corbo, F. The Tower of Babel of Pharma-Food Study on Extra Virgin Olive Oil Polyphenols. Foods 2022, 11, 1915. [Google Scholar] [CrossRef] [PubMed]


| Polyphenolic Compounds | Salicornia Species | Extraction Method | Experimental Condition | Amount | Biological Activity | Ref. |
|---|---|---|---|---|---|---|
| Chlorogenic acid | S. europea, S. ramosissima S. herbacea | CE CE UAE CE MAE | EtOH H2O, 100 °C, 5 h 80% EtOH, 25 °C, 1 h H2O, 100 °C, 5 min 72–94 °C, 300 W, 5–10 min | 0.22 mg/g 14.1 mg/g dw 53.19 ug/g fw 0.0758 mg/g dw 0.0342 mg/g dw | Antihypertensive Antimicrobic Reduction of neointimal hyperplasia | [24] [62] [90] [79] |
| Caffeoyl-5-dihydrocaffeoylquinic acid | S. herbacea | CE | MeOH, rt, 24 h | 75.6 ± 2.3 mg/100 g fw | Inhibiting CE-OOH formation | [40] |
| 3-caffeoyl-5-dihydrocaffeoylquinic acid methyl ester | S. herbacea | CE | MeOH, rt, 24 h | 69 ± 1.4 μg/100 g fw | Inhibiting CE-OOH formation | [40] |
| 3-caffeoyl-4-dihydrocaffeoylquinic acid methyl ester | S. herbacea | CE | MeOH, rt, 24 h | 71.9 ± 1.9 μg/100 g fw | Inhibiting CE-OOH formation | [40] |
| 3,5-dihydrocaffeoylquinic acid methyl ester | S. herbacea | CE | MeOH, rt, 24 h | 171.9 ± 1.5 μg/100 g fw | Inhibiting CE-OOH formation | [40] |
| 3-caffeoylquinic acid | S. herbacea | CE | MeOH, rt, 24 h | Inhibiting CE-OOH formation | [40] | |
| 3-caffeoylquinic acid methyl ester | S. herbacea | CE | MeOH, rt, 24 h | Inhibiting CE-OOH formation | [40] | |
| 3-caffeoyl-4-dicaffeoylquinic acid (tungtungmadic acid) | S. herbacea | CE | 80% MeOH, rt | 8 mg/kg dw | Protective effect on endothelial cell function | [43,63] |
| 3,5-dicaffeoylquinic acid | S. ramosissima | UAE CE MAE | 80% EtOH, 1 h, 25 °C H2O, 5 min,100 °C 300 W, 5–10 min, 72–9 °C, | 25.83 mg/g fw 0.0259 mg/g dw 0.0280 mg/g dw | Antiproliferative Antihypertensive | [90] [79] |
| 4,5-dicaffeoylquinic acid | S. ramosissima | UAE | 80% EtOH, 1 h, 25 °C | 11.75 mg/g fw | Antiproliferative Antihypertensive | [90] |
| trans-Ferulic acid | S. europea, S. ramosissima, S. herbacea | EAE CE UAE EAE + UAE CE CE MAE | H2O, 37 °C, 6 h H2O, 100 °C, 5 h 80% EtOH, 1 h, 25 °C 12 h, 40 °C and EtOH, 3 h MeOH, rt, 72 h H2O, 100 °C, 5 min 300 W, 72–94 °C, 5–10 min | 2.60 ± 0.33 ug/g 8.2 mg/g dw 4.21 mg/g fw 8.45 mg% dw 0.1346 mg/g dw 0.0578 mg/g dw | Antidiabetic Antihypertensive Inhibitor of CYP450 | [21] [62] [99] [63] [79] |
| p-Coumaric acid | S. europea, S. ramosissima S. herbacea | CE UAE CE CE MAE | H2O, 100 °C, 5 h 80% EtOH, 25 °C, 1 h MeOH, rt, 72 h H2O, 100 °C, 5 min 300 W, 72–94 °C, 5–10 min | 3.19 mg/g dw 0.32 mg/g dw 2.75 mg/g fw 0.0483 mg/g dw 0.0349 mg/g dw | Antihypertensive Inhibitor of CYP450 | [21] [62] [63] [79] |
| Pentadecylferulate | S. herbacea | CE | 80% acetone, rt, 24 h. | Anticancer | [11] | |
| Caffeic acid | S. europea, S. ramosissima S. herbacea | CE CE EAE + UAE CE MAE | EtOH H2O, 100 °C, 5 h 12 h, 40 °C + EtOH, 3 h H2O, 100 °C, 5 min 300 W, 72–94 °C, 5–10 min | 0.28 mg/g dw 9.5 mg/g dw 6.87 mg% dw 0.0144 mg/g dw 0.0032 mg/g dw | Antibacterial Inhibitor of CYP450 | [24] [62] [99] [79] |
| Gallic acid | S. europea | CE CE MAE | EtOH H2O, 100 °C, 5 min 300 W, 72–94 °C, 5–10 min | 0.8 mg/g dw 0.21 mg/g dw 0.15 mg/g dw | Antibacterial Inhibitor of CYP450 | [24] [79] |
| Protocathecuic acid | S. europea, S. ramosissima S. herbacea | CE EAE + UAE CE MAE | H2O, 100 °C, 5 h 12 h, 40 °C + EtOH, 3 h H2O, 100 °C, 5 min 300 W, 72–94 °C, 5–10 min | 8.4 mg/g dw 1.54 mg% dw 0.1275 mg/g dw 0.0929 mg/g dw | Amelioration and prevention of vascular diseases. | [62] [99] [79] |
| Rutin hydrate | S. europea, S. ramosissima | CE CE MAE | EtOH H2O, 100 °C, 5 min 300 W, 72–94 °C, 5–10 min | 10.05 mg/g dw 0.0999 mg/g dw 0.0781 mg/g dw | Antibacterial | [24] [79] |
| Catechin hydrate | S. europea, S. ramosissima | CE CE MAE | EtOH H2O, 100 °C, 5 min 300 W, 72–94 °C, 5–10 min | 1.17 mg/g dw 0.1116 mg/g dw 0.0046 mg/g dw | Antibacterial | [24] [79] |
| Isoquercitrin 6”-O-methyloxalate | S. herbacea | CE | MeOH, rt, 24 h | 0.47 mg/kg fw | Anti-inflammatory | [57] |
| Isorhamnetin 3-O-β-D-glucopyranoside | S. herbacea | CE CE | H2O, 100 °C, 5 h MeOH, rt, 24 h | 16.2 mg/g dw 1.25 mg/kg fw | Anti-inflammatory Amelioration and prevention of vascular diseases. | [62] [57] |
| Quercetin 3-O-β-D-glucopyranoside | S. herbacea | CE CE | H2O, 100 °C, 5 h MeOH, rt, 24 h | 3.4 mg/g dw 2.15 mg/kg fw | Amelioration and prevention of vascular diseases. | [62] [57] |
| Kaempferol | S. europea, S.ramosissima S. herbacea | UAE CE MAE CE | 80% EtOH, 25 °C, 1 h, H2O, 100 °C, 5 min 300 W, 72–94 °C, 5–10 min MeOH, rt, 24 h | 108.1–24.6 mg/100 g dw 10.90 mg/g 0.0052 mg/g dw 0.0047 mg/g dw | Inhibitor of CYP450 | [90] [79] [63] |
| Quercetin | S. europea, S.ramosissima S. herbacea | CE EAE + UAE CE MAE | H2O, 100 °C, 5 h 12 h, 40 °C + EtOH, 3 h H2O, 100 °C, 5 min 300 W, 72–94 °C, 5–10 min | 2.5 mg/g dw 12.63 mg%dw 0.0340 mg/g dw 0.0284 mg/g dw | Inhibitor of CYP450 Amelioration and prevention of vascular diseases. | [62] [99] [79] |
| Isorhamnetin | S. europea S. herbacea | CE EAE + UAE | H2O, 100 °C, 5 h 12 h, 40 °C + EtOH, 3 h | 18.4 mg/g dw 6.65 mg% dw | Amelioration and prevention of vascular diseases. | [62] [99] |
| Acanthoside B | S. europea | EAE + CE | 50 °C, 15 h + 50% EtOH, 3 h | 2.40 mg/g | Neuroproective | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limongelli, F.; Crupi, P.; Clodoveo, M.L.; Corbo, F.; Muraglia, M. Overview of the Polyphenols in Salicornia: From Recovery to Health-Promoting Effect. Molecules 2022, 27, 7954. https://doi.org/10.3390/molecules27227954
Limongelli F, Crupi P, Clodoveo ML, Corbo F, Muraglia M. Overview of the Polyphenols in Salicornia: From Recovery to Health-Promoting Effect. Molecules. 2022; 27(22):7954. https://doi.org/10.3390/molecules27227954
Chicago/Turabian StyleLimongelli, Francesco, Pasquale Crupi, Maria Lisa Clodoveo, Filomena Corbo, and Marilena Muraglia. 2022. "Overview of the Polyphenols in Salicornia: From Recovery to Health-Promoting Effect" Molecules 27, no. 22: 7954. https://doi.org/10.3390/molecules27227954
APA StyleLimongelli, F., Crupi, P., Clodoveo, M. L., Corbo, F., & Muraglia, M. (2022). Overview of the Polyphenols in Salicornia: From Recovery to Health-Promoting Effect. Molecules, 27(22), 7954. https://doi.org/10.3390/molecules27227954










