Callus Culture of Scorzonera radiata as a New, Highly Productive and Stable Source of Caffeoylquinic Acids
Abstract
:1. Introduction
2. Results
2.1. Callus Line Sr-L1
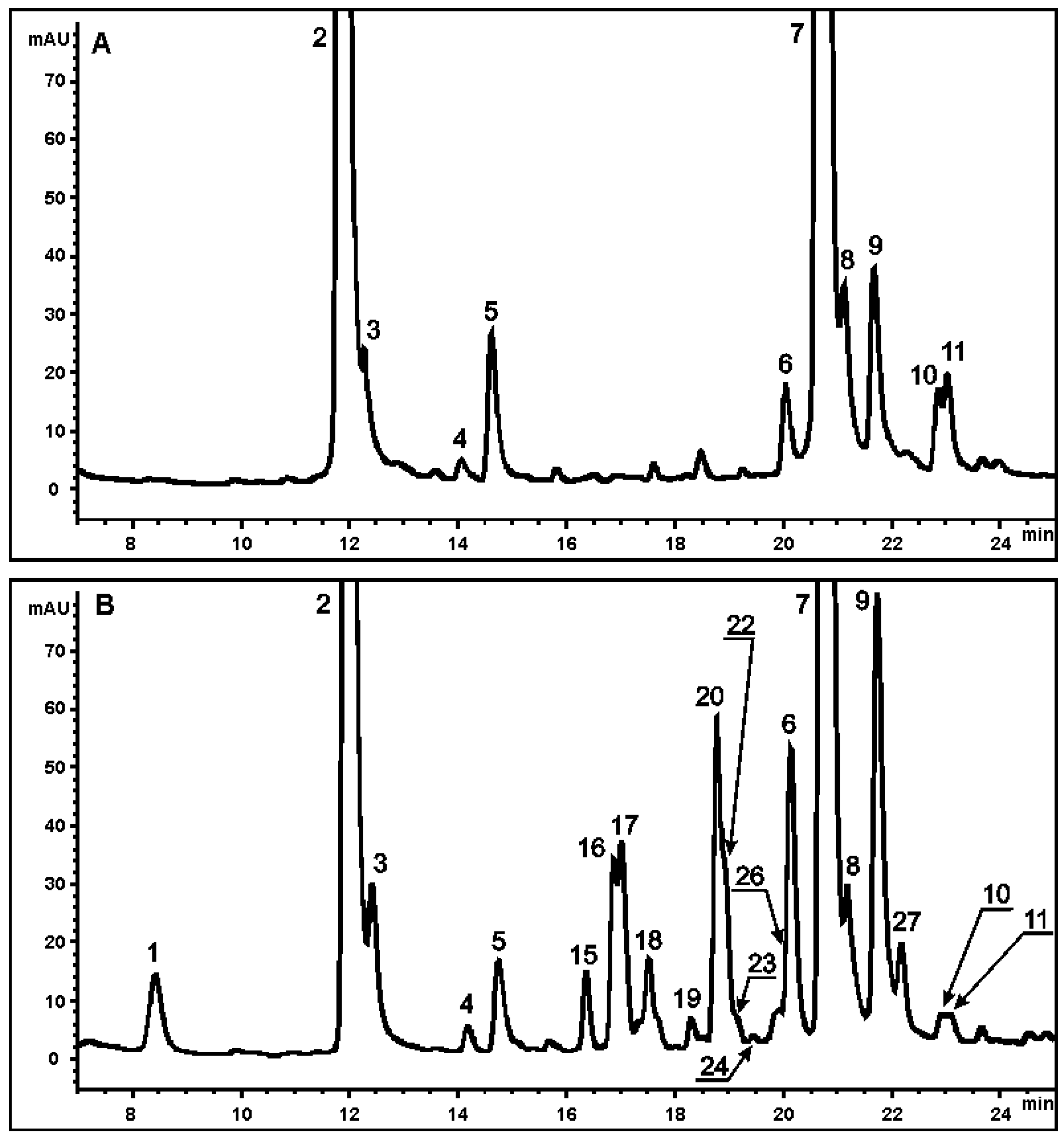
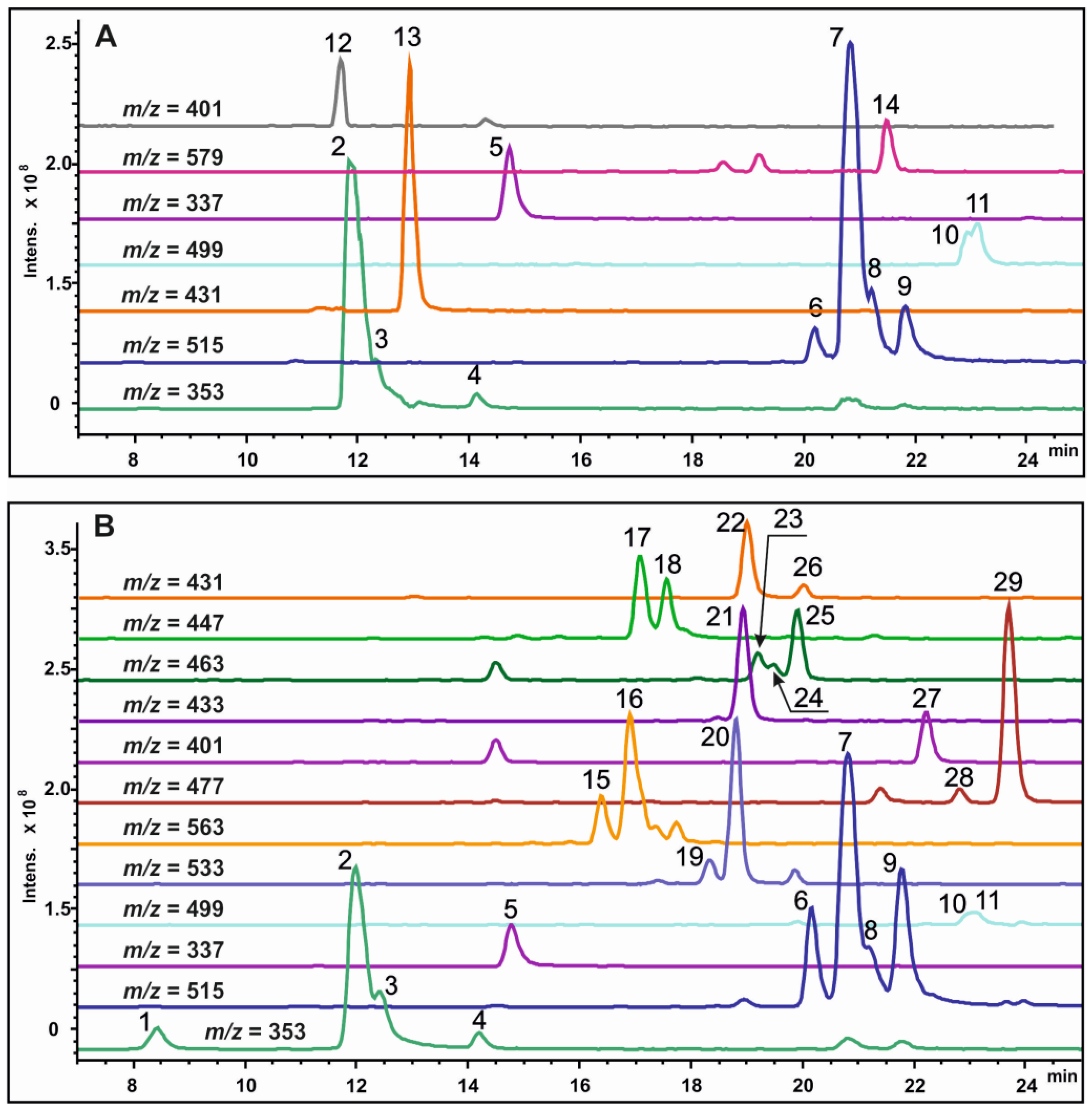
2.2. Analysis of Secondary Metabolites in the Sr-L1 Callus Line and Leaves of S. radiata Plants
| Peak No. | tR (min) | λmax (nm) | [M-H]− (m/z Detected) | [M-H]− (m/z Calculated) | Molecular Formula | MS2 Fragmentation (Precursor Ions [M-H]−) (% Base Peak) (m/z) | MS3 Fragmentation (% Base Peak) (m/z) ** | MS4 Fragmentation (% Base Peak) (m/z) ** | Assignment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8.4 | 324 | 353.0888 | 353.0878 | C16H18O9 | 191(100) *, 179(46), 135(6) | 173(91), 155(41), 127(82), 111(48), 93(73), 85(100) | - | 3-O-CQA |
| 2 | 11.9 | 325 | 353.0892 | 353.0878 | C16H18O9 | 191(100), 179(2) | 173(100), 127(80), 111(46), 109(48), 93(48), 85(94) | - | 5-O-CQA |
| 3 | 12.4 | 325 | 353.0889 | 353.0878 | C16H18O9 | 191(38), 179(62), 173(100), 135(5) | 155(35), 137(18), 111(30), 93(100) | - | 4-O-CQA |
| 4 | 14.2 | 320 | 353.0887 | 353.0878 | C16H18O9 | 191(100), 179(2) | 173(100), 127(75), 111(26), 109(27), 93(42), 85(92) | - | cis-5-O-CQA |
| 5 | 14.7 | 315 | 337.0939 | 337.0929 | C16H18O8 | 191(100), 163(3) | 173(93), 127(100), 111(40), 93(48), 85(88) | - | 5-O-p-coumaroylquinic acid |
| 6 | 20.1 | 326 | 515.1213 | 515.1195 | C25H24O12 | 353(100), 335(9), 299(1), 255(3), 203(5), 179(11), 173(30) | 191(50), 179(61), 173(100), 135(10) | 155(65), 111(23), 93(100) | 3,4-O-diCQA |
| 7 | 20.7 | 326 | 515.1207 | 515.1195 | C25H24O12 | 353(100), 191(5) | 191(100), 179(38), 173(4), 135(6) | 173(100), 127(75), 111(63), 85(83) | 3,5-O-diCQA |
| 8 | 21.1 | 325 | 515.1208 | 515.1195 | C25H24O12 | 353(100), 191(6) | 191(100), 179(32), 173(3), 135(5) | 173(100), 127(70), 111(55), 85(81) | cis-3,5-O-diCQA |
| 9 | 21.7 | 326 | 515.1206 | 515.1195 | C25H24O12 | 353(100), 335(3), 317(4), 299(5), 255(3), 203(11), 179(8), 173(10) | 191(29), 179(53), 173(100), 135(7) | 111(60), 93(100) | 4,5-O-diCQA |
| 10 | 22.9 | 316 | 499.1259 | 499.1246 | C25H24O11 | 353(5), 337(100), 335(3), 173(5), 163(12) | 173(59), 163(100), 119(6) | 119(100) | 3-O-p-coumaroyl-5-O-CQA |
| 11 | 23.1 | 317 | 499.1257 | 499.1246 | C25H24O11 | 353(100), 337(21), 191(5), 179(5) | 191(100), 179(16) | 173(100), 127(54), 111(68), 93(42), 85(64) | 3-O-caffeoyl-5-O-p-coumaroylquinic acid |
2.3. Determination and Quantification of CQAs
| Peak Number | Metabolite | Abbreviation | Sr-L1 | Leaves |
|---|---|---|---|---|
| 1 | 3-O-caffeoylquinic acid | 3-CQA | 0.02 ± 0.01 | 0.31 ± 0.04 |
| 2 | 5-O-caffeoylquinic acid | 5-CQA | 7.54 ± 0.80 | 7.29 ± 0.06 |
| 3 | 4-O-caffeoylquinic acid | 4-CQA | 0.04 ± 0.00 | 0.19 ± 0.03 |
| 4 | cis-5-O-caffeoylquinic acid | cis-5-CQA | 0.03 ± 0.01 | 0.07 ± 0.01 |
| 5 | 5-O-p-coumaroylquinic acid | 0.45 ± 0.03 | 0.26 ± 0.01 | |
| Total monoacyl derivatives | 8.05 ± 0.79 | 8.11 ± 0.07 | ||
| 6 | 3,4-O-dicaffeoylquinic acid | 3,4-diCQA | 0.29 ± 0.03 | 0.77 ± 0.05 |
| 7 | 3,5-O-dicaffeoylquinic acid | 3,5-diCQA | 18.52 ± 1.98 | 10.28 ± 0.37 |
| 8 | cis-3,5-O-dicaffeoylquinic acid | cis-3,5-diCQA | 0.10 ± 0.03 | 0.09 ± 0.01 |
| 9 | 4,5-O-dicaffeoylquinic acid | 4,5-diCQA | 0.40 ± 0.05 | 1.34 ± 0.12 |
| 10 | 3-O-p-coumaroyl-5-O-caffeoylquinic acid | 0.24 ± 0.04 | 0.06 ± 0.01 | |
| 11 | 3-O-caffeoyl-5-O-p-coumaroylquinic acid | 0.34 ± 0.03 | 0.06 ± 0.01 | |
| Total diacyl derivatives | 19.90 ± 2.03 | 12.60 ± 0.39 | ||
| Total | 27.95 ± 1.86 | 20.71 ± 0.31 |
2.4. Determination of Lignol Derivatives
| Peak No. * | tR (min) | λmax (nm) | Detected Ions Composition | m/z Detected | m/z Calculated | Molecular Formula | MS2 Fragmentation (% Base Peak) (m/z) | MS3 Fragmentation (% Base Peak) (m/z) | Assignment |
|---|---|---|---|---|---|---|---|---|---|
| 12 | 11.7 | 258 | [M+CH3COO]− | 401.1462 | 401.1453 | C16H22O8 | 341(46), 221(2), 179(100) **, 164(3), 161(3), 146(4) | 164(62), 161(100), 146(43) | Coniferyl alcohol O-hexoside (coniferin, coniferoside, and abietin) |
| [M+Na]+ | 365.1221 | 365.1207 | - | - | |||||
| 13 | 12.9 | 266 | [M+CH3COO]− | 431.1594 | 431.1573 | C17H24O9 | 371(16), 221(8), 209(100), 194(9), 179(3), 176(4), 161(2) | 194(100), 191(32), 179(3), 176(19), 161(3) | Sinapyl alcohol O-hexoside (syringin and eleutheroside B) |
| [M+Na]+ | 395.1329 | 395.1313 | - | - | |||||
| 14 | 21.3 | 275 | [M-H]− | 579.2096 | 579.2083 | C28H36O13 | 417(100), 181(5) | 402(40), 387(3), 371(3), 205(3), 181(100), 175(3), 166(38), 151(15) | Syringaresinol-O-hexoside (eleutheroside E1) |
| [M+Na]+ | 603.2082 | 603.2054 | - | - |
2.5. Determination of Flavonoid and Dihydrostilbene Derivatives
| Peak No. * | tR (min) | λmax (nm) | Detected Ions Composition | m/z Detected | m/z Calculated | Molecular Formula | MS2 Fragmentation (% Base Peak) (m/z) | Assignment |
|---|---|---|---|---|---|---|---|---|
| 15 | 16.4 | 271, 338 | [M-H]− | 563.1429 | 563.1406 | C26H28O14 | 503(11), 473(68), 443(98), 383(86), 353(100) | Apigenin-C-hexoside-C-pentoside I |
| [M+H]+ | 565.1531 | 565.1552 | - | |||||
| 16 | 16.9 | 271, 238 | [M-H]− | 563.1430 | 563.1406 | C26H28O14 | 503(5), 473(50), 443(95), 383(57), 353(100) | Apigenin-C-hexoside-C-pentoside II |
| [M+H]+ | 565.1542 | 565.1552 | - | |||||
| 17 | 17.0 | 257sh., 269, 348 | [M-H]− | 447.0953 | 447.0933 | C21H20O11 | 429(25), 357(82), 327(100) | Luteolin-6-C-glucoside (isoorientin) |
| [M+H]+ | 449.1075 | 449.1079 | - | |||||
| 18 | 17.5 | 256sh., 268, 350 | [M-H]− | 447.0942 | 447.0933 | C21H20O11 | 357(50), 327(100) | Luteolin-8-C-glucoside (Orientin) |
| [M+H]+ | 449.1093 | 449.1079 | - | |||||
| 19 | 18.3 | 270, 339 | [M-H]− | 533.1325 | 533.1300 | C25H26O13 | 515(15), 473(71), 443(100), 383(60), 353(56) | Apigenin-di-C-pentoside I |
| [M+H]+ | 535.1427 | 535.1446 | - | |||||
| 20 | 18.7 | 271, 338 | [M-H]− | 533.1323 | 533.1300 | C25H26O13 | 515(19), 473(85), 443(100), 413(16), 383(72), 353(68) | Apigenin-di-C-pentoside II |
| [M+H]+ | 535.1463 | 535.1446 | - | |||||
| 21 | 18.9 | 285 | [M-H]− | 433.1523 | 433.1504 | C22H26O9 | 271(100), 165(4) | Scorzodihydrostilbene C |
| [M-H-C6H10O5]− | 271.0968 | 271.0970 | 229(4), 165(100), 149(22) | |||||
| [M+Na]+ | 457.1458 | 457.1475 | - | |||||
| 22 | 19.0 | 271, 337 | [M-H]− | 431.0998 | 431.0984 | C21H20O10 | 341(6), 311(100) | Apigenin-8-C-glucoside (vitexin) |
| [M+H]+ | 433.1119 | 433.1129 | - | |||||
| 23 | 19.2 | 255, 265sh., 360 | [M-H]− | 463.0898 | 463.0882 | C21H20O12 | 301(100), 271(2), 179(3), 151(1) | Quercetin-3-O-galactoside (hyperoside) |
| [M+H]+ | 465.1011 | 465.1028 | - | |||||
| 24 | 19.5 | 255, 265sh., 358 | [M-H]− | 463.0901 | 463.0882 | C21H20O12 | 301(100), 271(1), 179(3), 151(2) | Quercetin-3-O-glucoside (isoquercitrin) |
| [M+H]+ | 465.1015 | 465.1028 | - | |||||
| 25 | 19.9 | 284 | [M-H]− | 463.1626 | 463.1610 | C23H28O10 | 301(100), 165(2) | Scorzodihydrostilbene A |
| [M-H-C6H10O5]− | 301.1072 | 301.1081 | 283(10), 259(4), 165(100), 149(48) | |||||
| [M+Na]+ | 487.1571 | 487.4580 | - | |||||
| 26 | 20.0 | Nd** | [M-H]− | 431.1005 | 431.0984 | C21H20O10 | 341(2), 311(100) | Apigenin-6-C-glucoside (isovitexin) |
| [M+H]+ | 433.1141 | 433.1129 | - | |||||
| 27 | 22.2 | 269, 340 | [M-H]− | 401.0891 | 401.0878 | C20H18O9 | 341(29), 311(100) | Apigenin-C-pentoside |
| [M+H]+ | 403.1037 | 403.1024 | - | |||||
| 28 | 22.8 | 280 | [M-H]− | 477.1777 | 477.1766 | C24H30O10 | 315(100) | Scorzodihydrostilbene D |
| [M+Na]+ | 501.1755 | 501.1737 | - | |||||
| 29 | 23.7 | 285 | [M-H]− | 477.1785 | 477.1766 | C24H30O10 | 357(23), 315(100), 163(1) | Scorzodihydrostilbene B |
| [M-H-C6H10O5]− | 315.1229 | 305.1238 | 299(22), 297(44), 281(25), 163(100), 149(31) | |||||
| [M+Na]+ | 501.1751 | 501.1737 | - |
3. Discussion
4. Materials and Methods
4.1. Plant Material and Callus Culture
4.2. Chemicals
4.3. Sample Preparation for Analytical Chromatography
4.4. UV Irradiation
4.5. Analytical Chromatography and Mass Spectrometry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kamelin, R.V.; Tagaev, I.U. A review of the Scorzonera species (Asteraceae). Bot. Zhurnal 1986, 71, 1672–1682. (In Russian) [Google Scholar]
- Zaika, M.A.; Kilian, N.; Jones, K.; Krinitsina, A.A.; Nilova, M.V.; Speranskaya, A.S.; Sukhorukov, A.P. Scorzonera sensu lato (Asteraceae, Cichorieae)—Taxonomic reassessment in the light of new molecular phylogenetic and carpological analyses. PhytoKeys 2020, 137, 1–85. [Google Scholar] [CrossRef] [PubMed]
- Zidorn, C.; Ellmerer, E.P.; Sturm, S.; Stuppner, H. Tyrolobibenzyls E and F from Scorzonera humilis and distribution of caffeic acid derivatives, lignans and tyrolobibenzyls in European taxa of the subtribe Scorzonerinae (Lactuceae, Asteraceae). Phytochemistry 2003, 63, 61–67. [Google Scholar] [CrossRef]
- Tsevegsuren, N.; Edrada, R.; Lin, W.; Ebel, R.; Torre, C.; Ortlepp, S.; Wray, V.; Proksch, P. Biologically active natural products from Mongolian medicinal plants Scorzonera divaricata and Scorzonera pseudodivaricata. J. Nat. Prod. 2007, 70, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Edrada-Ebel, R.; Tsevegsuren, N.; Sendker, J.; Braun, M.; Wray, V.; Lin, W.; Proksch, P.J. Dihydrostilbene derivatives from the mongolian medicinal plant Scorzonera radiata. J. Nat. Prod. 2009, 72, 671–675. [Google Scholar] [CrossRef]
- Bahadır, A.Ö.; Hošek, J.; Babula, P.; Cvačka, J.; Budešínský, M.; Dračinský, M.; Saltan, İ.G.; Kadlecová, D.; Ballová, L.; Šmejkal, K. Turkish. Scorzonera species extracts attenuate cytokine secretion via inhibition of NF-κB activation, showing anti-inflammatory effect in vitro. Molecules 2016, 21, 43. [Google Scholar] [CrossRef] [Green Version]
- Gil, M.; Wianowska, D. Chlorogenic acids—Their properties, occurrence and analysis. Ann. Univ. Mariae Curie-Sklodowska Sect. AA–Chem. 2017, LXXII, 61–104. [Google Scholar] [CrossRef]
- Lendzion, K.; Gornowicz, A.; Bielawski, K.; Bielawska, A. Phytochemical composition and biological activities of Scorzonera species. Int. J. Mol. Sci. 2021, 22, 5128. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Clifford, M.N.; Marks, S.; Knight, S.; Kuhnert, N. Characterization by LC-MSn of four new classes of p-coumaric acid-containing diacyl chlorogenic acids in green coffee beans. J. Agric. Food Chem. 2006, 54, 4095–4101. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Zheng, W.; Kuhnert, N. Profiling the chlorogenic acids of aster by HPLC-MSn. Phytochem. Anal. 2006, 17, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Kirkpatrick, J.; Kuhnert, N.; Roozendaal, H.; Salgado, P.R. LC-MSn analysis of the cis isomers of chlorogenic acids. Food Chem. 2008, 106, 379–385. [Google Scholar] [CrossRef]
- IUPAC. Nomenclature of cyclitols. Biochem. J. 1976, 153, 23–31. [Google Scholar]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Magaña, A.A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Q.; Hu, P.; Wu, W. Biguaiascorzolides A and B: Two novel dimeric guaianolides with a rare skeleton, from Scorzonera austriaca. Food Chem. 2009, 114, 1316–1320. [Google Scholar] [CrossRef]
- Wu, Q.X.; Su, Y.B.; Zhu, Y. Triterpenes and steroids from the roots of Scorzonera austriaca. Fitoterapia 2011, 82, 493–496. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, Q.-S.; Wang, G.-S. Preparative separation and purification of the total flavonoids in Scorzonera austriaca with macroporous resins. Molecules 2016, 21, 768. [Google Scholar] [CrossRef]
- Sezer, S.F.; Bahadir, A.O.; Saltan, C.G.; Erdogan, O.L.; Dall’Acqua, S.; Ozgokce, F. Prospective neurobiological effects of the aerial and root extracts and some pure compounds of randomly selected Scorzonera species. Pharm. Biol. 2014, 52, 873–882. [Google Scholar] [CrossRef]
- Zidorn, C.; Ellmerer-Müller, E.P.; Stuppner, H. Sesquiterpenoids from Scorzonera hispanica. Pharmazie 2000, 55, 550–555. [Google Scholar] [PubMed]
- Zidorn, C.; Ellmerer-Muller, E.P.; Stuppner, H. Tyrolobibenzyls—Novel secondary metabolites from Scorzonera humilis. Helv. Chim. Acta 2000, 83, 2920–2925. [Google Scholar] [CrossRef]
- Zidorn, C.; Petersen, B.O.; Udovičić, V.; Larsen, T.O.; Duus, J.O.; Rollinger, J.M.; Ongania, K.H.; Ellmerer, E.P.; Stuppner, H. Podospermic acid, 1,3,5-tri-O-(7,8-dihydrocaffeoyl)quinic acid from Podospermum laciniatum (Asteraceae). Tetrahedron Lett. 2005, 46, 1291–1294. [Google Scholar] [CrossRef]
- Paraschos, S.; Magiatis, P.; Kalpoutzakis, E.; Harvala, C.; Skaltsounis, A.L. Three new dihydroisocoumarins from the Greek endemic species Scorzonera cretica. J. Nat. Prod. 2001, 64, 1585–1587. [Google Scholar] [CrossRef]
- Sari, A.; Zidorn, C.; Ellmerer, E.P.; Ozgokce, F.; Ongania, K.H.; Stuppner, H. Phenolic compounds from Scorzonera tomentosa L. HeIv. Chim. Acta 2007, 90, 311–317. [Google Scholar] [CrossRef]
- Wang, Y.; Wray, V.; Tsevegsuren, N.; Lin, W.; Proksch, P. Phenolic compounds from the mongolian medicinal plant Scorzonera radiata. Z. Naturforsch. 2012, 67c, 135–143. [Google Scholar] [CrossRef]
- Tsevegsuren, N.; Proksch, P.; Wang, Y.; Davaakhuu, G. Bioactive phenolic acids from Scorzonera radiata Fisch. Mong. J. Chem. 2014, 12, 78–84. [Google Scholar] [CrossRef]
- Bahadır, A.Ö.; Ergene, Ö.B.; Bakar, F.; Saltan, Ç.G.; Nebioğlu, S. Evaluation of antioxidant activities and phenolic compounds of Scorzonera latifolia (Fisch. & Mey.) DC. collected from different geographic origins in Turkey. Turk. J. Pharm. Sci. 2017, 14, 179–184. [Google Scholar]
- Banerjee, S.K.; Bonde, C.G. Total phenolic content and antioxidant activity of extracts of Bridelia retusa Spreng bark: Impact of dielectric constant and geographical location. J. Med. Plants Res. 2011, 5, 817–822. [Google Scholar]
- Bryanskii, O.V.; Tolstikhina, V.V.; Semenov, A.A. A glycoside of syringaresinol from a tissue culture of Scorzonera hispanica. Chem. Nat. Compd. 1992, 25, 519–520. [Google Scholar] [CrossRef]
- Bryanskii, O.V.; Tolstikhina, V.V.; Zinchenko, S.V.; Semenov, A.A. A sesquiterpene glucoside from cultivated cells of Scorzonera hispanica. Chem. Nat. Compd. 1992, 28, 556–560. [Google Scholar] [CrossRef]
- Jaiswal, R.; Sovdat, T.; Vivan, F.; Kuhnert, N. Profiling and characterization by LC-MSn of the chlorogenic acids and hydroxycinnamoylshikimate esters in mate (Ilex paraguariensis). J. Agric. Food Chem. 2010, 58, 5471–5484. [Google Scholar] [CrossRef] [PubMed]
- Vereshchagina, Y.V.; Bulgakov, V.P.; Grigorchuk, V.P.; Veremeichik, G.N.; Tchernoded, G.K.; Gorpenchenko, T.Y.; Koren, O.G.; Phan, N.H.T.; Minh, N.T.; Chau, L.T.; et al. The rolC gene increases caffeoylquinic acid production in transformed artichoke cells. Appl. Microbiol. Biotechnol. 2014, 98, 7773–7780. [Google Scholar] [CrossRef] [PubMed]
- Eyles, A.; Jones, W.; Riedl, K.; Cipollini, D.; Schwartz, S.; Chan, K.; Herms, D.A.; Bonello, P. Comparative phloem chemistry of Manchurian (Fraxinus mandshurica) and two north American ash species (Fraxinus Americana and Fraxinus pennsylvanica). J. Chem. Ecol. 2007, 33, 1430–1448. [Google Scholar] [CrossRef]
- Sundaram, C.K.S.; Subramanian, I.P.I.; Subramanian, S.P.S. Isolation, characterization of syringin, phenylpropanoid glycosidefrom Musa paradisiaca tepal extract and evaluation of its antidiabeticeffect in streptozotocin-induced diabetic rats. Biomed. Prev. Nutr. 2014, 4, 105–111. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J.; Zhang, A.; Zhang, Y.; Meng, X.; Han, Y.; Zhang, Y.; Wang, X. Characterization of the multiple components of Acanthopanax senticosus stem by ultra high performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J. Sep. Sci. 2015, 39, 496–502. [Google Scholar] [CrossRef]
- Tchoumtchoua, J.; Mathiron, D.; Pontarin, N.; Gagneul, D.; van Bohemen, A.-I.; N’nang, E.O.; Mesnard, F.; Petit, E.; Fontaine, J.-X.; Molinié, R.; et al. Phenolic profiling of flax highlights contrasting patterns in winter and spring varieties. Molecules 2019, 24, 4303. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, F.; Hirai, M.Y.; Sasaki, E.; Akiyama, K.; Yonekura-Sakakibara, K.; Provart, N.J.; Sakurai, T.; Shimada, Y.; Saito, K. AtMetExpress development: A phytochemical atlas of Arabidopsis development. Plant Physiol. 2010, 152, 566–578. [Google Scholar] [CrossRef] [Green Version]
- Sanz, M.; de Simón, B.F.; Cadahía, E.; Esteruelas, E.; Muñoz, A.M.; Hernández, T.; Estrellac, I.; Pintod, E. LC-DAD/ESI-MS/MS study of phenolic compounds in ash (Fraxinus excelsior L. and F. americana L.) heartwood. Effect of toasting intensity at cooperage. J. Mass Spectrom. 2012, 47, 905–918. [Google Scholar] [CrossRef]
- Granica, S.; Lohwasser, U.; Jöhrer, K.; Zidorn, C. Qualitative and quantitative analyses of secondary metabolites in aerial and subaerial of Scorzonera hispanica L. (black salsify). Food Chem. 2015, 173, 321–331. [Google Scholar] [CrossRef]
- Granica, S.; Zidorn, C. Phenolic compounds from aerial parts as chemosystematic markers in the Scorzonerinae (Asteraceae). Biochem. Syst. Ecol. 2015, 58, 102–113. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I.; Nikolaev, V.M.; Kim, S.-W.; Vennos, C. Bioactive phenolics of the genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the Siberian species and their inhibitory potential against α-amylase and α-glucosidase. Front. Pharmacol. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Caristi, C.; Gargiulli, C.; Bellocco, E.; Toscano, G.; Leuzzi, U. Flavonoid glycosides in bergamot juice (Citrus bergamia Risso). J. Agric. Food Chem. 2006, 54, 3929–3935. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, P.B.; Valentão, P.; Gil-Izquierdo, A. Further knowledge on barley (Hordeum vulgare L.) leaves O-glycosyl-C-glycosyl flavones by liquid chromatography-UV diode-array detection-electrospray ionisation mass spectrometry. J. Chromatogr. A 2008, 1182, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Inyushkina, Y.V.; Fedoreyev, S.A. Rosmarinic acid and its derivatives: Biotechnology and applications. Crit. Rev. Biotechnol. 2012, 32, 203–217. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Vereshchagina, Y.V.; Veremeichik, G.N. Anticancer Polyphenols from Cultured Plant Cells: Production and New Bioengineering Strategies. Curr. Med. Chem. 2018, 25, 4671–4692. [Google Scholar] [CrossRef] [PubMed]
- Federici, E.; Touché, A.; Choquart, S.; Avanti, O.; Fay, L.; Offord, E.; Courtois, D. High isoflavone content and estrogenic activity of 25 year-old Glycine max tissue cultures. Phytochemistry 2003, 64, 717–724. [Google Scholar] [CrossRef]
- Veremeichik, G.N.; Bulgakov, V.P.; Shkryl, Y.N.; Silantieva, S.A.; Makhazen, D.S.; Tchernoded, G.K.; Mischenko, N.P.; Fedoreyev, S.A.; Vasileva, E.A. Activation of anthraquinone biosynthesis in long-cultured callus culture of Rubia cordifolia transformed with the rolA plant oncogene. J. Biotechnol. 2019, 306, 38–46. [Google Scholar] [CrossRef]
- Devrnja, N.; Krstić-Milošević, D.; Janošević, D.; Tešević, V.; Vinterhalter, B.; Savić, J.; Ćalić, D. In vitro cultivation of tansy (Tanacetum vulgare L.): A tool for the production of potent pharmaceutical agents. Protoplasma 2021, 258, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Raineri, A.; Campagnari, R.; Dal Toso, R.; Copetti, S.; Gomez-Lira, M.; Menegazzi, M. 3,5-Dicaffeoylquinic acid lowers 3T3-L1 mitotic clonal expansion and adipocyte differentiation by enhancing heme oxygenase-1 expression. Molecules 2021, 26, 5027. [Google Scholar] [CrossRef]
- Jang, Y.S.; Kim, H.-Y.; Zuo, G.; Lee, E.H.; Kang, S.K.; Lim, S.S. Constituents from Solidago virgaurea var. Gigantea and their inhibitory effect on lipid accumulation. Fitoterapia 2020, 146, 104683. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Park, S.K.; Guo, T.J.; Ha, J.S.; Lee, D.S.; Kim, J.M.; Lee, U.; Kim, D.O.; Heo, H.J. Reversal of Trimethyltin-Induced Learning and Memory Deficits by 3,5-dicaffeoylquinic acid. Oxidative Med. Cell. Longev. 2016, 2016, 6981595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Kim, Y.; Lee, C.; Kim, Y.T. 3,5-Dicaffeoylquinic acid attenuates microglial activation-mediated inflammatory pain by enhancing autophagy through the suppression of MCP3/JAK2/STAT3 signaling. Biomed. Pharmacother. 2022, 153, 113549. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Lin, Y.; Wang, X.; Wang, J.; Liu, Q.; Ding, N.; Huang, L.; Xiang, Q.; Fang, J.; Tan, G.; et al. Inhibition of abnormally activated HIF-1α-GLUT1/3-glycolysis pathway enhances the sensitivity of hepatocellular carcinoma to 5-caffeoylquinic acid and its derivatives. Eur. J. Pharmacol. 2022, 920, 174844. [Google Scholar] [CrossRef]
- Moglia, A.; Lanteri, S.; Comino, C.; Hill, L.; Knevitt, D.; Cagliero, C.; Rubiolo, P.; Bornemann, S.; Martin, C. Dual catalytic activity of hydroxycinnamoyl-coenzyme A quinate transferase from tomato allows it to moonlight in the synthesis of both mono- and dicaffeoylquinic acids. Plant Physiol. 2014, 166, 1777–1787. [Google Scholar] [CrossRef]
- Deshpande, S.; Jaiswal, R.; Matei, M.F.; Kuhnert, N. Investigation of acyl migration in mono- and dicaffeoylquinic acids under aqueous basic, aqueous acidic, and dry roasting conditions. J. Agric. Food Chem. 2014, 62, 9160–9170. [Google Scholar] [CrossRef]
- Xue, M.; Shi, H.; Zhang, J.; Liu, Q.-Q.; Guan, J.; Zhang, J.-Y.; Ma, Q. Stability and degradation of CQAs under different storage conditions studied by high-performance liquid chromatography with photo diode array detection and high-performance liquid chromatography with electrospray ionization collision-induced dissociation tandem mass spectrometry. Molecules 2016, 21, 948. [Google Scholar]
- Dawidowicz, A.L.; Typek, R. Transformation of chlorogenic acids during the coffee beans roasting process. Eur. Food Res. Technol. 2017, 243, 379–390. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Tchernoded, G.K.; Mischenko, N.P.; Khodakovskaya, M.; Glazunov, V.P.; Radchenko, S.V.; Zvereva, E.V.; Fedoreyev, S.A.; Zhuravlev, Y.N. Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC gene. J. Biotechnol. 2002, 97, 213–221. [Google Scholar] [CrossRef]


| Peak Number | Metabolite | Content |
|---|---|---|
| 15 | Apigenin-C-arabinoside-C-glucoside I | 0.61 ± 0.05 |
| 16 | Apigenin-C-arabinoside-C-glucoside II | 0.99 ± 0.07 |
| 17 | Luteolin-6-C-glucoside I | 1.32 ± 0.05 |
| 18 | Luteolin-8-C-glucoside II | 0.57 ± 0.09 |
| 19 | Apigenin-di-C-arabinoside I | 0.31 ± 0.03 |
| 20 | Apigenin-di-C-arabinoside II | 2.02 ± 0.19 |
| 22 | Apigenin-C-glucoside I | 0.79 ± 0.10 |
| 23 | Quercetin-O-glucoside I | 0.25 ± 0.02 |
| 24 | Quercetin-O-glucoside II | 0.15 ± 0.01 |
| 26 | Apigenin-C-glucoside II | 0.31 ± 0.02 |
| 27 | Apigenin-C-arabinoside | 0.67 ± 0.04 |
| Total | 7.98 ± 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grishchenko, O.V.; Grigorchuk, V.P.; Tchernoded, G.K.; Koren, O.G.; Bulgakov, V.P. Callus Culture of Scorzonera radiata as a New, Highly Productive and Stable Source of Caffeoylquinic Acids. Molecules 2022, 27, 7989. https://doi.org/10.3390/molecules27227989
Grishchenko OV, Grigorchuk VP, Tchernoded GK, Koren OG, Bulgakov VP. Callus Culture of Scorzonera radiata as a New, Highly Productive and Stable Source of Caffeoylquinic Acids. Molecules. 2022; 27(22):7989. https://doi.org/10.3390/molecules27227989
Chicago/Turabian StyleGrishchenko, Olga V., Valeria P. Grigorchuk, Galina K. Tchernoded, Olga G. Koren, and Victor P. Bulgakov. 2022. "Callus Culture of Scorzonera radiata as a New, Highly Productive and Stable Source of Caffeoylquinic Acids" Molecules 27, no. 22: 7989. https://doi.org/10.3390/molecules27227989
APA StyleGrishchenko, O. V., Grigorchuk, V. P., Tchernoded, G. K., Koren, O. G., & Bulgakov, V. P. (2022). Callus Culture of Scorzonera radiata as a New, Highly Productive and Stable Source of Caffeoylquinic Acids. Molecules, 27(22), 7989. https://doi.org/10.3390/molecules27227989







