Effect of Carbon Dots Concentration on Electrical and Optical Properties of Their Composites with a Conducting Polymer
Abstract
:1. Introduction
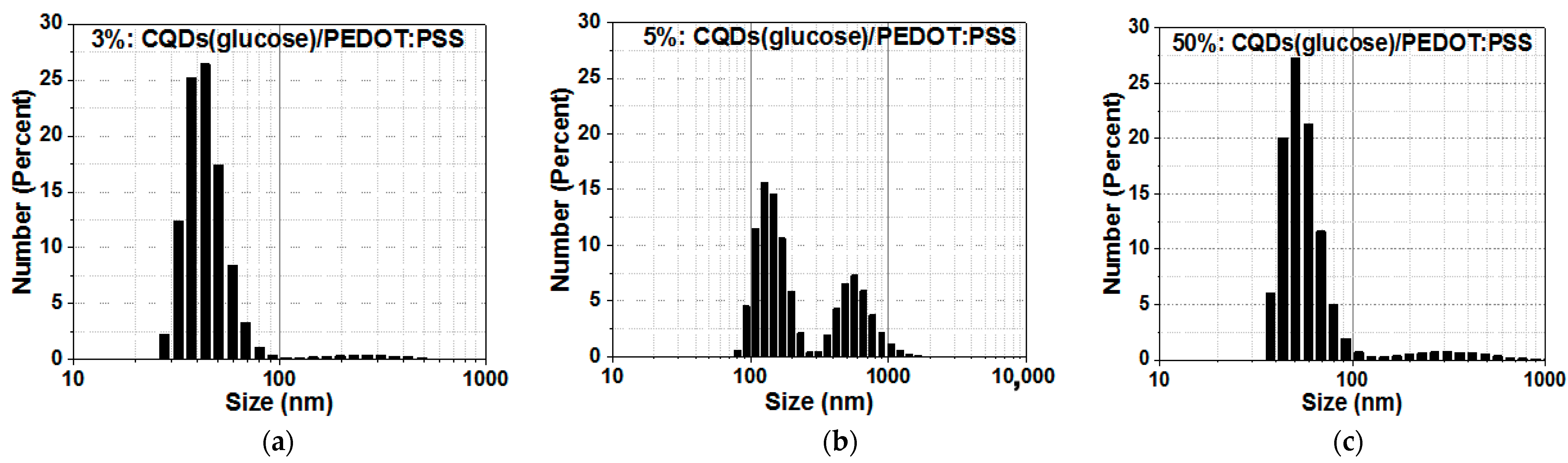
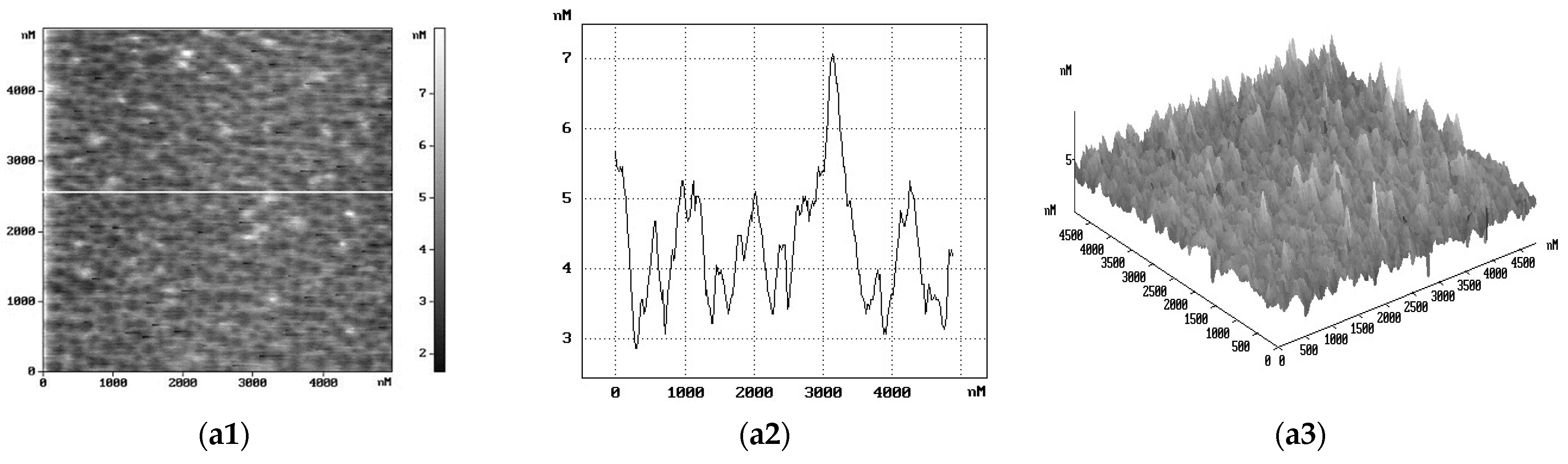
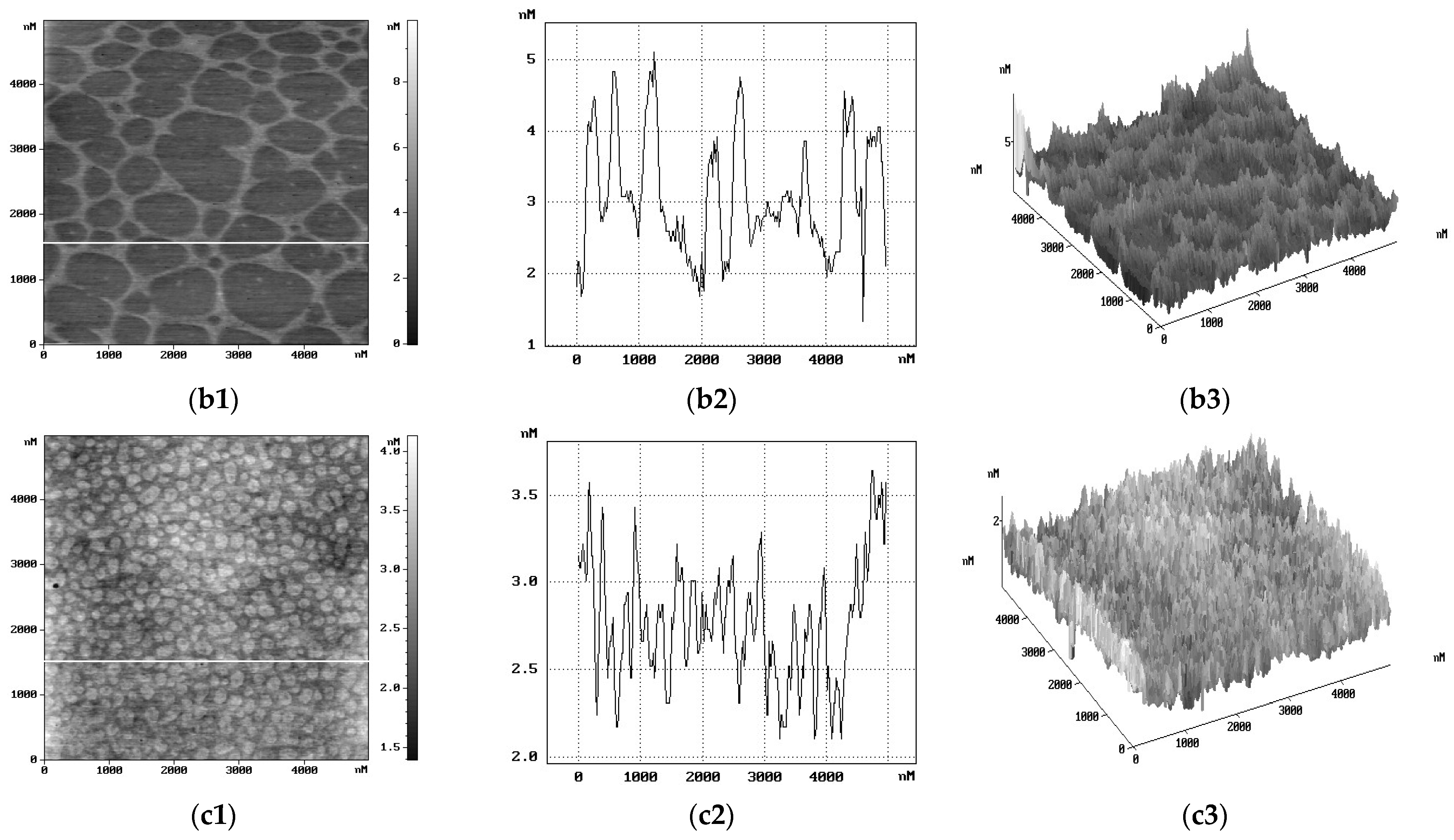
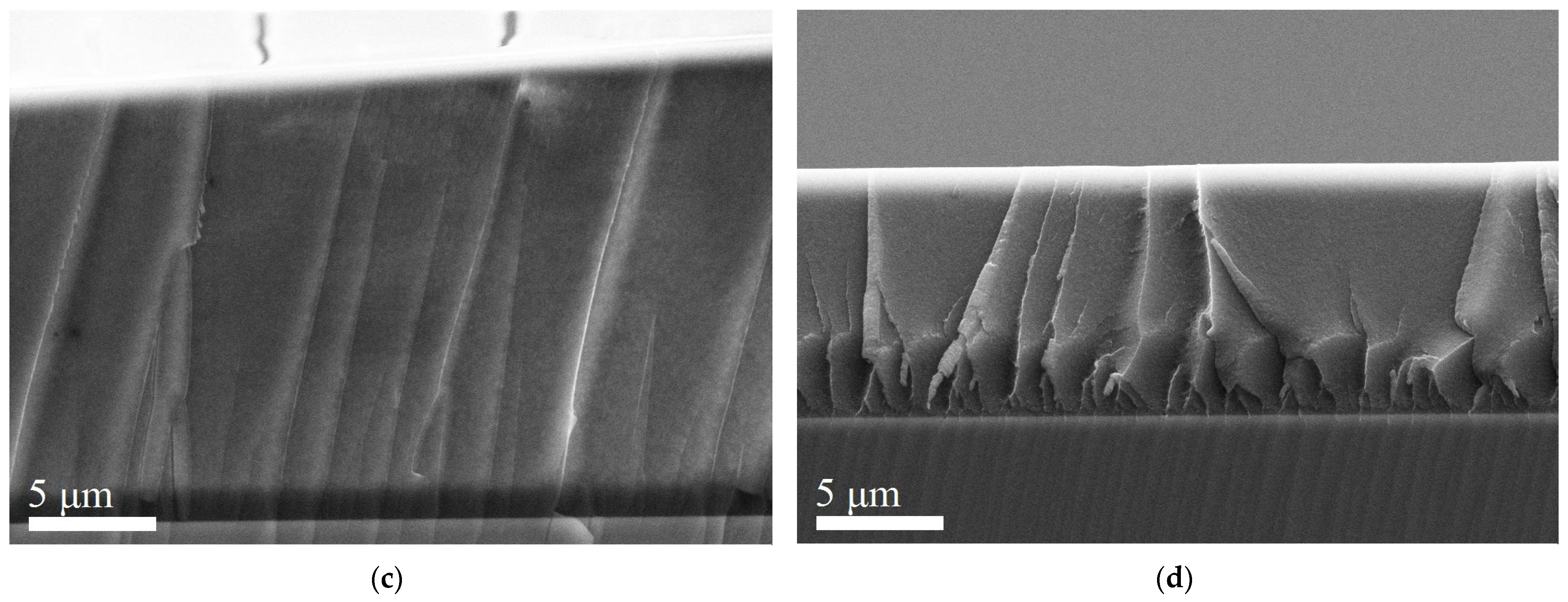
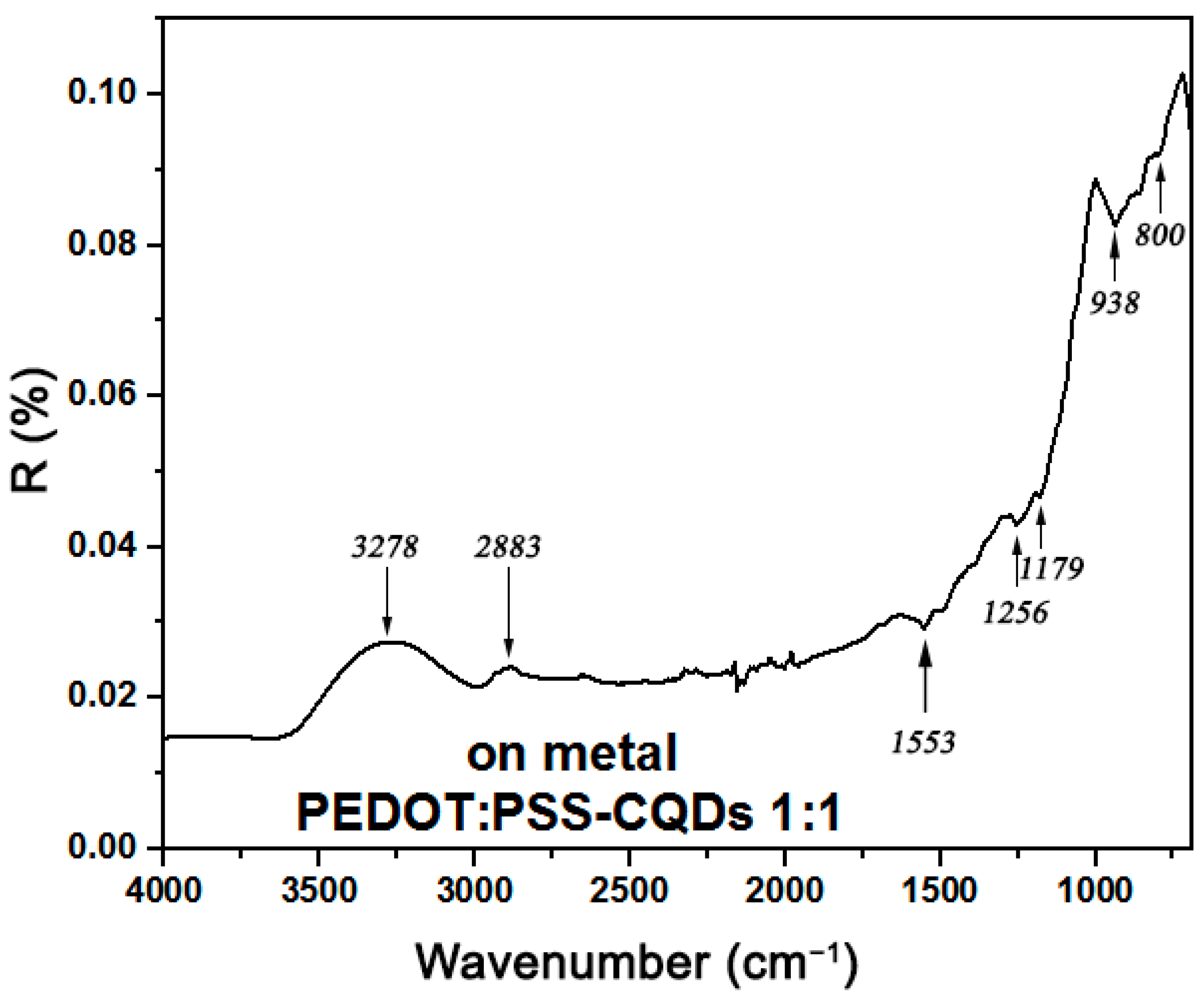
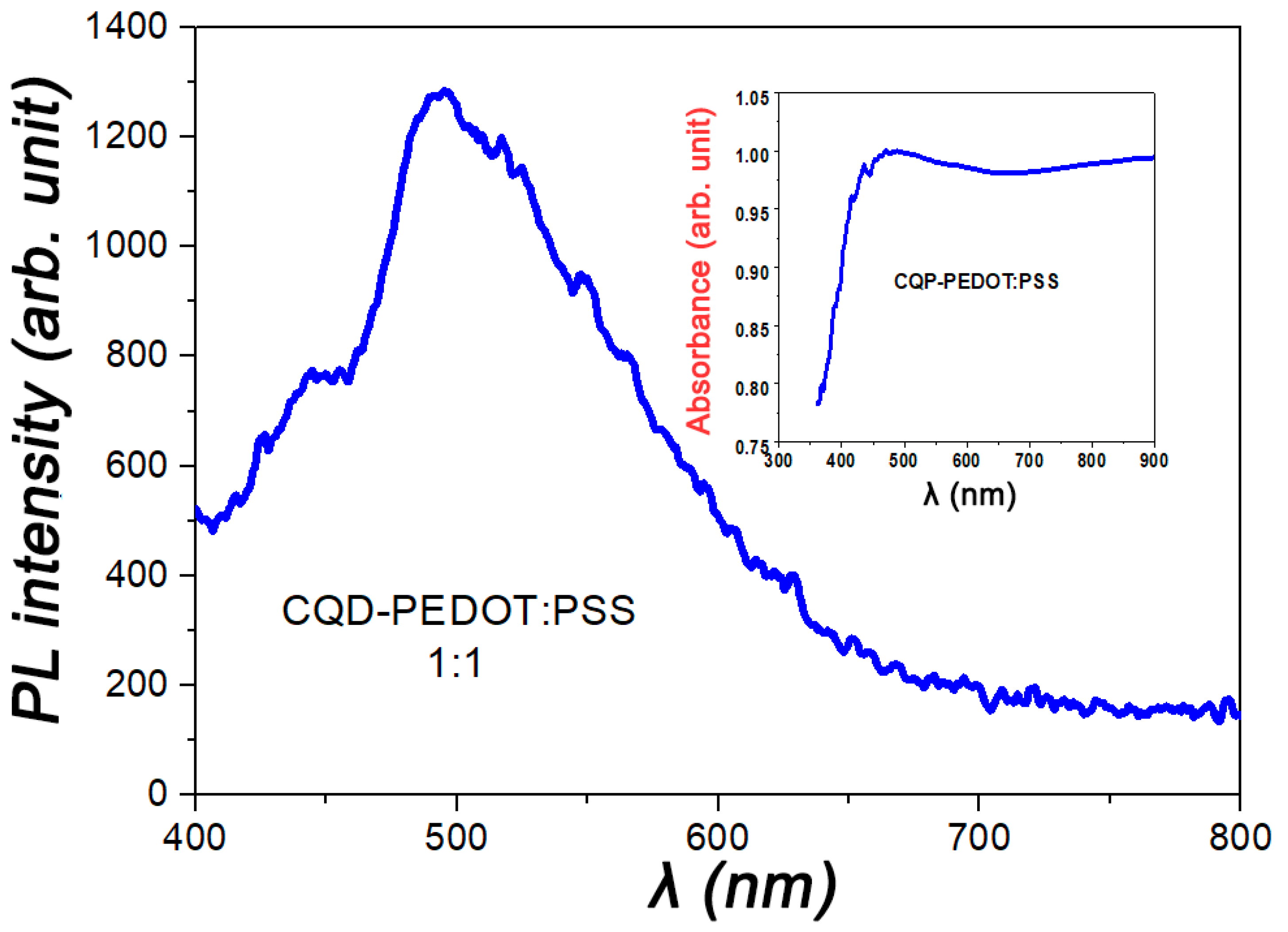
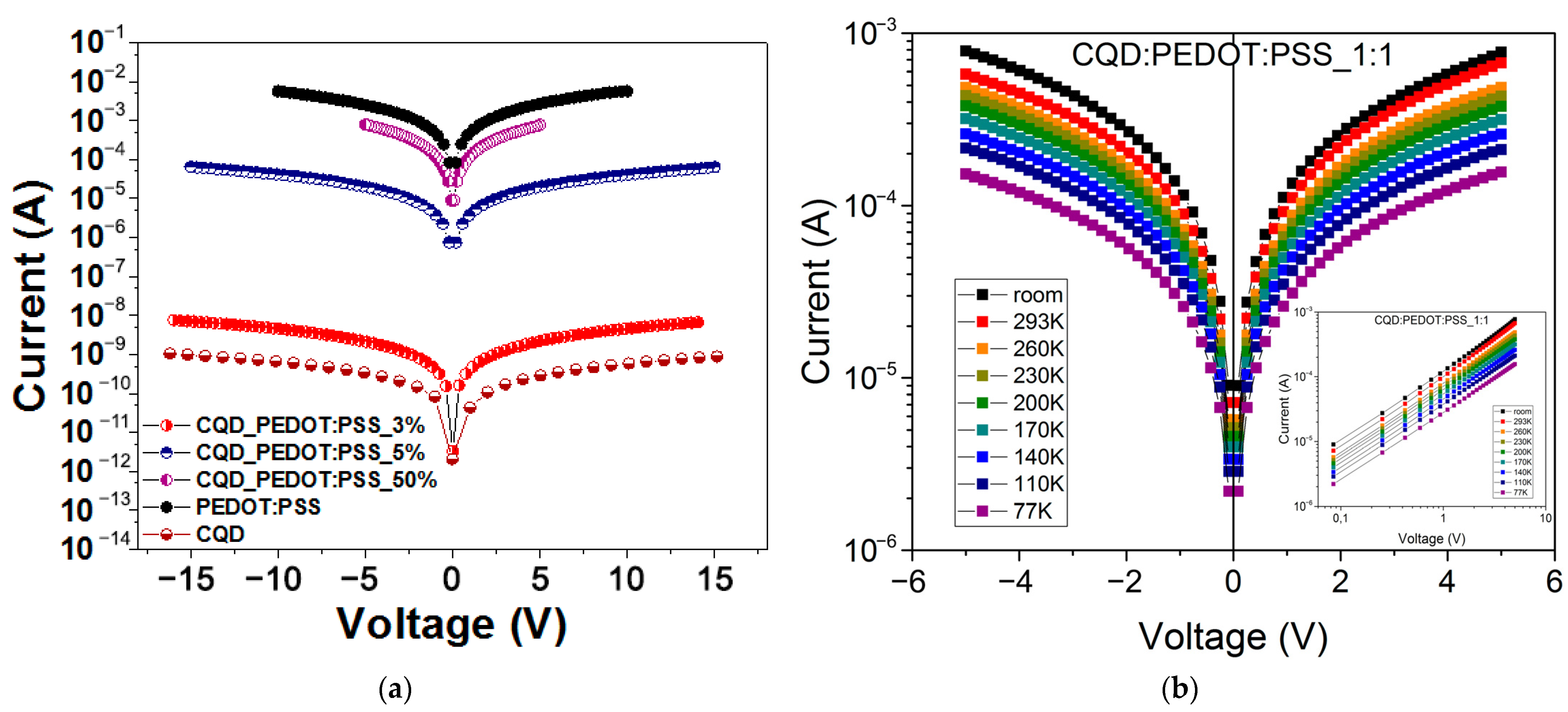
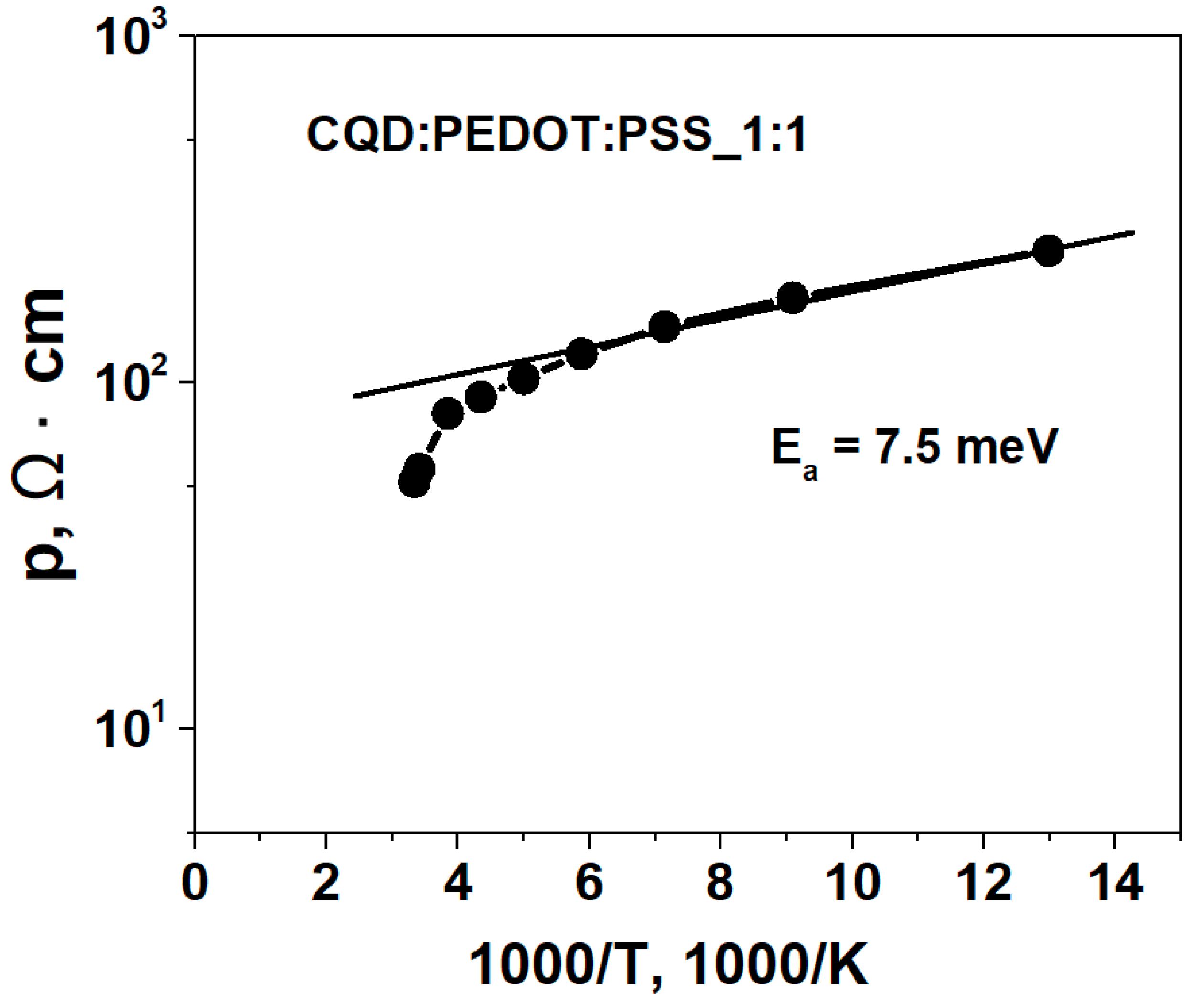
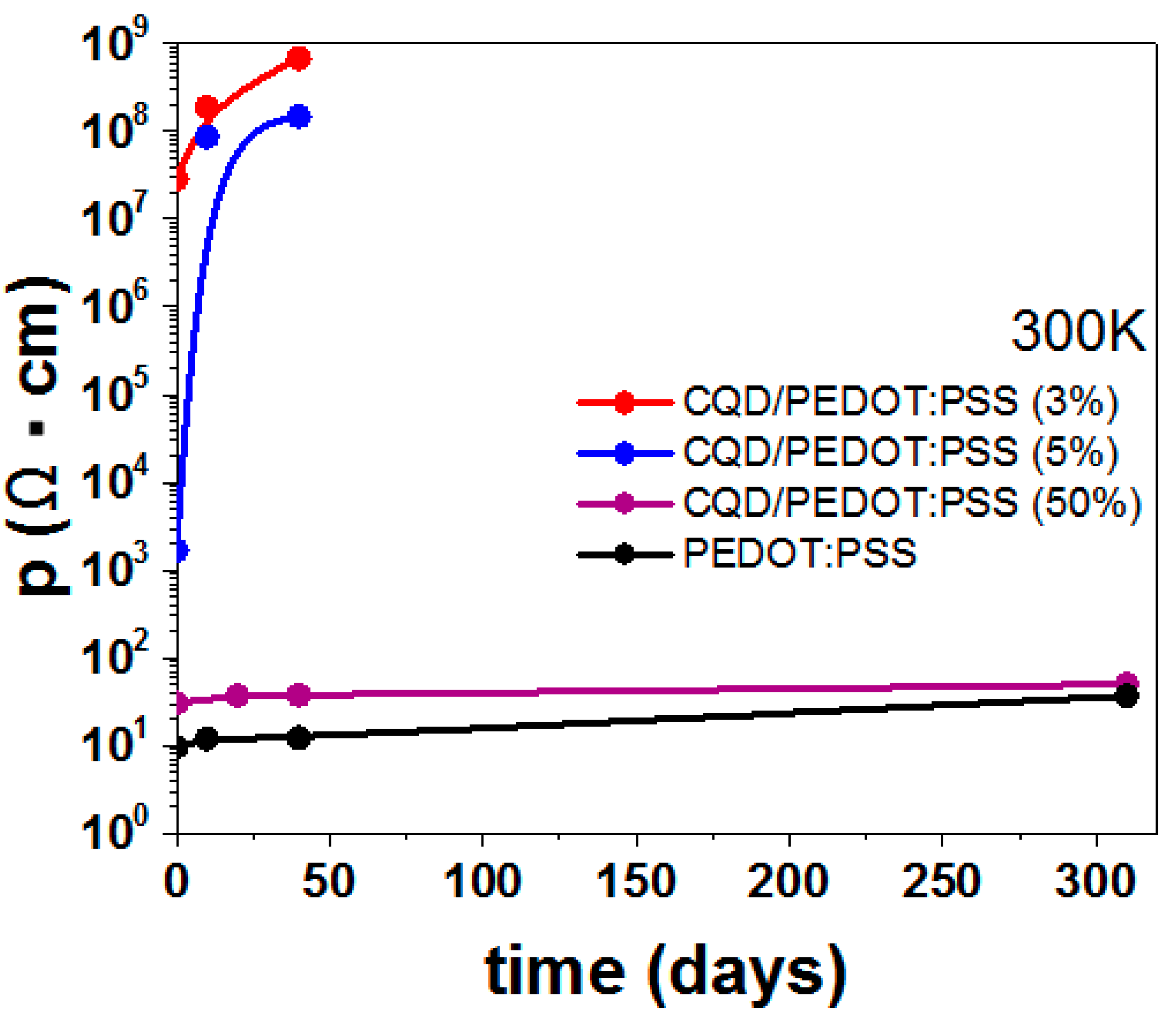
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of CQDs and CQD/PEDOT:PSS Composites
3.2. Characterization Techniques
3.3. Sample Preparation
3.4. Current–Voltage Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yuan, T.; Meng, T.; He, P.; Shi, Y.; Li, Y.; Li, X.; Fan, L.; Yang, S. Carbon quantum dots: An emerging material for optoelectronic applications. J. Mater. Chem. C 2019, 7, 6820–6835. [Google Scholar] [CrossRef]
- Nallayagari, A.R.; Sgreccia, E.; Pizzoferrato, R.; Cabibbo, M.; Kaciulis, S.; Bolli, E.; Pasquini, L.; Knauth, P.; Di Vona, M.L. Tuneable properties of carbon quantum dots by different synthetic methods. J. Nanostruct. Chem. 2022, 12, 565–580. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-sized carbon dots for bright and colorful photoluminescence. Am. J. Chem. Soc. 2006, 128, 7756. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, R.; Sheva, B. Carbon Quantum Dots: Synthesis, Properties and Applications, 1st ed.; Springer: Cham, Switzerland, 2017; pp. 1–3. [Google Scholar]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Karatutlu, A.; Patil, B.; Seker, I.; Istengir, S.; Bolat, A.; Yildirim, O.; Sevgen, Y.N.; Bakıs, Y.; Ortac, B.; Yilmaz, E.; et al. Structural, Optical, Electrical and Electrocatalytic Activity Properties of Luminescent Organic Carbon Quantum Dots. Chem. Sel. 2018, 3, 4730–4737. [Google Scholar] [CrossRef]
- Devi, P.; Saini, S.; Kim, K.-H. The advanced role of carbon quantum dots in nanomedical applications. Biosens. Bioelectron. 2019, 141, 111158. [Google Scholar] [CrossRef]
- Nazri, N.A.A.; Azeman, N.H.; Luo, Y.; Bakar, A.A.A. Carbon quantum dots for optical sensor applications: A review. Opt. Laser Technol. 2021, 139, 106928. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Badawi, A.; Alharthi, S.S.; Mostafa, N.Y.; Althobaiti, M.G.; Altalhi, T. Effect of carbon quantum dots on the optical and electrical properties of polyvinylidene fuoride polymer for optoelectronic applications. Appl. Phys. A 2019, 125, 858. [Google Scholar] [CrossRef]
- Kim, J.C.; Rahman, M.M.; Ju, M.J.; Lee, J.J. Highly conductive and stable graphene/PEDOT:PSS composite as a metal free cathode for organic dyesensitized solar cells. RSC Adv. 2018, 8, 19058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.; Yu, C. Highly Doped Carbon Nanotubes with Gold Nanoparticles and Their Influence on Electrical Conductivity and Thermopower of Nanocomposites. PLoS ONE 2012, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Nenashev, G.V.; Istomina, M.S.; Shcherbakov, I.P.; Shvidchenko, A.V.; Petrov, V.N.; Aleshin, A.N. Composite films based on carbon quantum dots in a matrix of PEDOT: PSS conductive polymer. Phys. Solid State 2021, 63, 1276–1282. [Google Scholar] [CrossRef]
- Kryukov, R.S.; Istomina, M.S.; Nenashev, G.V.; Podkovyrina, E.V.; Aleshin, A.N. Hydrothermal Synthesis of Carbon Quantum Dots (CQD’s) and Research of Composite Films Based on CQD’s. In 2022 Conference of Russian Young Researchers in Electrical and Electronic Engineering (ElConRus), 1st ed.; IEEE: Saint Petersburg, Russia, 2022; pp. 978–981. [Google Scholar]
- Zhang, L.; Jamal, R.; Zhao, Q.; Wang, M.; Abdiryim, T. Preparation of PEDOT/GO, PEDOT/MnO2, and PEDOT/GO/MnO2 nanocomposites and their application in catalytic degradation of methylene blue. Nanoscale Res. Lett. 2015, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Khazaei, A.; Rezaei, A.; Huajun, Z.; Xuwei, S. Ionic-Liquid-Modified Carbon Quantum Dots as a Support for the Immobilization of Tungstate Ions (WO42–): Heterogeneous Nanocatalysts for the Oxidation of Alcohols in Water. ACS Sustain. Chem. Eng. 2019, 7, 5283–5291. [Google Scholar] [CrossRef]
- Fayos, J. Possible 3D Carbon Structures as Progressive Intermediates in Graphite to Diamond Phase Transition. J. Solid State Chem. 1999, 148, 278–285. [Google Scholar] [CrossRef]
- Hassel, O.; Mark, H. Uber die Kristallstruktur des Graphis. Z. Phys. 1924, 25, 317–337. [Google Scholar] [CrossRef]
- Avinash, C.L.A.; Aditya, K.; Sandeep, P.; Vishnu, D.B.; Mimani, R.T.; Venkataramaniah, K. Carbon Quantum Dots from Coconut Husk: Evaluation for Antioxidant and Cytotoxic Activity. Mater. Focus 2016, 5, 55–61. [Google Scholar]
- Aleshin, A.N.; Berestennikov, A.S.; Krylov, P.S.; Shcherbakov, I.P.; Petrov, V.N.; Trapeznikova, I.N.; Mamalimov, R.I.; Khripunov, A.K.; Tkachenko, A.A. Electrical and optical properties of bacterial cellulose films modified with conductive polymer PEDOT/PSS. Synth. Met. 2015, 199, 147–151. [Google Scholar] [CrossRef]
- Sunita, R.; Prachi, S.; Verma, A.L. Synthesis of PEDOT:PSS (poly(3,4-ethylenedioxythiophene))/poly(4-styrene sulfonate))/ ngps (nanographitic platelets) nanocomposites as chemiresistive sensors for detection of nitroaromatics. Polym. Eng. Sci. 2013, 53, 2045–2052. [Google Scholar]
- Janus, Ł.; Piątkowski, M.; Radwan-Pragłowska, J.; Bogdał, D.; Matysek, D. Chitosan-Based Carbon Quantum Dots for Biomedical Applications: Synthesis and Characterization. Nanomaterials 2019, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Bibekananda, D.; Niranjan, K. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar]
- Woo, S.; Jeong, J.H.; Lyu, H.K.; Han, Y.S.; Kim, Y. In situ-prepared composite materials of PEDOT:PSS buffer layer-metal nanoparticles and their application to organic solar cells. Nanoexpress 2012, 7, 641. [Google Scholar]



| Resistivity, Ω cm | Conductivity, S/cm | Reference | |
|---|---|---|---|
| Graphene/PEDOT:PSS (0.02 wt%) | 6.25 | 0.16 | [13] |
| Graphene/PEDOT:PSS (0.1 wt%) | 0.77 | 1.29 | [13] |
| Graphene/PEDOT:PSS (0.5 wt%) | 0.39 | 2.58 | [13] |
| Graphene/PEDOT:PSS (1 wt%) | 0.16 | 6.24 | [13] |
| Carbon nanotubes/PEDOT:PSS/Au/polymer (60%, 20%, 10%, 10%) | 0.0004 | 2400 | [14] |
| Carbon nanotubes/PEDOT:PSS/Au/polymer (60%, 15%, 15%, 10%) | 0.0002 | 6000 | [14] |
| Metal nanoparticles (Au)/PEDOT:PSS | 0.15 | 6.67 | [26] |
| Carbon quantum dots/PEDOT:PSS | 30.03 | 0.03 | This article |
| Pure PEDOT:PSS | 9.54 | 0.1 | This article |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nenashev, G.V.; Istomina, M.S.; Kryukov, R.S.; Kondratev, V.M.; Shcherbakov, I.P.; Petrov, V.N.; Moshnikov, V.A.; Aleshin, A.N. Effect of Carbon Dots Concentration on Electrical and Optical Properties of Their Composites with a Conducting Polymer. Molecules 2022, 27, 8000. https://doi.org/10.3390/molecules27228000
Nenashev GV, Istomina MS, Kryukov RS, Kondratev VM, Shcherbakov IP, Petrov VN, Moshnikov VA, Aleshin AN. Effect of Carbon Dots Concentration on Electrical and Optical Properties of Their Composites with a Conducting Polymer. Molecules. 2022; 27(22):8000. https://doi.org/10.3390/molecules27228000
Chicago/Turabian StyleNenashev, Grigorii V., Maria S. Istomina, Roman S. Kryukov, Valeriy M. Kondratev, Igor P. Shcherbakov, Vasily N. Petrov, Vyacheslav A. Moshnikov, and Andrey N. Aleshin. 2022. "Effect of Carbon Dots Concentration on Electrical and Optical Properties of Their Composites with a Conducting Polymer" Molecules 27, no. 22: 8000. https://doi.org/10.3390/molecules27228000






