Preparation of Nitrogen and Sulfur Co-Doped Fluorescent Carbon Dots from Cellulose Nanocrystals as a Sensor for the Detection of Rutin
Abstract
:1. Introduction
2. Results and Discussion
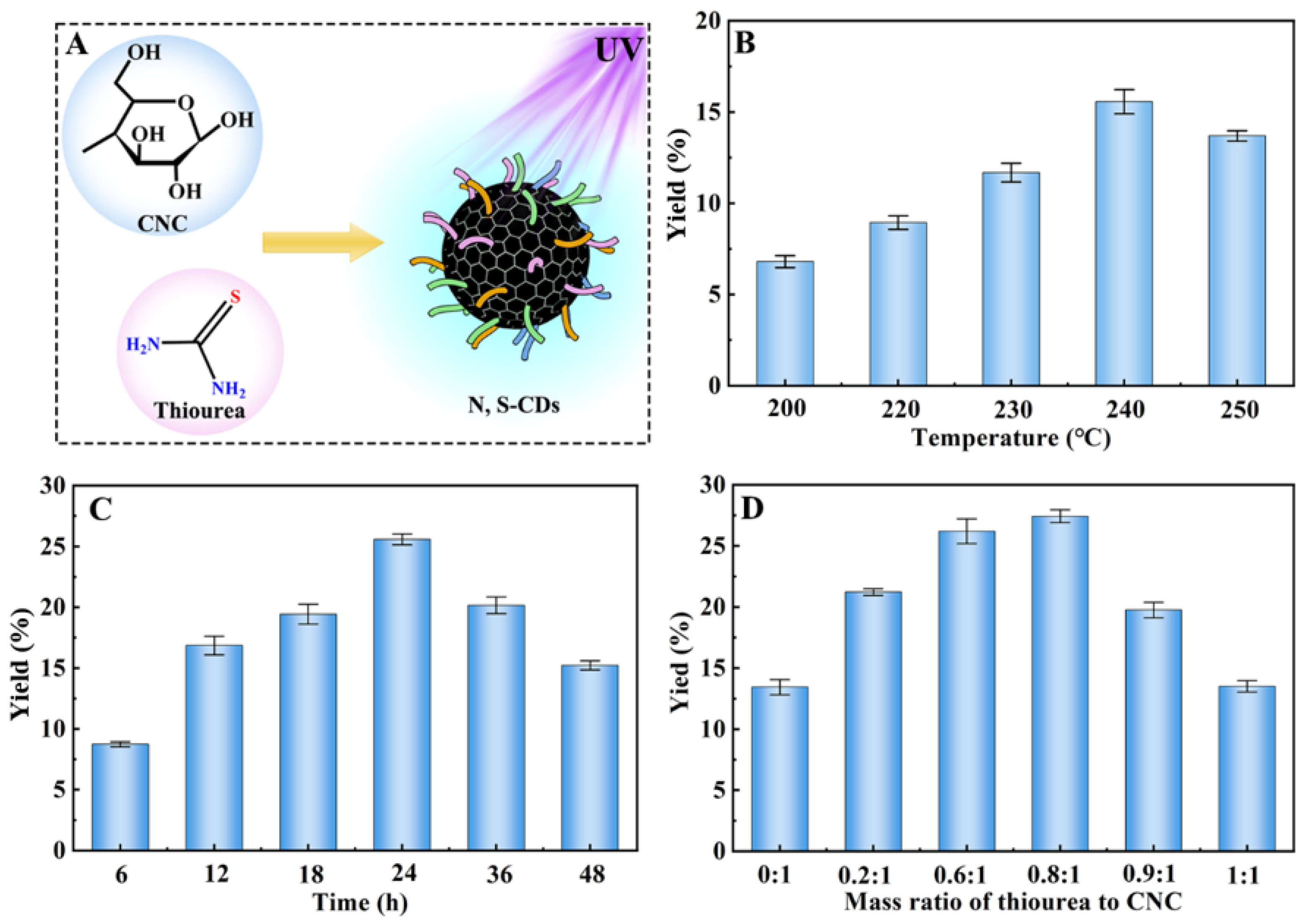
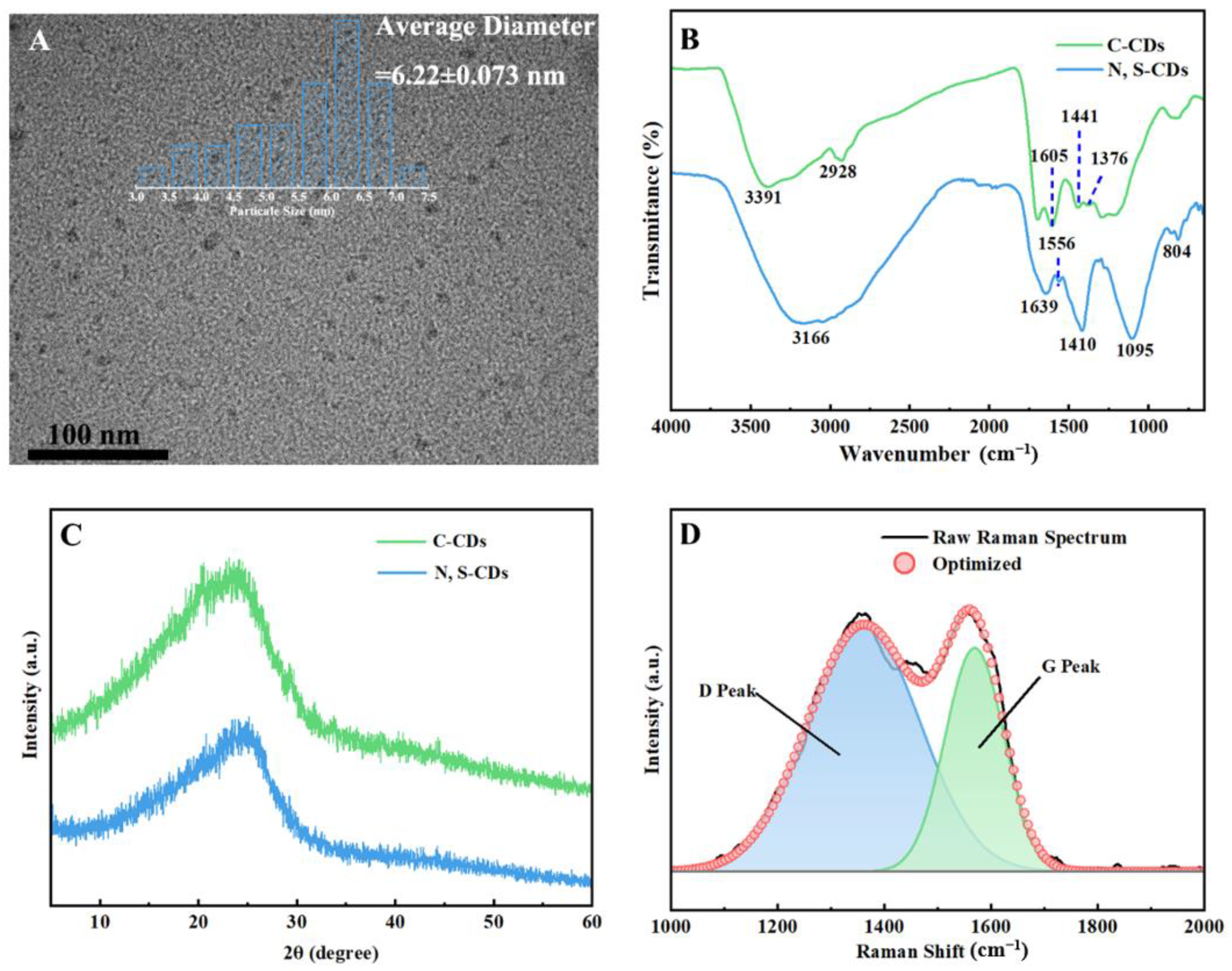
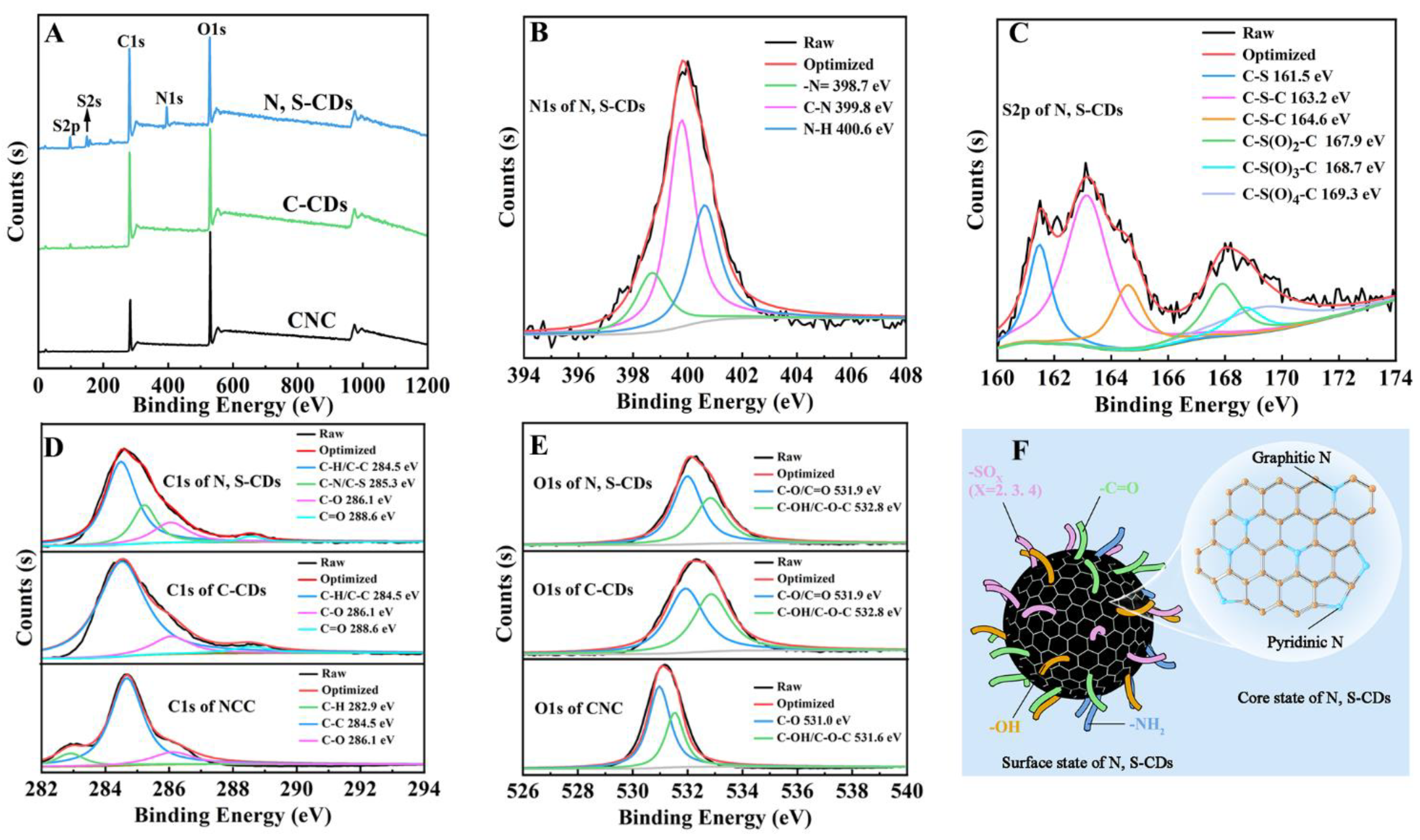
2.1. Synthesis and Characterization of N, S-CDs
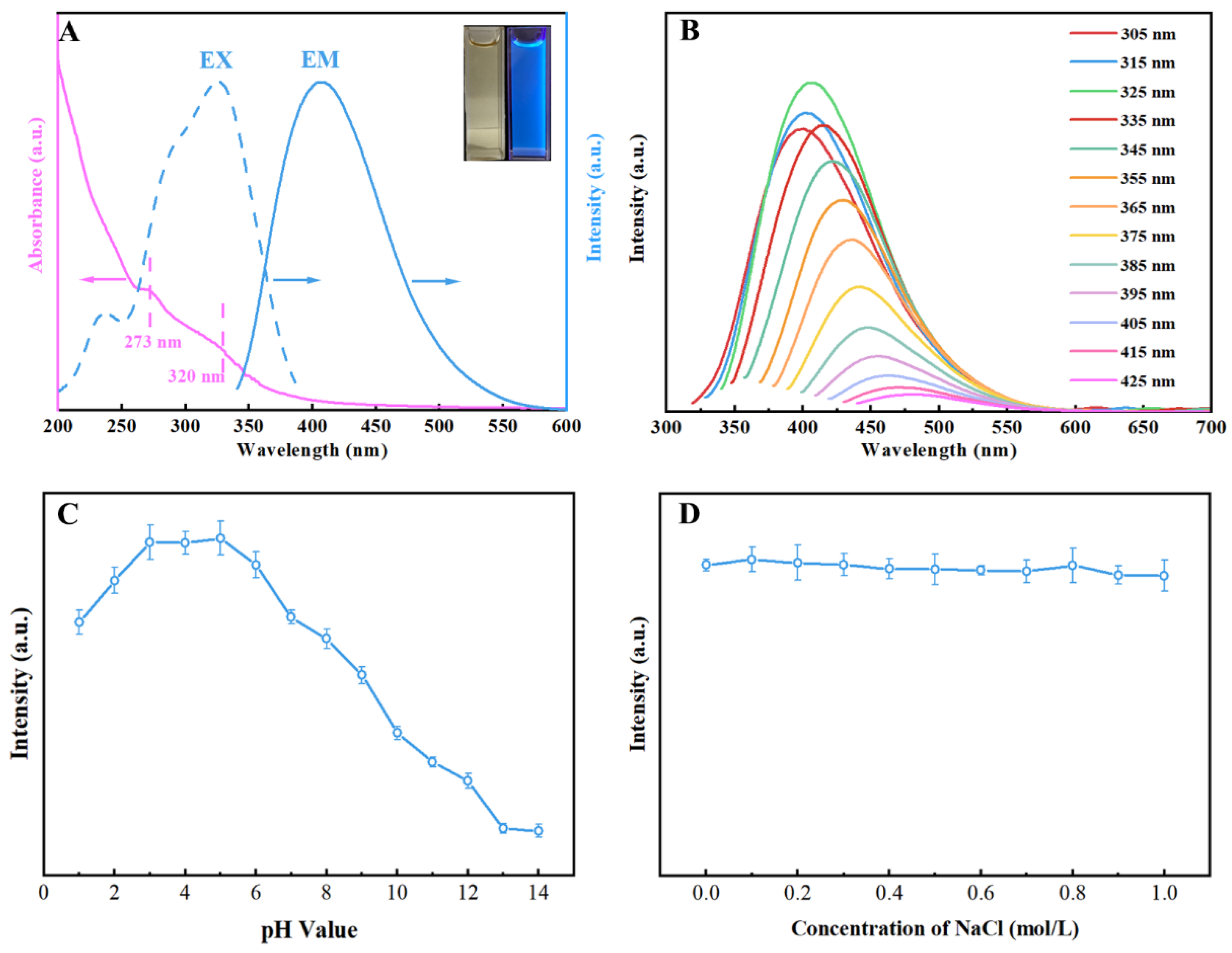
2.2. Optical Properties and Fluorescence Stability of N, S-CDs
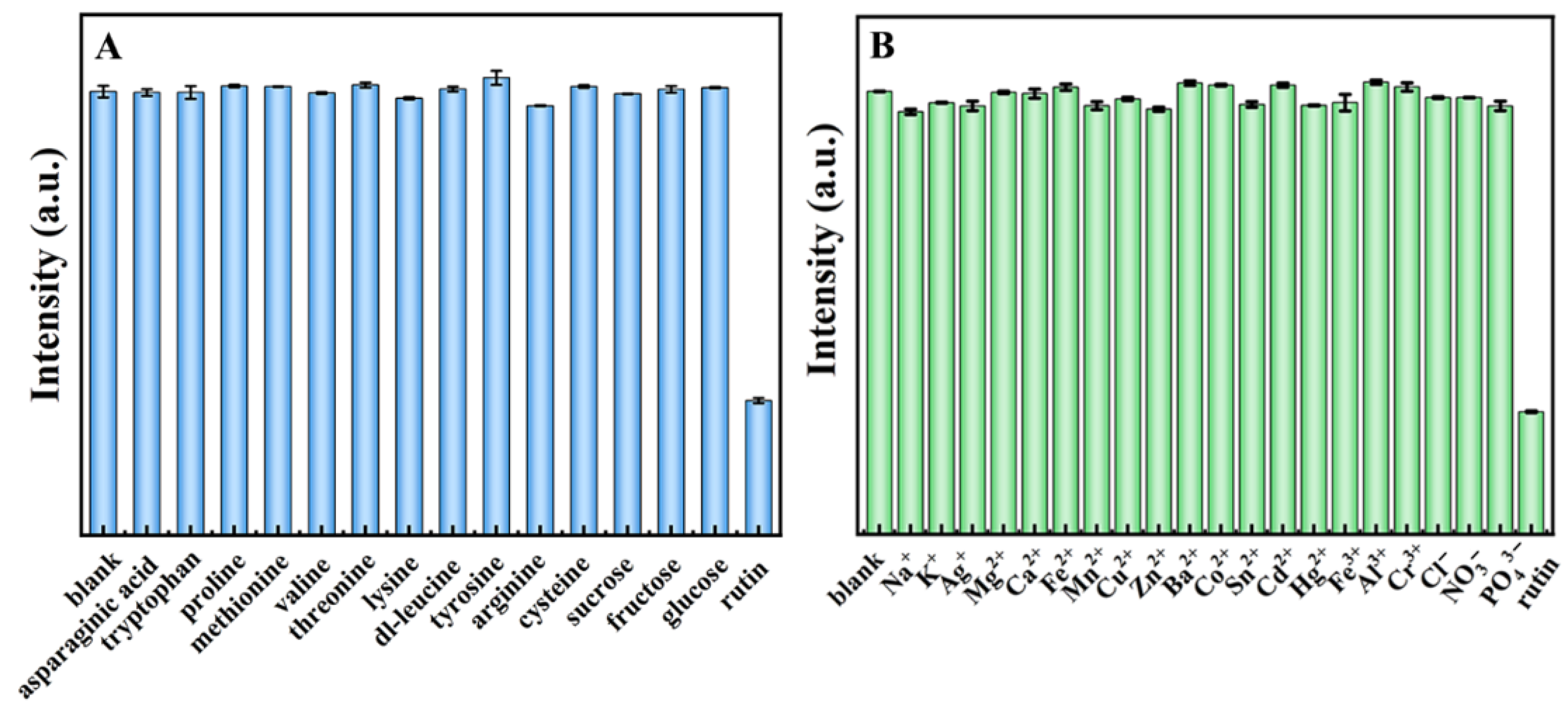
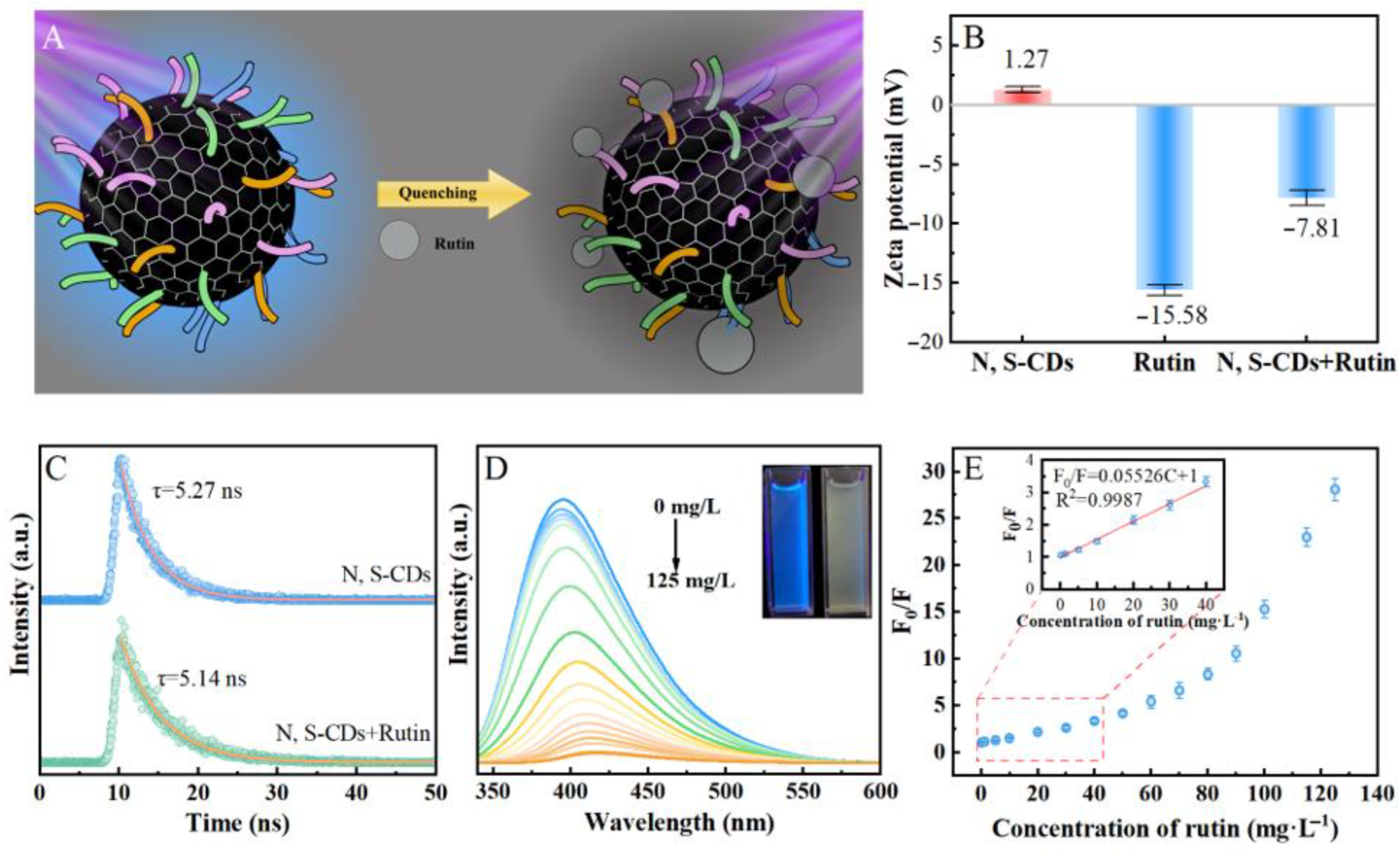
2.3. Fluorescence “Turn off” Detection of Rutin
2.4. Practical Application in Real Samples
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Cellulose Nanocrystal (CNC)
4.3. Preparation of N, S-CDs
4.4. Physicochemical Properties of CNC and N, S-CDs
4.5. Photoluminescence Properties of N, S-CDs
4.6. Fluorescent Detection of Rutin by N, S-CDs
4.7. Detection of Rutin in Real Samples
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.; Chen, Z. A review of carbon dots produced from biomass wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, B.; Li, C.; Huang, C. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, H.; Lai, J.; Peng, X.; Hu, Y.; Gu, W.; Ye, L. Carbon dots with red-shifted photoluminescence by fluorine doping for optical bio-imaging. Carbon 2018, 128, 78–85. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, Y.; Zhang, T.; Ma, J.; Li, H.; Zhou, J.; Wang, Z.; Sun, R. Preparation of carbon dots from waste cellulose diacetate as a sensor for tetracycline detection and fluorescence ink. Int. J. Biol. Macromol. 2020, 164, 4289–4298. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Song, X.; Hu, Z.; Feng, G. A carbon dots-based ratiometric fluorescence probe for monitoring intracellular pH and bioimaging. J. Photochem. Photobiol. A 2021, 409, 113129. [Google Scholar] [CrossRef]
- Wang, H.; Hou, W.; Hao, Y.; Jiang, W.; Chen, H.; Zhang, Q. Novel yellow solid-state fluorescent-emitting carbon dots with high quantum yield for white light-emitting diodes. Spectrochim. Acta A 2020, 250, 119340. [Google Scholar] [CrossRef] [PubMed]
- Gottam, R.; Zhang, X.; Chen, J.; Xue, X.; Zhang, Y. Sulfur Implanted Carbon Dots Embedded Graphene as Ultra-Stable Anode for Li-Ion Batteries. Energy Technol. 2021, 9, 2000899. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Zhang, S.; Fu, Y.; Wang, L.; Zhao, X.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Demirci, S.; McNally, A.B.; Ayyala, R.S.; Lawson, L.B.; Sahiner, N. Synthesis and characterization of nitrogen-doped carbon dots as fluorescent nanoprobes with antimicrobial properties and skin permeability. J. Drug Deliv. Sci. Technol. 2020, 59, 101889. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, X.; Liu, J.; Wang, Q.; Jin, L. One-step synthesis of yellow-emissive carbon dots with a large Stokes shift and their application in fluorimetric imaging of intracellular pH. Spectrochim. Acta A 2020, 227, 117677. [Google Scholar] [CrossRef]
- da Silva Souza, D.R.; Caminhas, L.D.; de Mesquita, J.P.; Pereira, F.V. Luminescent carbon dots obtained from cellulose. Mater. Chem. Phys. 2018, 203, 148–155. [Google Scholar] [CrossRef]
- Shen, P.; Gao, J.; Cong, J.; Liu, Z.; Li, C.; Yao, J. Synthesis of cellulose-based carbon dots for bioimaging. ChemistrySelect 2016, 1, 1314–1317. [Google Scholar] [CrossRef]
- Venkatesan, G.; Rajagopalan, V.; Chakravarthula, S.N. Boswellia ovalifoliolata bark extract derived carbon dots for selective fluorescent sensing of Fe3+. J. Environ. Chem. Eng. 2019, 7, 103013. [Google Scholar] [CrossRef]
- Jeong, Y.; Moon, K.; Jeong, S.; Koh, W.; Lee, K. Converting waste papers to fluorescent carbon dots in the recycling process without loss of ionic liquids and bioimaging applications. ACS Sustain. Chem. Eng. 2018, 6, 4510–4515. [Google Scholar] [CrossRef]
- Jin, K.; Zhang, J.; Tian, W.; Ji, X.; Yu, J.; Zhang, J. Facile Access to Solid-State Carbon Dots with High Luminescence Efficiency and Excellent Formability via Cellulose Derivative Coatings. ACS Sustain. Chem. Eng. 2020, 8, 5937–5945. [Google Scholar] [CrossRef]
- Nawaz, H.; Zhang, X.; Chen, S.; You, T.; Xu, F. Recent studies on cellulose-based fluorescent smart materials and their applications: A comprehensive review. Carbohydr. Polym. 2021, 267, 118135. [Google Scholar] [CrossRef]
- Nagarajan, S.; Skillen, N.C.; Irvine, J.T.; Lawton, L.A.; Robertson, P.K. Cellulose II as bioethanol feedstock and its advantages over native cellulose. Renew. Sustain. Energy Rev. 2017, 77, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Ragauskas, A. Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Curr. Opin. Biotechnol. 2014, 27, 150–158. [Google Scholar] [CrossRef]
- Shi, L.; Yang, J.; Zeng, H.; Chen, Y.; Yang, S.; Wu, C.; Zeng, H.; Yoshihito, O.; Zhang, Q. Carbon dots with high fluorescence quantum yield: The fluorescence originates from organic fluorophores. Nanoscale 2016, 8, 14374–14378. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, H.; Wang, W.; Jin, Z.; Liu, Q.; Yang, H.; Cao, Y.; Li, W.; Fakhri, A.; Gupta, V.K. Preparation of carbon dots-hematite quantum dots-loaded hydroxypropyl cellulose-chitosan nanocomposites for drug delivery, sunlight catalytic and antimicrobial application. J. Photochem. Photobiol. B 2021, 219, 112201. [Google Scholar] [CrossRef]
- Riahi, Z.; Rhim, J.; Bagheri, R.; Pircheraghi, G.; Lotfali, E. Carboxymethyl cellulose-based functional film integrated with chitosan-based carbon quantum dots for active food packaging applications. Prog. Org. Coat. 2022, 166, 106794. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Mu, X.; Gong, W.; Lv, D.; Hong, Y.; Si, C.; Li, B. Preparation and characterization of thermally stable cellulose nanocrystals via a sustainable approach of FeCl3-catalyzed formic acid hydrolysis. Cellulose 2016, 23, 2389–2407. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Liu, H.; Xie, H.; Xu, T.; Zhao, X.; Liu, Y.; Zhang, X.; Si, C. Highly Efficient and Sustainable Preparation of Carboxylic and Thermostable Cellulose Nanocrystals via FeCl3-Catalyzed Innocuous Citric Acid Hydrolysis. ACS Sustain. Chem. Eng. 2020, 8, 16691–16700. [Google Scholar] [CrossRef]
- Liu, K.; Wei, J.; Wang, C. Sensitive detection of rutin based on β-cyclodextrin@ chemically reduced graphene/Nafion composite film. Electrochim. Acta 2011, 56, 5189–5194. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, K.; Liu, J.; Song, G.; Ye, B. A supersensitive sensor for rutin detection based on multi-walled carbon nanotubes and gold nanoparticles modified carbon paste electrodes. Anal. Methods 2012, 4, 1350–1356. [Google Scholar] [CrossRef]
- Wu, H.; Chen, M.; Fan, Y.; Elsebaei, F.; Zhu, Y. Determination of rutin and quercetin in Chinese herbal medicine by ionic liquid-based pressurized liquid extraction–liquid chromatography-chemiluminescence detection. Talanta 2012, 88, 222–229. [Google Scholar] [CrossRef]
- Yalikun, N.; Mamat, X.; Li, Y.; Hu, X.; Wang, P.; Hu, G. Taraxacum-like Mg-Al-Si@porous carbon nanoclusters for electrochemical rutin detection. Microchim. Acta 2019, 186, 379. [Google Scholar] [CrossRef]
- Gackowski, M.; Przybylska, A.; Kruszewski, S.; Koba, M.; Mądra-Gackowska, K.; Bogacz, A. Recent Applications of Capillary Electrophoresis in the Determination of Active Compounds in Medicinal Plants and Pharmaceutical Formulations. Molecules 2021, 26, 4141. [Google Scholar] [CrossRef]
- Tan, S.; Hu, Y.; Gong, F.; Cao, Z.; Xia, J.; Zhang, L. A novel fluorescence sensor based on covalent immobilization of 3-amino-9-ethylcarbazole by using silver nanoparticles as bridges and carriers. Anal. Chim. Acta 2009, 636, 205–209. [Google Scholar] [CrossRef]
- Wang, B.; Gui, R.; Jin, H.; He, W.; Wang, Z. Red-emitting BSA-stabilized copper nanoclusters acted as a sensitive probe for fluorescence sensing and visual imaging detection of rutin. Talanta 2018, 178, 1006–1010. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, M.; Hou, Q.; Liu, R.; Wu, T.; Si, C. Further characterization of cellulose nanocrystal (CNC) preparation from sulfuric acid hydrolysis of cotton fibers. Cellulose 2016, 23, 439–450. [Google Scholar] [CrossRef]
- Younas, M.; Noreen, A.; Sharif, A.; Majeed, A.; Hassan, A.; Tabasum, S.; Mohammadi, A.; Zia, K.M. A review on versatile applications of blends and composites of CNC with natural and synthetic polymers with mathematical modeling. Int. J. Biol. Macromol. 2019, 124, 591–626. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Paul, W. Identification of Cellulosic Fibres by FTIR Spectroscopy. Stud. Conserv. 2003, 48, 269–275. [Google Scholar]
- Aslandaş, A.M.; Balcı, N.; Arık, M.; Şakiroğlu, H.; Onganer, Y.; Meral, K. Liquid nitrogen-assisted synthesis of fluorescent carbon dots from Blueberry and their performance in Fe3+ detection. Appl. Surf. Sci. 2015, 356, 747–752. [Google Scholar] [CrossRef]
- Muzyka, R.; Kwoka, M.; Smędowski, Ł.; Díez, N.; Gryglewicz, G. Oxidation of graphite by different modified Hummers methods. New Carbon Mater. 2017, 32, 15–20. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, D.; Wang, W.; Chen, J.; Zhou, L.; Zheng, Y.; Yu, K.; Huang, S.N. S co-doped carbon dots with orange luminescence synthesized through polymerization and carbonization reaction of amino acids. Appl. Surf. Sci. 2015, 342, 136–143. [Google Scholar] [CrossRef]
- Rai, S.; Singh, B.K.; Bhartiya, P.; Singh, A.; Kumar, H.; Dutta, P.K.; Mehrotra, G.K. Lignin derived reduced fluorescence carbon dots with theranostic approaches: Nano-drug-carrier and bioimaging. J. Lumin. 2017, 190, 492–503. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Q.; Wan, L.; You, X.; Chi, Y. Carbon based dot capped silver nanoparticles for efficient surface-enhanced Raman scattering. J. Mater. Chem. C 2016, 4, 7472–7477. [Google Scholar] [CrossRef]
- Li, L.; Yu, B.; You, T. Nitrogen and sulfur co-doped carbon dots for highly selective and sensitive detection of Hg (II) ions. Biosens. Bioelectron. 2015, 74, 263–269. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Saito, R. Characterizing Graphene, Graphite, and Carbon Nanotubes by Raman Spectroscopy. Annu. Rev. Condens. Matter. Phys. 2010, 1, 89–108. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, M.; Liu, Y.; He, F.; Gao, F.; Su, Y.; Wei, H.; Zhang, Y. Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale 2014, 6, 1890–1895. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Zhang, T.; Zhang, W.; Chen, W.; Cao, Y.; Chen, M.; Zhou, X. Orange-Emissive Carbon Quantum Dots: Toward Application in Wound pH Monitoring Based on Colorimetric and Fluorescent Changing. Small 2019, 15, e1902823. [Google Scholar] [CrossRef] [PubMed]
- ReddyPrasad, P.; Ofomaja, A.E.; Naidoo, E.B. RETRACTED: Preparation of CN-dots/F-NiO nanocomposite for enhanced photocatalytic degradation of selected organic pollutants. Appl. Surf. Sci. 2015, 327, 13–21. [Google Scholar] [CrossRef]
- Gong, X.; Lu, W.; Liu, Y.; Li, Z.; Shuang, S.; Dong, C.; Choi, M.M.F. Low temperature synthesis of phosphorous and nitrogen co-doped yellow fluorescent carbon dots for sensing and bioimaging. J. Mater. Chem. B 2015, 3, 6813–6819. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Huang, X.; Ni, J.; Han, Y.; Zhang, C.; Li, S.; Cao, J.; Li, J.; Chen, Z.; Han, S. One-step synthesis of self-quenching-resistant biomass-based solid-state fluorescent carbon dots with high yield for white lighting emitting diodes. Dyes Pigments 2021, 185, 108953. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, G.; Luo, Q.; Xiao, T.; Zuo, Y.; Guo, X.; Xu, D.; Wu, Y. A 3D net-like structured fluorescent aerogel based on carboxy-methylated cellulose nanofibrils and carbon dots as a highly effective adsorbent and sensitive optical sensor of Cr(vi). Environ. Sci. Nano 2020, 7, 773–781. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Zhao, L.; Ding, J.; Chen, M.; Chen, G.; Li, Y.; Ding, L. Synthesis of tiny carbon dots with high quantum yield using multi-walled carbon nanotubes as support for selective “turn-off-on” detection of rutin and Al3+. Carbon 2019, 143, 391–401. [Google Scholar] [CrossRef]
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef]
- Liang, Y.; Song, J. Electrochemiluminescence from direct electro-oxidations of organic analyte rutin and its analytical application. Electroanal. Chem. 2008, 624, 27–32. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef]
- Cai, Z.; Chen, S.; Ma, X.; Na, D.; Zhao, J.; Wu, T.; Zhang, C. Preparation and use of tyrosine-capped copper nanoclusters as fluorescent probe to determine rutin. J. Photochem. Photobiol. A 2021, 405, 112918. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, Z.; Gong, Y.; Zhang, Q.; Yan, G. Self-assembly of manganese doped zinc sulfide quantum dots/CTAB nanohybrids for detection of rutin. Biosens. Bioelectron. 2014, 52, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, N.; Wang, F.; Zhao, Q. Boronate affinity-based surface-imprinted quantum dots as novel fluorescent nanosensors for the rapid and efficient detection of rutin. Anal. Methods 2019, 11, 3212–3220. [Google Scholar] [CrossRef]
- Pan, C.; Qin, X.; Lu, M.; Ma, Q. Water Soluble Silicon Nanoparticles as a Fluorescent Probe for Highly Sensitive Detection of Rutin. ACS Omega 2022, 7, 28588–28596. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, S.; Xu, H.; Wang, L.; Zhu, X.; Chen, X.; Xu, W.; Xu, W.; Zhang, H.; Lin, Y. Masking quercetin: A simple strategy for selective detection of rutin by combination of bovine serum albumin and fluorescent silicon nanoparticles. Anal. Chim. Acta 2020, 1126, 7–15. [Google Scholar] [CrossRef]
- Su, H.; Bi, Z.; Ni, Y.; Yan, L. One-pot degradation of cellulose into carbon dots and organic acids in its homogeneous aqueous solution. Green Energy Environ. 2019, 4, 391–399. [Google Scholar] [CrossRef]
| Elements | Samples | ||
|---|---|---|---|
| CNC | C-CDs | N, S-CDs | |
| C | 54.89% | 66.28% | 58.24% |
| O | 44.05% | 28.99% | 26.90% |
| N | - | - | 7.90% |
| S | - | - | 6.96% |
| Materials | LODs(μM) | KSV (M−1) | Ref. |
|---|---|---|---|
| copper nanoclusters | 0.02 | 71,500 | [30] |
| copper nanoclusters | 0.01 | 38,000 | [51] |
| ZnS quantum dots | 0.06 | - | [52] |
| BA-CdTe@MIPs QDs | 0.06 | 95,000 | [53] |
| silicon nanoparticles | 0.01 | 4940 | [54] |
| silicon nanoparticles | 0.04 | - | [55] |
| N, S-CDs | 0.02 | 55,260 | This work |
| Samples | Rutin Spiked (mg·L−1) | Measured (mg·L−1, n = 5) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Lake water 1 | 1 | 0.97 | 96.5 | 2.8 |
| Lake water 2 | 15 | 15.24 | 101.6 | 2.4 |
| Lake water 3 | 40 | 38.85 | 97.1 | 3.2 |
| Human urine 1 | 1 | 1.04 | 104.3 | 1.9 |
| Human urine 2 | 15 | 14.68 | 97.9 | 2.1 |
| Human urine 3 | 40 | 40.93 | 1.02 | 1.6 |
| Serum 1 | 1 | 0.95 | 96.3 | 3.1 |
| Serum 2 | 15 | 15.83 | 104.2 | 3.4 |
| Serum 3 | 40 | 39.17 | 97.9 | 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Ji, Q.; Song, J.; Li, H.; Wang, X.; Shi, H.; Niu, M.; Chu, T.; Zhang, F.; Guo, Y. Preparation of Nitrogen and Sulfur Co-Doped Fluorescent Carbon Dots from Cellulose Nanocrystals as a Sensor for the Detection of Rutin. Molecules 2022, 27, 8021. https://doi.org/10.3390/molecules27228021
Zhang T, Ji Q, Song J, Li H, Wang X, Shi H, Niu M, Chu T, Zhang F, Guo Y. Preparation of Nitrogen and Sulfur Co-Doped Fluorescent Carbon Dots from Cellulose Nanocrystals as a Sensor for the Detection of Rutin. Molecules. 2022; 27(22):8021. https://doi.org/10.3390/molecules27228021
Chicago/Turabian StyleZhang, Tao, Qingxue Ji, Jiayi Song, Haiming Li, Xing Wang, Haiqiang Shi, Meihong Niu, Tingting Chu, Fengshan Zhang, and Yanzhu Guo. 2022. "Preparation of Nitrogen and Sulfur Co-Doped Fluorescent Carbon Dots from Cellulose Nanocrystals as a Sensor for the Detection of Rutin" Molecules 27, no. 22: 8021. https://doi.org/10.3390/molecules27228021






