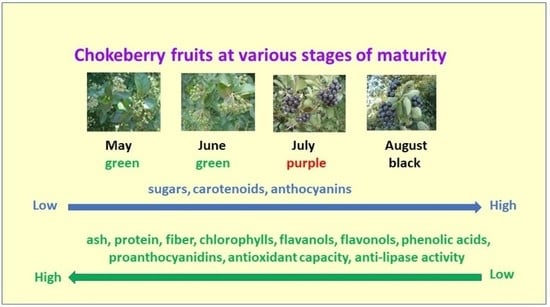
The Effect of Different Growth Stages of Black Chokeberry Fruits on Phytonutrients, Anti-Lipase Activity, and Antioxidant Capacity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Macronutrients of Chokeberry Fruits at Different Stages of Development
2.2. Antioxidants of Chokeberry Fruits at Different Stages of Development
2.3. In Vitro Inhibition of Pancreatic Lipase by Chokeberry Fruits at Different Stages of Development
2.4. Antioxidant Capacity of Chokeberry Fruits at Different Stages of Development
3. Conclusions
4. Materials and Methods
4.1. Standards and Reagents
4.2. Plant Material
4.3. Approximate Analysis
4.4. Determination of Dietary Fiber Components
4.5. Extraction and Analysis of Total Carotenoids
4.6. Extraction and Analysis of Total Chlorophylls
4.7. Extraction for Measurement of Phenolic Compounds, Antioxidant Potential, and Anti-Lipase Activity
4.8. Quantification of Phenolic Compounds Using Spectrophotometric Methods
4.9. Determination of Individual Phenolic Compounds Using UPLC/Q-TOF-MS Analysis
4.10. In Vitro Antioxidant Activity Assays
4.11. Pancreatic Lipase Activity Assay
4.12. Kinetics of Inhibition against Pancreatic Lipase
4.13. Combined Effect of Orlistat and Chokeberry Fruit Extracts
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olechno, E.; Puścion-Jakubik, A.; Zujko, M.E. Chokeberry (A. melanocarpa (Michx.) Elliott)—A natural product for metabolic disorders? Nutrients 2022, 14, 2688. [Google Scholar] [CrossRef] [PubMed]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa products and by-products for health and nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Drożdżyńska, A.; Gramza-Michałowska, A. Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors—An overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavová, J. Fruits of black chokeberry Aronia melanocarpa in the prevention of chronic diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [Green Version]
- Borowska, S.; Brzóska, M.M. Chokeberries (Aronia melanocarpa) and their products as a possible means for the prevention and treatment of noncommunicable diseases and unfavorable health effects due to exposure to xenobiotics. Compr. Rev. Food Sci. Food Saf. 2016, 15, 982–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Taheri, R.; Connolly, B.A.; Brand, M.H.; Bolling, B.W. Underutillized chokeberry (Aronia melanocarpa, Aronia arbutifolia, Aronia prunifolia) accessions are rich sources of anthocyanins, flavonoids, hydroxycinnamic acids, and proanthocyanidins. J. Agric. Food Chem. 2013, 61, 8581–8588. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Kubica, P.; Banaszczak, P.; Wojtanowska-Krośniak, A.; Krosniak, M.; Marzec-Wróblewska, U.; Badura, A.; Zagrodzki, P.; Bucinski, A.; et al. Comparative analysis of different groups of phenolic compounds in fruit and leaf extracts of Aronia sp.: A. melanocarpa, A. arbutifolia, and A.×prunifolia and their antioxidant activities. Eur. Food Res. Technol. 2017, 243, 1645–1657. [Google Scholar] [CrossRef] [Green Version]
- Wangensteen, H.; Bräunlich, M.; Nikolic, V.; Malterud, K.E.; Slimestad, R.; Barsett, H. Anthocyanins, proanthocyanidins and total phenolics in four cultivars of aronia: Antioxidant and enzyme inhibitory effects. J. Funct. Foods 2014, 7, 746–752. [Google Scholar] [CrossRef]
- Sosnowska, D.; Podsędek, A.; Redzynia, M.; Kucharska, A.Z. Inhibitory effect of black chokeberry fruit polyphenols on pancreatic lipase—Searching for most active inhibitors. J. Funct. Foods 2018, 49, 196–204. [Google Scholar] [CrossRef]
- Dudonné, S.; Dubé, P.; Anhê, F.F.; Pilon, G.; Marette, A.; Lemire, M.; Harris, C.; Dewailly, E.; Desjardins, Y. Comprehensive analysis of phenolic compounds and abscisic acid profiles of twelve native Canadian berries. J. Food Compost. Anal. 2015, 44, 214–224. [Google Scholar] [CrossRef]
- Jakobek, L.; Drenjančević, M.; Jukić, V.; Šeruga, M. Phenolic acids, flavonols, anthocyanins and antiradical activity of “Nero”,“Viking”,“Galicianka” and wild chokeberries. Sci. Hortic. 2012, 147, 56–63. [Google Scholar] [CrossRef]
- Ochmian, I.; Grajkowski, J.; Smolik, M. Comparison of some morphological features, quality and chemical content of four cultivars of chokeberry fruits (Aronia malanocarpa). Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Rop, O.; Mlcek, J.; Jurikova, T.; Valsikova, M.; Sochor, J.; Reznicek, V.; Kramarova, D. Phenolic content, antioxidant capacity, radical oxygen species scavenging and lipid peroxidation inhibiting activities of extracts of five black chokeberry (Aronia melanocarpa (Michx.) Elliot) cultivars. J. Med. Plant Res. 2010, 4, 2431–2437. [Google Scholar]
- Oszmiański, J.; Wojdyło, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Li, J.; Deng, Y.; Yuan, C.; Pan, L.; Chai, H.; Keller, W.J. Antioxidant and quinine reductase-inducing constituents of black chokeberry (Aronia melanocarpa) fruits. J. Agric. Food Chem. 2012, 60, 11551–11559. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Gralec, M.; Wawer, I.; Zawada, K. Aronia melanocarpa berries: Phenolics composition and antioxidant properties changes during fruit development and ripening. Emir. J. Food Agric. 2019, 31, 214–221. [Google Scholar]
- Yang, H.; Kim, Y.J.; Shin, Y. Influence of ripening stage and cultivar on physicochemical properties and antioxidant compositions of Aronia grown in South Korea. Foods 2019, 8, 598. [Google Scholar] [CrossRef] [Green Version]
- Sosnowska, D.; Podsędek, A.; Redzynia, M.; Żyżelewicz, D. Effects of fruit extracts on pancreatic lipase activity in lipid emulsions. Plant Foods Hum. Nutr. 2015, 70, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Podsędek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef]
- Jeppsson, N.; Johansson, R. Changes in fruit quality in black chokeberry (Aronia melanocarpa) during maturation. J. Hortic. Sci. Biotechnol. 2000, 75, 340–345. [Google Scholar] [CrossRef]
- Engin, S.P.; Mert, C. The effects of harvesting time on the physicochemical components of aronia berry. Turk. J. Agric. For. 2020, 44, 361–370. [Google Scholar] [CrossRef]
- King, E.S.; Cho, J.; Li, H.; Jiang, X.; Madler, A.K.; Weishair, M.K.; Glenn, S.; Brand, M.H.; Xu, C.; Bolling, B.W. Time of harvest affects United States-grown Aronia mitschurinii berry polyphenols,° Brix, and acidity. J. Agric. Food Res. 2021, 6, 100248. [Google Scholar] [CrossRef]
- Mazilu, I.E.; Paraschiv, M.; Diaconescu, D.M.; Cosmulescu, S.N. Biochemical changes in two Aronia melanocarpa cultivars’ berries during the harvest season. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12393. [Google Scholar] [CrossRef]
- Andrzejewska, J.; Sadowska, K.; Klóska, Ł.; Rogowski, L. The effect of plant age and harvest time on the content of chosen components and antioxidative potential of black chokeberry fruit. Acta Sci. Pol. Hortorum Cultus 2015, 14, 105–114. [Google Scholar]
- Denev, P.; Lojek, A.; Ciz, M.; Kratchanova, M. Antioxidant activity and polyphenol content of Bulgarian fruits. Bul. J. Agric. Sci. 2013, 19, 22–27. [Google Scholar]
- Tanaka, T.; Tanaka, A. Chemical components and characteristics of black chokeberry. J. Jpn. Soc. Food Sci. Technol. 2001, 48, 606–610. [Google Scholar] [CrossRef] [Green Version]
- Montagne, L.; Pluske, J.R.; Hampson, D. A review o interactions between dietary fiber and the intestinal mucus, and their consequences on digestive health in young-ruminant animals. Anim. Feed Sci. Technol. 2003, 108, 95–117. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Conterno, L.; Gasperotti, M.; Viola, R. Up-regulating the human intestinal microbiome using whole plant foods, polyphenols, and/or fiber. J. Agric. Food Chem. 2012, 60, 8776–8782. [Google Scholar] [CrossRef] [PubMed]
- Reißner, A.M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and physicochemical properties of dried berry pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.W.; Yu, D.J.; Lee, H.J. Changes in anthocyanidin and anthocyanin pigments in highbush blueberry (Vaccinium corymbosum cv. Bluecrop) fruits during ripening. Hortic. Environ. Biotechnol. 2016, 57, 424–430. [Google Scholar] [CrossRef]
- Patel, P.R.; Rao, T.V.R. Growth and ripening in black plum [Syzygium cumini (L.) Skeels]. Inter. J. Fruit Sci. 2014, 14, 147–156. [Google Scholar] [CrossRef]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Worsztynowicz, P.; Napierała, M.; Białas, W.; Grajek, W.; Olkowicz, M. Pancreatic α-amylase and lipase inhibitory activity of polyphenolic compounds present in the extract of black chokeberry (Aronia melanocarpa L). Process Biochem. 2014, 49, 1457–1463. [Google Scholar] [CrossRef]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Romeo, F.V.; Rapisarda, P. Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals. J. Sci. Food Agric. 2016, 96, 4713–4723. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Hypoglycemic, anti-glycation and antioxidant in vitro properties of two Vaccinium species from Macaronesia: A relation to their phenolic composition. J. Funct. Foods 2018, 40, 595–605. [Google Scholar] [CrossRef]
- Jo, Y.H.; Kim, S.B.; Liu, Q.; Do, S.G.; Hwang, B.Y.; Lee, M.K. Comparison of pancreatic lipase inhibitory isoflavonoids from unripe and ripe fruits of Cudrania tricuspidata. PLoS ONE 2017, 12, e0172069. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Nakagawa, T.; Kishikawa, A.; Ohnuki, K.; Shimizu, K. In vitro bioactivities and phytochemical profile of various parts of the strawberry (Fragaria x ananassa var. Amaou). J. Funct. Foods 2015, 13, 38–49. [Google Scholar] [CrossRef]
- Salvadó, M.J.; Casanova, E.; Fernández-Iglesias, A.; Arola, L.; Bladé, C. Roles of proanthocyanidins rich extracts in obesity. Food Funct. 2015, 6, 1053–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boath, A.S.; Grussu, D.; Stewart, D.; McDougall, G.J. Berry polyphenols inhibit digestive enzymes: A source of potential health benefits? Food Dig. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Sugiyama, H.; Akazome, Y.; Shoji, T.; Yamaguchi, A.; Yasue, M.; Kanda, T.; Ohtake, Y. Oligomeric procyanidins in apple polyphenol are main active components for inhibition of pancreatic lipase and triglyceride absorption. J. Agric. Food Chem. 2007, 55, 4604–4609. [Google Scholar] [CrossRef]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
- Ballinger, A.; Peikin, S.R. Orlistat: Its current status as an anti-obesity drug. Eur. J. Pharmacol. 2002, 440, 109–117. [Google Scholar] [CrossRef]
- Sosnowska, D.; Podsędek, A.; Kucharska, A.Z. Proantthocyanidins as the main pancreatic lipase inhibitors in chokeberry fruits. Food Funct. 2022, 13, 5616–5625. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Satora, P.; Michalik, J. Transformations of phenolic compounds in an in vitro model simulating the human alimentary tract. Food Technol. Biotechnol. 2009, 47, 456–463. [Google Scholar]
- Samoticha, J.; Wojdyło, A.; Lech, K. The influence of different the drying methods on chemical composition and antioxidant activity in chokeberries. LWT–Food Sci. Technol. 2016, 66, 484–489. [Google Scholar] [CrossRef]
- Nollet, L.M. Physical characterization and nutrient analysis. In Handbook of Food Analysis, 2nd ed.; Dekker, M., Ed.; CRC Press: New York, NY, USA, 2004; pp. 61–67, 77–78, 173–176. [Google Scholar]
- Cavender, G.; Liu, M.; Hobbs, D.; Frei, B.; Strik, B.; Zhao, Y. Effect of different organic weed management strategies on the physicochemical, sensory, and antioxidant properties of machine-harvested blackberry fruits. J. Food Sci. 2014, 79, S2107–S2116. [Google Scholar] [CrossRef]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients, LWT-Food Sci. Technol. 2017, 80, 136–144. [Google Scholar]
- Rajalakshmi, K.; Banu, N. Extraction and estimation of chlorophyll from medicinal plants. Internat. J. Sci. Res. 2015, 4, 209–212. [Google Scholar]
- Rösch, D.; Bergmann, M.; Knorr, D.; Kroh, L.W. Structure—Antioxidant efficiency relationships of phenolic compounds and their contribution to the antioxidant activity of sea buckthorn juice. J. Agric. Food Chem. 2003, 51, 4233–4239. [Google Scholar] [CrossRef] [PubMed]
- Sosnowska, D.; Podsędek, A.; Kucharska, A.Z.; Redzynia, M.; Opęchowska, M.; Koziołkiewicz, M. Comparison of in vitro anti-lipase and antioxidant activities, and composition of commercial chokeberry juices. Eur. Food Res. Technol. 2016, 242, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Podsędek, A.; Zakłos-Szyda, M.; Polka, D.; Sosnowska, D. 2020. Effects of Viburnum opulus fruit extracts on adipogrnesis of 3T3-L1 cells and lipase activity. J. Funct. Foods 2020, 73, 104111. [Google Scholar] [CrossRef]


| Chemical Compounds | Stage of Fruit Development | |||
|---|---|---|---|---|
| S1 | S2 | S3 | S4 | |
| Ash | 4.05 ± 0.05 c | 3.17 ± 0.06 b | 2.43 ± 0.21 a | 2.45 ± 0.03 a |
| Protein | 2.02 ± 0.00 d | 1.43 ± 0.01 c | 0.89 ± 0.01 b | 0.74 ± 0.01 a |
| Fat | 3.35 ± 0.04 a | 2.65 ± 0.13 b | 4.10 ± 0.14 c | 3.34 ± 0.05 a |
| Sugars | 17.28 ± 0.54 a | 19.30 ± 0.41 b | 32.32 ± 0.83 c | 33.84 ± 0.78 d |
| Total acidity | 5.41 ± 0.00 b | 4.18 ± 0.16 c | 5.26 ± 0.04 ab | 5.15 ± 0.09 a |
| Fiber total | 39.43 ± 0.65 a | 38.90 ± 0.77 a | 33.72 ± 0.21 c | 26.99 ± 0.62 b |
| SDF total | 0.84 ± 0.02 a | 0.83 ± 0.03 a | 0.77 ± 0.01 b | 0.81 ± 0.01 ab |
| IDF total | 38.59 ± 0.65 a | 38.07 ± 0.78 a | 32.95 ± 0.60 c | 26.18 ± 0.62 b |
| Phytocompounds | Stage of Fruit Development | |||
|---|---|---|---|---|
| S1 | S2 | S3 | S4 | |
| Carotenoids (mg β-carotene/100 g DW) | 1.70 ± 0.28 a | 1.27 ± 0.03 a | 6.33 ± 0.11 b | 8.35 ± 0.23 c |
| Chlorophylls total (mg/100 g DW) | 75.48 ± 0.87 d | 52.85 ± 0.50 c | 5.03 ± 0.08 b | 2.15 ± 0.12 a |
| Chlorophyll a (mg/100 g DW) | 48.39 ± 0.56 d | 32.94 ± 0.54 c | 4.13 ± 0.02 b | 1.56 ± 0.12 a |
| Chlorophyll b (mg/100 g DW) | 27.12 ± 0.39 c | 19.92 ± 0.27 b | 0.91 ±0.10 a | 0.59 ± 0.01 a |
| Phenolics total (g GAE/100 g DW) | 12.30 ± 0.17 d | 11.69 ± 0.49 c | 2.70 ± 0.11 a | 4.23 ± 0.22 b |
| Proanthocyanidins total (g CYE/100 g DW) | 6.83 ± 0.36 b | 6.76 ± 0.39 b | 0.74 ± 0.05 a | 0.94 ± 0.02 a |
| Anthocyanins total (g CGE/100 g DW) | - | - | 1.29 ± 0.03 a | 2.64 ± 0.10 b |
| Compound | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| caffeoylquinic acid A | 40.07 ± 0.07 | 34.38 ± 0.08 | 15.37 ± 0.57 | 3.82 ± 0.18 |
| 3-caffeoylquinic acid | 2381.86 ± 22.00 | 1956.56 ± 6.99 | 771.62 ± 1.40 | 591.33 ± 1.11 |
| p-coumaroylquinic acid A | 138.89 ± 0.52 | 85.47 ± 0.09 | 42.46 ± 0.04 | 26.47 ± 0.01 |
| vanillate hexoside B | 4.12 ± 0.16 | 27.52 ± 0.17 | - | - |
| 5-caffeoylquinic acid | 3583.17 ± 24.08 | 2015.30 ± 1.34 | 854.75 ± 0.56 | 717.80 ± 0.94 |
| 4-caffeoylquinic acid | 14.53 ± 0.62 | 17.37 ± 0.05 | 12.15 ± 0.08 | 11.23 ± 0.05 |
| caffeoylquinic acid A | 40.70 ± 0.56 | 37.18 ± 0.02 | - | - |
| feruoylquinic acid A | 49.26 ± 0.65 | - | - | - |
| caffeoylquinic acid A | 180.16 ± 1.72 | - | - | - |
| caffeoylquinic acid A | 36.93 ± 0.29 | - | - | - |
| p-coumaroylquinic acid A | 95.80 ± 0.19 | - | - | - |
| 3,5-dicaffeoylquinic acid | 8.61 ± 0.83 | 7.54 ± 0.51 | - | - |
| Total phenolic acids | 6574.10 ± 41.32 d | 4192.45 ± 13.20 c | 1687.36 ± 1.44 b | 1350.65 ± 2.26 a |
| procyanidin B1 | 11.16 ± 1.65 | 46.08 ± 1.32 | - | - |
| procyanidin trimer c | 50.40 ± 2.21 | - | - | 27.03 ± 0.58 |
| (+)-catechin | 81.38 ± 1.67 | - | - | - |
| procyanidin B2 | 33.60 ± 2.68 | 137.21 ± 0.35 | - | - |
| procyanidin trimer c | - | - | 79.72 ± 0.53 | 23.93 ± 0.46 |
| procyanidin C1 | 194.49 ± 9.35 | 135.87 ± 0.38 | - | - |
| tetramer procyjanidyny c | 192.06 ± 5.20 | - | - | - |
| Total flavanols | 563.09 ± 10.87 d | 319,17 ± 1.86 c | 79.72 ± 0.53 b | 50.96 ± 0.46 a |
| quercetin 3-O-dihexoside D | 24.59 ± 0.14 | 22.93 ± 0.04 | 23.03 ± 0.07 | 12.96 ± 0.03 |
| quercetin 3-O-dihexoside D | - | - | 7.04 ± 0.01 | 4.08 ± 0.02 |
| quercetin 3-O-vicianoside D | 72.92 ± 0.53 | 43.29 ± 0.01 | 33.80 ± 0.03 | 17.76 ± 0.02 |
| quercetin 3-O-galactoside D | 177.36 ± 4.23 | 116.64 ± 0.14 | 59.88 ± 0.44 | 51.66 ± 0.07 |
| quercetin 3-O-robinoside D | - | 17.95 ± 0.04 | 17.38 ± 0.12 | 21.93 ± 0.50 |
| quercetin 3-O-rutinoside | 168.15 ± 1.13 | 96.85 ± 1.99 | 66.94 ± 0.90 | 49.06 ± 0.92 |
| quercetin 3-O-glucoside | 69.38 ± 2.10 | 49.98 ± 0.32 | 46.55 ± 0.05 | 37.48 ± 0.47 |
| kaempferol 3-O-sophoroside E | 32.03 ± 0.18 | 11.93 ± 0.01 | 6.99 ± 0.28 | - |
| kaempferol 3-O-rutinoside E | 54.56 ± 0.58 | - | - | - |
| isorhamnetin 3-O-rutinoside | 9.18 ± 0.14 | 7.99 ± 0.03 | 3.94 ± 0.01 | 2.51 ± 0.04 |
| isorhamnetin 3-O-rhamnosylhexoside F | 15.39 ± 0.15 | 6.11 ± 0.03 | 5.74 ± 0.05 | 2.07 ± 0.03 |
| Total flavonols | 623.55 ± 5.31 d | 373.67 ± 2.43 c | 271.29 ± 1.58 b | 199.51 ± 0.87 a |
| cyanidin 3-O-galactoside | - | - | 668.97 ± 1.19 | 1271.98 ± 1.91 |
| cyanidin 3-O-glucoside | - | - | 65.52 ± 0.06 | 121.51 ± 0.12 |
| cyanidin 3-O-arabinoside | - | - | 995.67 ± 0.88 | 1375.07 ± 1.99 |
| cyanidin 3-O-xyloside G | - | - | 63.79 ± 0.58 | 177.13 ± 2.05 |
| Total anthocyanins | - | - | 1793.96 ± 2.61 a | 2945.69 ± 2.94 b |
| eriodictyol hexoside H | 30.79 ± 0.86 | 20.60 ± 0.30 | 7.95 ± 0.05 | - |
| eriodictyol 7-glucuronide H | 74.78 ± 2.43 | 24.64 ± 0.03 | 29.86 ± 0.08 | 26.13 ± 0.01 |
| naringinin hexoside H | - | - | - | 7.04 ± 0.08 |
| Total flavanones | 105.57 ± 1.94 d | 45.24 ± 0.27 c | 37.79 ± 0.09 b | 33.17 ± 0.08 a |
| Sum of phenolic compounds | 7866.30 ± 35.97 d | 4930.53 ± 17.62 c | 3870.11 ± 5.02 a | 4579.98 ± 3.94 b |
| Fruit | Fruit Concentration (mg/mL) | Km (mg/mL) | Vmax (OD/min) | Ki (mg/mL) | Mode of Inhibition 1 | IC50 (mg/mL) 2 |
|---|---|---|---|---|---|---|
| S1 | 0 | 32.69 | 0.06 | 0.74 | mixed | 1.99 ± 0.04 a |
| 1.0 | 55.78 | 0.07 | ||||
| 1.4 | 185.15 | 0.18 | ||||
| 1.8 | 382.13 | 0.24 | ||||
| S2 | 0 | 32.69 | 0.06 | 1.07 | mixed | 1.97 ± 0.12 a |
| 1.0 | 60.98 | 0.08 | ||||
| 1.4 | 81.00 | 0.08 | ||||
| 1.8 | 137.18 | 0.10 | ||||
| S3 | 0 | 32.69 | 0.06 | 11.22 | mixed | 23.47 ± 0.01 c |
| 14 | 74.10 | 0.08 | ||||
| 18 | 274.55 | 0.22 | ||||
| 22 | 478.73 | 0.32 | ||||
| S4 | 0 | 32.69 | 0.06 | 10.78 | mixed | 14.80 ± 0.29 b |
| 6 | 41.47 | 0.07 | ||||
| 10 | 64.22 | 0.08 | ||||
| 14 | 174.52 | 0.15 |
| ABTS | FRAP | |
|---|---|---|
| Total phenolics 1 | 0.971 | 0.995 |
| Proanthocyanidins 1 | 0.969 | 0.986 |
| Phenolic acids 2 | 0.802 | 0.877 |
| Flavanols 2 | 0.909 | 0.960 |
| Flavonols 2 | 0.660 | 0.758 |
| Flavanones 2 | 0.553 | 0.670 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosnowska, D.; Kajszczak, D.; Podsędek, A. The Effect of Different Growth Stages of Black Chokeberry Fruits on Phytonutrients, Anti-Lipase Activity, and Antioxidant Capacity. Molecules 2022, 27, 8031. https://doi.org/10.3390/molecules27228031
Sosnowska D, Kajszczak D, Podsędek A. The Effect of Different Growth Stages of Black Chokeberry Fruits on Phytonutrients, Anti-Lipase Activity, and Antioxidant Capacity. Molecules. 2022; 27(22):8031. https://doi.org/10.3390/molecules27228031
Chicago/Turabian StyleSosnowska, Dorota, Dominika Kajszczak, and Anna Podsędek. 2022. "The Effect of Different Growth Stages of Black Chokeberry Fruits on Phytonutrients, Anti-Lipase Activity, and Antioxidant Capacity" Molecules 27, no. 22: 8031. https://doi.org/10.3390/molecules27228031
APA StyleSosnowska, D., Kajszczak, D., & Podsędek, A. (2022). The Effect of Different Growth Stages of Black Chokeberry Fruits on Phytonutrients, Anti-Lipase Activity, and Antioxidant Capacity. Molecules, 27(22), 8031. https://doi.org/10.3390/molecules27228031