Nanocellulose: A Fundamental Material for Science and Technology Applications
Abstract
1. Introduction
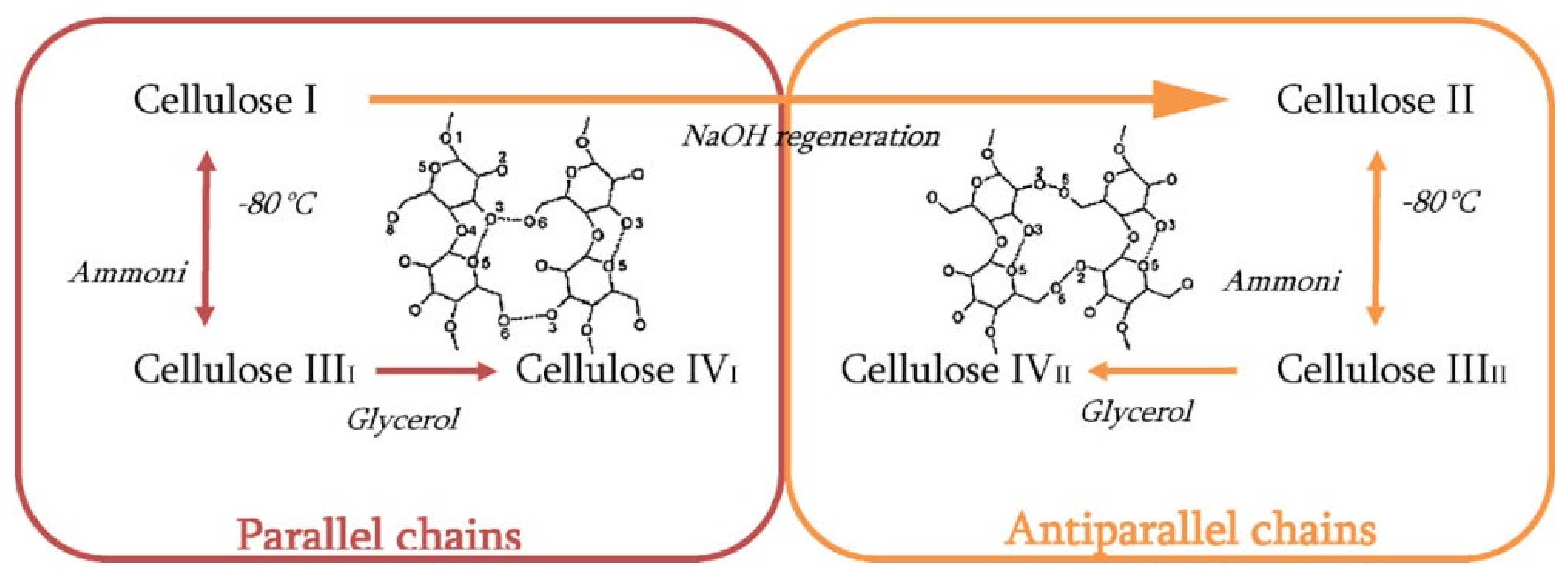
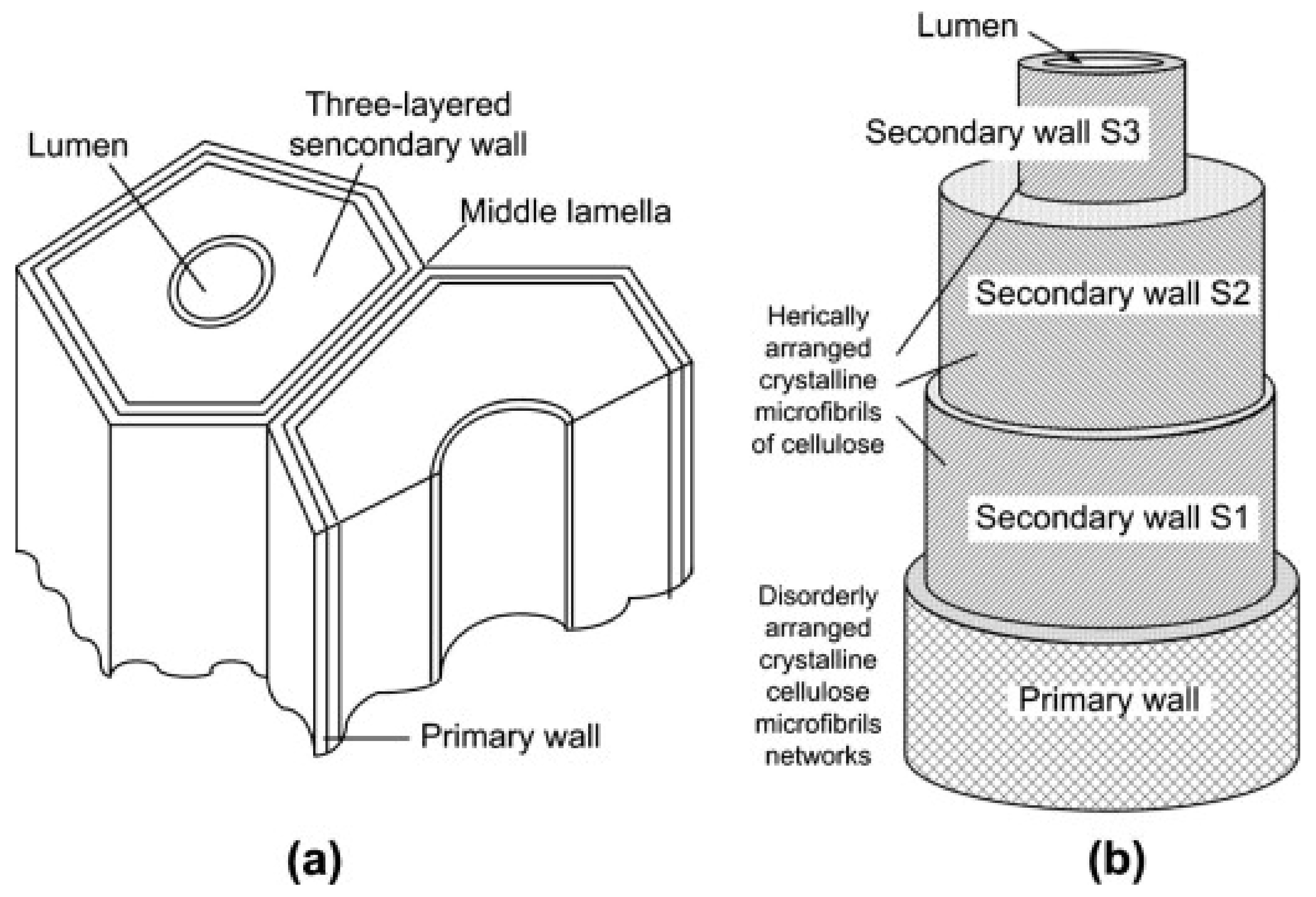
2. Chemical Structure of Cellulose
3. Source of Nanocellulose
4. Classification of Nanocellulose
4.1. Nanofibrillated Cellulose (NFC)
4.2. Cellulose Nanocrystal (CNC)
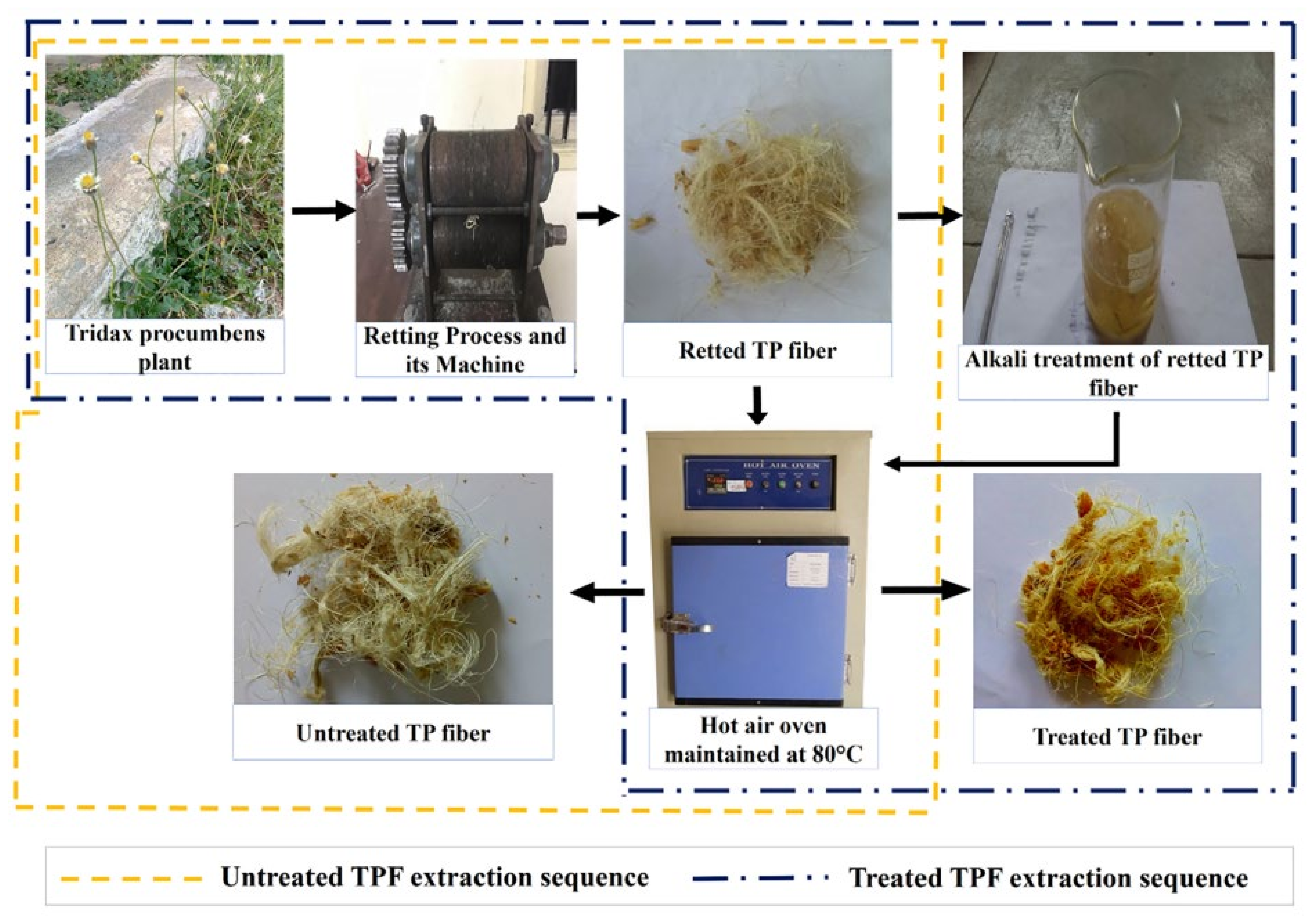
5. Pre-Treatments of Cellulose Sources
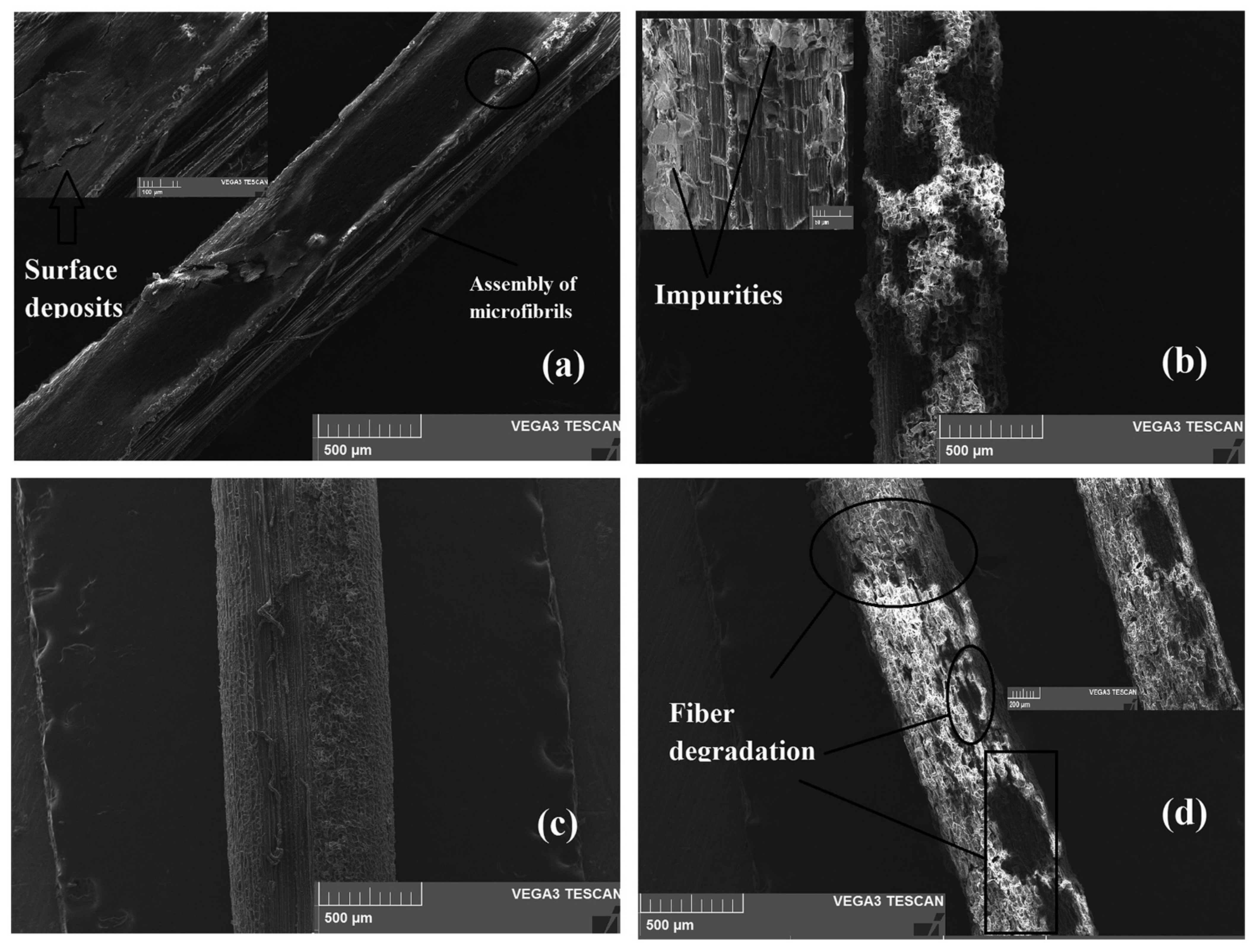
5.1. Alkali Treatment
5.2. Oxidation/Bleaching Treatment
6. Acid Hydrolysis
6.1. Mineral Acid Hydrolysis
6.2. Organic Acid Hydrolysis
7. Treatment Using Ionic Liquids (ILs)
8. Post Treatments
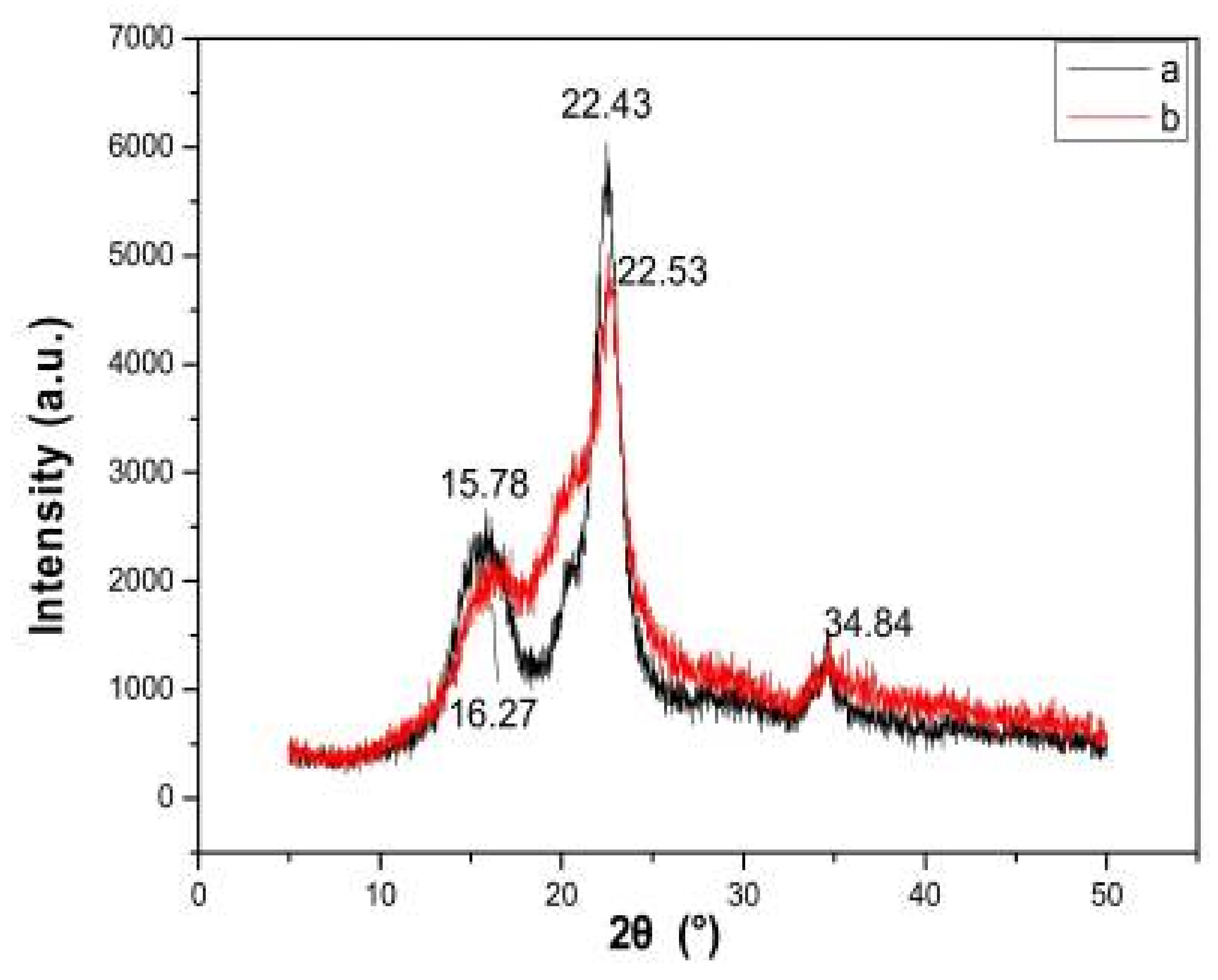
9. Current Reports on Nanocellulose and Their Applications
10. Fabrication Methods of Polymer Composites
11. Properties of Nanocellulose Reinforced Polymer Composites
12. Applications of Cellulose Fiber Reinforced Polymer Composites
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sienkiewicz, N.; Dominic, M.; Parameswaranpillai, J. Natural Fillers as Potential Modifying Agents for Epoxy Composition: A Review. Polymers 2022, 14, 265. [Google Scholar] [CrossRef]
- Rangappa, S.M.; Parameswaranpillai, J.; Siengchin, S.; Mohammad, J.; Togay, O. Bioepoxy based hybrid composites from nano-fillers of chicken feather and lignocellulose Ceiba pentandra. Sci. Rep. 2022, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Ngaowthong, C.; Borůvka, M.; Běhálek, L.; Lenfeld, P.; Švec, M.; Dangtungee, R.; Siengchin, S.; Rangappa, S.M.; Parameswaranpillai, J. Recycling of sisal fiber reinforced polypropylene and polylactic acid composites: Thermo-mechanical properties, morphology, and water absorption behavior. Waste Manag. 2019, 97, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kostag, M.; Jedvert, K.; El Seoud, O.A. Engineering of sustainable biomaterial composites from cellulose and silk fibroin: Fundamentals and applications. Int. J. Biol. Macromol. 2021, 167, 687–718. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Lyczko, N.; Gopakumar, D.; Maria, H.J.; Nzihou, A.; Thomas, S. Chitin and chitosan-based composites for energy and environmental applications: A Review. Waste Biomass Valorization 2021, 12, 4777–4804. [Google Scholar] [CrossRef]
- Bangar, S.; Whiteside, W.S. Nano-cellulose reinforced starch bio composite films—A review on green composites. Int. J. Biol. Macromol. 2021, 185, 849–860. [Google Scholar] [CrossRef]
- Aaliya, B.; Sunooj, K.V.; Lackner, M. Biopolymer composites: A review. Int. J. Biobased Plast. 2021, 3, 40–84. [Google Scholar] [CrossRef]
- Zhang, X.; Hua, H.; Shen, X.; Yang, Q. In vitro degradation and biocompatibility of poly (l-lactic acid)/chitosan fiber composites. Polymer 2007, 48, 1005–1011. [Google Scholar] [CrossRef]
- Mazzanti, V.; Pariante, R.; Bonanno, A.; de Ballesteros, O.R.; Mollica, F.; Filippone, G. Reinforcing mechanisms of natural fibers in green composites: Role of fibers morphology in a PLA/hemp model system. Compos. Sci. Technol. 2019, 180, 51–59. [Google Scholar] [CrossRef]
- Dufresne, A. Cellulose-based composites and nanocomposites. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 401–418. [Google Scholar]
- Bari, E.; Sistani, A.; Morrell, J.J.; Pizzi, A.; Akbari, M.R.; Ribera, J. Current strategies for the production of sustainable biopolymer composites. Polymers 2021, 13, 2878. [Google Scholar] [CrossRef]
- Hadi, A.E.; Siregar, J.; Cionita, T.; Norlaila, M.B.; Badari, M.A.M.; Irawan, A.; Jaafar, J.; Rihayat, T.; Junid, R.; Fitriyana, D.F. Potentiality of Utilizing Woven Pineapple Leaf Fibre for Polymer Composites. Polymers 2022, 14, 2744. [Google Scholar] [CrossRef] [PubMed]
- Pothan, L.A.; Oommen, Z.; Thomas, S. Dynamic mechanical analysis of banana fiber reinforced polyester composites. Compos. Sci. Technol. 2003, 63, 283–293. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Midhun Dominic, C.D.; Maheswary, S.; Neenu, K.V.; Sajadi, S.M.; dos Santos Rosa, D.; Sabura Begum, M.; Mathew, M.; Ajithkumar, T.G.; Parameswaranpillai, J.; George, T.S.; et al. Colocasia esculenta stems for the isolation of cellulose nanofibers: A chlorine-free method for the biomass conversion. Biomass Convers. Biorefinery 2022, 1–14. [Google Scholar] [CrossRef]
- Neenu, K.V.; Dominic, C.M.; Begum, S.; Parameswaranpillai, J.; Kanoth, B.; David, D.A.; Sajadi, S.M.; Dhanyasree, P.; Ajithkumar, T.G.; Badawi, M. Effect of oxalic acid and sulphuric acid hydrolysis on the preparation and properties of pineapple pomace derived cellulose nanofibers and nanopapers. Int. J. Biol. Macromol. 2022, 209, 1745–1759. [Google Scholar] [CrossRef]
- Dominic, C.M.; Raj, V.; Neenu, K.V.; Begum, S.; Formela, K.; Saeb, M.R.; Prabhu, D.D.; Vijayan, P.; Ajithkumar, T.G.; Parameswaranpillai, J. Chlorine-free extraction and structural characterization of cellulose nanofibers from waste husk of millet (Pennisetum glaucum). Int. J. Biol. Macromol. 2022, 206, 92–104. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Taupichai, S. Recent development of plant-derived nanocellulose in polymer nanocomposite foams and multifunctional applications: A mini-review. Express Polym. Lett. 2022, 16, 52–74. [Google Scholar] [CrossRef]
- Rao, A.; Divoux, T.; Owens, C.E.; Hart, A.J. Printable, castable, nanocrystalline cellulose-epoxy composites exhibiting hierarchical nacre-like toughening. Cellulose 2022, 29, 2387–2398. [Google Scholar] [CrossRef]
- Zhuo, X.; Liu, C.; Pan, R.; Dong, X.; Li, Y. Nanocellulose Mechanically Isolated from Amorpha fruticosa Linn. ACS Sustain. Chem. Eng. 2017, 5, 4414–4420. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Li, J.; Wang, F.; Wang, Q.; Zhang, Y.; Kong, L. Homogeneous isolation of nanocellulose from eucalyptus pulp by high pressure homogenization. Ind. Crops Prod. 2017, 104, 237–241. [Google Scholar] [CrossRef]
- Chakraborty, A.; Sain, M.; Kortschot, M. Cellulose microfibrils: A novel method of preparation using high shear refining and cryocrushing. Holzforschung 2022, 59, 102–107. [Google Scholar] [CrossRef]
- Sundari, M.T.; Ramesh, A. Isolation and characterization of cellulose nanofibers from the aquatic weed water hyacinth—Eichhornia crassipes. Carbohydr. Polym. 2012, 87, 1701–1705. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothen, L.A.; Cintil, J.; Thomas, S.; John, M.J.; Anandjiwala, R.; Narine, S.S. Environmental friendly method for the extraction of coir fibre and isolation of nanofibre. Carbohydr. Polym. 2013, 92, 1477–1483. [Google Scholar] [CrossRef]
- Rezanezhad, S.; Nazanezhad, N.; Asadpur, G. Isolation of Nanocellulose from Rice Waste via Ultrasonication. Lignocellulose 2013, 2, 282–291. [Google Scholar]
- Sisti, L.; Kalia, S.; Totaro, G.; Vannini, M.; Negroni, A.; Zanaroli, G.; Celli, A. Enzymatically treated curaua fibers in poly (butylene succinate)-based biocomposites. J. Environ. Chem. Eng. 2018, 6, 4452–4458. [Google Scholar] [CrossRef]
- Holland, C.; Perzon, A.; Cassland, R.; Jensen, J.; Langebeck, B.; Sørensen, O.B.; Whale, E.; Hepworth, D.; Plaice-Inglis, R.; Moestrup, Ø.; et al. Nanofibers produced from agro-industrial plant waste using entirely enzymatic pretreatments. Biomacromolecules 2018, 20, 443–453. [Google Scholar] [CrossRef]
- Mateo, S.; Peinado, S.; Morillas-Gutiérrez, F.; La Rubia, M.D.; Moya, A.J. Nanocellulose from agricultural wastes: Products and applications—A review. Processes 2021, 9, 1594. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Teramoto, Y. Recent advances in nanocellulose composites with polymers: A guide for choosing partners and how to incorporate them. Polymers 2018, 10, 517. [Google Scholar] [CrossRef]
- Halib, N.; Perrone, F.; Cemazar, M.; Dapas, B.; Farra, R.; Abrami, M.; Chiarappa, G.; Forte, G.; Zanconati, F.; Pozzato, G.; et al. Potential applications of nanocellulose-containing materials in the biomedical field. Materials 2017, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Salam, A.; Yahia, I.S. Physicochemical characterization of different cellulose polymorphs/graphene oxide composites and their antibacterial activity. Turk. J. Chem. 2017, 2018, 562–571. [Google Scholar] [CrossRef]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and opportunities in the characterization of cellulose–an important regulator of cell wall growth and mechanics. Front. Plant Sci. 2019, 9, 1894. [Google Scholar] [CrossRef] [PubMed]
- Chami Khazraji, A.; Robert, S. Interaction effects between cellulose and water in nanocrystalline and amorphous regions: A novel approach using molecular modeling. J. Nanomater. 2013, 2013, 409676. [Google Scholar] [CrossRef]
- Wada, M.; Ike, M.; Tokuyasu, K. Enzymatic hydrolysis of cellulose I is greatly accelerated via its conversion to the cellulose II hydrate form. Polym. Degrad. Stab. 2010, 95, 543–548. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose–Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for extraction of nanocellulose from various sources. Handb. Nanocellulose Cellul. Nanocompos. 2017, 1, 1–51. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Haafiz, M.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef]
- Neagu, R.C.; Gamstedt, E.K. Modelling of effects of ultrastructural morphology on the hygroelastic properties of wood fibres. J. Mater. Sci. 2007, 42, 10254–10274. [Google Scholar] [CrossRef]
- Owonubi, S.J.; Agwuncha, S.C.; Malima, N.M.; Shombe, G.B.; Makhatha, E.M.; Revaprasadu, N. Non-woody biomass as sources of nanocellulose particles: A review of extraction procedures. Front. Energy Res. 2021, 9, 608825. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Emmambux, M.N. Isolation, characterization, and application of nanocellulose from agro-industrial by-products: A review. Food Rev. Int. 2021, 1–29. [Google Scholar] [CrossRef]
- Dominic, M.; Joseph, R.; Begum, M.; Kumar, A.S.; Jeemol, A.; Jose, T.; Padmanabhan, D.; Formela, K.; Siengchin, S.; Parameswaranpillai, J.; et al. Cellulosic bionanocomposites based on acrylonitrile butadiene rubber and Cuscuta reflexa: Adjusting structure-properties balance for higher performance. Cellulose 2021, 28, 7053–7073. [Google Scholar] [CrossRef]
- Liu, K.; Takagi, H.; Osugi, R.; Yang, Z. Effect of physicochemical structure of natural fiber on transverse thermal conductivity of unidirectional abaca/bamboo fiber composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1234–1241. [Google Scholar] [CrossRef]
- Rojas, J.; Bedoya, M.; Ciro, Y. Current trends in the production of cellulose nanoparticles and nanocomposites for biomedical applications. Cellul. Fundam. Asp. Curr. Trends 2015, 193–228. [Google Scholar]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal cellulose, production and potential use in plastics: Challenges and opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Thomas, S.K.; Begum, S.; Midhun Dominic, C.D.; Salim, N.V.; Hameed, N.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Isolation and characterization of cellulose nanowhiskers from Acacia caesia plant. J. Appl. Polym. Sci. 2021, 138, 50213. [Google Scholar] [CrossRef]
- Rajala, S.; Siponkoski, T.; Sarlin, E.; Mettänen, M.; Vuoriluoto, M.; Pammo, A.; Juuti, J.; Rojas, O.J.; Franssila, S.; Tuukkanen, S. Cellulose nanofibril film as a piezoelectric sensor material. ACS Appl. Mater. Interfaces 2016, 8, 15607–15614. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.A.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.G. Isolation and utilization of cellulosic elements from the plant cell wall. Botany 2020, 98, 77–80. [Google Scholar] [CrossRef]
- Huang, P.; Wang, C.; Huang, Y.; Wu, M. Structure and properties of cellulose nanofibrils. Nanocellulose: Fundam. Adv. Mater. 2019, 53–80. [Google Scholar]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 7923068. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Ng, H.M.; Sin, L.T.; Tee, T.T.; Bee, S.T.; Hui, D.; Low, C.Y.; Rahmat, A.R. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Agu, O.S.; Tabil, L.G.; Meda, V.; Dumonceaux, T.; Mupondwav, E. Pretreatment of crop residues by application of microwave heating and alkaline solution for biofuel processing: A review. In Renewable Resources and Biorefineries; Jacob-Lopes, E., Zepka, L.Q., Eds.; IntechOpen: Rijeka, Croatia, 2018; pp. 47–67. [Google Scholar]
- Zimmermann, T.; Bordeanu, N.; Strub, E. Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential. Carbohydr. Polym. 2010, 79, 1086–1093. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef]
- Ferreira, D.; Cruz, J.; Fangueiro, R. Surface modification of natural fibers in polymer composites. In Green Composites for Automotive Applications; Woodhead Publishing: Sawston, UK, 2019; pp. 3–41. [Google Scholar]
- Suryanto, H.; Marsyahyo, E.; Irawan, Y.S.; Soenoko, R. Effect of alkali treatment on crystalline structure of cellulose fiber from mendong (Fimbristylis globulosa) straw in Key Engineering Materials. Trans Tech Publ. Ltd. 2014, 594, 720–724. [Google Scholar]
- Symington, M.C.; David-West, O.S.; Banks, W.M.; Pethrick, R.A.; Thomason, J.L. The effect of alkalisation on the mechanical properties of natural fibres. In Proceedings of the 13th European Conference on Composite Materials (EECM 13), Stockholm, Sweden, 2–5 June 2008; Volume 43, pp. 1083–1108. [Google Scholar] [CrossRef]
- Vijay, R.; Singaravelu, D.L.; Vinod, A.; Sanjay, M.R.; Siengchin, S.; Jawaid, M.; Khan, A.; Parameswaranpillai, J. Characterization of raw and alkali treated new natural cellulosic fibers from Tridax procumbens. Int. J. Biol. Macromol. 2019, 125, 99–108. [Google Scholar] [CrossRef]
- Prithiviraj, M.; Muralikannan, R. Investigation of optimal alkali-treated perotis indica plant fibers on physical, chemical, and morphological properties. J. Nat. Fibers 2020, 19, 2730–2743. [Google Scholar] [CrossRef]
- Cai, M.; Takagi, H.; Nakagaito, A.N.; Li, Y.; Waterhouse, G.I. Effect of alkali treatment on interfacial bonding in abaca fiber-reinforced composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 589–597. [Google Scholar] [CrossRef]
- Kaur, K.; Phutela, U.G. Morphological and structural changes in paddy straw influenced by alkali and microbial pretreatment. Detritus 2018, 3, 30–36. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Costa, L.A.; Fonseca, A.F.; Pereira, F.V.; Druzian, J.I. Extraction and characterization of cellulose nanocrystals from corn stover. Cell Chem. Technol. 2015, 49, 127–133. [Google Scholar]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012045. [Google Scholar] [CrossRef]
- Movva, M.; Kommineni, R. Extraction of cellulose from pistachio shell and physical and mechanical characterisation of cellulose-based nanocomposites. Mater. Res. Express 2017, 4, 045014. [Google Scholar] [CrossRef]
- Jayabal, S.; Sathiyamurthy, S.; Loganathan, K.T.; Kalyanasundaram, S. Effect of soaking time and concentration of NaOH solution on mechanical properties of coir–polyester composites. Bull. Mater. Sci. 2012, 35, 567–574. [Google Scholar] [CrossRef]
- Oushabi, A.; Sair, S.; Hassani, F.O.; Abboud, Y.; Tanane, O.; El Bouari, A. The effect of alkali treatment on mechanical, morphological and thermal properties of date palm fibers (DPFs): Study of the interface of DPF–Polyurethane composite. S. Afr. J. Chem. Eng. 2017, 23, 116–123. [Google Scholar] [CrossRef]
- Thomas, M.G.; Abraham, E.; Jyotishkumar, P.; Maria, H.J.; Pothen, L.A.; Thomas, S. Nanocelluloses from jute fibers and their nanocomposites with natural rubber: Preparation and characterization. Int. J. Biol. Macromol. 2015, 81, 768–777. [Google Scholar] [CrossRef]
- Ouyang, X.; Chen, L.; Zhang, S.; Yuan, Q.; Wang, W.; Linhardt, L.J. Effect of simultaneous steam explosion and alkaline depolymerization on corncob lignin and cellulose structure. Chem. Biochem. Eng. Q. 2018, 32, 177–189. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, Y.Y. Fractionation of corn stover by hot-water and aqueous ammonia treatment. Bioresour. Technol. 2006, 97, 224–232. [Google Scholar] [CrossRef]
- Guimarães, M.; Botaro, V.R.; Novack, K.M.; Flauzino Neto, W.; Mendes, L.M.; Tonoli, G.H. Preparation of cellulose nanofibrils from bamboo pulp by mechanical defibrillation for their applications in biodegradable composites. J. Nanosci. Nanotechnol. 2015, 15, 6751–6768. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Yang, F.; Tang, C.; Huang, Q. Effect of H2O2 bleaching treatment on the properties of finished transparent wood. Polymers 2019, 11, 776. [Google Scholar] [CrossRef]
- Hubbell, C.A.; Ragauskas, A.J. Effect of acid-chlorite delignification on cellulose degree of polymerization. Bioresour. Technol. 2010, 101, 7410–7415. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Puglia, D.; Petrucci, R.; Kenny, J.M.; Torre, L. Modulation of acid hydrolysis reaction time for the extraction of cellulose nanocrystals from Posidonia oceanica leaves. J. Renew. Mater. 2016, 4, 190–198. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Alanazi, H.H.; Alghamdi, A.A. Extraction and bleaching of olive tree branch cellulose. BioResources 2015, 10, 7136–7150. [Google Scholar] [CrossRef][Green Version]
- Sá, R.M.D.; Miranda, C.S.D.; José, N.M. Preparation and characterization of nanowhiskers cellulose from fiber arrowroot (Maranta arundinacea). Mater. Res. 2015, 18, 225–229. [Google Scholar] [CrossRef]
- Rehman, N.; Alam, S.; Amin, N.U.; Mian, I.; Ullah, H. Ecofriendly isolation of cellulose from Eucalyptus lenceolata: A novel approach. Int. J. Polym. Sci. 2018, 2018, 8381501. [Google Scholar] [CrossRef]
- Duan, L.; Yu, W.; Li, Z. Analysis of structural changes in jute fibers after peracetic acid treatment. J. Eng. Fibers Fabr. 2017, 12, 155892501701200104. [Google Scholar] [CrossRef]
- Grumo, J.C.; Jabber, L.J.Y.; Patricio, J.N.; Magdadaro, M.R.D.; Lubguban, A.A.; Alguno, A.C. Alkali and bleach treatment of the extracted cellulose from pineapple (Ananas comosus) leaves. J. Fundam. Appl. Sci. 2017, 9, 124–133. [Google Scholar]
- Mussatto, S.I.; Rocha, G.J.; Roberto, I.C. Hydrogen peroxide bleaching of cellulose pulps obtained from brewer’s spent grain. Cellulose 2008, 15, 641–649. [Google Scholar] [CrossRef]
- Yudha, V.; Rochardjo, H.S.B.; Jamasri, J.; Widyorini, R.; Yudhanto, F.; Darmanto, S. Isolation of cellulose from salacca midrib fibers by chemical treatments. IOP Conf. Ser. Mater. Sci. Eng. 2018, 434, 012078. [Google Scholar] [CrossRef]
- Valchev, I.; Yavorov, N.; Todorova, D. Producing bleached microcrystalline cellulose by two-stage dilute acid hydrolysis. Cellul. Chem. Technol. 2020, 54, 259–264. [Google Scholar]
- Punnadiyil, R.K.; Sreejith, M.; Purushothaman, E. Isolation of microcrystalline and nano cellulose from peanut shells. J. Chem. Pharm. Sci. 2016, 974, 2115. [Google Scholar]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Asri, S.E.A.M.; Ismail, A.F. Physicochemical properties of “green” nanocrystalline cellulose isolated from recycled newspaper. RSC Adv. 2015, 5, 29842–29849. [Google Scholar] [CrossRef]
- Campano, C.; Miranda, R.; Merayo, N.; Negro, C.; Blanco, A. Direct production of cellulose nanocrystals from old newspapers and recycled newsprint. Carbohydr. Polym. 2017, 173, 489–496. [Google Scholar] [CrossRef]
- Plermjai, K.; Boonyarattanakalin, K.; Mekprasart, W.; Pavasupree, S.; Phoohinkong, W.; Pecharapa, W. Extraction and characterization of nanocellulose from sugarcane bagasse by ball-milling-assisted acid hydrolysis. AIP Conf. Proc. 2018, 2010, 020005. [Google Scholar]
- Maaloul, N.; Ben Arfi, R.; Rendueles, M.; Ghorbal, A.; Diaz, M. Dialysis-free extraction and characterization of cellulose crystals from almond (Prunus dulcis) shells. J. Mater. Environ. Sci. 2017, 8, 4171–4181. [Google Scholar]
- Lu, P.; Hsieh, Y.L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Chandra, J.; George, N.; Narayanankutty, S.K. Isolation and characterization of cellulose nanofibrils from arecanut husk fibre. Carbohydr. Polym. 2016, 142, 158–166. [Google Scholar]
- Hastuti, N.; Kanomata, K.; Kitaoka, T. Hydrochloric acid hydrolysis of pulps from oil palm empty fruit bunches to produce cellulose nanocrystals. J. Polym. Environ. 2018, 26, 3698–3709. [Google Scholar] [CrossRef]
- Chen, Q.; Xiao, S.; Shi, S.; Cai, L. Isolation of cellulose from poplar wood by nitric acid-ethanol treatment and its effect on the quality of films cast from ionic liquid. BioRes 2018, 13, 8943–8955. [Google Scholar] [CrossRef]
- Hanani, A.N.; Zuliahani, A.; Nawawi, W.I.; Razif, N.; Rozyanty, A.R. The effect of various acids on properties of microcrystalline cellulose (MCC) extracted from rice husk (RH). IOP Conf. Ser. Mater. Sci. Eng. 2017, 204, 012025. [Google Scholar] [CrossRef]
- Trifol, J.; Sillard, C.; Plackett, D.; Szabo Bras, J.; Daugaard, A.E. Chemically extracted nanocellulose from sisal fibres by a simple and industrially relevant process. Cellulose 2017, 24, 107–118. [Google Scholar] [CrossRef]
- Lu, Q.; Cai, Z.; Lin, F.; Tang, L.; Wang, S.; Huang, B. Extraction of cellulose nanocrystals with a high yield of 88% by simultaneous mechanochemical activation and phosphotungstic acid hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 2165–2172. [Google Scholar] [CrossRef]
- Mhlongo, J.T.; Nuapia, Y.; Motsa, M.M.; Mahlangu, T.O.; Etale, A. Green chemistry approaches for extraction of cellulose nanofibers (CNFs): A comparison of mineral and organic acids. Mater. Today Proc. 2022, 10, 1150. [Google Scholar] [CrossRef]
- Erdogan, U.H.; Selli, F.; Duran, H. Banana plant waste as raw material for cellulose extraction. Fiber Text. 2017, 24, 48–52. [Google Scholar]
- Nazir, M.S.; Wahjoedi, B.A.; Yussof, A.W.; Abdullah, M.A. Eco-friendly extraction and characterization of cellulose from oil palm empty fruit bunches. BioRes. 2013, 8, 2161–2172. [Google Scholar] [CrossRef]
- Yeganeh, F.; Behrooz, R.; Rahimi, M. The effect of Sulfuric acid and Maleic acid on characteristics of nano-cellulose produced from waste office paper. Int. J. Nano Dimens. 2017, 8, 206–215. [Google Scholar]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef] [PubMed]
- Hameed, N.; Guo, Q. Blend films of natural wool and cellulose prepared from an ionic liquid. Cellulose 2010, 17, 803–813. [Google Scholar] [CrossRef]
- Hameed, N.; Bavishi, J.; Parameswaranpillai, J.; Salim, N.V.; Joseph, J.; Madras, G.; Fox, B.L. Thermally flexible epoxy/cellulose blends mediated by an ionic liquid. RSC Adv. 2015, 5, 52832–52836. [Google Scholar] [CrossRef]
- Hina, S.; Zhang, Y.; Wang, H. Role of ionic liquids in dissolution and regeneration of cellulose. Rev. Adv. Mater. Sci. 2015, 40, 215–226. [Google Scholar]
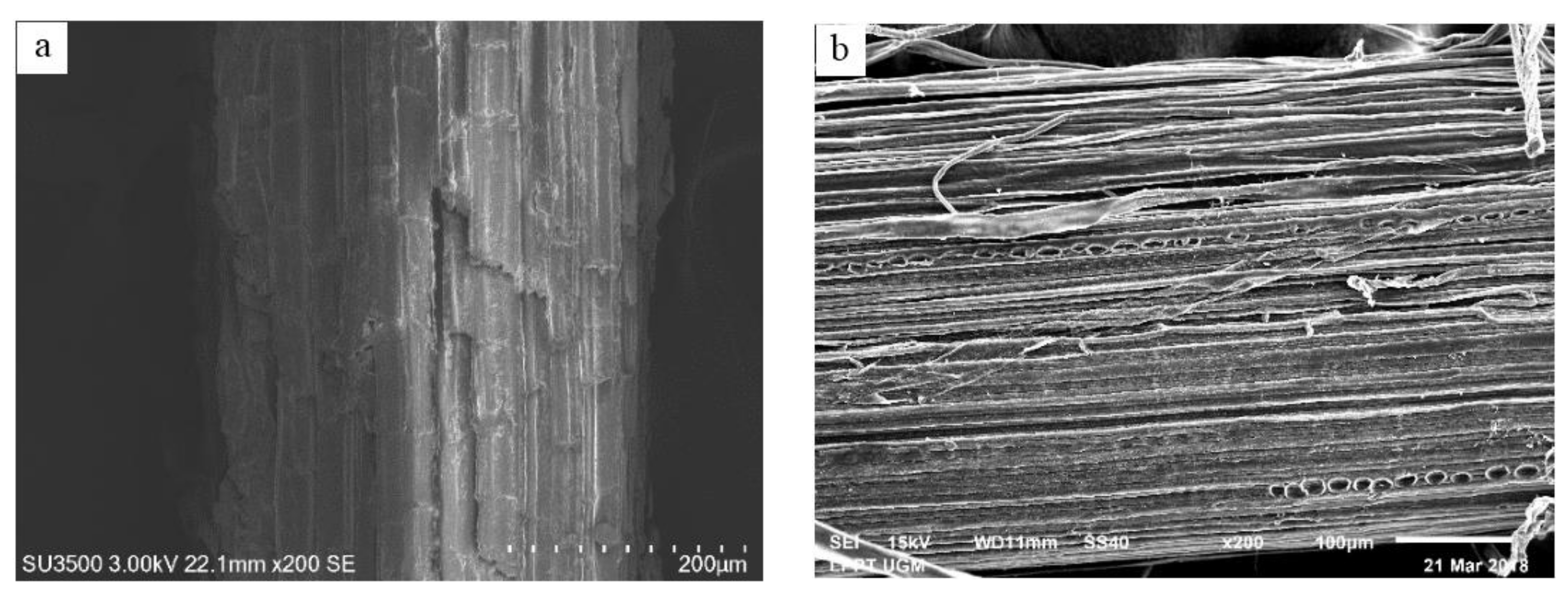
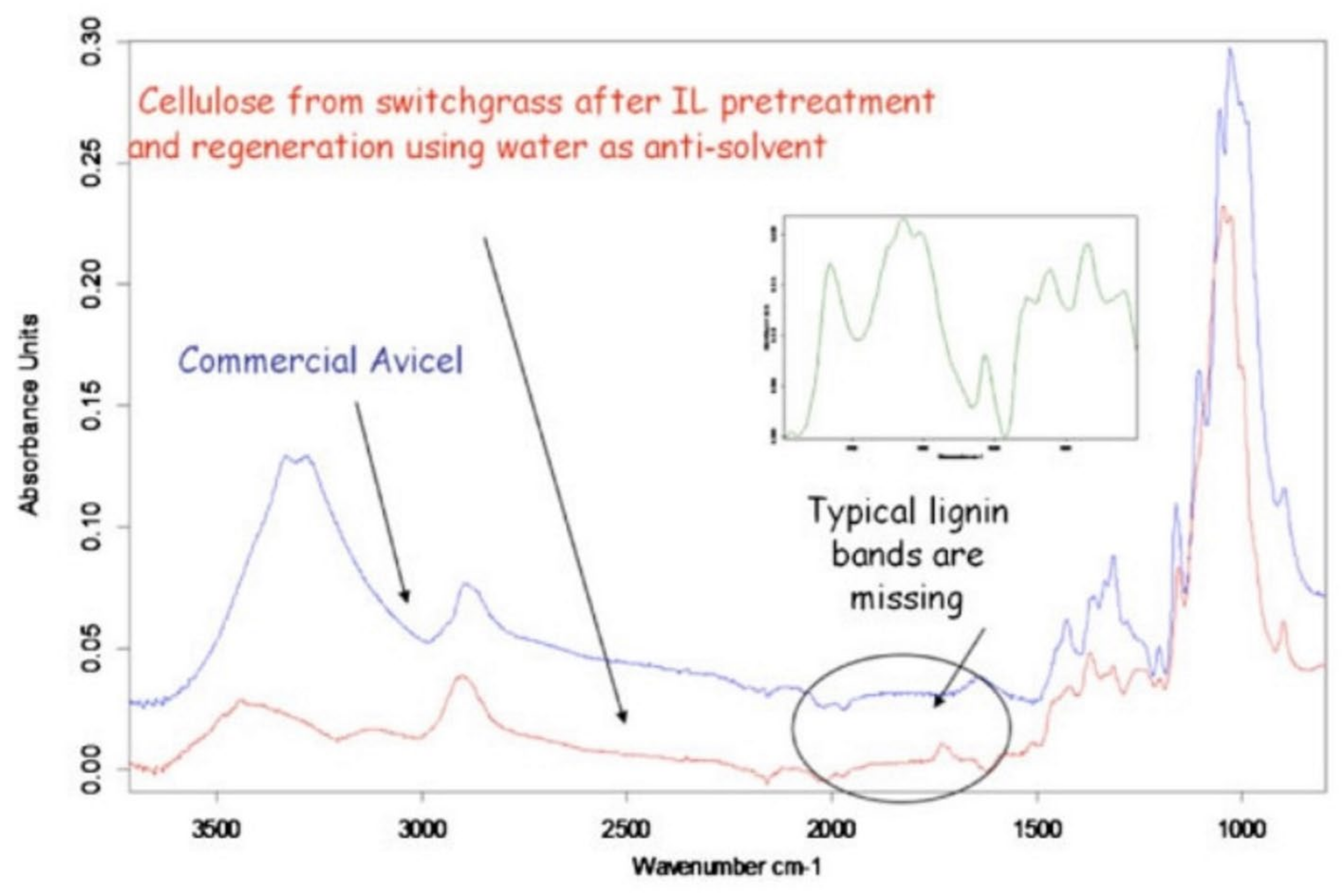
- Singh, S.; Simmons, B.A.; Vogel, K. Visualization of biomass solubilization and cellulose regeneration during ionic liquid pretreatment of switchgrass. Biotechnol. Bioeng. 2009, 104, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, Z.Z.; Chandran, R.R.R.; Jahan, A.; Khalid, K.; Rahman, M.M.; Al-Amin, M.; Akbarzadeh, O.; Badruddin, I.A.; Khan, T.Y.; Kamangar, S.; et al. Extraction of Cellulose Nano-Whiskers Using Ionic Liquid-Assisted Ultra-Sonication: Optimization and Mathematical Modelling Using Box–Behnken Design. Symmetry 2019, 11, 1148. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, J.; Lan, P.; Yang, H.; Lu, J.; Wang, Z. Study on the dissolution mechanism of cellulose by ChCl-based deep eutectic solvents. Materials 2020, 13, 278. [Google Scholar] [CrossRef]
- Kian, L.K.; Saba, N.; Jawaid, M.; Sultan, M.T.H. A review on processing techniques of bast fibers nanocellulose and its polylactic acid (PLA) nanocomposites. Int. J. Biol. Macromol. 2019, 121, 1314–1328. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Salaberria, A.M.; Andres, M.A.; Kaya, M.; Gunyakti, A.; Labidi, J. Utilization of flax (Linum usitatissimum) cellulose nanocrystals as reinforcing material for chitosan films. Int. J. Biol. Macromol. 2017, 104, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Orasugh, J.T.; Saha, N.R.; Rana, D.; Sarkar, G.; Mollick, M.M.R.; Chattoapadhyay, A.; Mitra, B.C.; Mondal, D.; Ghosh, S.K.; Chattopadhyay, D. Jute cellulose nano-fibrils/hydroxypropylmethylcellulose nanocomposite: A novel material with potential for application in packaging and transdermal drug delivery system. Ind. Crops Prod. 2018, 112, 633–643. [Google Scholar] [CrossRef]
- Peng, Y.; Gardner, D.J.; Han, Y. Drying cellulose nanofibrils: In search of a suitable method. Cellulose 2012, 19, 91–102. [Google Scholar] [CrossRef]
- Peng, Y.; Gardner, D.J.; Han, Y.; Kiziltas, A.; Cai, Z.; Tshabalala, M.A. Influence of drying method on the material properties of nanocellulose I: Thermostability and crystallinity. Cellulose 2013, 20, 2379–2392. [Google Scholar] [CrossRef]
- Srithep, Y.; Ellingham, T.; Peng, J.; Sabo, R.; Clemons, C.; Turng, L.S.; Pilla, S. Melt compounding of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/nanofibrillated cellulose nanocomposites. Polym. Degrad. Stab. 2013, 98, 1439–1449. [Google Scholar] [CrossRef]
- Zimmermann, M.V.; Borsoi, C.; Lavoratti, A.; Zanini, M.; Zattera, A.J.; Santana, R.M. Drying techniquees applied to cellulose nanofibers. J. Reinf. Plast. Compos. 2016, 35, 628–643. [Google Scholar] [CrossRef]
- Lim, H.J.; Cheng, W.K.; Tan, K.W.; Yu, L.J. Oil palm-based nanocellulose for a sustainable future: Where are we now? J. Environ. Chem. Eng. 2022, 34, 107271. [Google Scholar] [CrossRef]
- Colturato, L.; Goveia, D. Controlled release of vitamin D3 using a nanocellulose-based membrane. Sci Rep 2022, 12, 12411. [Google Scholar] [CrossRef]
- Alves, L.; Ramos, A.; Rasteiro, M.G.; Vitorino, C.; Ferraz, E.; Ferreira, J.; Puertas, M.L.; Gamelas, J.A. Composite Films of Nanofibrillated Cellulose with Sepiolite: Effect of Preparation Strategy. Coatings 2022, 12, 303. [Google Scholar] [CrossRef]
- Al-Hagar, O.E.; Abol-Fotouh, D. A turning point in the bacterial nanocellulose production employing low doses of gamma radiation. Sci. Rep. 2022, 12, 7012. [Google Scholar]
- Tanpichai, S.; Phoothong, F.; Boonmahitthisud, A. Superabsorbent cellulose-based hydrogels cross-liked with borax. Sci. Rep. 2022, 12, 8920. [Google Scholar]
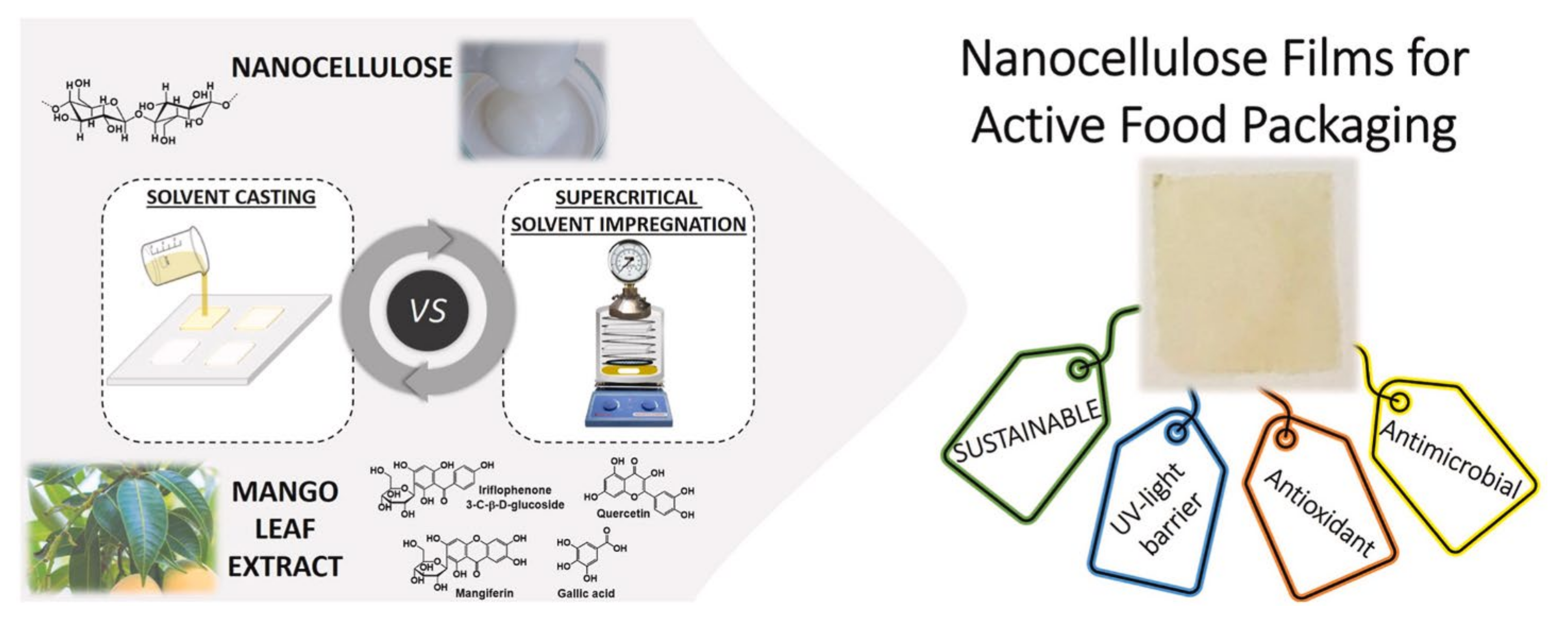
- Bastante, C.C.; Silva, N.H.; Cardoso, L.C.; Serrano, C.M.; de la Ossa, E.J.M.; Freire, C.S.; Vilela, C. Biobased films of nanocellulose and mango leaf extract for active food packaging: Supercritical impregnation versus solvent casting. Food Hydrocoll. 2021, 117, 106709. [Google Scholar] [CrossRef]
- Sardar, M.; Manna, M.; Maharana, M.; Sen, S. Remediation of dyes from industrial wastewater using low-cost adsorbents. In Green Adsorbents to Remove Metals, Dyes and Boron from Polluted Water; Springer: Cham, Switzerland, 2021; pp. 377–403. [Google Scholar]
- Tavakolian, M.; Wiebe, H.; Sadeghi, M.A.; van de Ven, T.G. Dye removal using hairy nanocellulose: Experimental and theoretical investigations. ACS Appl. Mater. Interfaces 2019, 12, 5040–5049. [Google Scholar] [CrossRef] [PubMed]
- Putro, J.N.; Santoso, S.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Nanocrystalline cellulose from waste paper: Adsorbent for azo dyes removal. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100260. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Lee, J.; Nandi, D.; Parameswaranpillai, J.; Pant, B.; Siengchin, S. Efficient Removal of Organic Dye from Aqueous Solution Using Hierarchical Zeolite-Based Biomembrane: Isotherm, Kinetics, Thermodynamics and Recycling Studies. Catalysts 2022, 12, 886. [Google Scholar] [CrossRef]
- Abdelaziz, R.M.; El-Maghraby, A.; Sadik, W.A.A.; El-Demerdash, A.G.M.; Fadl, E.A. Biodegradable cellulose nanocrystals hydrogels for removal of acid red 8 dye from aqueous solutions. Sci. Rep. 2022, 12, 6424. [Google Scholar]
- Babi, M.; Fatona, A.; Li, X.; Cerson, C.; Jarvis, V.M.; Abitbol, T.; Moran-Mirabal, J.M. Efficient labeling of nanocellulose for high-resolution fluorescence microscopy applications. Biomacromolecules 2022, 23, 1981–1994. [Google Scholar] [CrossRef]
- Shishehbor, M.; Son, H.; Nuruddin, M.; Youngblood, J.; Davis, C.; Zavattieri, D. Influence of alignment and microstructure features on the mechanical properties and failure mechanisms of cellulose nanocrystals (CNC) films. J. Mech. Behav. Biomed. Mater. 2021, 118, 104399. [Google Scholar]
- Heidarian, P.; Gharaie, S.; Yousefi, H.; Paulino, M.; Kaynak, A.; Varley, R.; Kouzani, A.Z. A 3D printable dynamic nanocellulose/nanochitin self-healing hydrogel and soft strain sensor. Carbohydr. Polym. 2022, 291, 119545. [Google Scholar] [CrossRef]
- Uetani, K.; Okada, T.; Oyama, H.T. Crystallite size effect on thermal conductive properties of nonwoven nanocellulose sheets. Biomacromolecules 2015, 16, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Chen, Y.; Sorieul, M. Wood-based flexible electronics. ACS Nano 2020, 14, 3528–3538. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J.D.; Shriver-Lake, L.C.; Walper, S.A.; Zabetakis, D.; Breger, J.C.; Stenger, D.A. Microbial nanocellulose printed circuit boards for medical sensing. Sensors 2020, 20, 2047. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.; Grant, I.; Dewey, M.; Bourque, M.; Neivandt, D.J. Production and Characterization of Cellulose Nanofiber Slurries and Sheets for Biomedical Applications. Front. Nanotechnol. 2022, 3, 729743. [Google Scholar] [CrossRef]
- Schiavi, D.; Francesconi, S.; Taddei, A.R.; Fortunati, E.; Balestra, G.M. Exploring cellulose nanocrystals obtained from olive tree wastes as sustainable crop protection tool against bacterial diseases. Sci. Rep. 2022, 12, 6149. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Siddique, R.; Huanfei, D.; Shereen, M.A.; Nabi, G.; Bai, Q.; Manan, S.; Xue, M.; Ullah, M.W.; Bowen, H. Perspective applications and associated challenges of using nanocellulose in treating bone-related diseases. Front. Bioeng. Biotechnol. 2021, 9, 616555. [Google Scholar] [CrossRef]
- Zielińska, D.; Szentner, K.; Waśkiewicz, A.; Borysiak, S. Production of nanocellulose by enzymatic treatment for application in polymer composites. Materials 2021, 14, 2124. [Google Scholar] [CrossRef]
- Bengtsson, M.; Le Baillif, M.; Oksman, K. Extrusion and mechanical properties of highly filled cellulose fibre–polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1922–1931. [Google Scholar] [CrossRef]
- Taheri, H.; Hietala, M.; Oksman, K. One-step twin-screw extrusion process of cellulose fibers and hydroxyethyl cellulose to produce fibrillated cellulose biocomposite. Cellulose 2020, 27, 8105–8119. [Google Scholar] [CrossRef]
- Forsgren, L.; Berglund, J.; Thunberg, J.; Rigdahl, M.; Boldizar, A. Injection molding and appearance of cellulose-reinforced composites. Polym. Eng. Sci. 2020, 60, 5–12. [Google Scholar] [CrossRef]
- Boufi, S.; Kaddami, H.; Dufresne, A. Mechanical performance and transparency of nanocellulose reinforced polymer nanocomposites. Macromol. Mater. Eng. 2014, 299, 560–568. [Google Scholar] [CrossRef]
- Somseemee, O.; Saeoui, P.; Schevenels, F.T.; Siriwong, C. Enhanced interfacial interaction between modified cellulose nanocrystals and epoxidized natural rubber via ultraviolet irradiation. Sci. Rep. 2022, 12, 6682. [Google Scholar]
- Ghasemi, S.; Behrooz, R.; Ghasemi, I.; Yassar, R.S.; Long, F. Development of nanocellulose-reinforced PLA nanocomposite by using maleated PLA (PLA-g-MA). J. Thermoplast. Compos. Mater. 2018, 31, 1090–1101. [Google Scholar] [CrossRef]
- De Souza, A.G.; Barbosa, R.F.; Rosa, D.S. Nanocellulose from industrial and agricultural waste for further use in PLA composites. J. Polym. Environ. 2020, 28, 1851–1868. [Google Scholar] [CrossRef]
- Lamm, M.E.; Li, K.; Ker, D.; Zhao, X.; Hinton, H.E.; Copenhaver, K.; Tekinalp, H.; Ozcan, S. Exploiting chitosan to improve the interface of nanocellulose reinforced polymer composites. Cellulose 2022, 29, 3859–3870. [Google Scholar] [CrossRef]
- Sultana, T.; Sultana, S.; Nur, H.; Khan, M.W. Studies on mechanical, thermal and morphological properties of betel nut husk nano cellulose reinforced biodegradable polymer composites. J. Compos. Sci. 2020, 4, 83. [Google Scholar] [CrossRef]
- Tang, C.; Liu, H. Cellulose nanofiber reinforced poly (vinyl alcohol) composite film with high visible light transmittance. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1638–1643. [Google Scholar] [CrossRef]
- Gong, G.; Pyo, J.; Mathew, A.; Oksman, K. Tensile behavior, morphology and viscoelastic analysis of cellulose nanofiber-reinforced (CNF) polyvinyl acetate (PVAc). Compos. Part A Appl. Sci. Manuf. 2011, 42, 1275–1282. [Google Scholar] [CrossRef]
- Nuruddin, M.; Hamlin, J.; Clarkson, C.M.; Howarter, J.A.; Szczepanski, C.R.; Youngblood, J. Processing and Characterization of Food-Grade Plasticizer-Compatibilized Cellulose Nanocrystals and Ethylene Vinyl Alcohol Copolymer Nanocomposites. ACS Appl. Polym. Mater. 2021, 3, 5000–5011. [Google Scholar] [CrossRef]
- Takagi, H.; Asano, A. Effects of processing conditions on flexural properties of cellulose nanofiber reinforced “green” composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 685–689. [Google Scholar] [CrossRef]
- Soni, R.; Asoh, T.A.; Uyama, H. Cellulose nanofiber reinforced starch membrane with high mechanical strength and durability in water. Carbohydr. Polym. 2020, 238, 116203. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.L.; Zhao, M.; Park, M.; Park, S.J. Recent trends of foaming in polymer processing: A review. Polymers 2019, 11, 953. [Google Scholar] [CrossRef]
- Ito, A.; Semba, T.; Kitagawa, K.; Okumura, H.; Yano, H. Cell morphologies and mechanical properties of cellulose nanofiber reinforced polypropylene foams. J. Cell. Plast. 2019, 55, 385–400. [Google Scholar] [CrossRef]
- Patil, S.; Bharimalla, A.K.; Nadanathangam, V.; Dhakane-Lad, J.; Mahapatra, A.; Jagajanantha, P.; Saxena, S. Nanocellulose reinforced corn starch-based biocomposite films: Composite optimization, characterization and storage studies. Food Packag. Shelf Life 2022, 33, 100860. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, Y.; Mei, C.; Cai, L.; Nadda, A.; van Le, Q.; Peng, Y.; Lam, S.S.; Sonne, C.; Xia, C. Advanced nanocellulose-based gas barrier materials: Present status and prospects. Chemosphere 2022, 286, 131891. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulose, A.; Parameswaranpillai, J.; George, J.J.; Gopi, J.A.; Krishnasamy, S.; Dominic C. D., M.; Hameed, N.; Salim, N.V.; Radoor, S.; Sienkiewicz, N. Nanocellulose: A Fundamental Material for Science and Technology Applications. Molecules 2022, 27, 8032. https://doi.org/10.3390/molecules27228032
Poulose A, Parameswaranpillai J, George JJ, Gopi JA, Krishnasamy S, Dominic C. D. M, Hameed N, Salim NV, Radoor S, Sienkiewicz N. Nanocellulose: A Fundamental Material for Science and Technology Applications. Molecules. 2022; 27(22):8032. https://doi.org/10.3390/molecules27228032
Chicago/Turabian StylePoulose, Aiswarya, Jyotishkumar Parameswaranpillai, Jinu Jacob George, Jineesh Ayippadath Gopi, Senthilkumar Krishnasamy, Midhun Dominic C. D., Nishar Hameed, Nisa V. Salim, Sabarish Radoor, and Natalia Sienkiewicz. 2022. "Nanocellulose: A Fundamental Material for Science and Technology Applications" Molecules 27, no. 22: 8032. https://doi.org/10.3390/molecules27228032
APA StylePoulose, A., Parameswaranpillai, J., George, J. J., Gopi, J. A., Krishnasamy, S., Dominic C. D., M., Hameed, N., Salim, N. V., Radoor, S., & Sienkiewicz, N. (2022). Nanocellulose: A Fundamental Material for Science and Technology Applications. Molecules, 27(22), 8032. https://doi.org/10.3390/molecules27228032






