The Impact of Nitrile-Specifier Proteins on Indolic Carbinol and Nitrile Formation in Homogenates of Arabidopsis thaliana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Chemical Synthesis of Indolic Standard Compounds
2.4. Derivatization of Standard Compounds
2.5. GC-MS and GC-FID
2.6. Determination of Response Factors
2.7. Analysis of Indole Glucosinolate Breakdown Products in Root and Leaf Homogenates
2.8. Generation of Di(indol-3-yl)methane Derivatives
2.9. Analysis of Glucosinolates
2.10. Statistics
3. Results
3.1. Optimized Derivatization Makes Indolic Carbinols and Nitriles Accessible for GC
3.2. Quantitative Analysis of Indolic Carbinol and Nitrile Standards
3.3. The Method Is Applicable to Plant Homogenates
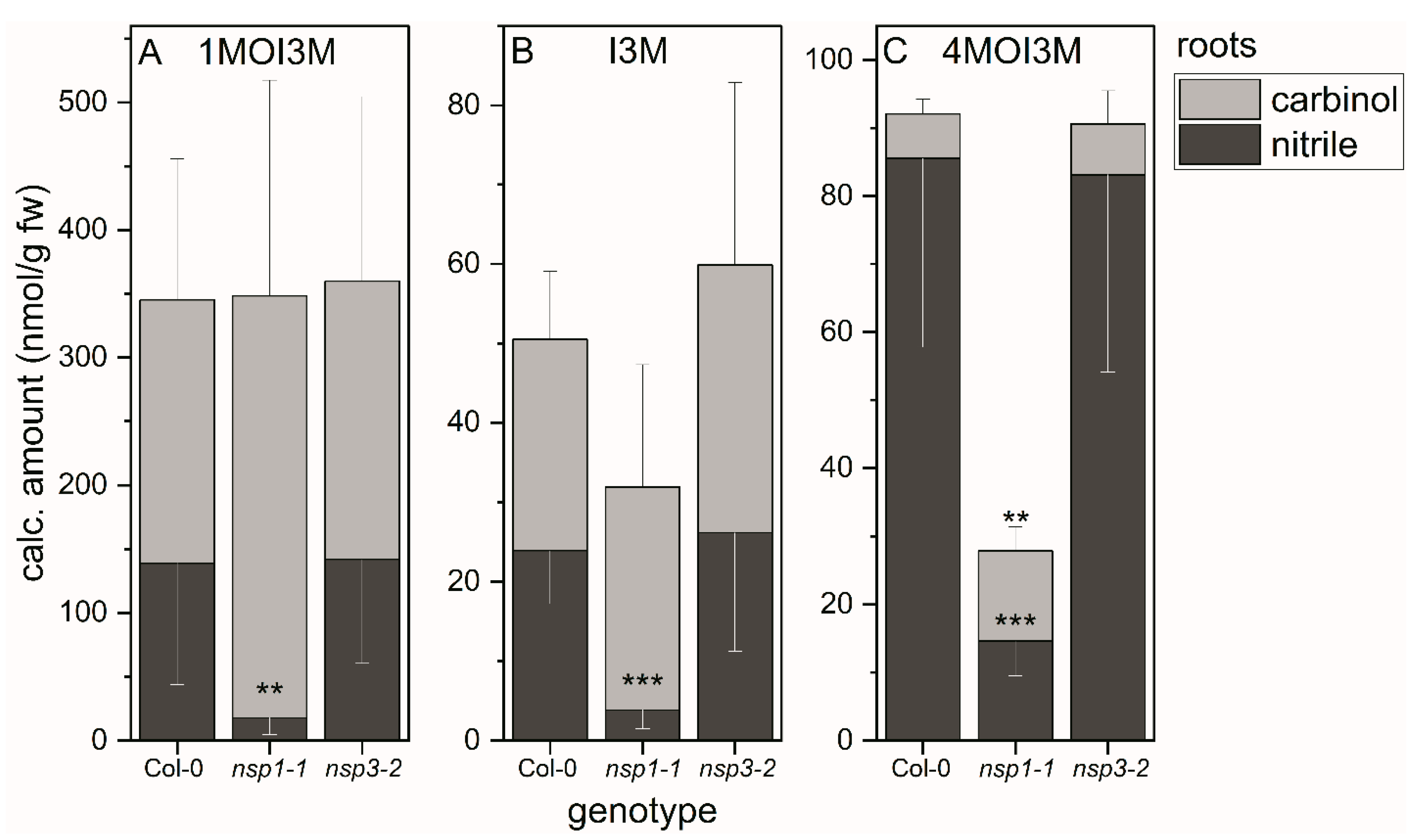
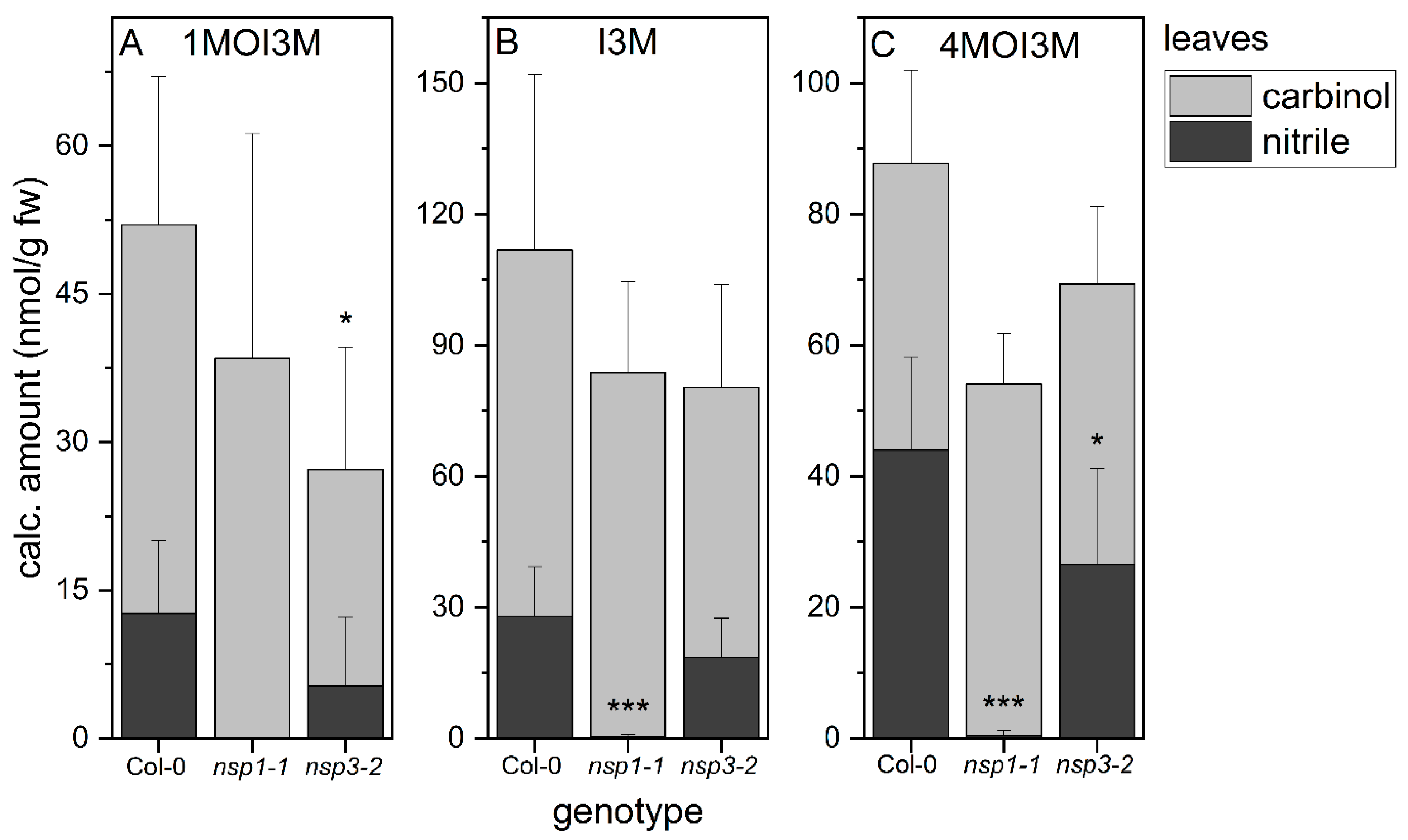
3.4. Indole Glucosinolate Substitution Patterns and NSP Deficiency Affect Indolic Carbinol/Nitrile Proportion in A. thaliana Root Homogenates
3.5. Methyl Ethers of Carbinols Are Formed in Root Homogenates and in an Aqueous Carbinol Standard Mixture
3.6. NSP1 Is Solely Responsible for Indolic Nitrile Formation in Rosette Homogenates of A. thaliana
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and stability of glucosinolates and their breakdown products in foods. Angew. Chem. Int. Ed. 2014, 53, 11430–11450. [Google Scholar] [CrossRef] [PubMed]
- Harun, S.; Abdullah-Zawawi, M.-R.; Goh, H.-H.; Mohamed-Hussein, Z.-A. A comprehensive gene inventory for glucosinolate biosynthetic pathway in Arabidopsis thaliana. J. Agric. Food Chem. 2020, 68, 7281–7297. [Google Scholar] [CrossRef]
- Traka, M.; Mithen, R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Yim, B.; Hanschen, F.S.; Wrede, A.; Nitt, H.; Schreiner, M.; Smalla, K.; Winkelmann, T. Effects of biofumigation using Brassica juncea and Raphanus sativus in comparison to disinfection using Basamid on apple plant growth and soil microbial communities at three field sites with replant disease. Plant Soil 2016, 406, 389–408. [Google Scholar] [CrossRef]
- Giamoustaris, A.; Mithen, R. The effect of modifying the glucosinolate content of leaves of oilseed rape (Brassica napus ssp. oleifera) on its interaction with specialist and generalist pests. Ann. Appl. Biol. 1995, 126, 347–363. [Google Scholar] [CrossRef]
- Li, Q.; Eigenbrode, S.D.; Stringam, G.R.; Thiagarajah, M.R. Feeding and growth of Plutella xylostella and Spodoptera eridania on Brassica juncea with varying glucosinolate concentrations and myrosinase activities. J. Chem. Ecol. 2000, 26, 2401–2419. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Rohn, S. Advanced research on glucosinolates in food products. Foods 2021, 10, 3148. [Google Scholar] [CrossRef] [PubMed]
- Chhajed, S.; Mostafa, I.; He, Y.; Abou-Hashem, M.; El-Domiaty, M.; Chen, S. Glucosinolate biosynthesis and the glucosinolate–myrosinase system in plant defense. Agronomy 2020, 10, 1786. [Google Scholar] [CrossRef]
- Lv, Q.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. The cellular and subcellular organization of the glucosinolate-myrosinase system against herbivores and pathogens. Int. J. Mol. Sci. 2022, 23, 1577. [Google Scholar] [CrossRef]
- Wittstock, U.; Kurzbach, E.; Herfurth, A.-M.; Stauber, E.J. Glucosinolate breakdown. In Glucosinolates. Advances in Botanical Research; Kopriva, S., Ed.; Academic Press: London, UK, 2016; Volume 80, pp. 125–169. [Google Scholar]
- Nakano, R.T.; Piślewska-Bednarek, M.; Yamada, K.; Edger, P.P.; Miyahara, M.; Kondo, M.; Böttcher, C.; Mori, M.; Nishimura, M.; Schulze-Lefert, P.; et al. PYK10 myrosinase reveals a functional coordination between endoplasmic reticulum bodies and glucosinolates in Arabidopsis thaliana. Plant J. 2017, 89, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, P.; Piślewska-Bednarek, M.; Svatoš, A.; Schneider, B.; Doubský, J.; Mansurova, M.; Humphry, M.; Consonni, C.; Panstruga, R.; Sanchez-Vallet, A.; et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 2009, 323, 101–106. [Google Scholar] [CrossRef]
- Koroleva, O.A.; Cramer, R. Single-cell proteomic analysis of glucosinolate-rich S-cells in Arabidopsis thaliana. Methods 2011, 54, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Andini, S.; Araya-Cloutier, C.; Sanders, M.; Vincken, J.-P. Simultaneous analysis of glucosinolates and isothiocyanates by Reversed-Phase Ultra-High-Performance Liquid Chromatography-Electron Spray Ionization-Tandem Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 3121–3131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burow, M.; Zhang, Z.-Y.; Ober, J.A.; Lambrix, V.M.; Wittstock, U.; Gershenzon, J.; Kliebenstein, D.J. ESP and ESM1 mediate indol-3-acetonitrile production from indol-3-ylmethyl glucosinolate in Arabidopsis. Phytochemistry 2008, 69, 663–671. [Google Scholar] [CrossRef]
- Lambrix, V.; Reichelt, M.; Mitchell-Olds, T.; Kliebenstein, D.J.; Gershenzon, J. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell 2001, 13, 2793–2807. [Google Scholar] [CrossRef] [Green Version]
- Buskov, S.; Olsen, C.E.; Sørensen, H.; Sørensen, S. Supercritical fluid chromatography as basis for identification and quantitative determination of indol-3-ylmethyl oligomers and ascorbigens. J. Biochem. Biophys. Meth. 2000, 43, 175–195. [Google Scholar] [CrossRef]
- Agerbirk, N.; de Vos, M.; Kim, J.H.; Jander, G. Indole glucosinolate breakdown and its biological effects. Phytochem. Rev. 2009, 8, 101–120. [Google Scholar] [CrossRef]
- Virtanen, A.I. Studies on organic sulphur compounds and other labile substances in plants. Phytochemistry 1965, 4, 207–228. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E.; Sørensen, H. Initial and final products, nitriles, and ascorbigens produced in myrosinase-catalyzed hydrolysis of indole glucosinolates. J. Agric. Food Chem. 1998, 46, 1563–1571. [Google Scholar] [CrossRef]
- Wittstock, U.; Burow, M. Tipping the scales—Specifier proteins in glucosinolate hydrolysis. IUBMB Life 2007, 59, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Mocniak, L.E.; Elkin, K.; Bollinger, J.M. Lifetimes of the aglycone substrates of specifier proteins, the autonomous iron enzymes that dictate the products of the glucosinolate-myrosinase defense system in Brassica plants. Biochemistry 2020, 59, 2432–2441. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, U.; Meier, K.; Dörr, F.; Ravindran, B.M. NSP-dependent simple nitrile formation dominates upon breakdown of major aliphatic glucosinolates in roots, seeds, and seedlings of Arabidopsis thaliana Columbia-0. Front. Plant Sci. 2016, 7, 1821. [Google Scholar] [CrossRef] [Green Version]
- Matusheski, N.V.; Swarup, R.; Juvik, J.A.; Mithen, R.; Bennett, M.; Jeffery, E.H. Epithiospecifier protein from broccoli (Brassica oleracea L. ssp. italica) inhibits formation of the anticancer agent sulforaphane. J. Agric. Food Chem. 2006, 54, 2069–2076. [Google Scholar] [CrossRef]
- Wittstock, U.; Kliebenstein, D.J.; Lambrix, V.; Reichelt, M.; Gershenzon, J. Glucosinolate hydrolysis and its impact on generalist and specialist insect herbivores. In Integrative Phytochemistry: From Ethnobotany to Molecular Ecology; Romeo, J.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 101–125. [Google Scholar]
- Plaszkó, T.; Szűcs, Z.; Vasas, G.; Gonda, S. Interactions of fungi with non-isothiocyanate products of the plant glucosinolate pathway: A review on product formation, antifungal activity, mode of action and biotransformation. Phytochemistry 2022, 200, 113245. [Google Scholar] [CrossRef]
- Capuano, E.; Dekker, M.; Verkerk, R.; Oliviero, T. Food as pharma? The case of glucosinolates. Curr. Pharm. Des. 2017, 23, 2697–2721. [Google Scholar] [CrossRef]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
- Kissen, R.; Bones, A.M. Nitrile-specifier proteins involved in glucosinolate hydrolysis in Arabidopsis thaliana. J. Biol. Chem. 2009, 284, 12057–12070. [Google Scholar] [CrossRef] [Green Version]
- Burow, M.; Losansky, A.; Müller, R.; Plock, A.; Kliebenstein, D.J.; Wittstock, U. The genetic basis of constitutive and herbivore-induced ESP-independent nitrile formation in Arabidopsis. Plant Physiol. 2009, 149, 561–574. [Google Scholar] [CrossRef]
- Petersen, B.L.; Chen, S.; Hansen, C.H.; Olsen, C.E.; Halkier, B.A. Composition and content of glucosinolates in developing Arabidopsis thaliana. Planta 2002, 214, 562–571. [Google Scholar]
- Klopsch, R.; Witzel, K.; Artemyeva, A.; Ruppel, S.; Hanschen, F.S. Genotypic variation of glucosinolates and their breakdown products in leaves of Brassica rapa. J. Agric. Food Chem. 2018, 66, 5481–5490. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Pfitzmann, M.; Witzel, K.; Stützel, H.; Schreiner, M.; Zrenner, R. Differences in the enzymatic hydrolysis of glucosinolates increase the defense metabolite diversity in 19 Arabidopsis thaliana accessions. Plant Physiol. Biochem. 2018, 124, 126–135. [Google Scholar] [CrossRef]
- Zhao, Y.; Hull, A.K.; Gupta, N.R.; Goss, K.A.; Alonso, J.; Ecker, J.R.; Normanly, J.; Chory, J.; Celenza, J.L. Trp-dependent auxin biosynthesis in Arabidopsis: Involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 2002, 16, 3100–3112. [Google Scholar] [CrossRef] [Green Version]
- Sønderby, I.E.; Hansen, B.G.; Bjarnholt, N.; Ticconi, C.; Halkier, B.A.; Kliebenstein, D.J. A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS ONE 2007, 2, e1322. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Sønderby, I.E.; Halkier, B.A.; Jander, G.; de Vos, M. Non-volatile intact indole glucosinolates are host recognition cues for ovipositing Plutella xylostella. J. Chem. Ecol. 2009, 35, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Gibeaut, D.M.; Hulett, J.; Cramer, G.R.; Seemann, J.R. Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol. 1997, 115, 317–319. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, M.M.; Tholl, D.; Tokuhisa, J.G. An aeroponic culture system for the study of root herbivory on Arabidopsis thaliana. Plant Methods 2011, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Pedras, M.S.C.; Yaya, E.E. Tenualexin, other phytoalexins and indole glucosinolates from wild cruciferous species. Chem. Biodivers. 2014, 11, 910–918. [Google Scholar] [CrossRef]
- Kronbak, R.; Duus, F.; Vang, O. Effect of 4-methoxyindole-3-carbinol on the proliferation of colon cancer cells in vitro, when treated alone or in combination with indole-3-carbinol. J. Agric. Food Chem. 2010, 58, 8453–8459. [Google Scholar] [CrossRef]
- Labrière, C.; Talapatra, S.K.; Thoret, S.; Bougeret, C.; Kozielski, F.; Guillou, C. New MKLP-2 inhibitors in the paprotrain series: Design, synthesis and biological evaluations. Bioorg. Med. Chem. 2016, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Buchner, R. Approach to determination of HPLC response factors for glucosinolates. In Glucosinolates in Rapeseeds: Analytical Aspects; Wathelet, J.-P., Ed.; Martinus Nijhoff Publishers: Boston, MA, USA, 1987; pp. 50–58. [Google Scholar]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Kataoka, H. Derivatization reactions for the determination of amines by gas chromatography and their applications in environmental analysis. J. Chromatogr. A 1996, 733, 19–34. [Google Scholar] [CrossRef]
- Müller, T.M.; Böttcher, C.; Glawischnig, E. Dissection of the network of indolic defence compounds in Arabidopsis thaliana by multiple mutant analysis. Phytochemistry 2019, 161, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lahrmann, U.; Strehmel, N.; Langen, G.; Frerigmann, H.; Leson, L.; Ding, Y.; Scheel, D.; Herklotz, S.; Hilbert, M.; Zuccaro, A. Mutualistic root endophytism is not associated with the reduction of saprotrophic traits and requires a noncompromised plant innate immunity. New Phytol. 2015, 207, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Kudjordjie, E.N.; Hooshmand, K.; Sapkota, R.; Darbani, B.; Fomsgaard, I.S.; Nicolaisen, M. Fusarium oxysporum disrupts microbiome-metabolome networks in Arabidopsis thaliana roots. Microbiol. Spectr. 2022, 10, e0122622. [Google Scholar] [CrossRef]
- Pastorczyk, M.; Bednarek, P. The function of glucosinolates and related metabolites in plant innate immunity. In Glucosinolates. Advances in Botanical Research; Kopriva, S., Ed.; Academic Press: London, UK, 2016; Volume 80, pp. 171–198. [Google Scholar]
- Jeschke, V.; Weber, K.; Moore, S.S.; Burow, M. Coordination of glucosinolate biosynthesis and turnover under different nutrient conditions. Front. Plant Sci. 2019, 10, 1560. [Google Scholar] [CrossRef]
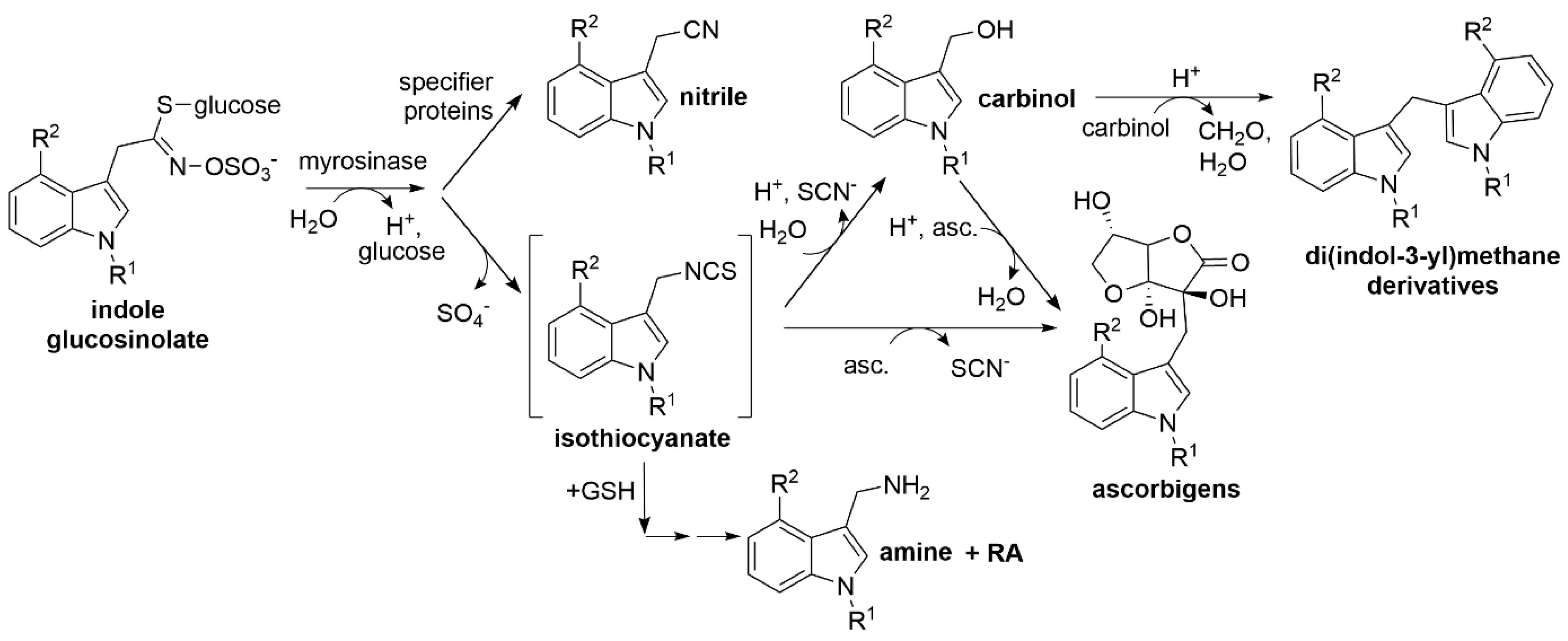


| R1 | R2 | Glucosinolate | Carbinol | Nitrile |
|---|---|---|---|---|
| H | H | I3M: indol-3-ylmethyl glucosinolate (glucobrassicin) | 1, I3C: indole-3-carbinol | 2, I3ACN: indole-3-acetonitrile |
| OCH3 | H | 1MOI3M: 1-methoxyindol-3-ylmethyl glucosinolate (neoglucobrassicin) | 3, 1MOI3C: 1-methoxyindole-3-carbinol | 4, 1MOI3ACN: 1-methoxyindole-3-acetonitrile |
| H | OCH3 | 4MOI3M: 4-methoxyindol-3-ylmethyl glucosinolate | 5, 4MOI3C: 4-methoxyindole-3-carbinol | 6, 4MOI3ACN: 4-methoxyindole-3-acetonitrile |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chroston, E.C.M.; Hielscher, A.; Strieker, M.; Wittstock, U. The Impact of Nitrile-Specifier Proteins on Indolic Carbinol and Nitrile Formation in Homogenates of Arabidopsis thaliana. Molecules 2022, 27, 8042. https://doi.org/10.3390/molecules27228042
Chroston ECM, Hielscher A, Strieker M, Wittstock U. The Impact of Nitrile-Specifier Proteins on Indolic Carbinol and Nitrile Formation in Homogenates of Arabidopsis thaliana. Molecules. 2022; 27(22):8042. https://doi.org/10.3390/molecules27228042
Chicago/Turabian StyleChroston, Eleanor C. M., Annika Hielscher, Matthias Strieker, and Ute Wittstock. 2022. "The Impact of Nitrile-Specifier Proteins on Indolic Carbinol and Nitrile Formation in Homogenates of Arabidopsis thaliana" Molecules 27, no. 22: 8042. https://doi.org/10.3390/molecules27228042
APA StyleChroston, E. C. M., Hielscher, A., Strieker, M., & Wittstock, U. (2022). The Impact of Nitrile-Specifier Proteins on Indolic Carbinol and Nitrile Formation in Homogenates of Arabidopsis thaliana. Molecules, 27(22), 8042. https://doi.org/10.3390/molecules27228042







