Borate-Based Compounds as Mixed Polyanion Cathode Materials for Advanced Batteries
Abstract
:1. Introduction
2. Development and Classifications of Cathode Materials for Secondary Alkali Ion Batteries
2.1. Layered Oxides
2.2. Spinel Oxides
2.3. Polyanion Oxides
2.4. Advantages and Disadvantages of Oxide Cathodes
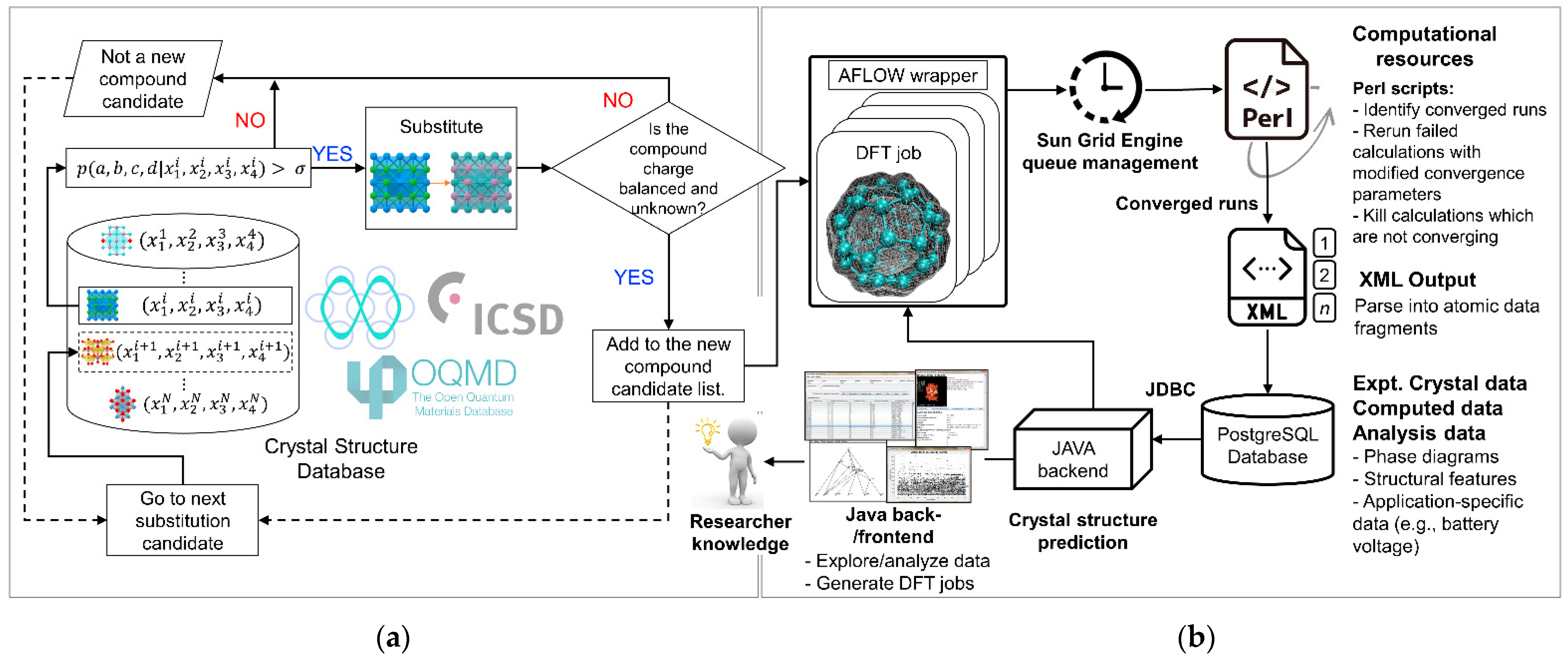
3. Combined Data Mining and High-Throughput ab Initio Computing Methodology for Novel Cathode Materials Development
4. Borate-Based Compounds as Cathodes
5. Borophosphates (BPO)
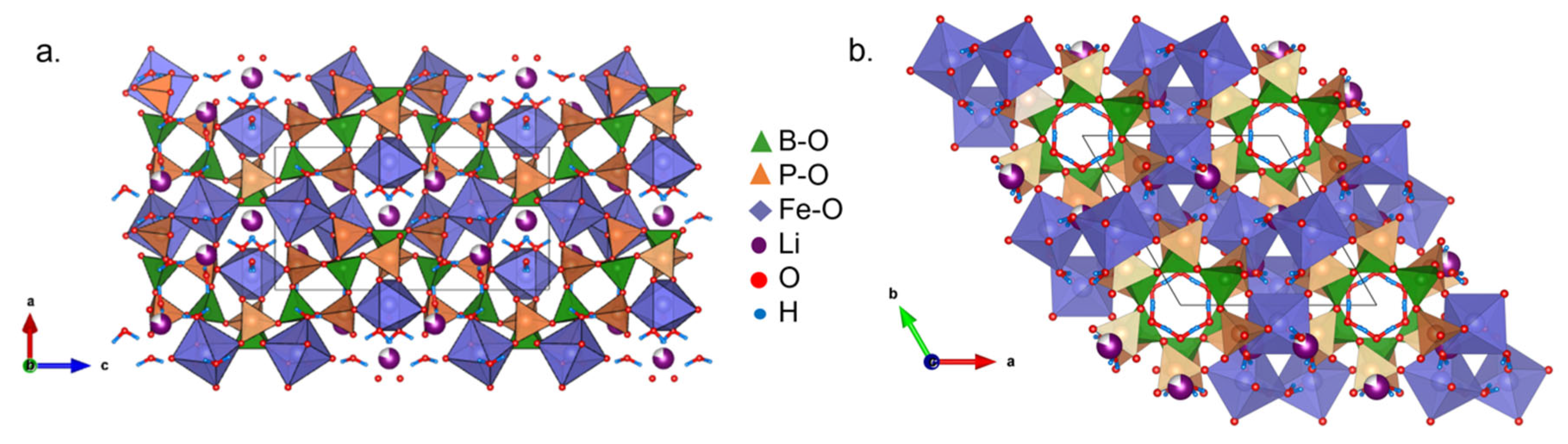
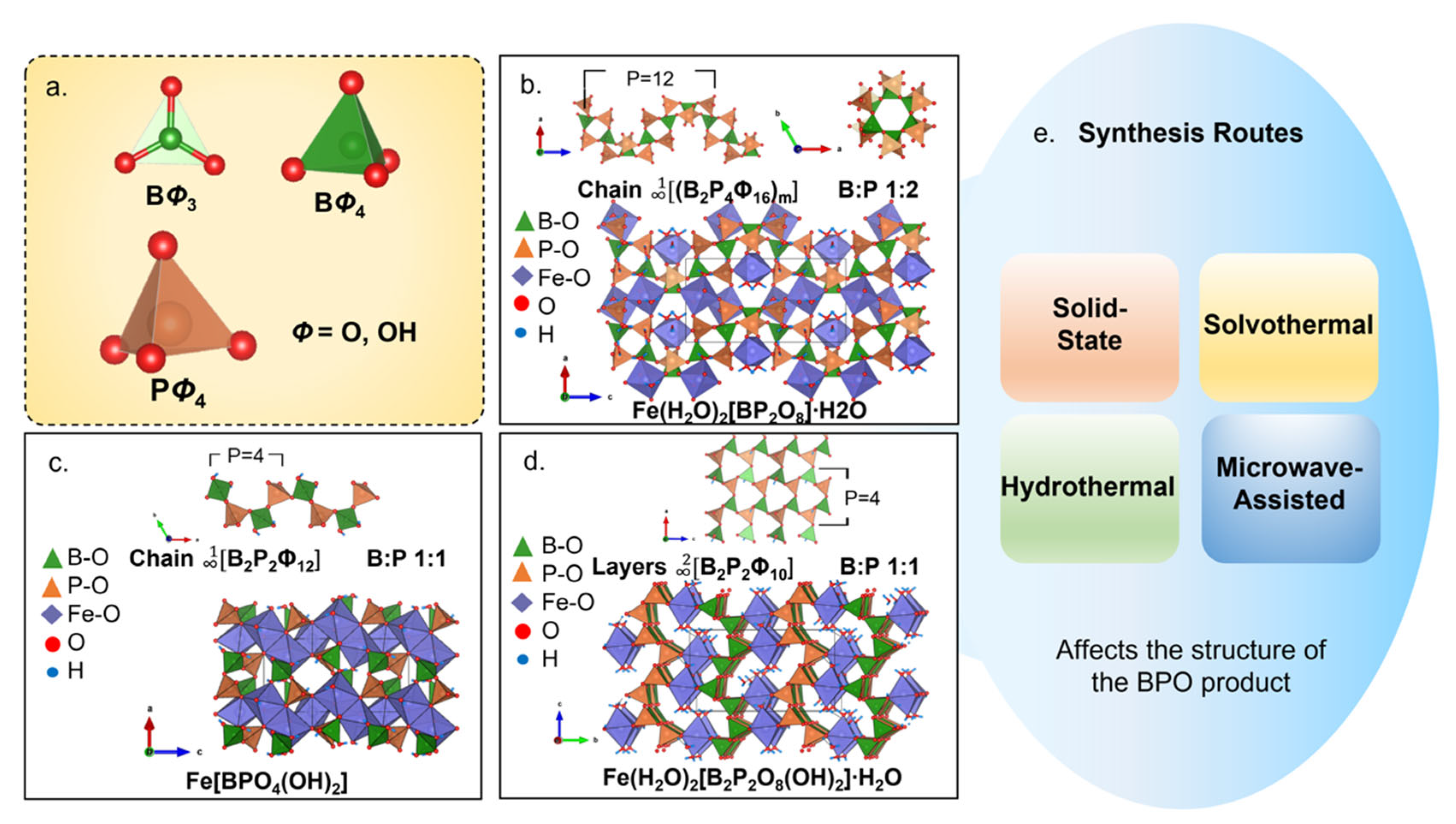
5.1. Structures
5.2. Properties
5.3. Synthesis Routes
6. Borosilicates (BSiO)
6.1. Structures
6.2. Properties
6.3. Synthesis Routes
7. Borosulfates (BSO)
7.1. Structures
7.2. Properties
7.3. Synthesis Routes
8. Outlook and Target Structures
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Economic Forum. A Vision for a Sustainable Battery Value Chain in 2030: Unlocking the Full Potential to Power Sustainable Development and Climate Change Mitigation; Insight Report; World Economic Forum: Cologny, Switzerland, 2019; Available online: https://www.weforum.org/reports/a-vision-for-a-sustainable-battery-value-chain-in-2030/ (accessed on 18 August 2022).
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Bhutada, G. Breaking Down the Cost of an EV Battery Cell. Available online: https://www.visualcapitalist.com/breaking-down-the-cost-of-an-ev-battery-cell/ (accessed on 18 August 2022).
- Islam, M.S.; Fisher, C.A.J. Lithium and Sodium Battery Cathode Materials: Computational Insights into Voltage, Diffusion and Nanostructural Properties. Chem. Soc. Rev. 2014, 43, 185–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manthiram, A. A Reflection on Lithium-Ion Battery Cathode Chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurzweil, P. Lithium Battery Energy Storage: State of the Art Including Lithium-Air and Lithium-Sulfur Systems. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Moseley, P.T., Garche, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 269–307. ISBN 978-0-444-62616-5. [Google Scholar]
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-Ion and Na-Ion Batteries as “Beyond Li-Ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x ≤ 1): A New Cathode Material for Batteries of High Energy Density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar] [CrossRef]
- Thackeray, M.M.; David, W.I.F.; Goodenough, J.B. Structural Characterization of the Lithiated Iron Oxides LixFe3O4 and LixFe2O3 (0 < x < 2). Mater. Res. Bull. 1982, 17, 785–793. [Google Scholar] [CrossRef]
- Thackeray, M.M.; David, W.I.F.; Bruce, P.G.; Goodenough, J.B. Lithium Insertion into Manganese Spinels. Mater. Res. Bull. 1983, 18, 461–472. [Google Scholar] [CrossRef]
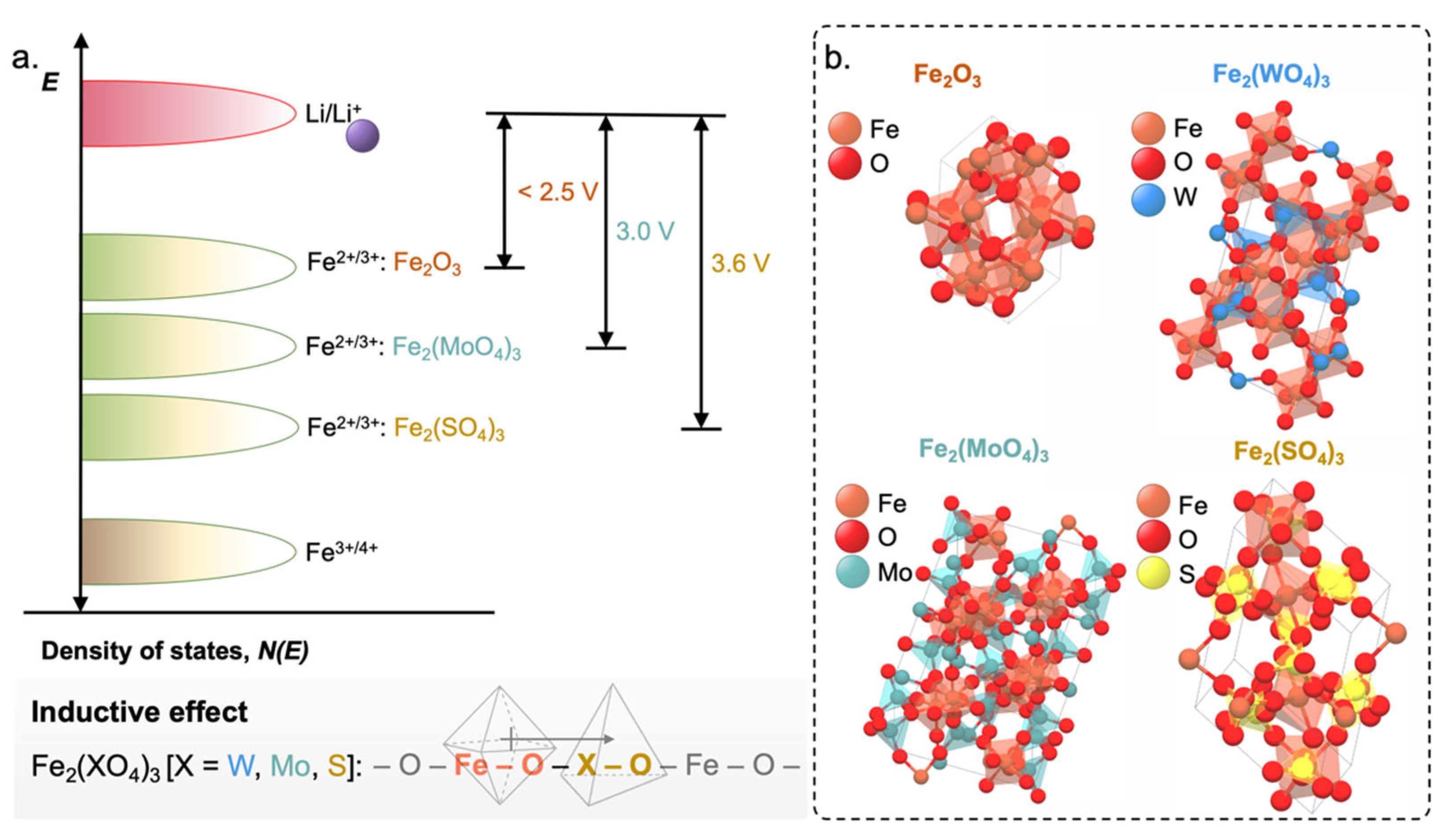
- Manthiram, A.; Goodenough, J.B. Lithium Insertion into Fe2(MO4)3 Frameworks: Comparison of M = W with M = Mo. J. Solid State Chem. 1987, 71, 349–360. [Google Scholar] [CrossRef]
- Manthiram, A.; Goodenough, J.B. Lithium Insertion into Fe2(SO4)3 Frameworks. J. Power Sources 1989, 26, 403–408. [Google Scholar] [CrossRef]
- Deng, J.; Bae, C.; Marcicki, J.; Masias, A.; Miller, T. Safety Modelling and Testing of Lithium-Ion Batteries in Electrified Vehicles. Nat. Energy 2018, 3, 261–266. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Q. Recent Progress in Multivalent Metal (Mg, Zn, Ca, and Al) and Metal-Ion Rechargeable Batteries with Organic Materials as Promising Electrodes. Small 2019, 15, 1805061. [Google Scholar] [CrossRef] [PubMed]
- Ponrouch, A.; Bitenc, J.; Dominko, R.; Lindahl, N.; Johansson, P.; Palacin, M.R. Multivalent Rechargeable Batteries. Energy Storage Mater. 2019, 20, 253–262. [Google Scholar] [CrossRef]
- Ping Ong, S. Accelerating Materials Science with High-Throughput Computations and Machine Learning. Comput. Mater. Sci. 2019, 161, 143–150. [Google Scholar] [CrossRef]
- Li, J.; Lim, K.; Yang, H.; Ren, Z.; Raghavan, S.; Chen, P.Y.; Buonassisi, T.; Wang, X. AI Applications through the Whole Life Cycle of Material Discovery. Matter 2020, 3, 393–432. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Lee, S.; Kang, H.; Sambandam, B.; Mathew, V.; Hwang, J.Y.; Kim, J. Recent Achievements in Experimental and Computational Studies of Positive Electrode Materials for Nonaqueous Ca- and Al-Ion Batteries. J. Phys. Chem. C 2022, 126, 9209–9227. [Google Scholar] [CrossRef]
- Hautier, G.; Jain, A.; Chen, H.; Moore, C.; Ong, S.P.; Ceder, G. Novel Mixed Polyanions Lithium-Ion Battery Cathode Materials Predicted by High-Throughput Ab Initio Computations. J. Mater. Chem. 2011, 21, 17147. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, S.; Luo, J. The Role of Cations in Second-Order Nonlinear Optical Materials Based on π-Conjugated [BO3]3− Groups. Coord. Chem. Rev. 2018, 366, 1–28. [Google Scholar] [CrossRef]
- Ling, J.; Karuppiah, C.; Krishnan, S.G.; Reddy, M.V.; Misnon, I.I.; Ab Rahim, M.H.; Yang, C.C.; Jose, R. Phosphate Polyanion Materials as High-Voltage Lithium-Ion Battery Cathode: A Review. Energy Fuels 2021, 35, 10428–10450. [Google Scholar] [CrossRef]
- Bao, L.; Gao, W.; Su, Y.; Wang, Z.; Li, N.; Chen, S.; Wu, F. Progression of the Silicate Cathode Materials Used in Lithium Ion Batteries. Chin. Sci. Bull. 2013, 58, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Barpanda, P. Sulfate Chemistry for High-Voltage Insertion Materials: Synthetic, Structural and Electrochemical Insights. Isr. J. Chem. 2015, 55, 537–557. [Google Scholar] [CrossRef]
- Yamada, A.; Iwane, N.; Harada, Y.; Nishimura, S.; Koyama, Y.; Tanaka, I. Lithium Iron Borates as High-Capacity Battery Electrodes. Adv. Mater. 2010, 22, 3583–3587. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Touzain, P. Graphite Intercalation Compounds as Cathode Materials. Mater. Sci. Eng. 1977, 31, 319–329. [Google Scholar] [CrossRef]
- Steele, B.C.H. Chemical Diffusion. In Fast ion transport in solids. Solid state batteries and devices. In Proceedings of the NATO sponsored Advanced Study Institute on Fast Ion Transport in Solids, Solid State Batteries and Devices, Belgirate, Italy, 5–15 September 1972; van Gool, W., Ed.; North Holland Publish Co.: Amsterdam, The Netherlands, 1973; pp. 103–122, ISBN 0444104321. [Google Scholar]
- Gamble, F.R.; Osiecki, J.H.; Cais, M.; Plsharody, R.; DiSalvo, F.J.; Geballe, T.H. Intercalation Complexes of Lewis Bases and Layered Sulfides: A Large Class of New Superconductors. Science 1971, 174, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Dines, M.B. Lithium Intercalation via -Butyllithium of the Layered Transition Metal Dichalcogenides. Mater. Res. Bull. 1975, 10, 287–291. [Google Scholar] [CrossRef]
- Dines, M.B. Intercalation of Metallocenes in the Layered Transition-Metal Dichalcogenides. Science 1975, 188, 1210–1211. [Google Scholar] [CrossRef]
- Rao, G.V.S.; Tsang, J.C. Electrolysis Method of Intercalation of Layered Transition Metal Dichalcogenides. Mater. Res. Bull. 1974, 9, 921–926. [Google Scholar] [CrossRef]
- Leblanc-Soreau, A.; Danot, M.; Trichet, L.; Rouxel, J. Les Intercalaires AxTiS2 et AxZrS2. Structure et Liaisons. (A = Li, Na, K, Rb, Cs). Mater. Res. Bull. 1974, 9, 191–197. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef]
- Brandt, K. Historical Development of Secondary Lithium Batteries. Solid State Ion. 1994, 69, 173–183. [Google Scholar] [CrossRef]
- Whittingham, M.S. Batteries à Base de Chalcogénures. Belgian Patent 819,672, 10 March 1975. [Google Scholar]
- Whittingham, M.S. Chalcogenide Batteries. U.S. Patent 4,009,052, 22 February 1977. [Google Scholar]
- Py, M.A.; Haering, R.R. Structural Destabilization Induced by Lithium Intercalation in MoS2 and Related Compounds. Can. J. Phys. 1983, 61, 76–84. [Google Scholar] [CrossRef]
- Koch, V.R. Status of the Secondary Lithium Electrode. J. Power Sources 1981, 6, 357–370. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Allred, A.L. Electronegativity Values from Thermochemical Data. J. Inorg. Nucl. Chem. 1961, 17, 215–221. [Google Scholar] [CrossRef]
- Goodenough, J.B. Perspective on Engineering Transition-Metal Oxides. Chem. Mater. 2014, 26, 820–829. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A Materials Genome Approach to Accelerating Materials Innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Kosuge, K.; Kachi, S. Electric and Magnetic Properties of LixV2−xO2. Mater. Res. Bull. 1969, 4, 95–106. [Google Scholar] [CrossRef]
- Rüdorff, W.; Becker, H. Notizen: Die Strukturen von LiVO2, NaVO2, LiCrO2 Und NaCrO2. Z. Nat. B J. Chem. Sci. 1954, 9, 614–615. [Google Scholar] [CrossRef] [Green Version]
- Johnston, W.D.; Heikes, R.R.; Sestrich, D. The Preparation, Crystallography, and Magnetic Properties of the LixCo(1−x)O System. J. Phys. Chem. Solids 1958, 7, 1–13. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Wickham, D.G.; Croft, W.J. Some Ferrimagnetic Properties of the System LixNi1−xO. J. Appl. Phys. 1958, 29, 382–383. [Google Scholar] [CrossRef]
- Chebiam, R.V.; Kannan, A.M.; Prado, F.; Manthiram, A. Comparison of the Chemical Stability of the High Energy Density Cathodes of Lithium-Ion Batteries. Electrochem. Commun. 2001, 3, 624–627. [Google Scholar] [CrossRef]
- Venkatraman, S.; Shin, Y.; Manthiram, A. Phase Relationships and Structural and Chemical Stabilities of Charged Li1−xCoO2−δ and Li1−xNi0.85Co0.15O2−δ Cathodes. Electrochem. Solid State Lett. 2003, 6, A9. [Google Scholar] [CrossRef]
- Dutta, G.; Manthiram, A.; Goodenough, J.B.; Grenier, J.-C. Chemical Synthesis and Properties of Li1−δ−xNi1+δO2 and Li[Ni2]O4. J. Solid State Chem. 1992, 96, 123–131. [Google Scholar] [CrossRef]
- Rougier, A.; Gravereau, P.; Delmas, C. Optimization of the Composition of the Li1−zNi1+zO2 Electrode Materials: Structural, Magnetic, and Electrochemical Studies. J. Electrochem. Soc. 1996, 143, 1168–1175. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Bruce, P.G. Synthesis of Layered LiMnO2 as an Electrode for Rechargeable Lithium Batteries. Nature 1996, 381, 499–500. [Google Scholar] [CrossRef]
- de Picciotto, L.A.; Thackeray, M.M.; David, W.I.F.; Bruce, P.G.; Goodenough, J.B. Structural Characterization of Delithiated LiVO2. Mater. Res. Bull. 1984, 19, 1497–1506. [Google Scholar] [CrossRef]
- He, L.-P.; Li, K.; Zhang, Y.; Liu, J. Structural and Electrochemical Properties of Low-Cobalt-Content LiNi0.6+xCo0.2-XMn0.2O2 (0.0 ≤ x ≤ 0.1) Cathodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 28253–28263. [Google Scholar] [CrossRef]
- Aryal, S.; Durham, J.L.; Lipson, A.L.; Pupek, K.Z.; Kahvecioglu, O. Roles of Mn and Co in Ni-Rich Layered Oxide Cathodes Synthesized Utilizing a Taylor Vortex Reactor. Electrochim. Acta 2021, 391, 138929. [Google Scholar] [CrossRef]
- Qiao, R.; Liu, J.; Kourtakis, K.; Roelofs, M.G.; Peterson, D.L.; Duff, J.P.; Deibler, D.T.; Wray, L.A.; Yang, W. Transition-Metal Redox Evolution in LiNi0.5Mn0.3Co0.2O2 Electrodes at High Potentials. J. Power Sources 2017, 360, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Liu, Z.; Shen, J.; Xu, X.; Zeng, L.; Zhang, B.; Zhu, H.; Liu, Q.; Liu, J.; Zhu, M. A Nanorod-like Ni-Rich Layered Cathode with Enhanced Li+ Diffusion Pathways for High-Performance Lithium-Ion Batteries. J. Mater. Chem. A 2021, 9, 2830–2839. [Google Scholar] [CrossRef]
- Liao, C.; Li, F.; Liu, J. Challenges and Modification Strategies of Ni-Rich Cathode Materials Operating at High-Voltage. Nanomaterials 2022, 12, 1888. [Google Scholar] [CrossRef]
- Julien, C.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries. In Lithium Batteries; Springer International Publishing: Cham, Switzerland, 2016; pp. 29–68. ISBN 978-3-319-19108-9. [Google Scholar]
- Thackeray, M.M.; Coetzer, J. A Preliminary Investigation of the Electrochemical Performance of α-Fe2O3 and Fe3O4 Cathodes in High-Temperature Cells. Mater. Res. Bull. 1981, 16, 591–597. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Johnson, P.J.; de Picciotto, L.A.; Bruce, P.G.; Goodenough, J.B. Electrochemical Extraction of Lithium from LiMn2O4. Mater. Res. Bull. 1984, 19, 179–187. [Google Scholar] [CrossRef]
- Hunter, J.C. Preparation of a New Crystal Form of Manganese Dioxide: λ-MnO2. J. Solid State Chem. 1981, 39, 142–147. [Google Scholar] [CrossRef]
- Zhan, C.; Lu, J.; Jeremy Kropf, A.; Wu, T.; Jansen, A.N.; Sun, Y.-K.; Qiu, X.; Amine, K. Mn(II) Deposition on Anodes and Its Effects on Capacity Fade in Spinel Lithium Manganate–Carbon Systems. Nat. Commun. 2013, 4, 2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Picciotto, L.; Thackeray, M. Insertion/Extraction Reactions of Lithium with LiV2O4. Mater. Res. Bull. 1985, 20, 1409–1420. [Google Scholar] [CrossRef]
- Zhong, Q.; Bonakdarpour, A.; Zhang, M.; Gao, Y.; Dahn, J.R. Synthesis and Electrochemistry of LiNixMn2-xO4. J. Electrochem. Soc. 1997, 144, 205–213. [Google Scholar] [CrossRef]
- Manthiram, A.; Chemelewski, K.; Lee, E.-S. A Perspective on the High-Voltage LiMn1.5Ni0.5O4 Spinel Cathode for Lithium-Ion Batteries. Energy Environ. Sci. 2014, 7, 1339. [Google Scholar] [CrossRef]
- Drozhzhin, O.A.; Shevchenko, V.A.; Bobyleva, Z.V.; Alekseeva, A.M.; Antipov, E.V. Rational Screening of High-Voltage Electrolytes and Additives for Use in LiNi0.5Mn1.5O4-Based Li-Ion Batteries. Molecules 2022, 27, 3596. [Google Scholar] [CrossRef] [PubMed]
- Dahn, J.R.; Obrovac, M. Thermal Stability of LixCoO2, LixNiO2 and 2-MnO2 and Consequences for the Safety of Li-Ion Cells. Solid State Ion. 1994, 69, 6. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Guyomard, D. The Li1+xMn2O4/C Rocking-Chair System: A Review. Electrochim. Acta 1993, 38, 1221–1231. [Google Scholar] [CrossRef]
- Thackeray, M.M.; de Kock, A.; Rossouw, M.H.; Liles, D.; Bittihn, R.; Hoge, D. Spinel Electrodes from the Li-Mn-O System for Rechargeable Lithium Battery Applications. J. Electrochem. Soc. 1992, 139, 363–366. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Molenda, J.; Mole, M. Composite Cathode Material for Li-Ion Batteries Based on LiFePO4 System. In Metal, Ceramic and Polymeric Composites for Various Uses; Cuppoletti, J., Ed.; InTech: Osaka, Japan, 2011; ISBN 978-953-307-353-8. [Google Scholar]
- Ahuja, G. An Investigation of Some Lithium Insertion Compounds. Ph.D. Dissertation, University of Texas at Austin, Austin, TX, USA, 1991. [Google Scholar]
- Masquelier, C.; Croguennec, L. Polyanionic (Phosphates, Silicates, Sulfates) Frameworks as Electrode Materials for Rechargeable Li (or Na) Batteries. Chem. Rev. 2013, 113, 6552–6591. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bates, J.B.; Hart, F.X.; Sales, B.C.; Zuhr, R.A.; Robertson, J.D. Characterization of Thin-Film Rechargeable Lithium Batteries with Lithium Cobalt Oxide Cathodes. J. Electrochem. Soc. 1996, 143, 3203–3213. [Google Scholar] [CrossRef]
- Xu, J.; Hu, E.; Nordlund, D.; Mehta, A.; Ehrlich, S.N.; Yang, X.-Q.; Tong, W. Understanding the Degradation Mechanism of Lithium Nickel Oxide Cathodes for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 31677–31683. [Google Scholar] [CrossRef]
- Välikangas, J.; Laine, P.; Hietaniemi, M.; Hu, T.; Tynjälä, P.; Lassi, U. Precipitation and Calcination of High-Capacity LiNiO2 Cathode Material for Lithium-Ion Batteries. Appl. Sci. 2020, 10, 8988. [Google Scholar] [CrossRef]
- Gu, M.; Belharouak, I.; Zheng, J.; Wu, H.; Xiao, J.; Genc, A.; Amine, K.; Thevuthasan, S.; Baer, D.R.; Zhang, J.-G.; et al. Formation of the Spinel Phase in the Layered Composite Cathode Used in Li-Ion Batteries. ACS Nano 2013, 7, 760–767. [Google Scholar] [CrossRef]
- Bruce, P.G.; Armstrong, A.R.; Gitzendanner, R.L. New Intercalation Compounds for Lithium Batteries: Layered LiMnO2. J. Mater. Chem. 1999, 9, 193–198. [Google Scholar] [CrossRef]
- Che, G.; Jirage, K.B.; Fisher, E.R.; Martin, C.R.; Yoneyama, H. Chemical-Vapor Deposition-Based Template Synthesis of Microtubular TiS2 Battery Electrodes. J. Electrochem. Soc. 1997, 144, 4296–4302. [Google Scholar] [CrossRef]
- Gummow, R.J.; de Kock, A.; Thackeray, M.M. Improved Capacity Retention in Rechargeable 4 V Lithium/Lithium-Manganese Oxide (Spinel) Cells. Solid State Ion. 1994, 69, 59–67. [Google Scholar] [CrossRef]
- Shui, J.L.; Jiang, G.S.; Xie, S.; Chen, C.H. Thin Films of Lithium Manganese Oxide Spinel as Cathode Materials for Secondary Lithium Batteries. Electrochim. Acta 2004, 49, 2209–2213. [Google Scholar] [CrossRef]
- Colbow, K.M.; Dahn, J.R.; Haering, R.R. Structure and Electrochemistry of the Spinel Oxides LiTi2O4 and Li43Ti53O4. J. Power Sources 1989, 26, 397–402. [Google Scholar] [CrossRef]
- Feng, C.Q.; Li, L.; Guo, Z.P.; Shi, D.Q.; Zeng, R.; Zhu, X.J. Synthesis and Properties of Li–Ti–O Spinel (LiTi2O4). J. Alloys Compd. 2009, 478, 767–770. [Google Scholar] [CrossRef]
- Pistoia, G.; Pasquali, M.; de Picciotto, L.A.; Thackeray, M.M. Behaviour of the Spinel LiV2O4 as a Positive Electrode for Secondary Li Cells. Solid State Ion. 1988, 28–30, 879–885. [Google Scholar] [CrossRef]
- Choi, S.; Manthiram, A. Synthesis and Electrochemical Properties of LiCo2O4 Spinel Cathodes. J. Electrochem. Soc. 2002, 149, A162. [Google Scholar] [CrossRef]
- Hassoun, J.; Bonaccorso, F.; Agostini, M.; Angelucci, M.; Betti, M.G.; Cingolani, R.; Gemmi, M.; Mariani, C.; Panero, S.; Pellegrini, V.; et al. An Advanced Lithium-Ion Battery Based on a Graphene Anode and a Lithium Iron Phosphate Cathode. Nano Lett. 2014, 14, 4901–4906. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Garcia-Araez, N.; Hector, A.L. Understanding and Development of Olivine LiCoPO4 Cathode Materials for Lithium-Ion Batteries. J. Mater. Chem. A 2018, 6, 14483–14517. [Google Scholar] [CrossRef]
- Li, G.; Azuma, H.; Tohda, M. LiMnPO4 as the Cathode for Lithium Batteries. Electrochem. Solid State Lett. 2002, 5, A135. [Google Scholar] [CrossRef]
- Kim, M.; Jung, Y.; Kang, B. High Electrochemical Performance of 3.9 V LiFeSO4F Directly Synthesized by a Scalable Solid- State Reaction within 1 h. J. Mater. Chem. A 2015, 3, 7583–7590. [Google Scholar] [CrossRef] [Green Version]
- Nytén, A.; Abouimrane, A.; Armand, M.; Gustafsson, T.; Thomas, J.O. Electrochemical Performance of Li2FeSiO4 as a New Li-Battery Cathode Material. Electrochem. Commun. 2005, 7, 156–160. [Google Scholar] [CrossRef]
- Hou, X.; Liang, J.; Zhang, T.; Li, Y.; Tang, S.; Sun, H.; Zhang, J.; Xie, H. Insights into Electrochemistry and Mechanical Stability of α- And β-Li2MnP2O7 for Lithium-Ion Cathode Materials: First-Principles Comparison. J. Phys. Chem. C 2017, 121, 22656–22664. [Google Scholar] [CrossRef]
- Okada, S.; Ueno, M.; Uebou, Y.; Yamaki, J. Fluoride Phosphate Li2CoPO4F as a High-Voltage Cathode in Li-Ion Batteries. J. Power Sources 2005, 146, 565–569. [Google Scholar] [CrossRef]
- Chen, H.; Hautier, G.; Jain, A.; Moore, C.; Kang, B.; Doe, R.; Wu, L.; Zhu, Y.; Tang, Y.; Ceder, G. Carbonophosphates: A New Family of Cathode Materials for Li-Ion Batteries Identified Computationally. Chem. Mater. 2012, 24, 2009–2016. [Google Scholar] [CrossRef]
- Hautier, G.; Fischer, C.; Ehrlacher, V.; Jain, A.; Ceder, G. Data Mined Ionic Substitutions for the Discovery of New Compounds. Inorg. Chem. 2011, 50, 656–663. [Google Scholar] [CrossRef]
- Hautier, G.; Fischer, C.C.; Jain, A.; Mueller, T.; Ceder, G. Finding Natures Missing Ternary Oxide Compounds Using Machine Learning and Density Functional Theory. Chem. Mater. 2010, 22, 3762–3767. [Google Scholar] [CrossRef]
- Ceder, G.; Morgan, D.; Fischer, C.; Tibbetts, K.; Curtarolo, S. Data-Mining-Driven Quantum Mechanics for the Prediction of Structure. MRS Bull. 2006, 31, 981–985. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.C.; Tibbetts, K.J.; Morgan, D.; Ceder, G. Predicting Crystal Structure by Merging Data Mining with Quantum Mechanics. Nat. Mater. 2006, 5, 641–646. [Google Scholar] [CrossRef]
- Setyawan, W.; Curtarolo, S. High-Throughput Electronic Band Structure Calculations: Challenges and Tools. Comput. Mater. Sci. 2010, 49, 299–312. [Google Scholar] [CrossRef] [Green Version]
- Altair Grid Engine. Available online: https://www.altair.com/grid-engine/ (accessed on 12 August 2022).
- The Perl Programming Language. Available online: https://www.perl.org/ (accessed on 12 August 2022).
- Stonebraker, M.; Rowe, L.A. The Design of POSTGRES. ACM Sigmod Rec. 1986, 15, 340–355. [Google Scholar] [CrossRef]
- Stonebraker, M.; Kemnitz, G. The POSTGRES next Generation Database Management System. Commun. ACM 1991, 34, 78–92. [Google Scholar] [CrossRef]
- Jain, A.; Hautier, G.; Moore, C.J.; Ping Ong, S.; Fischer, C.C.; Mueller, T.; Persson, K.A.; Ceder, G. A High-Throughput Infrastructure for Density Functional Theory Calculations. Comput. Mater. Sci. 2011, 50, 2295–2310. [Google Scholar] [CrossRef]
- Khomyakov, A.P. Sidorenkite, Na3Mn(PO4)(CO3), a New Mineral. Int. Geol. Rev. 1980, 22, 811–814. [Google Scholar] [CrossRef]
- Chen, H.; Hao, Q.; Zivkovic, O.; Hautier, G.; Du, L.-S.; Tang, Y.; Hu, Y.-Y.; Ma, X.; Grey, C.P.; Ceder, G. Sidorenkite (Na3MnPO4CO3): A New Intercalation Cathode Material for Na-Ion Batteries. Chem. Mater. 2013, 25, 2777–2786. [Google Scholar] [CrossRef]
- Wang, C.; Sawicki, M.; Emani, S.; Liu, C.; Shaw, L.L. Na3MnCO3PO4—A High Capacity, Multi-Electron Transfer Redox Cathode Material for Sodium Ion Batteries. Electrochim. Acta 2015, 161, 322–328. [Google Scholar] [CrossRef]
- Delmas, C.; Braconnier, J.J.; Hagenmuller, P. A New Variety of LiCoO2 with an Unusual Oxygen Packing Obtained by Exchange Reaction. Mater. Res. Bull. 1982, 17, 117–123. [Google Scholar] [CrossRef]
- Gaubicher, J.; Wurm, C.; Goward, G.; Masquelier, C.; Nazar, L. Rhombohedral Form of Li3V2(PO4)3 as a Cathode in Li-Ion Batteries. Chem. Mater. 2000, 12, 3240–3242. [Google Scholar] [CrossRef]
- Gurbanova, O.A.; Belokoneva, E.L. Comparative Crystal Chemical Analysis of Borophosphates and Borosilicates. Crystallogr. Rep. 2007, 52, 624–633. [Google Scholar] [CrossRef]
- Yaghoobnejad Asl, H.; Stanley, P.; Ghosh, K.; Choudhury, A. Iron Borophosphate as a Potential Cathode for Lithium- and Sodium-Ion Batteries. Chem. Mater. 2015, 27, 7058–7069. [Google Scholar] [CrossRef]
- Tao, L.; Rousse, G.; Sougrati, M.T.; Chotard, J.-N.; Masquelier, C. (NH4)0.75Fe(H2O)2[BP2O8 ]·0.25H2O, a Fe3+/Fe2+ Mixed Valence Cathode Material for Na Battery Exhibiting a Helical Structure. J. Phys. Chem. C 2015, 119, 4540–4549. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Schäfer, G.; Carrillo-Cabrera, W.; Cardoso, R.; Schnelle, W.; Zhao, J.-T.; Kniep, R. Open-Framework Borophosphates: (NH4)0.4FeII0.55FeIII0.5(H2O)2[BP2O8]·0.6H2O and NH4FeIII[BP2O8(OH)]. Chem. Mater. 2001, 13, 4348–4354. [Google Scholar] [CrossRef]
- Boy, I.; Schäfer, G.; Kniep, R. Crystal Structure of Sodium Iron(II) Diaqua Catena-[Monoboro-Diphosphate] Monohydrate, NaFe(H2O)2[BP2O8]·H2O, and of Potassium Iron(II) Diaqua Catena-[Monoboro-Diphosphate] Hemihydrate, KFe(H2O)2[BP2O8]·0.5H2O. Z. Kristallogr. New Cryst. Struct. 2001, 216, 13–14. [Google Scholar] [CrossRef]
- Islam, M.S.; Dominko, R.; Masquelier, C.; Sirisopanaporn, C.; Armstrong, A.R.; Bruce, P.G. Silicate Cathodes for Lithium Batteries: Alternatives to Phosphates? J. Mater. Chem. 2011, 21, 9811–9818. [Google Scholar] [CrossRef] [Green Version]
- Aravindan, V.; Karthikeyan, K.; Kang, K.S.; Yoon, W.S.; Kim, W.S.; Lee, Y.S. Influence of Carbon towards Improved Lithium Storage Properties of Li2MnSiO4 Cathodes. J. Mater. Chem. 2011, 21, 2470–2475. [Google Scholar] [CrossRef]
- Dominko, R. Li2MSiO4 (M = Fe and/or Mn) Cathode Materials. J. Power Sources 2008, 184, 462–468. [Google Scholar] [CrossRef]
- Kokalj, A.; Dominko, R.; Mali, G.; Meden, A.; Gaberscek, M.; Jamnik, J. Beyond One-Electron Reaction in Li Cathode Materials: Designing Li2MnxFe1−XSiO4. Chem. Mater. 2007, 19, 3633–3640. [Google Scholar] [CrossRef]
- Li, L.; Han, E.; Jia, T.; Yang, X.; Yuan, W.; Zhang, Y. The Doping Modification of PO43− or BO33− on the Electrochemical Performance of Li2Fe0.98Mg0.02SiO4/C Cathode Materials. Ionics 2020, 26, 5961–5970. [Google Scholar] [CrossRef]
- Li, L.; Jia, T.; Liu, C.; Han, E.; Yang, Y.; Qin, Y.; Zhang, M. The Modification of Li2FeSiO4 Materials by Dual Doping with Ag and PO43− or BO33−. Ionics 2021, 27, 1887–1898. [Google Scholar] [CrossRef]
- Netzsch, P. On Silicate-Analogous Materials-Synthesis and Characterisation of Novel Borosulfates. Ph.D. Dissertation, Augsburg University, Minneapolis, MN, USA, 2021. [Google Scholar]
- Pasqualini, L.C.; Huppertz, H.; Bruns, J. M[B2(SO4)4] (M = Mn, Zn)—Syntheses and Crystal Structures of Two New Phyllosilicate Analogue Borosulfates. Inorganics 2019, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Bruns, J.; Höppe, H.A.; Daub, M.; Hillebrecht, H.; Huppertz, H. Borosulfates—Synthesis and Structural Chemistry of Silicate Analogue Compounds. Chem. Eur. J. 2020, 26, 7966–7980. [Google Scholar] [CrossRef] [Green Version]
- Pauling, L. The Principles Determining the Structure of Complex Ionic Crystals. J. Am. Chem. Soc. 1929, 51, 1010–1026. [Google Scholar] [CrossRef]
- Kniep, R.; Gözel, G.; Eisenmann, B.; Röhr, C.; Asbrand, M.; Kizilyalli, M. Borophosphates—A Neglected Class of Compounds: Crystal Structures of MII[BPO5](MII = Ca, Sr) and Ba3[BP3O12]. Angew. Chem. Int. Ed. 1994, 33, 749–751. [Google Scholar] [CrossRef]
- Bauer, H. Uber eine Reihe isotyper Erdalkaliboratphosphate und -arsenate vom Typus 2 MeO·X2O5·B2O3. Z. Anorg. Allg. Chem. 1965, 337, 183–190. [Google Scholar] [CrossRef]
- Ramamoorthy, P.; Rockett, T.J. Phase Relations in the System CaO-B2O3-P2O5 at 900 °C. J. Am. Ceram. Soc. 1974, 57, 501. [Google Scholar] [CrossRef]
- Ozdil, Y. Synthesis and Characterization of Rare-Earth Borophosphates. Master’s Thesis, The Middle East Technical University, Çankaya, Turkey, 2004. [Google Scholar]
- Li, M.; Verena-Mudring, A. New Developments in the Synthesis, Structure, and Applications of Borophosphates and Metalloborophosphates. Cryst. Growth Des. 2016, 16, 2441–2458. [Google Scholar] [CrossRef] [Green Version]
- Liebau, F. Structural Chemistry of Silicates: Structure, Bonding, and Classification; Springer: Berlin, Germany, 2012; ISBN 9783540137474. [Google Scholar]
- Kniep, R.; Engelhardt, H.; Hauf, C. A First Approach to Borophosphate Structural Chemistry. Chem. Mater. 1998, 10, 2930–2934. [Google Scholar] [CrossRef]
- Ewald, B.; Huang, Y.-X.; Kniep, R. Structural Chemistry of Borophosphates, Metalloborophosphates, and Related Compounds. Z. Anorg. Allg. Chem. 2007, 633, 1517–1540. [Google Scholar] [CrossRef]
- Yilmaz, A.; Bu, X.; Kizilyalli, M.; Stucky, G.D. Fe(H2O)2BP2O8·H2O, a First Zeotype Ferriborophosphate with Chiral Tetrahedral Framework Topology. Chem. Mater. 2000, 12, 3243–3245. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Ewald, B.; Schnelle, W.; Prots’, Y.; Kniep, R. CCDC 603865: Experimental Crystal Structure Determination 2019. Inorg. Chem. 2006, 45, 7578. [Google Scholar] [CrossRef]
- Menezes, P.W.; Hoffmann, S.; Prots, Y.; Schnelle, W.; Kniep, R. Preparation and Characterization of the Layered Borophosphates MII(H2O)2[B2P2O8(OH)2]·H2O (MII = Fe, Co, Ni). Inorg. Chim. Acta 2010, 363, 4299–4306. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered Porous Materials for Emerging Applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef]
- Sevov, S.C. Synthesis and Structure of CoB2P3O12(OH)·C2H10N2: The First Metal Borophosphate with an Open Framework Structure. Angew. Chem. Int. Ed. 1996, 35, 2630–2632. [Google Scholar] [CrossRef]
- Huang, Y.X.; Ewald, B.; Schnelle, W.; Prots, Y.; Kniep, R. Chirality and Magnetism in a Novel Series of Isotypic Borophosphates: MII[BPO4(OH)2] (MII = Mn, Fe, Co). Inorg. Chem. 2006, 45, 7578–7580. [Google Scholar] [CrossRef] [PubMed]
- Kniep, R.; Will, H.G.; Boy, I.; Röhr, C. 61 Helices from Tetrahedral Ribbons [BP2O]: Isostructural Borophosphates MIMII(H2O)2[BP2O8]·H2O (MI = Na, K; MII = Mg, Mn, Fe, Co, Ni, Zn) and Their Dehydration to Microporous Phases MIMII(H2O)[BP2O8]. Angew. Chem. Int. Ed. 1997, 36, 1013–1014. [Google Scholar] [CrossRef]
- Zhang, W.L.; Lin, C.S.; Geng, L.; Li, Y.Y.; Zhang, H.; He, Z.Z.; Cheng, W.D. Synthesis and Characterizations of Two Anhydrous Metal Borophosphates: MIII2BP3O12 (M = Fe, In). J. Solid State Chem. 2010, 183, 1108–1113. [Google Scholar] [CrossRef]
- West, A. Synthesis, Processing and Fabrication Methods. In Solid State Chemistry and Its Applications; Wiley: Oxford, UK, 2014; pp. 187–270. ISBN 978-1-119-94294-8. [Google Scholar]
- Stein, A.; Keller, S.W.; Mallouk, T.E. Turning down the Heat: Design and Mechanism in Solid-State Synthesis. Science 1993, 259, 1558–1564. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, S.; Han, S.; Zhang, B.; Dong, L.; Zhang, M.; Yang, Z. K7B2P5O19: A Novel Alkali Metal Borophosphate with Zero Dimensional [B2P5O19]7− Anionic Units. Cryst. Eng. Comm. 2014, 16, 6848–6851. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yamane, H. Synthesis, Crystal Structure and Lithium Ion Conduction of Li3BP2O8. Dalton Trans. 2014, 43, 2294–2300. [Google Scholar] [CrossRef]
- Lei, B.-H.; Jing, Q.; Yang, Z.; Zhang, B.; Pan, S. Anomalous Second-Harmonic Generation Response in SrBPO5 and BaBPO5. J. Mater. Chem. C 2015, 3, 1557–1566. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, J. (NH4)[B3PO6(OH)3]·0.5H2O. Acta Crystallogr. Sect. E Crystallogr. Commun. 2007, 63, i185. [Google Scholar] [CrossRef]
- Ewald, B.; Prots, Y.; Menezes, P.; Natarajan, S.; Zhang, H.; Kniep, R. Chain Structures in Alkali Metal Borophosphates: Synthesis and Characterization of K3[BP3O9(OH)3] and Rb3[B2P3O11(OH)2]. Inorg. Chem. 2005, 44, 6431–6438. [Google Scholar] [CrossRef]
- Yang, M.; Yu, J.; Chen, P.; Li, J.; Fang, Q.; Xu, R. Synthesis and Characterization of Metalloborophosphates with Zeotype ANA Framework by the Boric Acid ‘Flux’ Method. Microporous Mesoporous Mater. 2005, 87, 124–132. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Na, T.; Xu, J.; Wang, L.; Yu, J.; Xu, R. (NH4)6[Mn3B6P9O36(OH)3]·4H2O: A New Open-Framework Manganese Borophosphate Synthesized by Using Boric Acid Flux Method. Dalton Trans. 2011, 40, 2549. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, X.; Yu, J.; Zhu, J.; Liu, X.; Chen, G.; Yan, Y. LiCu2[BP2O8(OH)2]: A Chiral Open-Framework Copper Borophosphate via Spontaneous Asymmetrical Crystallization. Dalton Trans. 2013, 42, 6298. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mudring, A.-V. A New Open-Framework Iron Borophosphate from Ionic Liquids: KFe[BP2O8(OH)]. Crystals 2011, 1, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Shan, Y.; He, M.; Liu, Y. Impetus for Solvothermal Synthesis Technique: Synthesis and Structure of a Novel 1-D Borophosphate Using Ionic Liquid as Medium. J. Solid State Chem. 2003, 176, 33–36. [Google Scholar] [CrossRef]
- Feng, S.H.; Li, G.H. Hydrothermal and Solvothermal Syntheses. In Modern Inorganic Synthetic Chemistry: Second Edition; Elsevier: Amsterdam, The Netherlands, 2017; pp. 73–104. ISBN 9780444635914. [Google Scholar]
- Liu, W.; Ge, M.; Yang, X.; Chen, H.; Li, M.; Zhao, J. Hydrothermal Synthesis and Characterization of Two Organically Templated Cadmium Borophosphates with Novel Structures. Inorg. Chem. 2004, 43, 3910–3914. [Google Scholar] [CrossRef]
- Yang, T.; Sun, J.; Li, G.; Eriksson, L.; Zou, X.; Liao, F.; Lin, J. Na8[Cr4B12P8O44(OH)4][P2O7]⋅nH2O: A 3D Borophosphate Framework with Spherical Cages. Chem. Eur. J. 2008, 14, 7212–7217. [Google Scholar] [CrossRef]
- Shi, H.; Feng, Y.; Huang, Q.; Qiu, D.; Li, M.; Liu, K. Ti[BP2O7(OH)3]: The First Titanium Borophosphate Containing Novel Anionic Partial Structure. CrystEngComm 2011, 13, 7185. [Google Scholar] [CrossRef]
- Baykal, A.; Kizilyalli, M.; Toprak, M.; Kniep, R. Hydrothermal and Microwave Synthesis of Boron Phosphate, BPO4. Turk. J. Chem. 2001, 25, 425–432. Available online: https://dergipark.org.tr/tr/download/article-file/124377 (accessed on 12 August 2022).
- Hauf, C.; Yilmaz, A.; Kizilyalli, M.; Kniep, R. Borophosphates: Hydrothermal and Microwave-Assisted Synthesis of Na5[B2P3O13]. J. Solid State Chem. 1998, 140, 154–156. [Google Scholar] [CrossRef]
- Song, Y.; Ding, L.; An, Q.; Zhai, S.; Song, X. Synthesis and Characterization of Zinc Borophosphates with ANA-Zeotype Framework by the Microwave Method. J. Solid State Chem. 2013, 202, 300–304. [Google Scholar] [CrossRef]
- Millini, R.; Perego, G.; Bellussi, G. Synthesis and Characterization of Boron-containing Molecular Sieves. Top. Catal. 1999, 9, 13–34. [Google Scholar] [CrossRef]
- Whittam, T.V.; Youll, B. Zeolite Nu-1. U.S. Patent 4060590, 29 November 1977. [Google Scholar]
- Taramasso, M.; Manara, G.; Fattore, V.; Notari, B. Crystalline Silica. G.B. Patent 2024790A, 16 January 1980. [Google Scholar]
- Bellussi, G.; Millini, R.; Carati, A.; Maddinelli, G.; Gervasini, A. Synthesis and Comparative Characterization of Al, B, Ga, and Fe Containing Nu-1-Type Zeolitic Framework. Zeolites 1990, 10, 642–649. [Google Scholar] [CrossRef]
- Ehrt, D. Structure, Properties and Applications of Borate Glasses. Glass Technol. 2000, 41, 182–185. Available online: https://www.ingentaconnect.com/content/sgt/gt/2000/00000041/00000006/4106182 (accessed on 12 August 2022).
- Möncke, D.; Tricot, G.; Winterstein-Beckmann, A.; Wondraczek, L.; Kamitsos, E. On the Connectivity of Borate Tetrahedra in Borate and Borosilicate Glasses. Phys. Chem. Glasses Eur. J. Glass Sci. Technol. Part B 2015, 56, 203–211. [Google Scholar] [CrossRef]
- Szumera, M.; Łagowska, B.; Sułowska, J.; Jeleń, P.; Olejniczak, Z.; Lach, R.; Berezicka, A.; Kijo-Kleczkowska, A. Investigating the Crystallization Process of Boron-Bearing Silicate-Phosphate Glasses by Thermal and Spectroscopic Methods. Molecules 2022, 27, 867. [Google Scholar] [CrossRef] [PubMed]
- Appleman, D.E.; Clark, J.R. Crystal Structure of Reedmergnerite, a Boron Albite, and Its Relation to Feldspar Crystal Chemistry1. Am. Mineral. 1965, 50, 1827–1850. Available online: https://pubs.geoscienceworld.org/msa/ammin/article-abstract/50/11-12/1827/540217/Crystal-structure-of-reedmergnerite-a-boron-albite (accessed on 12 August 2022).
- Phillips, M.W.; Gibbs, G.V.; Ribbe, P.H. The Crystal Structure of Danburite: A Comparison with Anorthite, Albite, and Reedmergnerite. Am. Mineral. 1974, 59, 79–85. Available online: https://pubs.geoscienceworld.org/msa/ammin/article-abstract/59/1-2/79/541016/The-Crystal-Structure-of-Danburite-A-Comparison (accessed on 12 August 2022).
- Ito, T.; Mori, H. The Crystal Structure of Datolite. Acta Crystallogr. 1953, 6, 24–32. [Google Scholar] [CrossRef]
- Testa, F.; Chiappetta, R.; Crea, F.; Aiello, R.; Fonseca, A.; Nagy, J.B. Synthesis of Borosilicalite-1 with High Boron Content from Fluoridecontaining Media. Stud. Surf. Sci. Catal. 1995, 94, 349–356. [Google Scholar] [CrossRef]
- Cichocki, A.; Parasiewicz-Kaczmarska, J.; Michalik, M.; Buś, M. Synthesis and Characterization of Boralites with the MFI Structure. Zeolites 1990, 10, 577–582. [Google Scholar] [CrossRef]
- Shvanskii, E.V.; Leonyuk, N.I.; Bocelli, G.; Righi, L. Crystallization and Structural Characteristics of New Borosilicates. J. Solid State Chem. 2000, 154, 312–316. [Google Scholar] [CrossRef]
- Shia, Y.; Liang, J.K.; Zhang, H.; Yang, J.L.; Zhuang, W.D.; Rao, G.H. Crystal Structure and Vibrational Spectra Studies of Stillwellite Compounds NdBSiO5. J. Alloys Compd. 1997, 259, 163–169. [Google Scholar] [CrossRef]
- Foit, F.F.; Phillips, M.W.; Gibbs, G.V. A Refinement of the Crystal Structure of Datolite, CaBSiO4(OH). Am. Mineral. 1973, 58, 909–914. Available online: https://pubs.geoscienceworld.org/msa/ammin/article-abstract/58/9-10/909/542888/A-Refinement-of-the-Crystal-Structure-of-Datolite (accessed on 12 August 2022).
- Johansson, G. A Refinement of the Crystal Structure of Danburite. Acta Crystallogr. 1959, 12, 522–525. [Google Scholar] [CrossRef]
- Mason, R.A. The Ordering Behaviour of Reedmergnerite, NaBSi3O8. Contrib. Mineral. Petrol. 1980, 72, 329–333. [Google Scholar] [CrossRef]
- Millini, R.; Montanari, L.; Bellussi, G. Synthesis and Characterization of a Potassium Borosilicate with ANA Framework Type Structure. Microporous Mater. 1993, 1, 9–15. [Google Scholar] [CrossRef]
- Millini, R.; Carati, A.; Bellussi, G. Synthesis of a New Porous Borosilicate with the Levyne Structure. Zeolites 1992, 12, 265–268. [Google Scholar] [CrossRef]
- Hölderich, W. New Horizons in Catalysis Using Modified and Unmodified Pentasil Zeolites. In Studies in Surface Science and Catalysis; Murakami, Y., Iijima, A., Ward, J.W., Eds.; New Developments in Zeolite Science and Technology; Elsevier: Amsterdam, The Netherlands, 1986; Volume 28, pp. 827–834. [Google Scholar]
- Sha, H.; Su, R.; Xiong, Z.; Wang, Z.; Shan, P.; He, C.; Yang, X.; Long, X. Borosilicate Crystal LaBSiO5: A New Promising Ultraviolet Quasiphase Matching Material. Adv. Opt. Mater. 2021, 9, 2100080. [Google Scholar] [CrossRef]
- Krzhizhanovskaya, M.G.; Bubnova, R.S.; Krivovichev, S.V.; Belousova, O.L.; Filatov, S.K. Synthesis, Crystal Structure and Thermal Behavior of Sr3B2SiO8 Borosilicate. J. Solid State Chem. 2010, 183, 2352–2357. [Google Scholar] [CrossRef]
- Rinaldi, R.; Gatta, G.D.; Angel, R.J. Crystal Chemistry and Low-Temperature Behavior of Datolite: A Single-Crystal X-ray Diffraction Study. Am. Mineral. 2010, 95, 1413–1421. [Google Scholar] [CrossRef]
- Miao, Z.; Yang, Y.; Wei, Z.; Yang, Z.; Yu, S.; Pan, S. NaCa5BO3(SiO4)2 with Interesting Isolated [BO3] and [SiO4] Units in Alkali- and Alkaline-Earth-Metal Borosilicates. Inorg. Chem. 2019, 58, 3937–3943. [Google Scholar] [CrossRef]
- Gorelova, L.A.; Filatov, S.K.; Krzhizhanovskaya, M.G.; Bubnova, R.S. High Temperature Behaviour of Danburite-like Borosilicates MB2Si2O8 (M = Ca, Sr, Ba). Phys. Chem. Glasses: Eur. J. Glass Sci. Technol. Part B 2015, 56, 189–196. [Google Scholar] [CrossRef]
- Vortmann, S.; Marler, B.; Gies, H.; Daniels, P. Synthesis and Crystal Structure of the New Borosilicate Zeolite RUB-13. Microporous Mater. 1995, 4, 111–121. [Google Scholar] [CrossRef]
- Höppe, H.A.; Kazmierczak, K.; Daub, M.; Förg, K.; Fuchs, F.; Hillebrecht, H. The First Borosulfate K5[B(SO4)4]. Angew. Chem. Int. Ed. 2012, 51, 6255–6257. [Google Scholar] [CrossRef]
- Netzsch, P.; Pielnhofer, F.; Glaum, R.; Höppe, H.A. Synthesis-Controlled Polymorphism and Optical Properties of Phyllosilicate-Analogous Borosulfates M[B2(SO4)4] (M =Mg, Co). Chem. Eur. J. 2020, 26, 14745–14753. [Google Scholar] [CrossRef] [PubMed]
- Hämmer, M.; Bayarjargal, L.; Höppe, H.A. The First Bismuth Borosulfates Comprising Oxonium and a Tectosilicate-Analogous Anion. Angew. Chem. Int. Ed. 2021, 60, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Netzsch, P.; Gross, P.; Takahashi, H.; Höppe, H.A. Synthesis and Characterization of the First Borosulfates of Magnesium, Manganese, Cobalt, Nickel, and Zinc. Inorg. Chem. 2018, 57, 8530–8539. [Google Scholar] [CrossRef]
- Netzsch, P.; Pielnhofer, F.; Glaum, R.; Höppe, H.A. CSD 2014319: Experimental Crystal Structure Determination 2020. Chem. Eur. J. 2020, 26, 14745. [Google Scholar] [CrossRef] [PubMed]
- Hämmer, M.; Bayarjargal, L.; Höppe, H.A. CCDC 2025944: Experimental Crystal Structure Determination 2020. Angew. Chem. Int. Ed. 2020, 60, 1503. [Google Scholar] [CrossRef] [PubMed]
- Netzsch, P.; Gross, P.; Takahashi, H.; Höppe, H.A. CCDC 1842797: Experimental Crystal Structure Determination 2018. Inorg. Chem. 2018, 57, 8530. [Google Scholar] [CrossRef]
- Yoon, G.; Kim, D.H.; Park, I.; Chang, D.; Kim, B.; Lee, B.; Oh, K.; Kang, K. Using First-Principles Calculations for the Advancement of Materials for Rechargeable Batteries. Adv. Funct. Mater. 2017, 27, 1702887. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Prots, Y.; Kniep, R. Crystal Structure of Cobalt Manganese Monoaqua Catena-[Monohydrogenborate- Tris(Hydrogenphosphate)], (Co0.6Mn0.4)2(H2O)[BP3O9(OH)4]. Z. Kristallogr. New Cryst. Struct. 2009, 224, 371–372. [Google Scholar] [CrossRef]
- Ewald, B.; Öztan, Y.; Prots, Y.; Kniep, R. Structural Patterns and Dimensionality in Magnesium Borophosphates: The Crystal Structures of Mg2(H2O)[BP3O9(OH)4] and Mg(H2O)2[B2P2O8(OH)2]·H2O. Z. Anorg. Allg. Chem. 2005, 631, 1615–1621. [Google Scholar] [CrossRef]
- Bontchev, R.P.; Sevov, S.C. Co5BP3O14: The First Borophosphate with Planar BO3 Groups Connected to PO4 Tetrahedra. Inorg. Chem. 1996, 35, 6910–6911. [Google Scholar] [CrossRef]
- Duan, R.; Liu, W.; Cao, L.; Su, G.; Xu, H.; Zhao, C. A New Copper Borophosphate with Novel Polymeric Chains and Its Structural Correlation with Raw Materials in Molten Hydrated Flux Synthesis. J. Solid State Chem. 2014, 210, 60–64. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Prots, Y.; Kniep, R. Zn[BPO4(OH)2]: A Zinc Borophosphate with the Rare Moganite-Type Topology. Chem. Eur. J. 2008, 14, 1757–1761. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, A. An Open-Framework Borophosphate, LiCu2BP2O8(OH)2. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, i40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Yu, J.; Di, J.; Li, J.; Chen, P.; Fang, Q.; Chen, Y.; Xu, R. Syntheses, Structures, Ionic Conductivities, and Magnetic Properties of Three New Transition-Metal Borophosphates Na5(H3O{MII3[B3O3(OH)]3(PO4)6}·2H2O (MII = Mn, Co, Ni). Inorg. Chem. 2006, 45, 3588–3593. [Google Scholar] [CrossRef] [PubMed]
- Boy, I.; Cordier, G.; Eisenmann, B.; Kniep, R. Oligomere Tetraeder-Anionen in Borophosphaten: Darstellung und Kristallstrukturen von NaFe[BP2O7(OH)3] und K2Fe2[B2P4O16(OH)2. Z. Naturforsch. B J. Chem. Sci. 1998, 53, 165–170. [Google Scholar] [CrossRef]
- Menezes, P.W.; Hoffmann, S.; Prots, Y.; Kniep, R. Synthesis and Crystal Structure of CaCo(H2O)[BP2O8(OH)]·H2O. Z. Anorg. Allg. Chem. 2009, 635, 614–617. [Google Scholar] [CrossRef]
- Menezes, P.W.; Hoffmann, S.; Prots, Y.; Kniep, R. Crystal Structure of Calcium Iron(II) Hydrogenmonophosphatedihydrogenmonoborate- Monophosphate, CaFe[BP2O7(OH)3]. Z. Kristallogr. New Cryst. Struct. 2008, 223, 335–336. [Google Scholar] [CrossRef]
- Barrer, R.M.; Freund, E.F. Hydrothermal Chemistry of Silicates. Part XVIII. Reactions in the System CaO–B2O3–SiO2–H2O. J. Chem. Soc. Dalton Trans. 1974, 19, 2060–2065. [Google Scholar] [CrossRef]
- Saradhi, M.P.; Boudin, S.; Varadaraju, U.V.; Raveau, B. A New BaB2Si2O8:Eu2+/Eu3+, Tb3+ Phosphor—Synthesis and Photoluminescence Properties. J. Solid State Chem. 2010, 183, 2496–2500. [Google Scholar] [CrossRef]
- Xue, Z.; Qi, X.; Li, L.; Li, W.; Xu, L.; Xie, Y.; Lai, X.; Hu, G.; Peng, Z.; Cao, Y.; et al. Sodium Doping to Enhance Electrochemical Performance of Overlithiated Oxide Cathode Materials for Li-Ion Batteries via Li/Na Ion-Exchange Method. ACS Appl. Mater. Interfaces 2018, 10, 27141–27149. [Google Scholar] [CrossRef]
- Liu, B.; Wang, D.; Avdeev, M.; Shi, S.; Yang, J.; Zhang, W. High-Throughput Computational Screening of Li-Containing Fluorides for Battery Cathode Coatings. ACS Sustain. Chem. Eng. 2020, 8, 948–957. [Google Scholar] [CrossRef]
- Ma, L.; Li, N.; Long, C.; Dong, B.; Fang, D.; Liu, Z.; Zhao, Y.; Li, X.; Fan, J.; Chen, S.; et al. Achieving Both High Voltage and High Capacity in Aqueous Zinc-ion Battery for Record High Energy Density. Adv. Funct. Mater. 2019, 29, 1906142. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Li, H.; Ruan, Z.; Tang, Z.; Liu, Z.; Wang, Z.; Huang, Y.; Pei, Z.; Zapien, J.A.; et al. Initiating a Mild Aqueous Electrolyte Co3O4/Zn Battery with 2.2 V-High Voltage and 5000-Cycle Lifespan by a Co(III) Rich-Electrode. Energy Environ. Sci. 2018, 11, 2521–2530. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Zhang, X. Challenges and Perspectives for Manganese-based Oxides for Advanced Aqueous Zinc-ion Batteries. InfoMat 2020, 2, 237–260. [Google Scholar] [CrossRef]
- Geng, L.; Lv, G.; Xing, X.; Guo, J. Reversible Electrochemical Intercalation of Aluminum in Mo6S8. Chem. Mater. 2015, 27, 4926–4929. [Google Scholar] [CrossRef]
- Li, Z.; Mu, X.; Zhao-Karger, Z.; Diemant, T.; Behm, R.J.; Kübel, C.; Fichtner, M. Fast Kinetics of Multivalent Intercalation Chemistry Enabled by Solvated Magnesium-Ions into Self-Established Metallic Layered Materials. Nat. Commun. 2018, 9, 5115. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Yan, M.; Zhang, G.; Sun, R.; Chen, L.; An, Q.; Mai, L. Layered VS2 Nanosheet-Based Aqueous Zn Ion Battery Cathode. Adv. Energy Mater. 2017, 7, 1601920. [Google Scholar] [CrossRef]
- Chen, C.; Shi, F.; Zhang, S.; Su, Y.; Xu, Z.-L. Ultrastable and High Energy Calcium Rechargeable Batteries Enabled by Calcium Intercalation in a NASICON Cathode. Small 2022, 18, e2107853. [Google Scholar] [CrossRef]
- Ko, J.S.; Paul, P.P.; Wan, G.; Seitzman, N.; DeBlock, R.H.; Dunn, B.S.; Toney, M.F.; Nelson Weker, J. NASICON Na3V2(PO4)3 Enables Quasi-Two-Stage Na+ and Zn2+ Intercalation for Multivalent Zinc Batteries. Chem. Mater. 2020, 32, 3028–3035. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Li, N.; Liu, Z.; Tang, Z.; Zapien, J.A.; Chen, S.; Fan, J.; Zhi, C. Hydrogen-Free and Dendrite-Free All-Solid-State Zn-Ion Batteries. Adv. Mater. 2020, 32, e1908121. [Google Scholar] [CrossRef]
- Canepa, P.; Sai Gautam, G.; Hannah, D.C.; Malik, R.; Liu, M.; Gallagher, K.G.; Persson, K.A.; Ceder, G. Odyssey of Multivalent Cathode Materials: Open Questions and Future Challenges. Chem. Rev. 2017, 117, 4287–4341. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Yao, W.; He, X.; Song, T.; Tang, Y. Mixed Polyanionic Compounds as Positive Electrodes for Low-Cost Electrochemical Energy Storage. Angew. Chem. Int. Ed. 2020, 59, 9255–9262. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, G.; Jiang, H.; Parkin, I.P.; Shearing, P.R.; Brett, D.J.L. Cathode Design for Aqueous Rechargeable Multivalent Ion Batteries: Challenges and Opportunities. Adv. Funct. Mater. 2021, 31, 2010445. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, L.; Cao, X.; Zhou, J.; Pan, A.; Fang, G.; Wang, S.; Liang, S. Ion Migration and Defect Effect of Electrode Materials in Multivalent-Ion Batteries. Prog. Mater. Sci. 2022, 125, 100911. [Google Scholar] [CrossRef]




| Cathode Material | Reversible Range (Δx) | Specific Capacity (Ah kg−1) | Capacity Density (Ah I−1) |
|---|---|---|---|
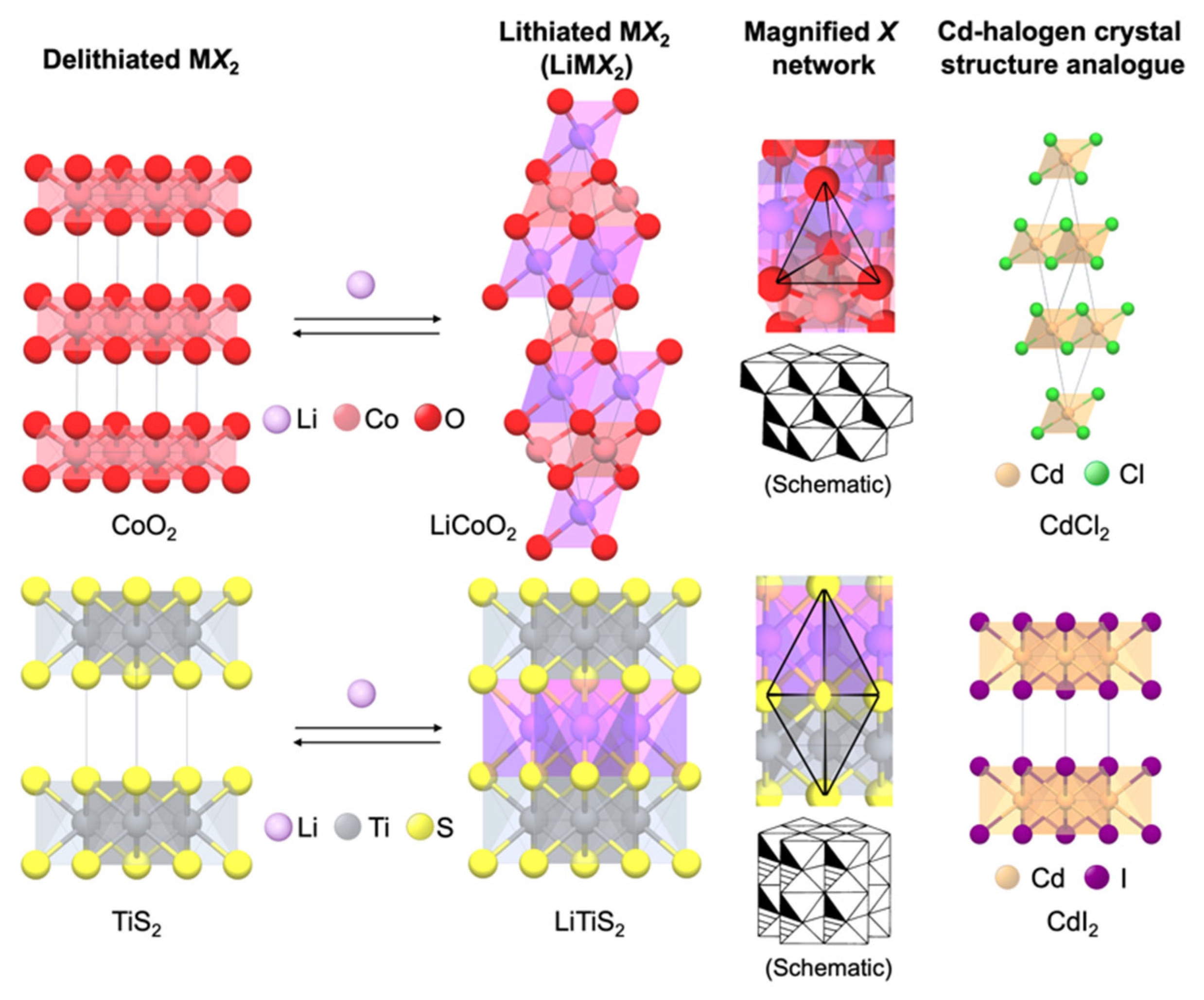
| LixTiS2 | 1.0 | 239 | 782 |
| LixMoS2 | 0.8 | 134 | 678 |
| LixNbSe3 | 3.0 | 244 | 2121 |
| Type of Oxide Cathode | Electronic Conductivity 1 | Structure | Stability | Sustainability 1 | Ref. |
|---|---|---|---|---|---|
| Layered | (+) | close-packed; high density | acid leaching of transition metals | (−) | [59,60] |
| Spinel | (+) | close-packed; high density | (−) | [59,60] | |
| Polyanion | (−) | low density | better safety due to tightly bound O to P, S, or Si | (+) | [5] |
| Crystal Structure | Compound | Cell Voltage (V) | Specific Capacity (mAh g−1) Theoretical/Actual | Remarks | Ref. |
|---|---|---|---|---|---|
| Layered | LiCoO2 | 3.8 | 274/137 | Excellent cycling performance | [73] |
| High working voltage | |||||
| LiNiO2 | 3.7 | 275/160 | High specific capacity | [74] | |
| Low thermal stability | [75] | ||||
| LiMnO2 | 3.3 | 285/130 | Low structural stability | [76] | |
| Favorable transformation to spinel structure | [77] | ||||
| LiNi0.65Co0.15Mn0.2O2 | 4.3 | 186.5 | High working voltage and capacity Poor cycling efficiency, rate performance, and thermal stability | [52] | |
| LiTiS2 | 1.9 | 239/235 | Significant capacity fade | [78] | |
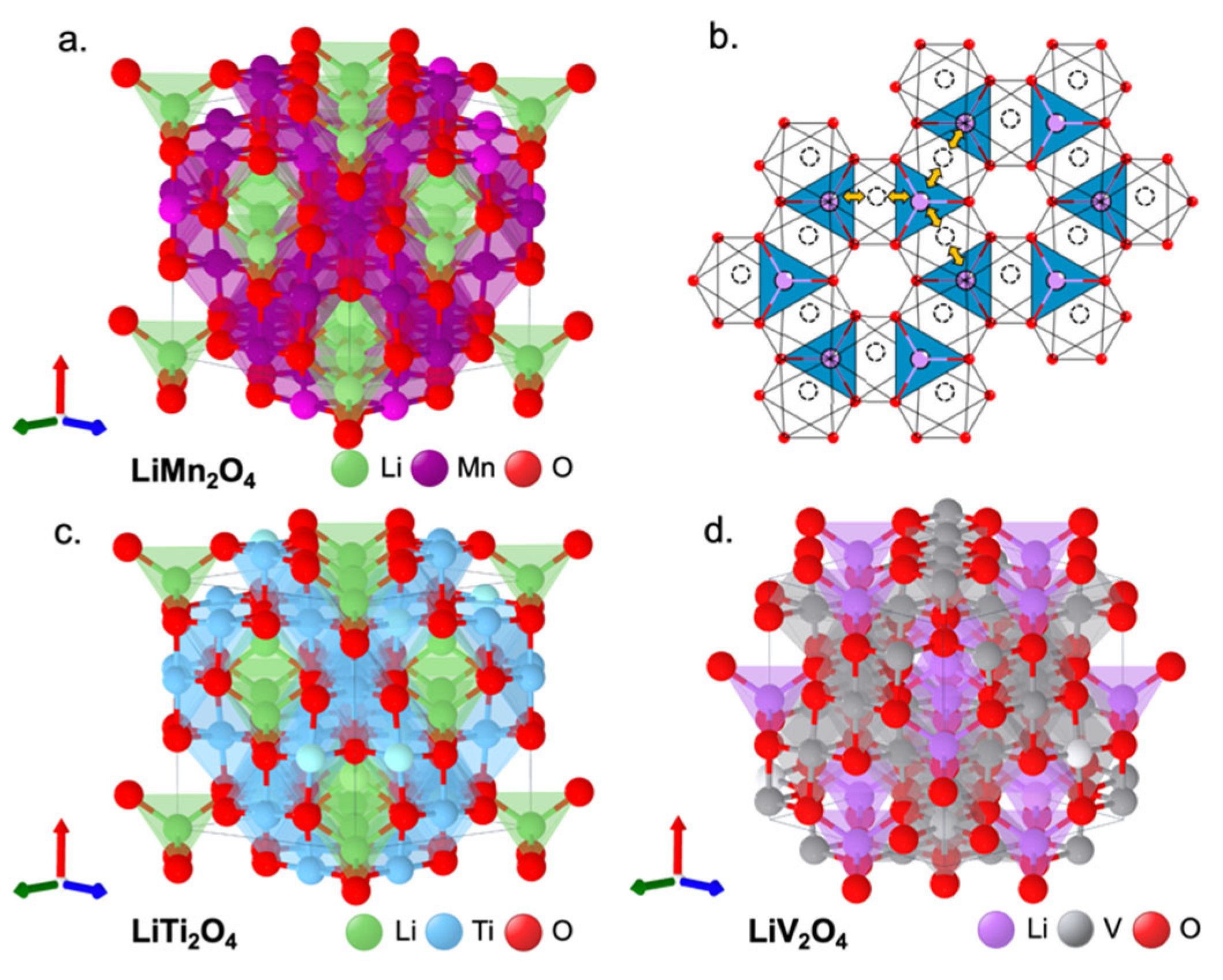
| Spinel | LiMn2O4 | 4 | 145/120 | Significant capacity fade | [79] |
| Poor cycling stability | [80] | ||||
| LiTi2O4 | 1.5 | 240 | Low operating voltage | [81] | |
| Good superconductivity | [82] | ||||
| LiV2O4 | 1.2 | 155/100 | Low structural stability | [83] | |
| Significant capacity fade at different voltages | |||||
| LiCo2O4 | 3.9 | 142 | Low structural stability | [84] | |
| Favorable transformation to layered structure | |||||
| Olivine/ polyanion | LiFePO4 | 3.5 | 170/ 165 | Good cycling stability | [69] |
| High rate capability | [85] | ||||
| Excellent thermal stability | |||||
| LiCoPO4 | 4.8 | 167 | Poor cycling stability | [86] | |
| Low coulombic efficiency | |||||
| Poor ionic conductivity | |||||
| LiMnPO4 | 4.1 | 171/140 | High capacity fade at different discharge rates | [87] | |
| LiFeSO4F | 3.9 | 151/140 | High rate capability | [88] | |
| Li2FeSiO4 | 2.8 | 166/140 | Low operating voltage | [89] | |
| Good electrochemical process reversibility | |||||
| LiMnP2O7 | 4.0 | 120 | High mechanical stability | [90] | |
| Li2CoPO4F | 5.0 | 310 | High electrolyte decomposition | [91] | |
| Li3FeCO3PO4 | 3.3 | 115/110 | Capacity increase after ball milling with carbon | [92] |
| Compound | Formula | Voltage (V) | Capacity (mAh g−1) | Specific Energy (Wh kg−1) | Energy Density (Wh I−1) | Change in Volume (% per e−) |
|---|---|---|---|---|---|---|
| (CO3)(PO4) | Li3Mn(CO3)(PO4) | 3.3; 4.1 | 232 | 859 | 2375 | 1.20 |
| Li2V(CO3)(PO4) | 3.5; 4.4 | 243 | 969 | 2604 | 0.90 | |
| (CO3)(SiO4) | Li3V(CO3)(SiO4) | 3.0; 3.7 | 239 | 799 | 2183 | 0.32 |
| Li3Mo(CO3)(SiO4) | 2.6; 3.5; 3.5 | 299 | 966 | 2989 | 0.24 | |
| BO3-based | Li3Mn(BO3)(PO4) | 4.1 | 117 | 473 | 1315 | 3.50 |
| Li3Mo(BO3)(PO4) | 2.9; 3.7; 3.7 | 296 | 1024 | 3200 | 0.21 | |
| Li3Cr(BO3)(PO4) | 4.2; 5.1; 5.1 | 354 | 1705 | 4814 | 0.59 | |
| Li3V(BO3)(PO4) | 3.4; 4.3 | 237 | 904 | 2487 | 0.44 | |
| Li3Mo(BO3)(SiO4) | 3.2; 3.7 | 200 | 692 | 2167 | 0.62 | |
| Li3Fe(BO3)(PO4) | 4 | 116 | 468 | 1330 | 0.61 | |
| Li3Bi(BO3)(PO4) | 4.3; 4.6 | 140 | 624 | 2444 | 0.01 | |
| Li3V(BO3)(SiO4) | 3.6 | 120 | 428 | 1172 | 1.30 | |
| Li3Bi(BO3)(SiO4) | 3.6 | 70 | 286 | 1039 | 2.60 | |
| Control | LiFePO4 | 3.4 | 170 | 544 | 1959 | 6.80 |
| LiCoO2 | 4 | 155 | 620 | 3100 | 1.80 |
| Cathode Material | Space Group | Intercalating Ion | Cathode Potential (V) | Specific Capacity (mAh g−1) | C-Rate | Remarks | Ref. |
|---|---|---|---|---|---|---|---|
| LiFe-BPO | P6122 | Li+ | 3.06 | 67.5 | C/50 | Higher reversibility compared to LiFe-BPO versus. Na+/Na. Achieved ~80% of theoretical capacity. | [109] |
| LiFe-BPO | P6122 | Na+ | 2.76 | 66.5 | C/50 | Achieved ~82% of its theoretical capacity. Observed a capacity loss of 9% after the third cycle. | [109] |
| NaFe-BPO | P6122 | Na+ | 2.9 | 66 | C/20 | Became almost electrochemically inactive by its 10th cycle. | [110] |
| NHFe-BPO | P6522 | Na+ | 2.9 | 80 | Capacity drops 60% after the 40th cycle. | [110] |
| Cathode | Space Group | Initial Capacity (mAh g−1) | Cathode Potential (V) | Charge Transfer Impedance Rct (Ω) | Li Diffusion Coefficient DLi+ (cm2 s−1) | Ref. |
|---|---|---|---|---|---|---|
| Li2Fe0.98Mg0.02(SiO4)0.97(BO3)0.03/C | P21/n | 138 | ~3 | 3546.0 | 2.68 × 10−15 | [117] |
| Li2Fe0.98Ag0.02(SiO4)0.99(BO3)0.01/C | P21/n | 150.8 | ~3 | 933.6 | 3.09 × 10−16 | [118] |
| Compound | X-ray Diffraction | Thermal Analyses | Magnetic Susceptibility | Infrared Spectroscopy | Application | Ref. |
|---|---|---|---|---|---|---|
| M [BPO4(OH)2] (M = Mn, Fe, Co) | Chiral space group P3221 or P3121 Helical MO6-chains along [001] Vierer BPO single chains perpendicular to [001] | One-step dehydration thermally stable at least up to ~458.85 to 492 °C | µeff values typical for pure (high-spin) MII compounds at lower temperatures, ø(T) curves indicate low-dimensional antiferromagnetic correlations | Nonlinear optics | [136] | |
| MIMII(H2O)2[BP2O8] ∙H2O (MI = Na, K; MII = Mg, Mn, Fe, Co, Ni, Zn) | Space group P6 hexagonal PO4 and BO4 tetrahedral helical ribbons through common vertices | Two-step dehydration thermal stability varies between 180 °C to 305 °C | Catalysis, separation | [137] | ||
| Fe(H2O)2BP2O8∙H2O | Space group P6522 zeolite-type, tetrahedral, chiral framework topology | First dehydration step at 100–235 °C and second at 500 °C Structure is crystalline at 235 °C and amorphous at 400 °C Unit cell volume decreases during heating | Paramagnetic down to 5 K of the Curie-Weiss type antiferromagnetic interactions between iron centers | Catalysis, separation, ion exchange | [131] | |
| M(H2O)2[B2P2O8 (OH)2]∙H2O (M = Fe, Co, Ni) | n space group P21/c wavy 63 net 2D arrangement of distorted corner sharing PO4 and HBO4 | Mass loss is between ~97 to 327 °C. Framework starts to decompose at ~247 °C | Magnetic behavior below 40 K (zero-field splitting and/or high-spin/low-spin transition) | Sorption, separation, catalysis, optics | [133] | |
| MIII2BP3O12 (M = Fe, In) | Space group P63/m hexagonal 3D architectures of corner-sharing M2O9 and B(PO4)3 units | Strong antiferromagnetic coupling dominates the exchange between iron atoms | Transparent range of 4000–1700 cm−1 optical band gaps of 5.39 eV (In2BP3O12) and 3.52 eV (Fe2BP3O12) | Sorption, separation, catalysis, ion exchange, optics | [138] |
| Compound | X-ray Diffraction | Thermal Analysis | Nuclear Magnetic Resonance | Application | Ref. |
|---|---|---|---|---|---|
| LaBSiO5 | Space group P31 Six-membered rings composed of BO4 and SiO4 tetrahedra | Ultraviolet nonlinear optical applications | [178] | ||
| Sr3B2Si2O8 | Space group Pnma Chain of SiO4, BO4, and BO3 polyhedra | Decomposition at 1043 K Maximal thermal expansion along [010] | Fabrication of glaze glass coatings | [179] | |
| CaBSiO4(OH) | Space group P21/c Two sets of alternating layers; first layer of 8- and 4-membered rings; second layer of Ca polyhedral in 6-membered ring | Axial expansion along [100] and [[010]] Volume thermal expansion coefficient of 1.5 × 10−5 K−1 | Geochemical marker | [180] | |
| NaCa5(BO3)(SiO4)2 | Space group P21/c Framework of isolated BO3 and SiO4 polyhedra connected by NaO7 polyhedra | No significant weight loss until 1513 K | Birefringent and nonlinear optical applications | [181] | |
| SrB2Si2O8 | Space group Pnma No phase transformation until 1173 K | Expands isotropically Volume thermal expansion coefficient of 25.4 × 10−6 °C−1 | Phosphors | [182] | |
| K16[B16Si32O96] | Space group ANA structure type Isostructural with leucite | Weight loss at 443–504 K due to hydrated phase and adsorbed water High thermal stability compared to other zeolite-type borosilicates due to its potassium ions | Sharp signal on the 11B spectrum showed symmetric tetrahedral (BO4) units Calcination did not change 11B spectrum Broad signal on 29Si spectrum due to short T2 of the nuclei | [175] | |
| Na5B5Si49O108 | Space group , hexagonal Levyne structure type Small structural collapse after calcination at 823 K | Three-step weight loss at 541 K, 775 K, and 845 K due to elimination of organic material in the framework | Symmetric tetrahedral unit BO4 from sharp signal Calcination at 823 K led to transition from tetrahedral to trigonal symmetry | [176] |
| Compounds | X-ray Diffraction | Thermal Analysis | Optical Spectroscopy | Infrared Spectroscopy | Applications | Ref. |
|---|---|---|---|---|---|---|
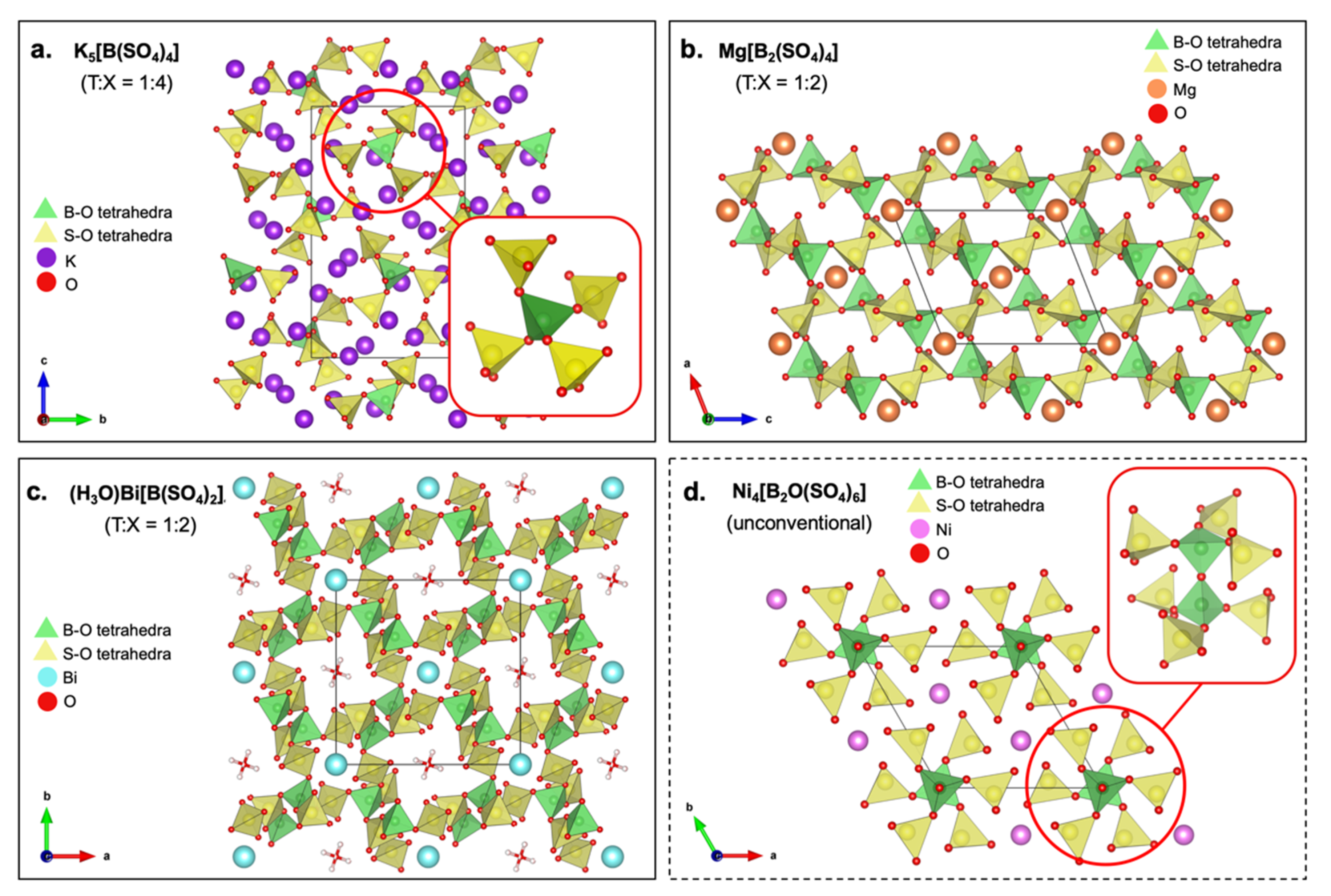
| M[B2(SO4)4] M = Mg, Co | Space group C2/c Infinite anionic layers parallel to (100) plane | Start to decompose above 450 °C | Reflectance spectra similar and typical of Co2+ ions in an almost undistorted oxoanionic coordination. | 1195 cm−1: asymmetric stretching (S-O terminal) 1170–1014 cm−1: asymmetric and symmetric stretching (B-O) 696–553 cm−1: asymmetric bending (O-S-O, O-B-O, S-O-B) | Used in optical materials | [185] |
| (H3O)Bi[B(SO4)2]4 | Space group 3D network consists of SO4 supertetrahedra sharing all four corners with other SO4 | Stable up to 180 °C before decomposition | UV region absorption edge | 1400–400 cm−1: borate and sulfate tetrahedra presence 840–1040 cm−1: B-O stretching >1100 cm−1: S-O stretching | Used in luminescent materials | [186] |
| M4[B2O(SO4)6] M = Mg, Mn, Co, Ni, Zn | Space group Layers of edge-sharing [B2O(SO4)6]8− anions and MO6 octahedra | Starts to decompose around 500 °C | High reflectance around 620–420 nm; pink body color of the compound | 1150–1420 cm−1: asymmetric SO4 stretching 980–1080 cm−1: asymmetric BO4 stretching 750–820 cm−1: S-O-B and B-O-B stretching 430–660 cm−1: BO4 and SO4 bending | Used as host material for phosphors | [187] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanglay, G.D.D.; Garcia, J.S.; Palaganas, M.S.; Sorolla, M., II; See, S.; Limjuco, L.A.; Ocon, J.D. Borate-Based Compounds as Mixed Polyanion Cathode Materials for Advanced Batteries. Molecules 2022, 27, 8047. https://doi.org/10.3390/molecules27228047
Sanglay GDD, Garcia JS, Palaganas MS, Sorolla M II, See S, Limjuco LA, Ocon JD. Borate-Based Compounds as Mixed Polyanion Cathode Materials for Advanced Batteries. Molecules. 2022; 27(22):8047. https://doi.org/10.3390/molecules27228047
Chicago/Turabian StyleSanglay, Giancarlo Dominador D., Jayson S. Garcia, Mecaelah S. Palaganas, Maurice Sorolla, II, Sean See, Lawrence A. Limjuco, and Joey D. Ocon. 2022. "Borate-Based Compounds as Mixed Polyanion Cathode Materials for Advanced Batteries" Molecules 27, no. 22: 8047. https://doi.org/10.3390/molecules27228047
APA StyleSanglay, G. D. D., Garcia, J. S., Palaganas, M. S., Sorolla, M., II, See, S., Limjuco, L. A., & Ocon, J. D. (2022). Borate-Based Compounds as Mixed Polyanion Cathode Materials for Advanced Batteries. Molecules, 27(22), 8047. https://doi.org/10.3390/molecules27228047