Photoelectron Properties and Organic Molecules Photodegradation Activity of Titania Nanotubes with CuxO Nanoparticles Heat Treated in Air and Argon
Abstract
1. Introduction
2. Results
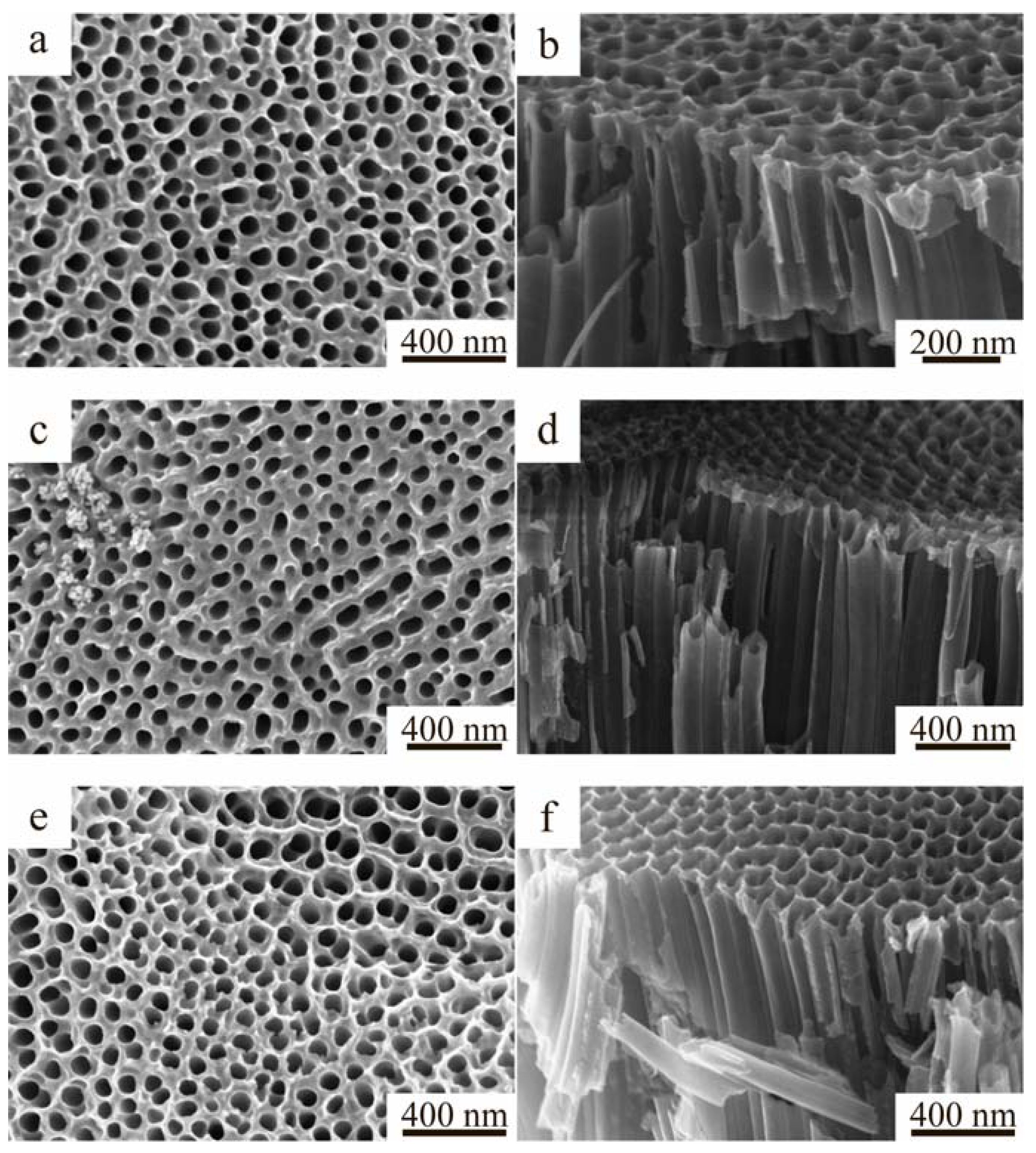
2.1. Morphology
2.2. Structural Properties
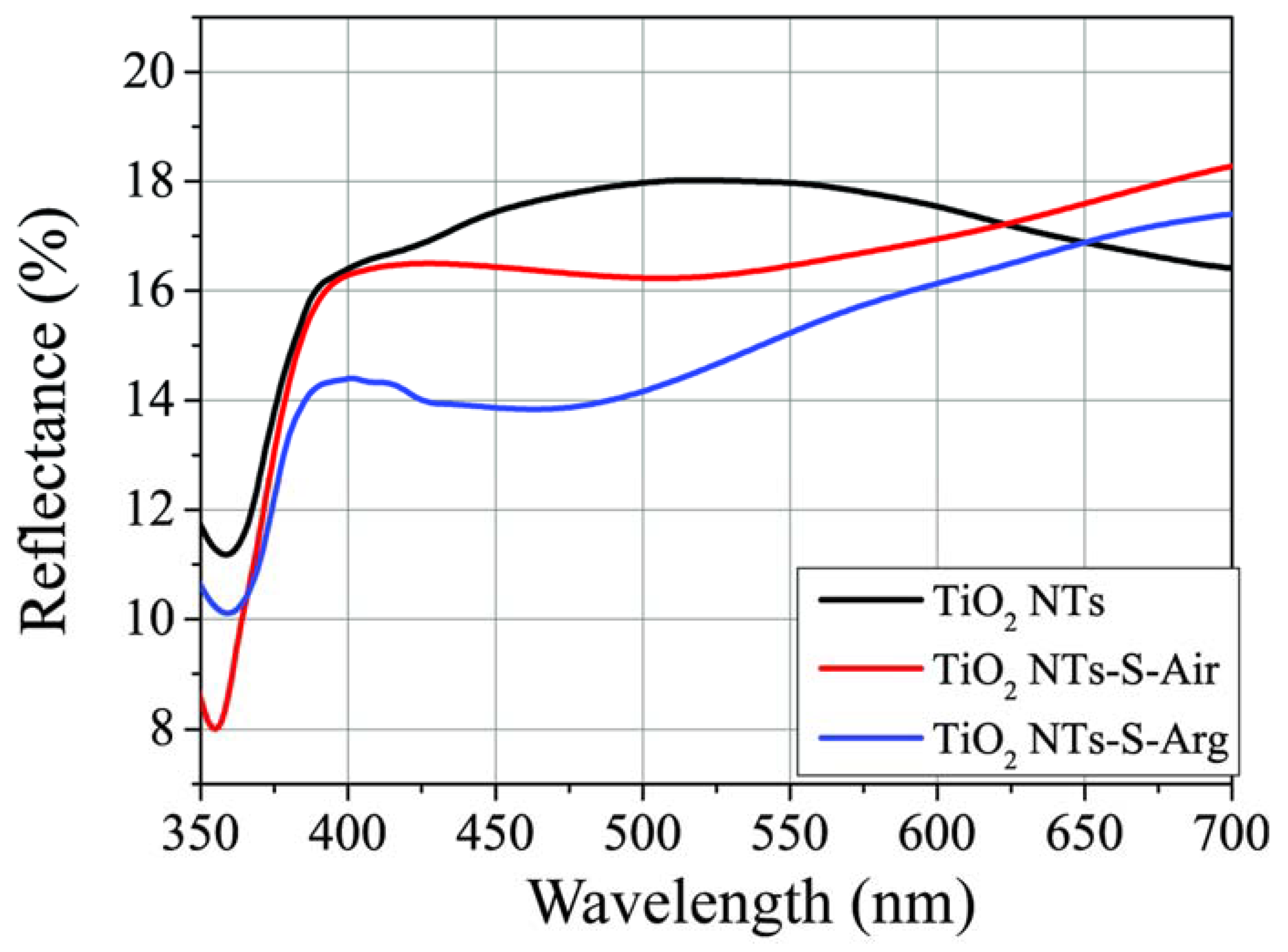
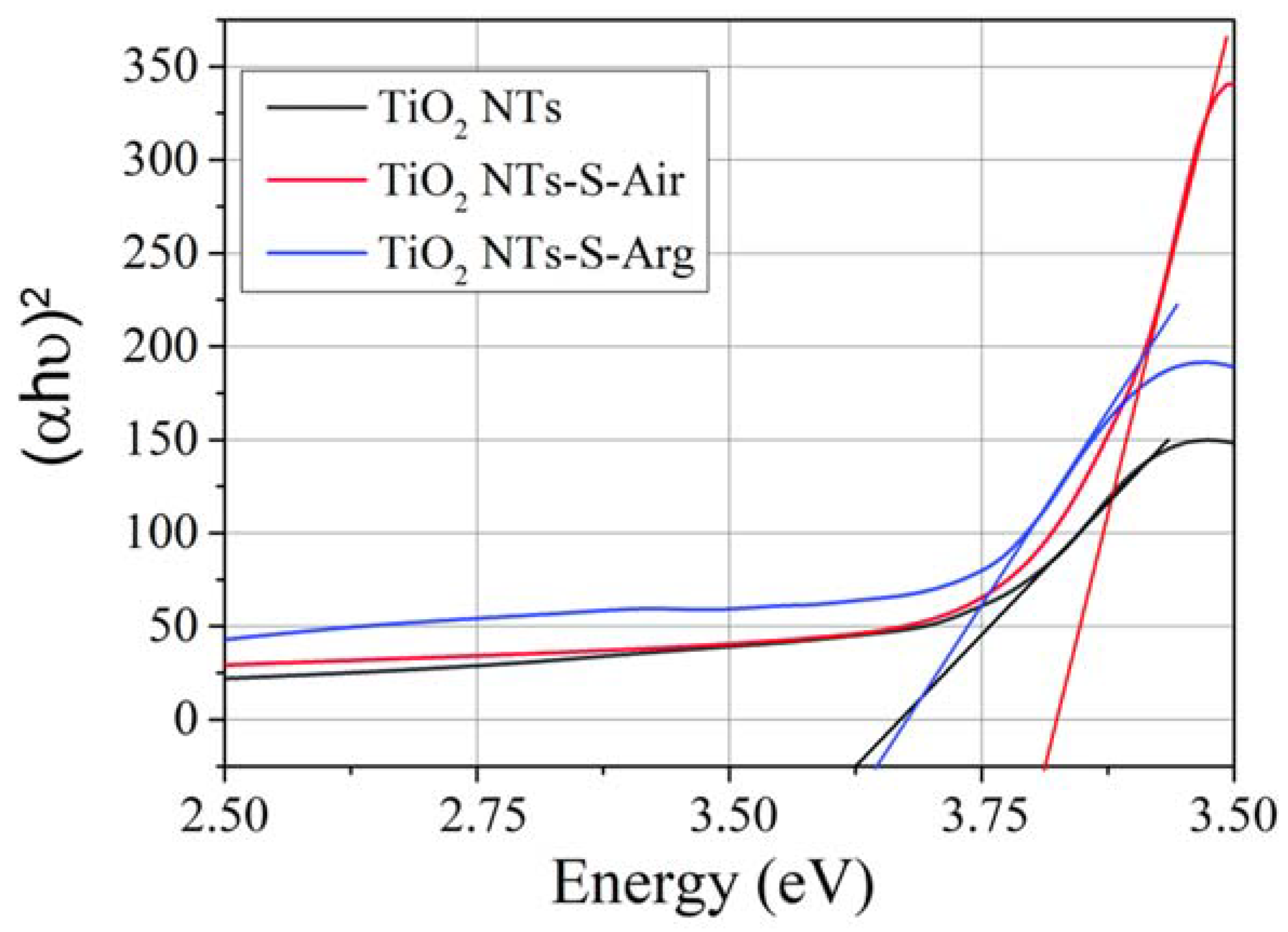
2.3. Optical Properties
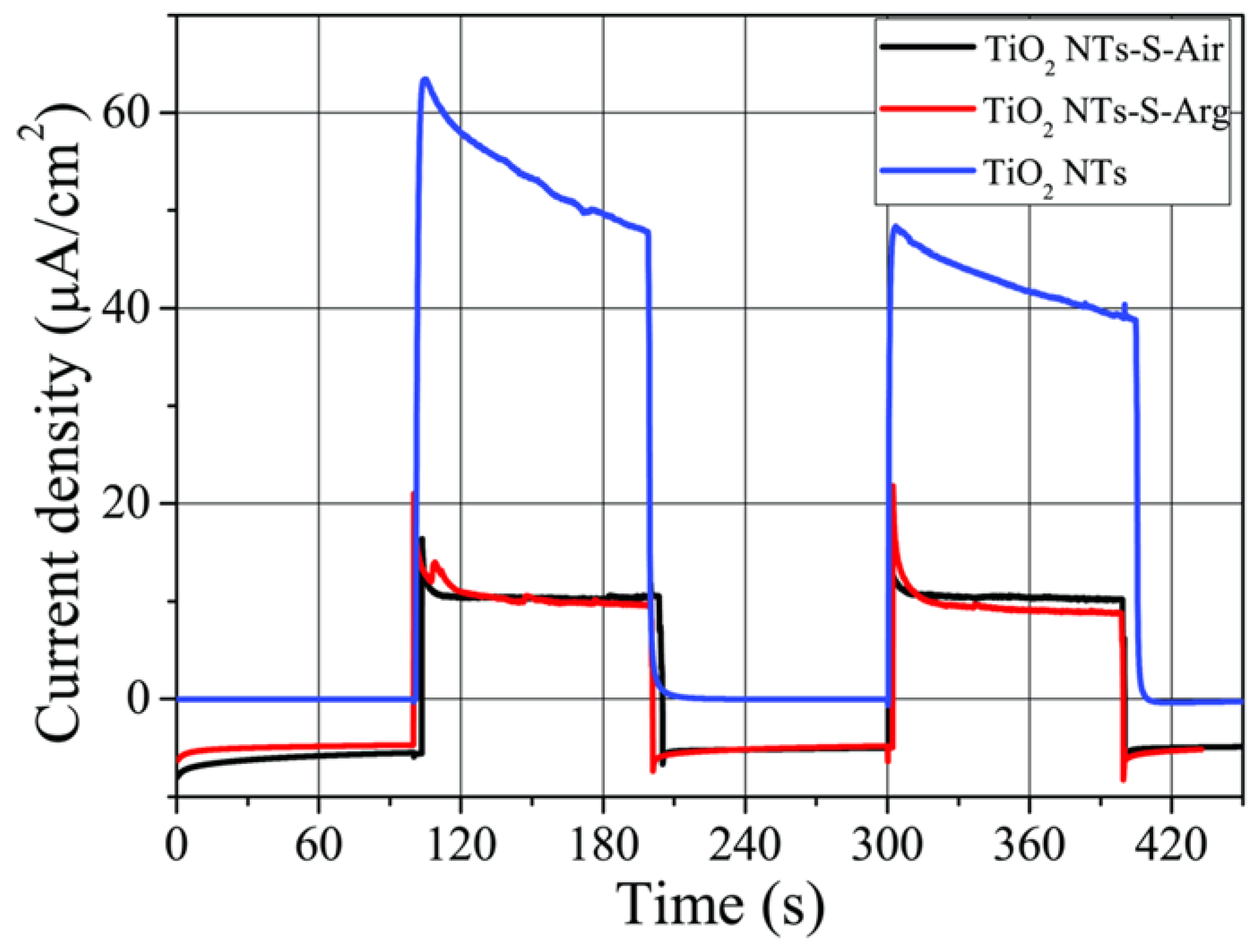
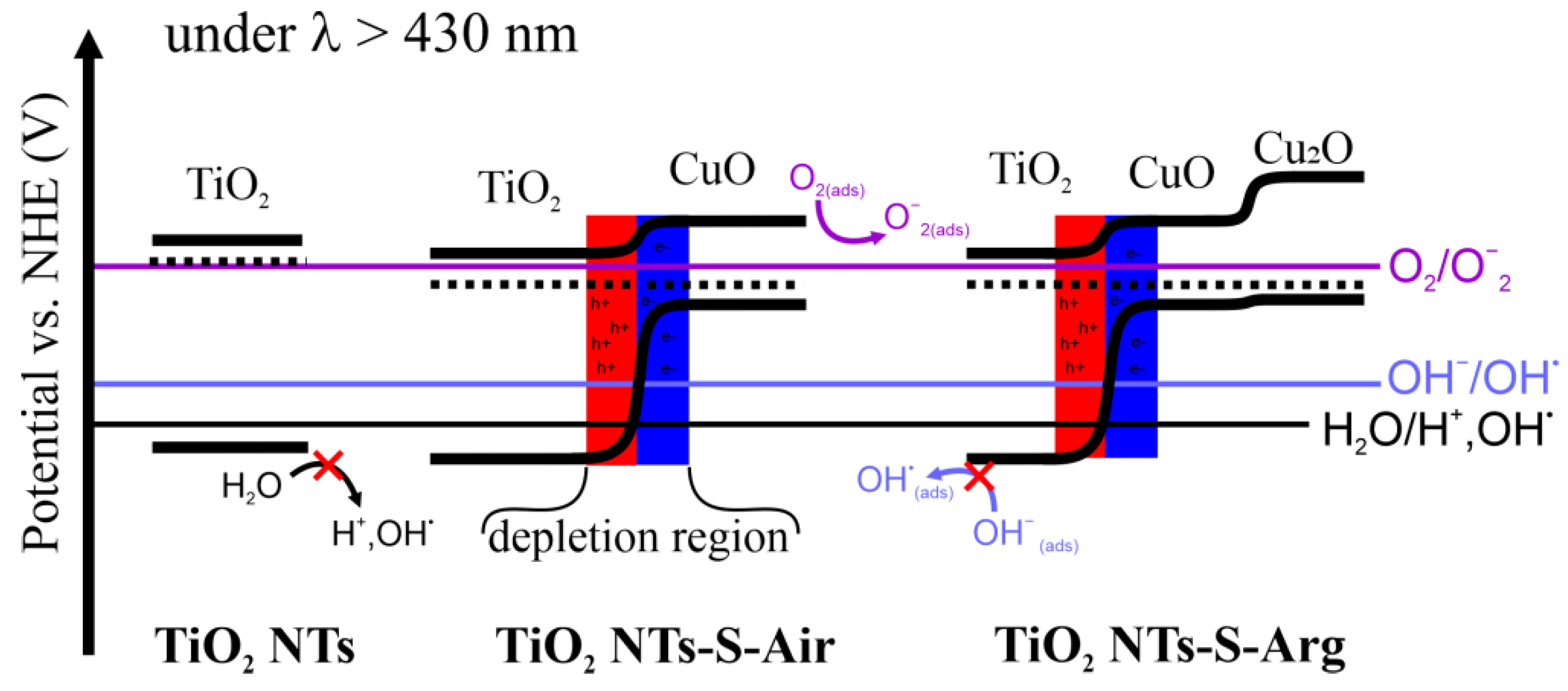
2.4. Photoelectrochemical Properties
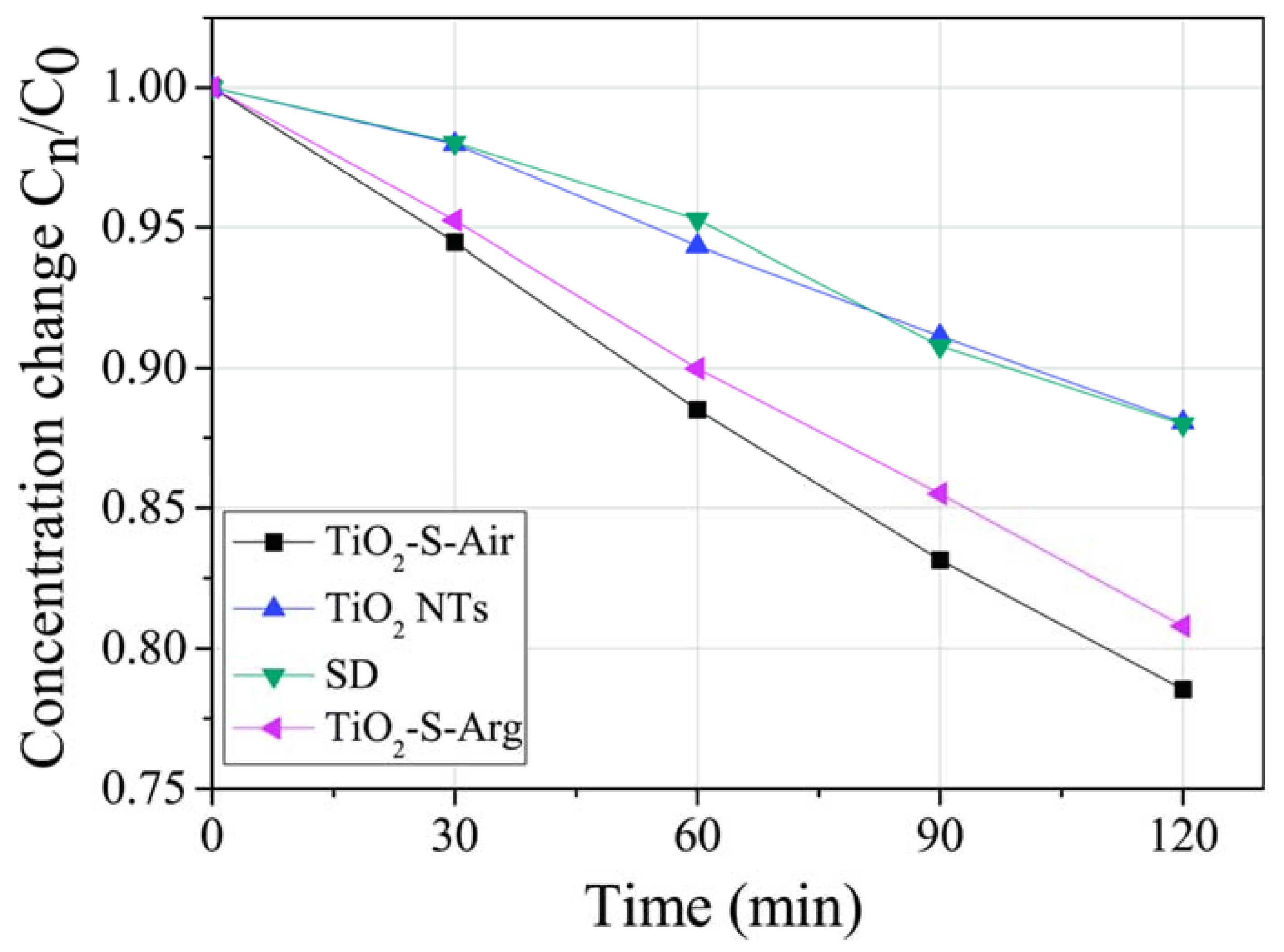
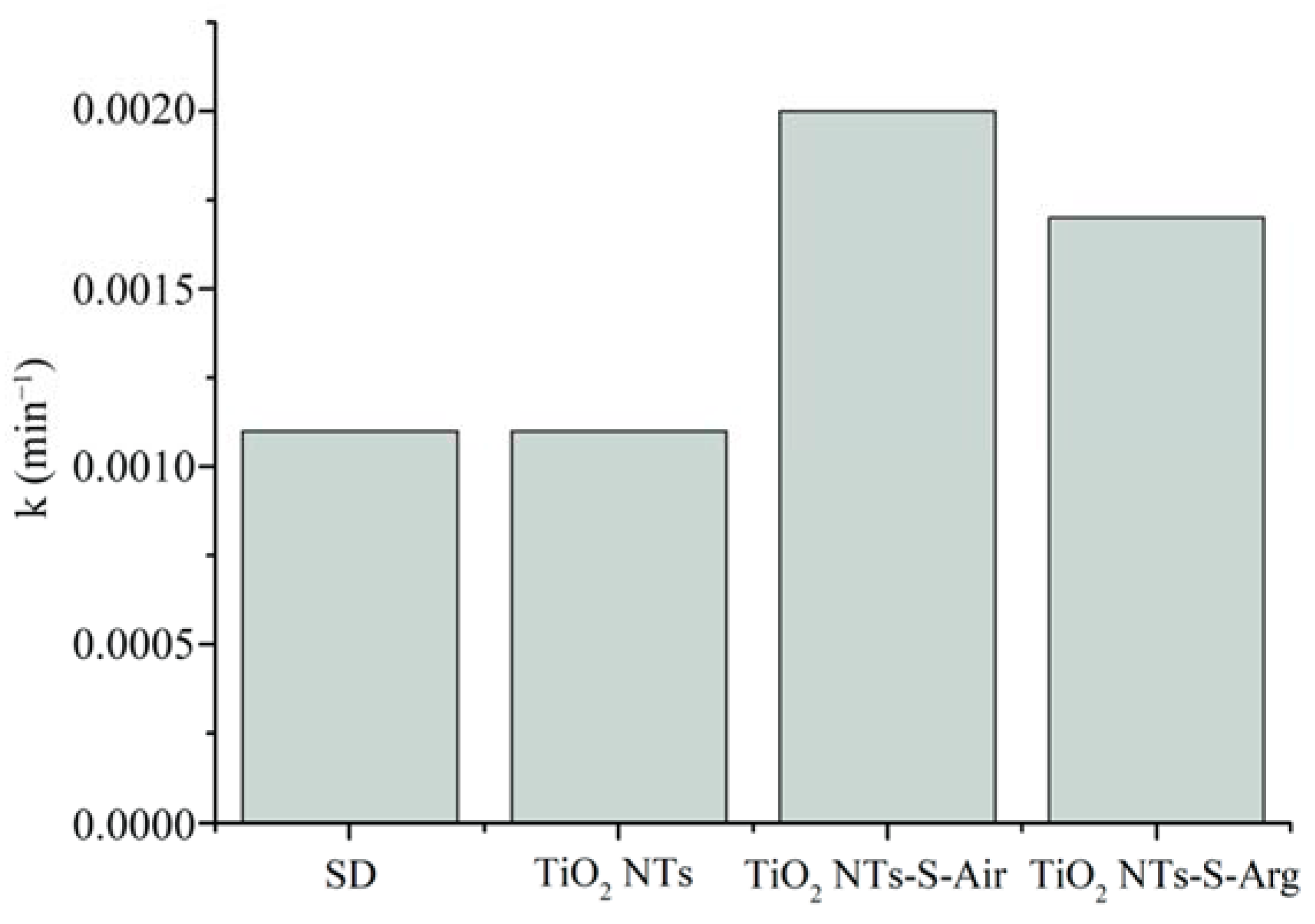
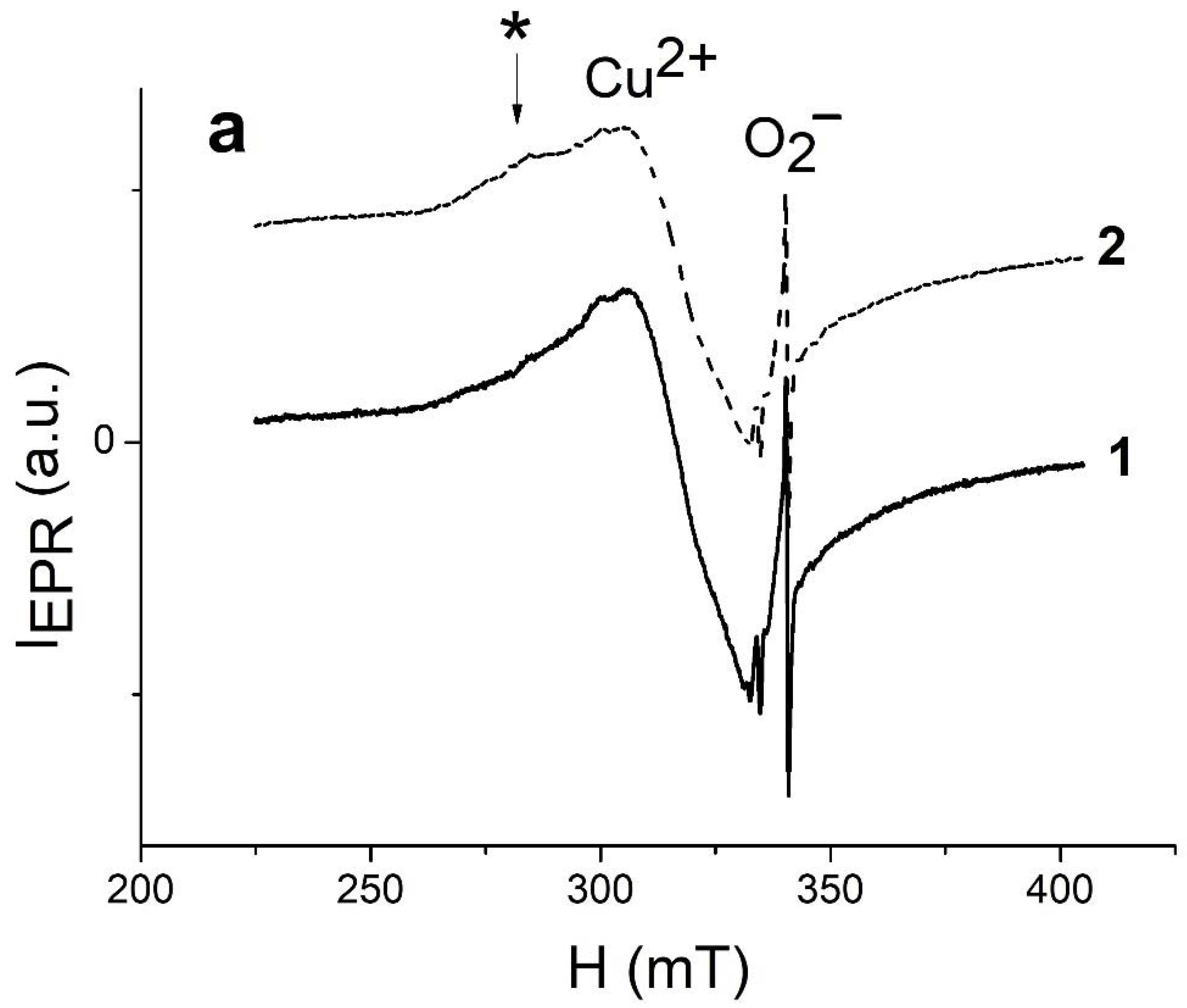
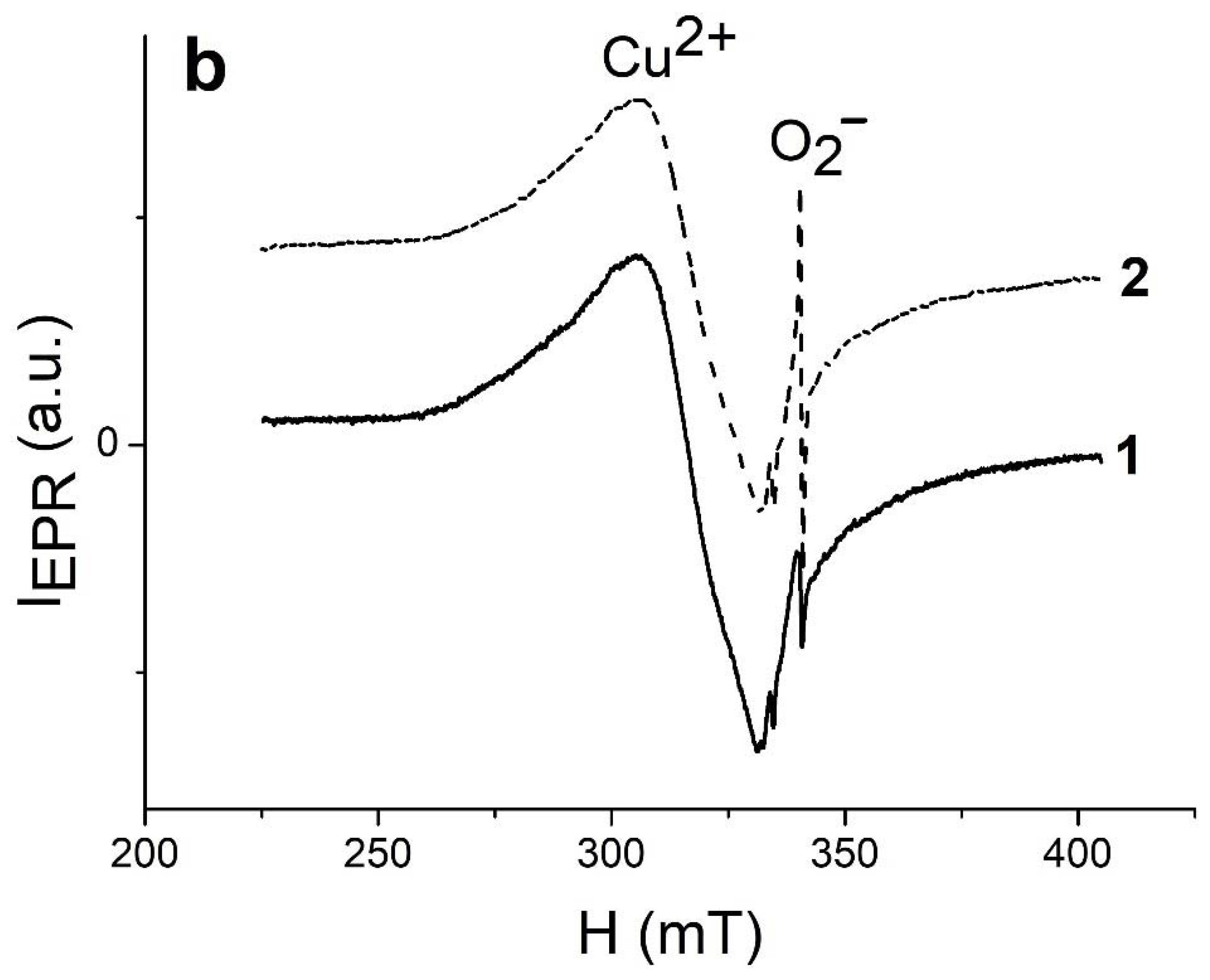
2.5. Photocatalytic Activity and EPR Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Synthesis of TiO2 NTs
4.2. Synthesis of TiO2 NTs/CuxO Heterostructures
4.3. Microscopy
4.4. Time-of-Flight Secondary Ion Mass Spectrometry
4.5. Investigation of Optical Properties
4.6. Photoelectrochemical Properties
4.7. Photocatalysis
4.8. EPR Spectroscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic Carbon-Nanotube–TiO2 Composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Zubaira, M.; Kim, H.-R.; Razzaq, A.; Grimes, C.A.; In, S.-I. Solar spectrum photocatalytic conversion of CO2 to CH4 utilizing TiO2 nanotube arrays embedded with graphene quantum dots. J. CO2 Util. 2018, 26, 70–79. [Google Scholar] [CrossRef]
- Gavrilin, I.; Dronov, A.; Volkov, R.; Savchuk, T.; Dronova, D.; Borgardt, N.; Pavlikov, A.; Gavrilov, S.; Gromov, D. Differences in the local structure and composition of anodic TiO2 nanotubes annealed in vacuum and air. Appl. Surf. Sci. 2020, 516, 146120. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Chen, W.-T.; Jovic, V.; Sun-Waterhouse, D.; Idriss, H.; Waterhouse, G.I.N. The role of CuO in promoting photocatalytic hydrogen production over TiO2. Int. J. Hydrogen Energy 2013, 38, 15036–15048. [Google Scholar] [CrossRef]
- Arifin, K.; Yunus, R.M.; Minggu, L.J.; Kassim, M.B. Improvement of TiO2 Nanotubes for Photoelectrochemical Water Splitting: Review. Int. J. Hydrogen Energy 2021, 46, 4998–5024. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Z.; Mao, X.; Dong, Y.; Peng, L.; Sun-Waterhouse, D.; Kennedy, J.V.; Waterhouse, G.I.N. Recent Advances in Self-Supported Semiconductor Heterojunction Nanoarrays as Efficient Photoanodes for Photoelectrochemical Water Splitting. Small 2022, e2204553. [Google Scholar] [CrossRef]
- Faisal, M.; Harraz, F.A.; Ismail, A.A.; El-Toni, A.M.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Novel mesoporous NiO/TiO2 nanocomposites with enhanced photocatalytic activity under visible light illumination. Ceram. Int. 2018, 44, 7047–7056. [Google Scholar] [CrossRef]
- Hua, Z.; Dai, Z.; Bai, X.; Ye, Z.H.; Wang, P.; Gu, H.; Huang, X. Copper nanoparticles sensitized TiO2 nanotube arrays electrode with enhanced photoelectrocatalytic activity for diclofenac degradation. Chem. Eng. J. 2016, 283, 514–523. [Google Scholar] [CrossRef]
- Muiva, C.M.; Maabong, K.; Moditswe, C.H. CuO nanostructured thin films synthesized by chemical bath deposition on seed layers deposited by successive ionic layer adsorption and reaction and chemical spray pyrolysis techniques. Thin Solid Film. 2016, 616, 48–54. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Feng, J.; Zhang, M. Shape-controlled synthesis of single-crystalline cupric oxide by microwave heating using an ionic liquid. Mater. Lett. 2008, 62, 2787–2790. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.-Z.; Zhu, J.-J.; Chen, H.-Y. Preparation of CuO nanoparticles by microwave irradiation. J. Cryst. Growth 2002, 244, 88–94. [Google Scholar] [CrossRef]
- Chen, Z.-Z.; Shi, E.-W.; Zheng, Y.-Q.; Li, W.-J.; Xiao, B.; Zhuang, J.-Y. Growth of hex-pod-like Cu2O whisker under hydrothermal conditions. J. Cryst. Growth 2003, 249, 294–300. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, X.; Zhang, M. Hydrothermal synthesis of sheaf-like CuO via ionic liquids. Mater. Lett. 2008, 62, 385–388. [Google Scholar] [CrossRef]
- Tang, X.-L.; Ren, L.; Sun, L.-N.; Tian, W.-G.; Cao, M.-H.; Hu, C.H.-W. A Solvothermal Route to Cu2O Nanocubes and Cu Nanoparticles. Chem. Res. Chin. Univ. 2006, 22, 547–551. [Google Scholar] [CrossRef]
- Xu, H.Y.; Chen, C.; Xu, L.; Dong, J.K. Direct growth and shape control of Cu2O film via one-step chemical bath deposition. Thin Solid Film. 2013, 527, 76–80. [Google Scholar] [CrossRef]
- Muhibbullah, M.; Ichimura, M. Fabrication of copper oxide thin films by the drop chemical deposition technique. Mater. Res. Bull. 2012, 47, 1968–1972. [Google Scholar] [CrossRef]
- Lamri Zeggar, M.; Chabane, L.; Aida, M.S.; Attaf, N.; Zebbar, N. Solution flow rate influence on properties of copper oxide thin films deposited by ultrasonic spray pyrolysis. Mater. Sci. Semicond. Process. 2015, 30, 645–650. [Google Scholar] [CrossRef]
- Saravanan, V.; Shankar, P.; Mani, G.K.; Balaguru Rayappan, J.B. Growth and characterization of spray pyrolysis deposited copper oxide thin films: Influence of substrate and annealing temperatures. J. Anal. Appl. Pyrolysis 2015, 111, 272–277. [Google Scholar] [CrossRef]
- Bulakhe, R.N.; Shinde, N.M.; Thorat, R.D.; Nikam, S.S.; Lokhande, C.D. Deposition of copper iodide thin films by chemical bath deposition (CBD) and successive ionic layer adsorption and reaction (SILAR) methods. Curr. Appl. Phys. 2013, 13, 1661–1667. [Google Scholar] [CrossRef]
- Bayansal, F.; Sahin, B.; Yüksel, M.; Biyikli, N.; Cetinkara, H.A.; Güder, H.S. Influence of coumarin as an additive on CuO nanostructures prepared by successive ionic layer adsorption and reaction (SILAR) method. J. Alloys Compd. 2013, 566, 78–82. [Google Scholar] [CrossRef]
- Ravichandran, A.; Dhanabalan, K.; Valanarasu, S.; Vasuhi, A.; Kathalingam, A. Role of immersion time on the properties of SILAR deposited CuO thin films. J. Mater. Sci. Mater. Electron. 2015, 26, 921–926. [Google Scholar] [CrossRef]
- Shinde, S.K.; Dubal, D.P.; Ghodake, G.S.; Kim, D.Y.; Fulari, V.J. Morphological tuning of CuO nanostructures by simple preparative parameters in SILAR method and their consequent effect on supercapacitors. Nano Struct. Nano Objects 2016, 6, 5–13. [Google Scholar] [CrossRef]
- Patil, A.S.; Patil, M.D.; Lohar, G.M.; Jadhav, S.T.; Fulari, V.J. Supercapacitive properties of CuO thin films using modified SILAR method. Ionics 2017, 23, 1259–1266. [Google Scholar] [CrossRef]
- Gençyılmaz, O.; Taşköprü, T. Effect of pH on the synthesis of CuO films by SILAR method. J. Alloys Compd. 2017, 695, 1205–1212. [Google Scholar] [CrossRef]
- Jang, J.; Chung, S.; Kang, H.; Subramanian, V. P-type CuO and Cu2O transistors derived from a sol–gel copper (II) acetate monohydrate precursor. Thin Solid Film. 2016, 600, 157–161. [Google Scholar] [CrossRef]
- Yu, R.-S.; Hu, D.-H. Formation and characterization of p-type semiconductor CuCrO2 thin films prepared by a sol–gel method. Ceram. Int. 2015, 41, 9383–9391. [Google Scholar] [CrossRef]
- Balık, M.; Bulut, V.; Erdogan, I.Y. Optical, structural and phase transition properties of Cu2O, CuO and Cu2O/CuO: Their photoelectrochemical sensor applications. Int. J. Hydrogen Energy 2019, 44, 18744–18755. [Google Scholar] [CrossRef]
- Daoudi, O.; Elmadani, A.; Lharch, M.; Fahoume, M. A new efficient synthesis of CuO thin films using modified SILAR method. Opt. Quantum Electron. 2020, 52, 413. [Google Scholar] [CrossRef]
- Pelawi, L.F.; Slamet, S.; Elysabeth, T. Combination of electrocoagulation and photocatalysis for hydrogen production and decolorization of tartrazine dyes using CuO-TiO2 nanotubes photocatalysts. AIP Conf. Proc. 2020, 2223, 040001. [Google Scholar] [CrossRef]
- De Brito, J.F.; Tavella, F.; Genovese, C.H.; Ampelli, C.; Zanoni, M.V.B.; Centi, G.; Perathoner, S. Role of CuO in the modification of the photocatalytic water splitting behavior of TiO2 nanotube thin films. Appl. Catal. B Environ. 2018, 224, 136–145. [Google Scholar] [CrossRef]
- Konstantinova, E.A.; Zaitsev, V.B.; Kytina, E.V.; Marikutsa, A.V. Photoaccumulating nanoheterostructures based on titanium dioxide. Semiconductors 2021, 55, 219–227. [Google Scholar] [CrossRef]
- Kim, B.-H.; Park, M.; Kim, G.; Hermansson, K.; Broqvist, P.; Choi, H.-J.; Lee, K.-R. Indirect-to-Direct Band Gap Transition of Si Nanosheets: Effect of Biaxial Strain. J. Phys. Chem. C 2018, 122, 15297–15303. [Google Scholar] [CrossRef]
- Martín-Gomez, J.; Hidalgo-Carrillo, J.; Montes, V.; Estevez-Toledano, R.C.; Escamilla, J.C.; Marinas, A.; Urbano, F.J. EPR and CV studies cast further light on the origin of the enhanced hydrogen production through glycerol photoreforming on CuO:TiO2 physical mixtures. J. Environ. Chem. Eng. 2021, 9, 105336. [Google Scholar] [CrossRef]
- Kokorin, A.; Bahnemann, D. Chemical Physics of Nanostructured Semiconductors; CRC Press: London, UK, 2003; pp. 203–261. [Google Scholar]
- Savchuk, T.P.; Kytina, E.V.; Konstantinova, E.A.; Kytin, V.G.; Pinchuk, O.; Tarhanov, A.K.; Zaitsev, V.B.; Maniecki, T. Photocatalytic CO2 conversion using anodic TiO2 nanotube-CuxO composites. Catalysts 2022, 12, 1011. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rodriguez, J.A.; Hanson, J.C.; Frenkel, A.I.; Lee, P.L. Reduction of CuO and Cu2O with H2: H embedding and kinetic effects in the formation of suboxides. J. Am. Chem. Soc. 2003, 125, 10684–10692. [Google Scholar] [CrossRef] [PubMed]
- Savchuk, T.; Gavrilin, I.; Konstantinova, E.A.; Dronov, A.; Volkov, R.L.; Borgardt, N.; Maniecki, T.; Gavrilov, S.A.; Zaitsev, V.B. Anodic TiO2 nanotube arrays for photocatalytic CO2 conversion: Comparative photocatalysis and EPR study. Nanotechnology 2022, 33, 055706. [Google Scholar] [CrossRef] [PubMed]
- Sathya, M.; Brahmari, H.; Ashil, A.; Mariyappan, S.H.; Chitiphon, C.H.; Keiko, S.; Boopathy, R.; Karthikeyan, S. A Critical Study of Cu2O: Synthesis and Its Application in CO2 Reduction by Photochemical and Electrochemical Approaches. Catalysts 2022, 12, 445. [Google Scholar] [CrossRef]
- Yoshio, N.; Atsuko, N. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
- Li, Z.; Luan, Y.; Qu, Y.; Jing, L. Modification strategies with inorganic acids for efficient photocatalysts by promoting the adsorption of O2. ACS Appl. Mater. Interfaces 2015, 7, 22727–22740. [Google Scholar] [CrossRef]
- Masahiro, M.; Kayano, S.; Kazuhito, H. Antiviral Effect of Visible Light-Sensitive CuxO/TiO2 Photocatalyst. Catalysts 2020, 10, 1093. [Google Scholar] [CrossRef]
- Jun, M.; Furong, W.; Mehran, M. Ultrafast Chemistry of Water Radical Cation, H2O•+, in Aqueous Solutions. Molecules 2018, 23, 244. [Google Scholar] [CrossRef]
- Wardman, P. Reduction Potentials of One-Electron Couples Involving Free Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef]
- Shekofteh-Gohari, M.; Habibi-Yangjeh, A.; Abitorabi, M.; Rouhi, A. Magnetically separable nanocomposites based on ZnO and their applications in photocatalytic processes: A review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 806–857. [Google Scholar] [CrossRef]
- Idrees, K.H.; Khalid, S.; Ivar, Z.; Baoliang, Z.H.; Abdulmajeed, H.; Ashfaq, A.; Shujaat, A.; Zada Hanif, A.; Luqman, S.H.; Tariq, S.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinova, E.; Savchuk, T.; Pinchuk, O.; Kytina, E.; Ivanova, E.; Volkova, L.; Zaitsev, V.; Pavlikov, A.; Elizarova, E. Photoelectron Properties and Organic Molecules Photodegradation Activity of Titania Nanotubes with CuxO Nanoparticles Heat Treated in Air and Argon. Molecules 2022, 27, 8080. https://doi.org/10.3390/molecules27228080
Konstantinova E, Savchuk T, Pinchuk O, Kytina E, Ivanova E, Volkova L, Zaitsev V, Pavlikov A, Elizarova E. Photoelectron Properties and Organic Molecules Photodegradation Activity of Titania Nanotubes with CuxO Nanoparticles Heat Treated in Air and Argon. Molecules. 2022; 27(22):8080. https://doi.org/10.3390/molecules27228080
Chicago/Turabian StyleKonstantinova, Elizaveta, Timofey Savchuk, Olga Pinchuk, Ekaterina Kytina, Elizaveta Ivanova, Lidiya Volkova, Vladimir Zaitsev, Alexander Pavlikov, and Elena Elizarova. 2022. "Photoelectron Properties and Organic Molecules Photodegradation Activity of Titania Nanotubes with CuxO Nanoparticles Heat Treated in Air and Argon" Molecules 27, no. 22: 8080. https://doi.org/10.3390/molecules27228080
APA StyleKonstantinova, E., Savchuk, T., Pinchuk, O., Kytina, E., Ivanova, E., Volkova, L., Zaitsev, V., Pavlikov, A., & Elizarova, E. (2022). Photoelectron Properties and Organic Molecules Photodegradation Activity of Titania Nanotubes with CuxO Nanoparticles Heat Treated in Air and Argon. Molecules, 27(22), 8080. https://doi.org/10.3390/molecules27228080







