Synthesis, In Silico and In Vivo Toxicity Assessment of Functionalized Pyridophenanthridinones via Sequential MW-Assisted Intramolecular Friedel-Crafts Alkylation and Direct C–H Arylation
Abstract
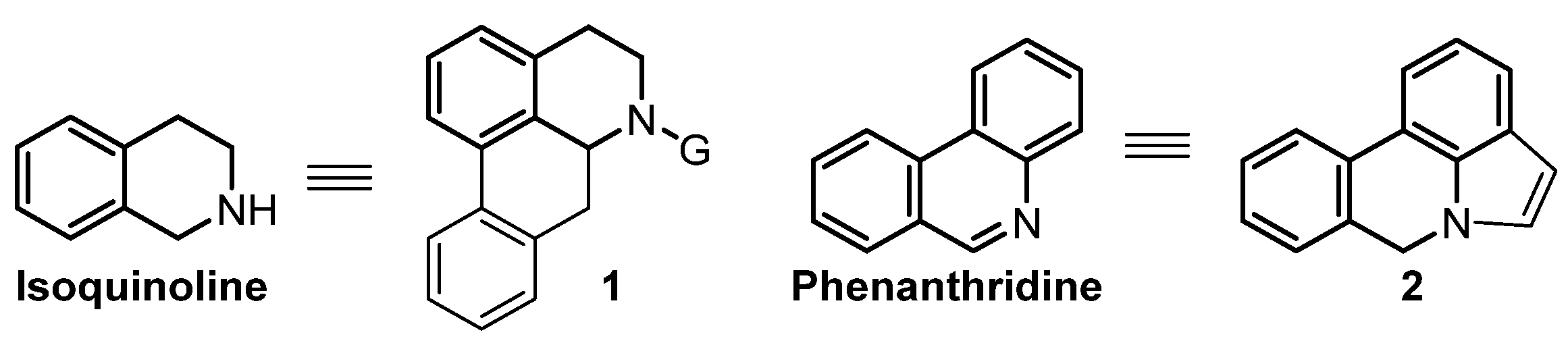
1. Introduction
2. Results and Discussion
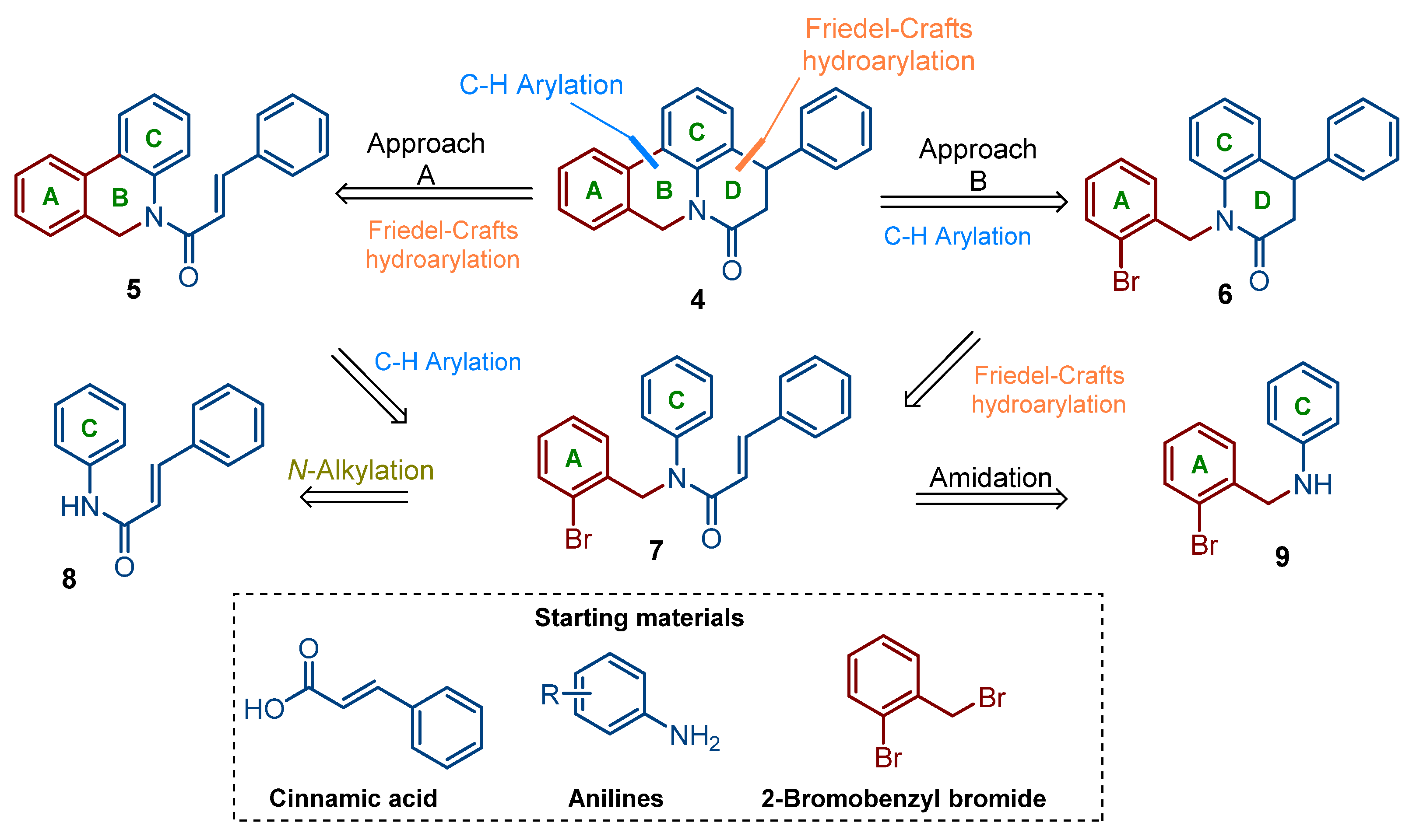
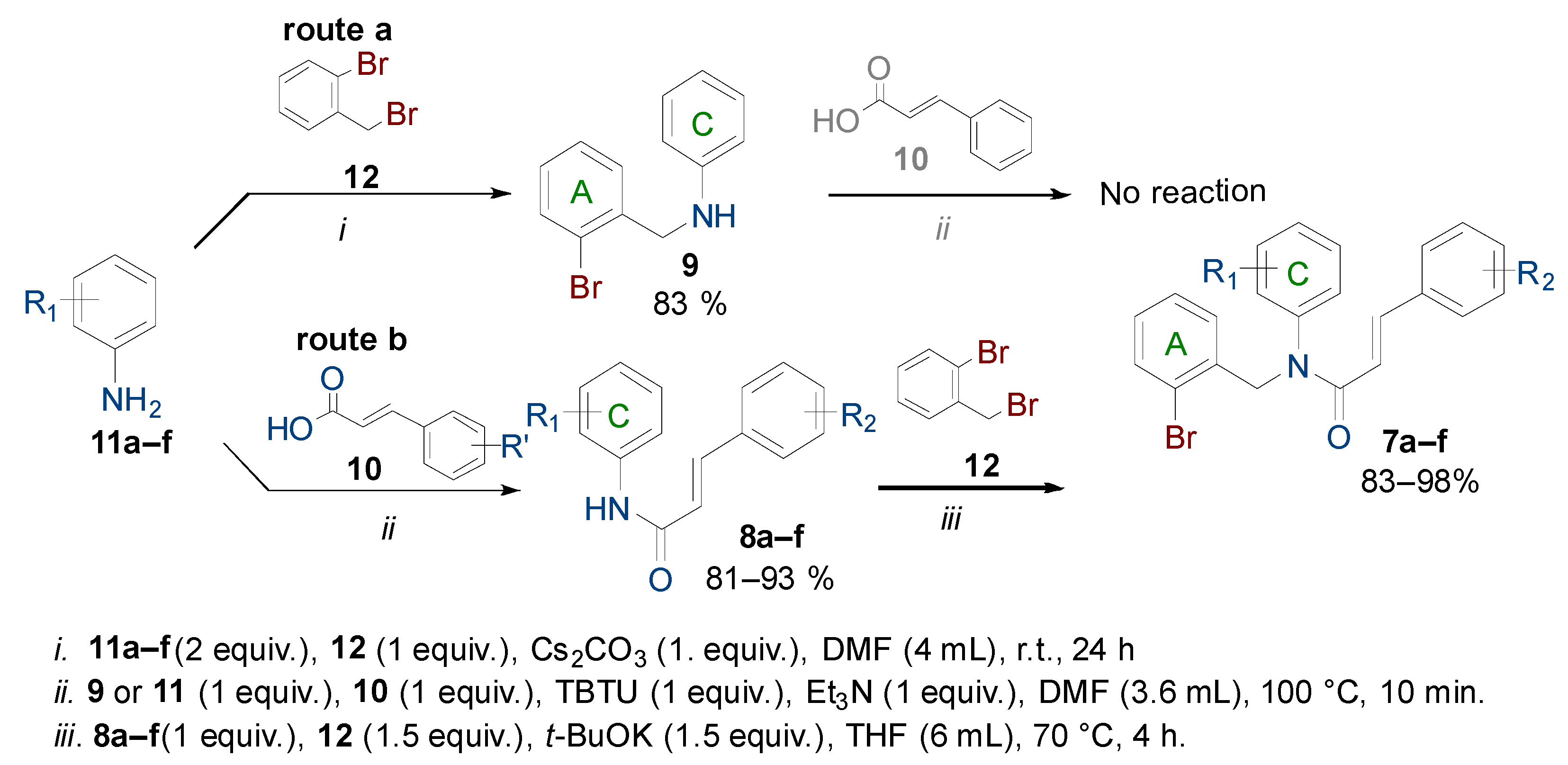
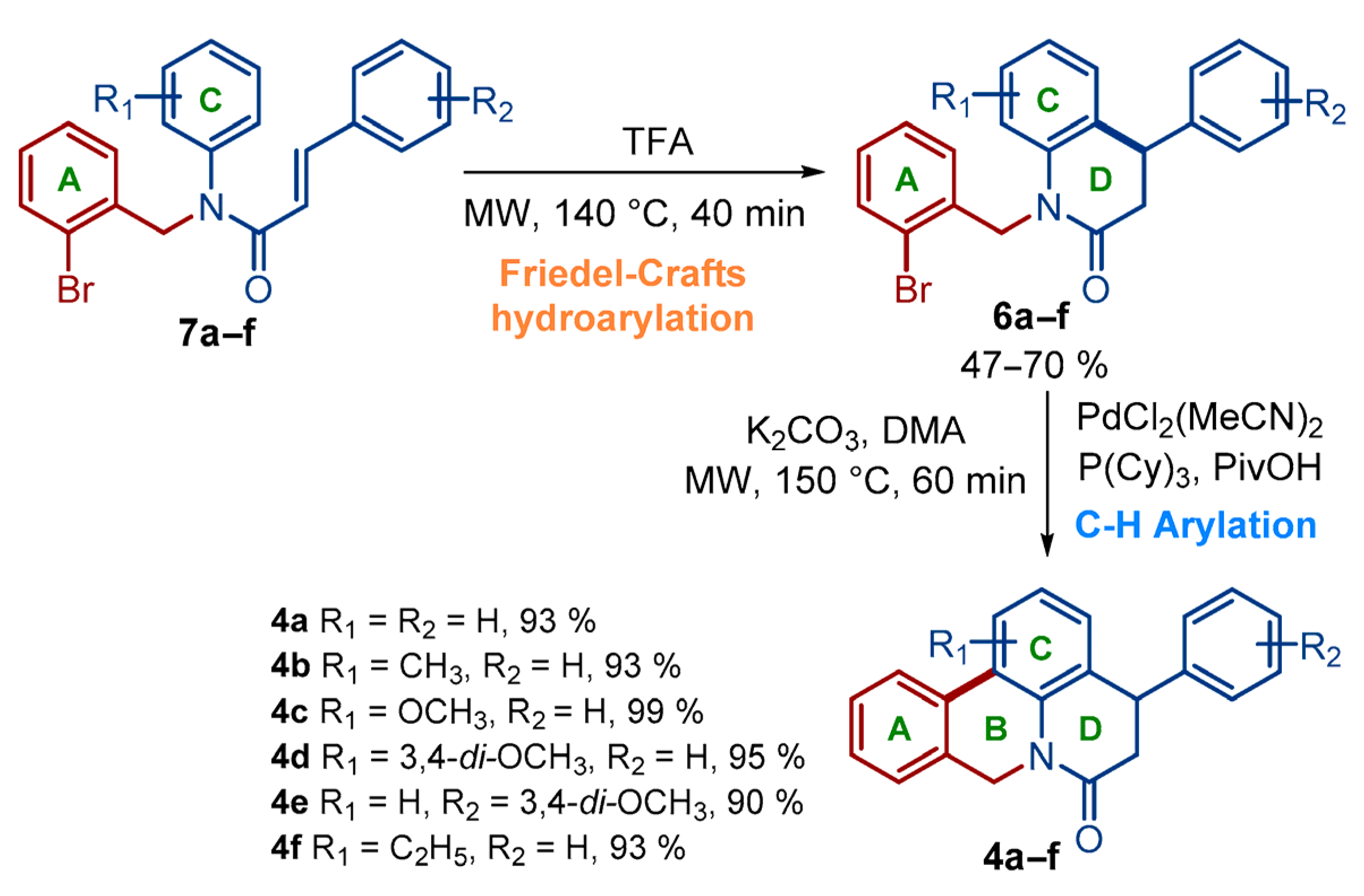
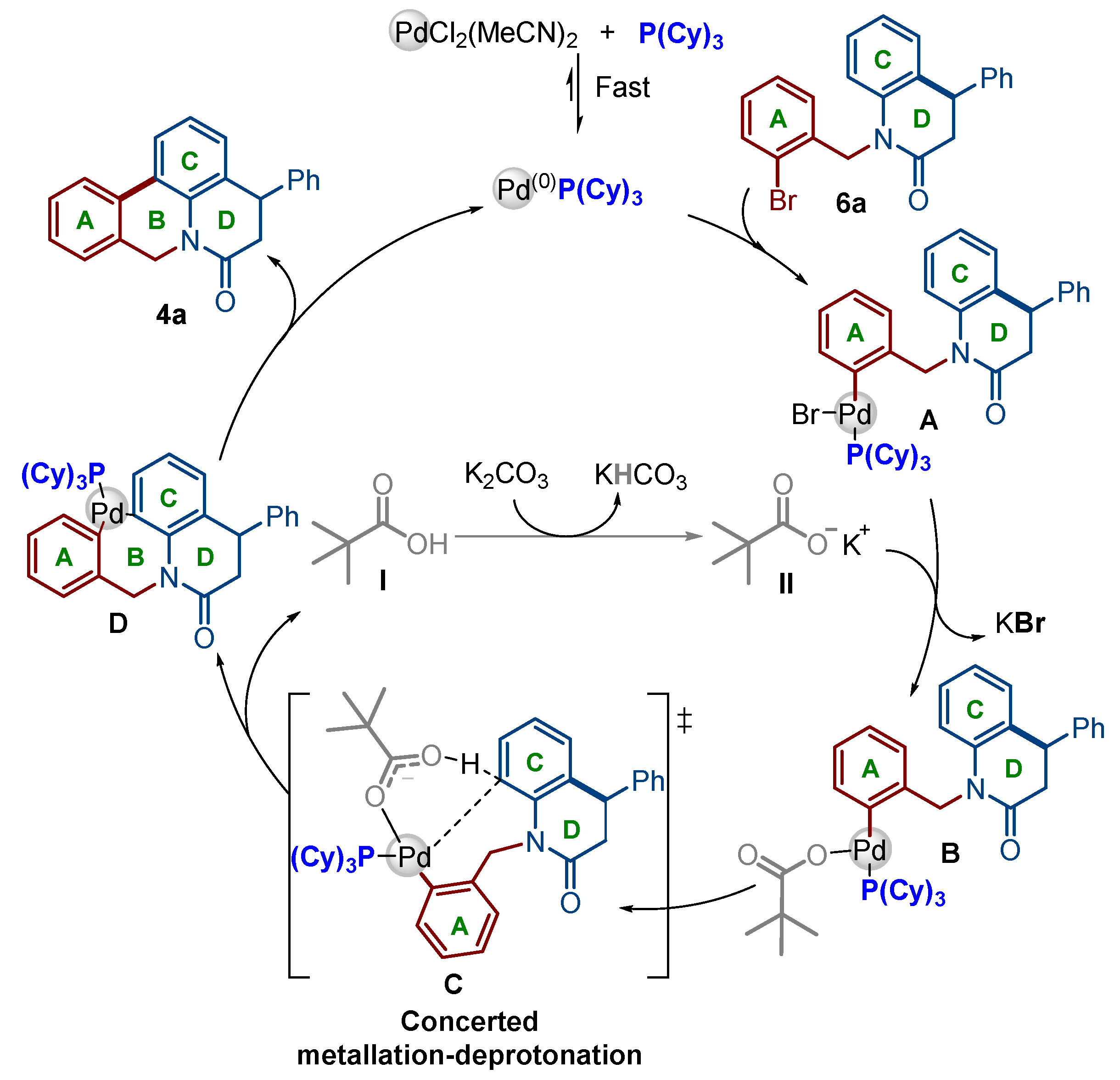
2.1. Chemistry
2.2. Computational Studies
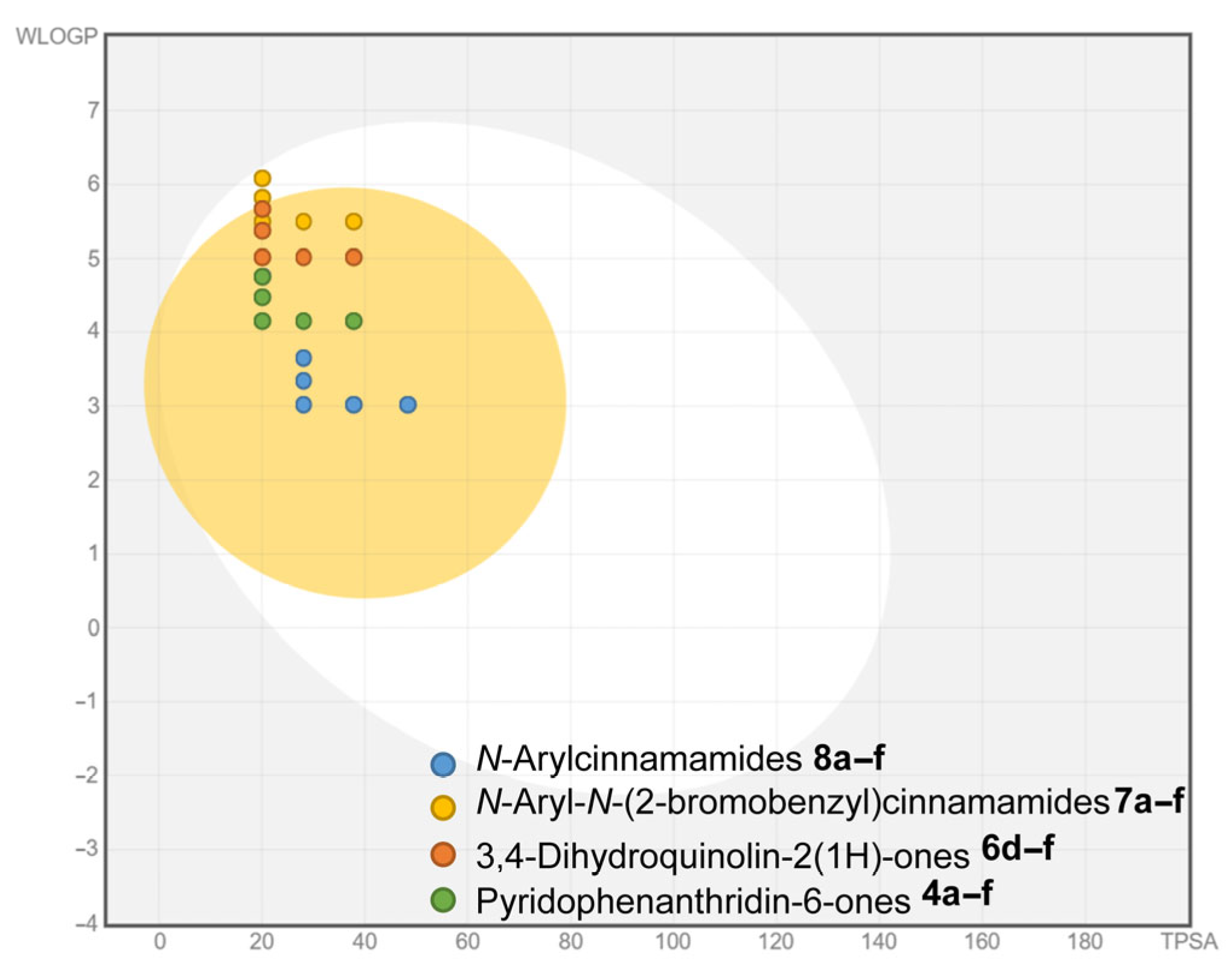
2.2.1. In Silico Prediction of Physicochemical Properties
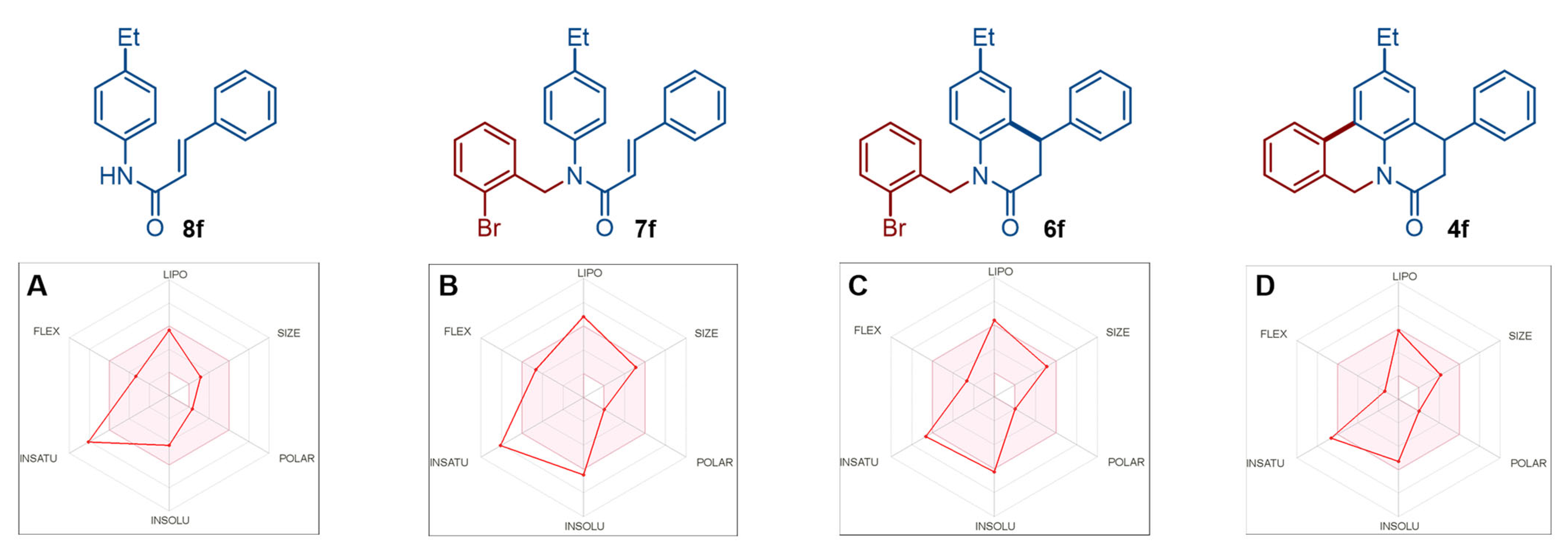
2.2.2. Bioavailability Radar
2.2.3. ADMET Study
2.3. In Vivo Toxicity Assessment in Zebrafish Embryos
3. Materials and Methods
3.1. General Procedures
3.2. General Procedure for the Synthesis of N-Aryl Cinnamamides 8a–f
3.3. General Procedure for the Synthesis of N-(2-Bromobenzyl)-N-Phenyl Cinnamamides 7a–f
3.4. General Procedure for the Synthesis of 4-Phenyl-3,4-Dihydroquinolin-2(1H)-Ones 6a–f
3.5. General Procedure for the Synthesis of Pyrido[3,2,1-de]Phenanthridin-6-Ones 4a–f
3.6. In Silico and Bioinformatic Studies
3.7. Toxicity Assessment of the Pyridophenanthridin-6-One 4f and Its Corresponding Precursors 6f–8f Using the Zebrafish Embryo Model
3.8. Determination of Zebrafish Embryo LC50
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Oderinde, M.S.; Mao, E.; Ramirez, A.; Pawluczyk, J.; Jorge, C.; Cornelius, L.A.M.; Kempson, J.; Vetrichelvan, M.; Pitchai, M.; Gupta, A.; et al. Synthesis of Cyclobutane-Fused Tetracyclic Scaffolds via Visible-Light Photocatalysis for Building Molecular Complexity. J. Am. Chem. Soc. 2020, 142, 3094–3103. [Google Scholar] [CrossRef]
- Yokosaka, T.; Nemoto, T.; Nakayama, H.; Shiga, N.; Hamada, Y. Synthesis of nitrogen-containing fused-polycyclic compounds from tyramine derivatives using phenol dearomatization and cascade cyclization. Chem. Commun. 2014, 50, 12775–12778. [Google Scholar] [CrossRef] [PubMed]
- Aulenta, F.; Wefelscheid, U.K.; Brüdgam, I.; Reißig, H.-U. Nitrogen-Containing Tricyclic and Tetracyclic Compounds by Stereoselective Samarium Diiodide Promoted Cyclizations of Quinolyl-Substituted Ketones—A New Access to Azasteroids. Eur. J. Org. Chem. 2008, 2008, 2325–2335. [Google Scholar] [CrossRef]
- Ponnala, S.; Chaudhary, S.; González-Sarrias, A.; Seeram, N.P.; Harding, W.W. Cytotoxicity of aporphines in human colon cancer cell lines HCT-116 and Caco-2: An SAR study. Bioorg. Med. Chem. Lett. 2011, 21, 4462–4464. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Di, D.; Li, M.; Fen, Y. Structural and mechanistic bases of the anticancer activity of natural aporphinoid alkaloids. Curr. Top. Med. Chem. 2013, 13, 2116–2126. [Google Scholar] [CrossRef]
- Chapleur, Y.; Chretien, F.; Ibn Ahmed, S.; Khaldi, M. Chemistry and Synthesis of Highly Oxygenated Alkaloids from Amaryllidaceae: Lycoricidine, Narciclasine, Pancratistatin and Analogs. Curr. Org. Synth. 2006, 3, 341–378. [Google Scholar] [CrossRef]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2016, 33, 1318–1343. [Google Scholar] [CrossRef]
- Achten, C.; Andersson, J.T. Overview of Polycyclic Aromatic Compounds (PAC). Polycycl. Aromat. Compd. 2015, 35, 177–186. [Google Scholar] [CrossRef]
- Ranjbar, S.; Edraki, N.; Firuzi, O.; Khoshneviszadeh, M.; Miri, R. 5-Oxo-hexahydroquinoline: An attractive scaffold with diverse biological activities. Mol. Divers. 2019, 23, 471–508. [Google Scholar] [CrossRef]
- Aleti, R.R.; Festa, A.A.; Voskressensky, L.G.; Van der Eycken, E.V. Synthetic Strategies in the Preparation of Phenanthridinones. Molecules 2021, 26, 5560–5581. [Google Scholar] [CrossRef]
- Fukuda, H.; Karaki, F.; Dodo, K.; Noguchi-Yachide, T.; Ishikawa, M.; Hashimoto, Y.; Ohgane, K. Phenanthridin-6-one derivatives as the first class of non-steroidal pharmacological chaperones for Niemann-Pick disease type C1 protein. Bioorganic Med. Chem. Lett. 2017, 27, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.-Q.; Li, L.-C.; Song, J.-R.; Li, H.-Y.; Li, D.-P. Structurally Simple Phenanthridine Analogues Based on Nitidine and Their Antitumor Activities. Molecules 2019, 24, 437. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Kamath, S.; Sanchez, T.; Neamati, N.; Schinazi, R.F.; Buolamwini, J.K. Synthesis and biological evaluation of novel 5(H)-phenanthridin-6-ones, 5(H)-phenanthridin-6-one diketo acid, and polycyclic aromatic diketo acid analogs as new HIV-1 integrase inhibitors. Bioorg. Med. Chem. 2007, 15, 1212–1228. [Google Scholar] [CrossRef] [PubMed]
- Weltin, D.; Picard, V.; Aupeix, K.; Varin, M.; Oth, D.; Marchal, J.; Dufour, P.; Bischoff, P. Immunosuppressive activities of 6(5h)-phenanthridinone, a new poly(adp-ribose)polymerase inhibitor. Int. J. Immunopharmacol. 1995, 17, 265–271. [Google Scholar] [CrossRef]
- Harayama, T.; Sato, T.; Hori, A.; Abe, H.; Takeuchi, Y. Palladium-assisted Biaryl Coupling Reaction of 1-(2-Iodobenzoyl)-1,2,3,4-tetrahydroquinoline. Heterocycles 2005, 66, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; LaPorte, M.G.; McIntosh, M.C.; Weinreb, S.M.; Parvez, M. Exploratory Synthetic Studies of the α-Methoxylation of Amides via Cuprous Ion-Promoted Decomposition of o-Diazobenzamides. J. Org. Chem. 1996, 61, 9483–9493. [Google Scholar] [CrossRef]
- Puerto Galvis, C.E.; Kouznetsov, V.V. Recent Advances for the C–C and C–N Bond Formation in the Synthesis of 1-Phenethyl-tetrahydroisoquinoline, Aporphine, Homoaporphine, and β-Carboline Alkaloids. Synthesis 2017, 49, 4535–4561. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Jiao, N. Rh-Catalyzed Construction of Quinolin-2(1H)-ones via C–H Bond Activation of Simple Anilines with CO and Alkynes. J. Am. Chem. Soc. 2015, 137, 9246–9249. [Google Scholar] [CrossRef]
- Wu, J.; Xiang, S.; Zeng, J.; Leow, M.; Liu, X.-W. Practical Route to 2-Quinolinones via a Pd-Catalyzed C–H Bond Activation/C–C Bond Formation/Cyclization Cascade Reaction. Org. Lett. 2015, 17, 222–225. [Google Scholar] [CrossRef]
- Qi, C.; Guo, T.; Xiong, W.; Wang, L.; Jiang, P.H. Silver-Promoted Coupling of Carbon Dioxide,o-Alkynylanilines and Diaryliodonium Salts: Straightforward Access to 4-Aryloxy-2-quinolinones. ChemistrySelect 2017, 2, 4691–4695. [Google Scholar] [CrossRef]
- Zhang, B.; Studer, A. Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Feng, X.; Hu, B.-L.; Deng, C.-L.; Zhang, X.-G. Synthesis of 6-(Trifluoromethyl)phenanthridines via Palladium-Catalyzed Tandem Suzuki/C–H Arylation Reactions. J. Org. Chem. 2013, 78, 6025–6030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Mück-Lichtenfeld, C.; Daniliuc, C.G.; Studer, A. 6-Trifluoromethyl-Phenanthridines through Radical Trifluoromethylation of Isonitriles. Angew. Chem. Int. Ed. 2013, 52, 10792–10795. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, Y.; Li, W.; Xie, Q.; Shao, L. Microwave-Assisted Synthesis of Phenanthridines by Radical Insertion/Cyclization of Biphenyl Isocyanides. J. Org. Chem. 2016, 81, 8426–8435. [Google Scholar] [CrossRef]
- Jiang, H.; An, X.; Tong, K.; Zheng, T.; Zhang, Y.; Yu, S. Visible-Light-Promoted Iminyl-Radical Formation from Acyl Oximes: A Unified Approach to Pyridines, Quinolines, and Phenanthridines. Angew. Chem. Int. Ed. 2015, 54, 4055–4059. [Google Scholar] [CrossRef]
- Lafrance, M.; Blaquière, N.; Fagnou, K. Direct intramolecular arylation of unactivated arenes: Application to the synthesis of aporphine alkaloids. Chem. Commun. 2004, 24, 2874–2875. [Google Scholar] [CrossRef]
- Hellal, M.; Singh, S.; Cuny, G.D. Monoligated Pd(0)-catalyzed intramolecular ortho- and para-arylation of phenols for the synthesis of aporphine alkaloids. Synthesis of (−)-lirinine. Tetrahedron 2012, 68, 1674–1681. [Google Scholar] [CrossRef]
- Cuny, G.D. Synthesis of (±)-aporphine utilizing Pictet–Spengler and intramolecular phenol ortho-arylation reactions. Tetrahedron Lett. 2004, 45, 5167–5170. [Google Scholar] [CrossRef]
- Lafrance, M.; Blaquiere, N.; Fagnou, K. Aporphine Alkaloid Synthesis and Diversification via Direct Arylation. Eur. J. Org. Chem. 2007, 2007, 811–825. [Google Scholar] [CrossRef]
- Pedroni, J.; Saget, T.; Donets, P.A.; Cramer, N. Enantioselective palladium(0)-catalyzed intramolecular cyclopropane functionalization: Access to dihydroquinolones, dihydroisoquinolones and the BMS-791325 ring system. Chem. Sci. 2015, 6, 5164–5171. [Google Scholar] [CrossRef]
- Kong, W.-X.; Xie, S.-J.; Cao, C.-Y.; Zhang, C.-W.; Wang, C.; Duan, W.-L. Asymmetric construction of quaternary α-nitro amides by palladium-catalyzed C(sp3)–H arylation. Chem. Commun. 2020, 56, 2292–2295. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Foresee, A.L.N.; Tunge, J.A. Trifluoroacetic Acid-Mediated Hydroarylation: Synthesis of Dihydrocoumarins and Dihydroquinolones. J. Org. Chem. 2005, 70, 2881–2883. [Google Scholar] [CrossRef]
- Angibaud, P.R.; Venet, M.G.; Filliers, W.; Broeckx, R.; Ligny, Y.A.; Muller, P.; Poncelet, V.S.; End, D.W. Synthesis Routes Towards the Farnesyl Protein Transferase Inhibitor ZARNESTRATM. Eur. J. Org. Chem. 2004, 2004, 479–486. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, J.Y.; Kwak, J.; Chang, S. Recent advances in the transition metal-catalyzed twofold oxidative C–H bond activation strategy for C–C and C–N bond formation. Chem. Soc. Rev. 2011, 40, 5068–5083. [Google Scholar] [CrossRef]
- Gómez-Lor, A.B.; Echavarren, A.M. Synthesis of a Triaza Analogue of Crushed-Fullerene by Intramolecular Palladium-Catalyzed Arylation. Org. Lett. 2004, 6, 2993–2996. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, M.; Sharma, S.; Nayal, O.S.; Kumar, N.; Singh, B.; Sharma, U. Microwave assisted synthesis of phenanthridinones and dihydrophenanthridines by vasicine/KOtBu promoted intramolecular C–H arylation. Org. Biomol. Chem. 2016, 14, 8536–8544. [Google Scholar] [CrossRef] [PubMed]
- Ortiz Villamizar, M.C.; Puerto Galvis, C.E.; Kouznetsov, V.V. The A3 Redox-Neutral C1-Alkynylation of Tetrahydroisoquinolines: A Comparative Study between Visible Light Photocatalysis and Transition-Metal Catalysis. Synthesis 2020, 53, 547–556. [Google Scholar] [CrossRef]
- Puerto Galvis, C.E.; Kouznetsov, V.V. Biomimetic Total Synthesis of Dysoxylum Alkaloids. J. Org. Chem. 2019, 84, 15294–15308. [Google Scholar] [CrossRef] [PubMed]
- Safina, L.Y.; Selivanova, G.A.; Koltunov, K.Y.; Shteingarts, V.D. Synthesis of polyfluorinated 4-phenyl-3,4-dihydroquinolin-2-ones and quinolin-2-ones via superacidic activation of N-(polyfluorophenyl)cinnamamides. Tetrahedron Lett. 2009, 50, 5245–5247. [Google Scholar] [CrossRef]
- Inglis, S.R.; Stojkoski, C.; Branson, K.M.; Cawthray, J.F.; Fritz, D.; Wiadrowski, E.; Pyke, S.M.; Booker, G.W. Identification and Specificity Studies of Small-Molecule Ligands for SH3 Protein Domains. J. Med. Chem. 2004, 47, 5405–5417. [Google Scholar] [CrossRef]
- Koltunov, K.Y.; Prakash, G.K.S.; Rasul, G.; Olah, G.A. Superacidic Activation of Maleimide and Phthalimide and Their Reactions with Cyclohexane and Arenes. Eur. J. Org. Chem. 2006, 2006, 4861–4866. [Google Scholar] [CrossRef]
- de Figueiredo, R.M.; Suppo, J.-S.; Campagne, J.-M. Nonclassical Routes for Amide Bond Formation. Chem. Rev. 2016, 116, 12029–12122. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Porras, A.; Gamba-Sánchez, D. Recent Developments in Amide Synthesis Using Nonactivated Starting Materials. J. Org. Chem. 2016, 81, 11548–11555. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, R.M.; Sheppard, T.D. Recent Developments in Amide Synthesis: Direct Amidation of Carboxylic Acids and Transamidation Reactions. Eur. J. Org. Chem. 2013, 2013, 7453–7465. [Google Scholar] [CrossRef]
- Ojeda-Porras, A.; Hernández-Santana, A.; Gamba-Sánchez, D. Direct amidation of carboxylic acids with amines under microwave irradiation using silica gel as a solid support. Green Chem. 2015, 17, 3157–3163. [Google Scholar] [CrossRef]
- Puerto Galvis, C.E.; Kouznetsov, V.V. Synthesis of zanthoxylamide protoalkaloids and their in silico ADME-Tox screening and in vivo toxicity assessment in zebrafish embryos. Eur. J. Pharm. Sci. 2018, 127, 291–299. [Google Scholar] [CrossRef]
- Dunetz, J.R.; Magano, J.; Weisenburger, G.A. Large-Scale Applications of Amide Coupling Reagents for the Synthesis of Pharmaceuticals. Org. Process. Res. Dev. 2016, 20, 140–177. [Google Scholar] [CrossRef]
- Ortiz Villamizar, M.C.; Zubkov, F.I.; Puerto Galvis, C.E.; Vargas Méndez, L.Y.; Kouznetsov, V.V. The study of metal-free and palladium-catalysed synthesis of benzochromenes via direct C–H arylation using unactivated aryl benzyl ethers derived from essential oils as raw materials. Org. Chem. Front. 2017, 4, 1736–1744. [Google Scholar] [CrossRef]
- Rueping, M.; Nachtsheim, B.J. A review of new developments in the Friedel-Crafts alkylation—From green chemistry to asymmetric catalysis. Beilstein J. Org. Chem. 2010, 6, 6. [Google Scholar] [CrossRef]
- Mierina, I.; Jure, M.; Stikute, A. Synthetic approaches to 4-(het)aryl-3,4-dihydroquinolin-2(1H)-ones. Chem. Heterocycl. Compd. 2016, 52, 509–523. [Google Scholar] [CrossRef]
- López, S.E.; Salazar, J. Trifluoroacetic acid: Uses and recent applications in organic synthesis. J. Fluor. Chem. 2013, 156, 73–100. [Google Scholar] [CrossRef]
- Pardo, L.M.; Prendergast, A.M.; Nolan, M.-T.; Muimhneacháin, E.; McGlacken, G.P. Pd/Pivalic Acid Mediated Direct Arylation of 2-Pyrones and Related Heterocycles. Eur. J. Org. Chem. 2015, 2015, 3540–3550. [Google Scholar] [CrossRef]
- García-Cuadrado, D.; Braga, A.A.C.; Maseras, F.; Echavarren, A.M. Proton Abstraction Mechanism for the Palladium-Catalyzed Intramolecular Arylation. J. Am. Chem. Soc. 2006, 128, 1066–1067. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves first drug under new antibacterial and antifungal drug programme. Nat. Rev. Drug Discov. 2018, 17, 777. [Google Scholar] [CrossRef]
- Ewes, W.A.; Elmorsy, M.A.; El-Messery, S.M.; Nasr, M.N. Synthesis, biological evaluation and molecular modeling study of [1,2,4]-Triazolo[4,3-c]quinazolines: New class of EGFR-TK inhibitors. Bioorg. Med. Chem. 2020, 28, 115373. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, I.H.; Kim, H.J.; Chang, G.S.; Chung, J.E.; No, K.T. The PreADME Approach: Web-based program for rapid. Prediction of physico-chemical, drug absorption and drug-like properties. In EuroQSAR 2002–Designing Drugs and Crop Protectants: Processes, Problems and Solutions; Blackwell Publishing: Boston, MA, USA, 2003; Volume 2003, pp. 418–420. [Google Scholar]
- Wang, N.-N.; Huang, C.; Dong, J.; Yao, Z.-J.; Zhu, M.-F.; Deng, Z.-K.; Lv, B.; Lu, A.-P.; Chen, A.F.; Cao, D.-S. Predicting human intestinal absorption with modified random forest approach: A comprehensive evaluation of molecular representation, unbalanced data, and applicability domain issues. RSC Adv. 2017, 7, 19007–19018. [Google Scholar] [CrossRef]
- Lambrinidis, G.; Vallianatou, T.; Tsantili-Kakoulidou, A. In vitro, in silico and integrated strategies for the estimation of plasma protein binding. A review. Adv. Drug Deliv. Rev. 2015, 86, 27–45. [Google Scholar] [CrossRef]
- Banks, W.A. From blood–brain barrier to blood–brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X. QSPR model for Caco-2 cell permeability prediction using a combination of HQPSO and dual-RBF neural network. RSC Adv. 2020, 10, 42938–42952. [Google Scholar] [CrossRef] [PubMed]
- Fredlund, L.; Winiwarter, S.; Hilgendorf, C. In Vitro Intrinsic Permeability: A Transporter-Independent Measure of Caco-2 Cell Permeability in Drug Design and Development. Mol. Pharm. 2017, 14, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.-N.; Dong, J.; Deng, Y.-H.; Zhu, M.-F.; Wen, M.; Yao, Z.-J.; Lu, A.-P.; Wang, J.-B.; Cao, D.-S. ADME Properties Evaluation in Drug Discovery: Prediction of Caco-2 Cell Permeability Using a Combination of NSGA-II and Boosting. J. Chem. Inf. Model. 2016, 56, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Inoue, A.; Sasagawa, S.; Koiwa, J.; Kawaguchi, K.; Kawase, R.; Maruyama, T.; Kim, S.; Tanaka, T. Using zebrafish in systems toxicology for developmental toxicity testing. Congenit. Anom. 2016, 56, 18–27. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jang, G.H.; Byun, C.H.; Jeun, M.; Searson, P.C.; Lee, K.H. Zebrafish models for functional and toxicological screening of nanoscale drug delivery systems: Promoting preclinical applications. Biosci. Rep. 2017, 37, BSR20170199. [Google Scholar] [CrossRef]
- OECD. Section 2: Effects on biotic systems test no. 236: Fish embryo acute toxicity (FET) test. In Guidelines for the Testing of Chemicals; Organization for Economic Cooperation and Development: Paris, France, 2013. [Google Scholar]
- Ali, S.; Van Mil, H.G.J.; Richardson, M.K. Large-Scale Assessment of the Zebrafish Embryo as a Possible Predictive Model in Toxicity Testing. PLoS ONE 2011, 6, e21076. [Google Scholar] [CrossRef]

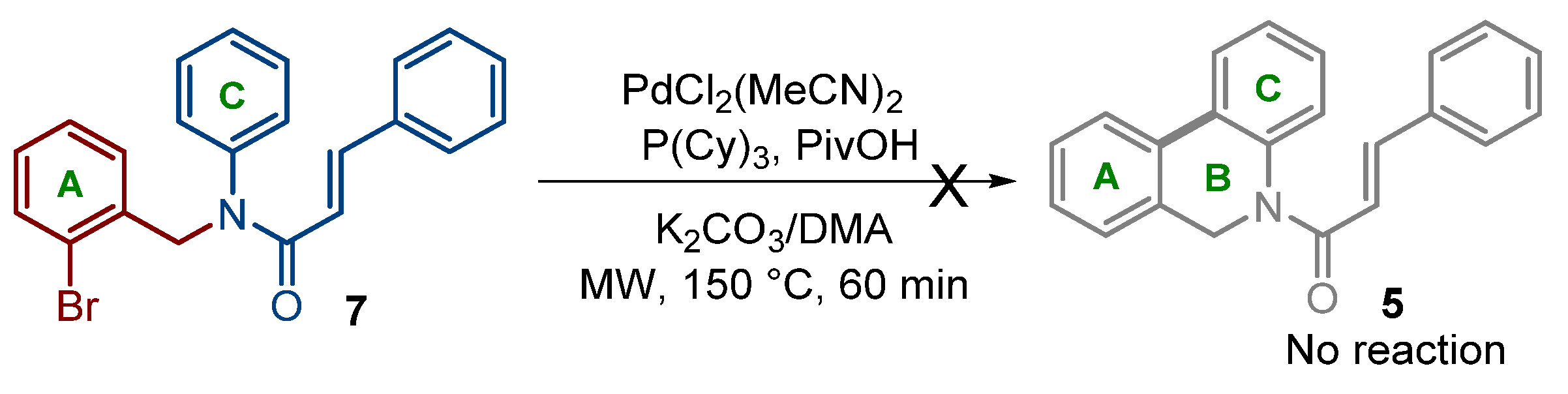

| Comp. | Physicochemical Properties | ADMET Profile | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MW a | CLogP b | CLogS c | HBA d | HBD e | nRB f | TPSA g | HIA h | PPB i | BBB j | PCaco-2 k | LogKp l | AMES m | CarGen n | |
| 8a | 223.27 | 3.07 | −5.01 | 1 | 1 | 4 | 29.10 | 97.61 | 100.0 | 2.0112 | 34.884 | −5.10 | Muta | − |
| 8b | 237.30 | 3.41 | −5.40 | 1 | 1 | 4 | 29.10 | 97.66 | 100.0 | 3.0328 | 35.831 | −4.93 | Muta | − |
| 8c | 253.30 | 3.08 | −5.14 | 2 | 1 | 5 | 38.33 | 95.86 | 84.71 | 0.6735 | 44.974 | −5.30 | Muta | + |
| 8d | 283.32 | 2.92 | −5.26 | 3 | 1 | 6 | 47.56 | 95.55 | 85.45 | 0.2240 | 49.428 | −5.89 | Muta | + |
| 8e | 283.32 | 3.07 | −5.26 | 3 | 1 | 6 | 47.56 | 95.55 | 81.27 | 0.2494 | 50.605 | −5.44 | Muta | + |
| 8f | 251.32 | 3.72 | −5.80 | 1 | 1 | 5 | 29.10 | 97.71 | 100.0 | 4.6416 | 39.151 | −4.70 | Muta | − |
| 7a | 392.29 | 4.97 | −7.99 | 1 | 0 | 6 | 20.31 | 100.0 | 97.94 | 1.5997 | 56.681 | −4.75 | Non-Mut | + |
| 7b | 406.32 | 5.36 | −8.37 | 1 | 0 | 6 | 20.31 | 100.0 | 96.14 | 2.2080 | 56.659 | −4.58 | Non-Mut | + |
| 7c | 422.31 | 5.01 | −8.09 | 2 | 0 | 2 | 29.54 | 100.0 | 94.41 | 0.7264 | 56.381 | −4.96 | Non-Mut | + |
| 7d | 452.34 | 4.96 | −8.19 | 3 | 0 | 8 | 38.77 | 98.57 | 91.61 | 0.3783 | 56.102 | −5.16 | Non-Mut | + |
| 7e | 452.34 | 4.93 | −8.19 | 3 | 0 | 8 | 38.77 | 98.57 | 91.00 | 0.4802 | 56.074 | −5.16 | Non-Mut | + |
| 7f | 420.34 | 5.67 | −8.76 | 1 | 0 | 1 | 20.31 | 100.0 | 99.43 | 3.3945 | 56.887 | −4.36 | Non-Mut | + |
| 6a | 392.29 | 4.67 | −8.44 | 1 | 0 | 3 | 20.31 | 100.0 | 97.52 | 1.3153 | 54.707 | −5.22 | Muta | + |
| 6b | 406.32 | 4.99 | −8.82 | 1 | 0 | 3 | 20.31 | 100.0 | 96.66 | 1.4601 | 54.821 | −5.05 | Muta | + |
| 6c | 422.31 | 4.62 | −8.54 | 2 | 0 | 4 | 29.54 | 100.0 | 94.41 | 0.6261 | 54.269 | −5.43 | Muta | + |
| 6d | 452.34 | 4.60 | −8.64 | 3 | 0 | 5 | 38.77 | 98.55 | 91.62 | 0.3588 | 53.881 | −5.63 | Muta | − |
| 6e | 452.34 | 4.59 | −8.64 | 1 | 0 | 3 | 38.77 | 98.55 | 91.59 | 0.5076 | 53.864 | −5.63 | Muta | − |
| 6f | 420.34 | 5.29 | −9.21 | 1 | 0 | 4 | 20.31 | 100.0 | 99.84 | 2.0731 | 55.379 | −4.83 | Muta | + |
| 4a | 311.38 | 4.01 | −7.51 | 1 | 0 | 1 | 20.31 | 100.0 | 100.0 | 2.8198 | 45.687 | −5.43 | Muta | − |
| 4b | 325.40 | 4.34 | −7.89 | 1 | 0 | 1 | 20.31 | 100.0 | 98.46 | 2.1787 | 46.537 | −5.25 | Muta | − |
| 4c | 341.40 | 3.98 | −7.62 | 2 | 0 | 2 | 29.54 | 100.0 | 94.61 | 3.6464 | 49.158 | −5.63 | Muta | − |
| 4d | 371.43 | 3.95 | −7.73 | 3 | 0 | 3 | 38.77 | 98.27 | 93.47 | 1.4389 | 50.886 | −5.84 | Muta | − |
| 4e | 371.43 | 3.93 | −7.73 | 3 | 0 | 3 | 38.77 | 98.27 | 89.78 | 0.5770 | 50.913 | −5.84 | Muta | − |
| 4f | 339.43 | 4.63 | −8.29 | 1 | 0 | 2 | 20.31 | 100.0 | 96.84 | 1.8388 | 48.352 | −5.03 | Muta | − |
| Comp. | Zebrafish LC50 a | Aquatic Animal Acute Toxicity b | |
|---|---|---|---|
| μmol/L ± SEM | mg/L ± SEM | ||
| 8f | 221.20 ± 4.39 | 43.9 ± 1.7 | ST |
| 7f | 136.91± 5.24 | 51.2 ± 1.7 | ST |
| 6f | 151.20 ± 8.53 | 86.5 ± 2.0 | ST |
| 4f | 67.81 ± 3.58 | 60.2 ± 1.2 | ST |
| Camptothecin 13 | 3.84 ± 0.21 c | 0.001 ± 0.1 | T |
| Imiquimod 14 | 350.85 ± 4.99 | 84.3 ± 1.2 | ST |
| Orlistat 15 | 86.99 ± 2.49 | 43.1 ± 1.2 | ST |
| Ribavirin 16 | 344.58 ± 4.23 | 84.1 ± 1.0 | ST |
| Propranolol 17 | 17.92 ± 0.79 | 4,6 ± 0.2 | MT |
| BHA 18 | 90.25 ± 1.15 | 16,3 ± 0.2 | ST |
| Eugenol 19 | 113.88 ± 0.54 | 18.7 ± 0.1 | ST |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz Villamizar, M.C.; Puerto Galvis, C.E.; Pedraza Rodríguez, S.A.; Zubkov, F.I.; Kouznetsov, V.V. Synthesis, In Silico and In Vivo Toxicity Assessment of Functionalized Pyridophenanthridinones via Sequential MW-Assisted Intramolecular Friedel-Crafts Alkylation and Direct C–H Arylation. Molecules 2022, 27, 8112. https://doi.org/10.3390/molecules27238112
Ortiz Villamizar MC, Puerto Galvis CE, Pedraza Rodríguez SA, Zubkov FI, Kouznetsov VV. Synthesis, In Silico and In Vivo Toxicity Assessment of Functionalized Pyridophenanthridinones via Sequential MW-Assisted Intramolecular Friedel-Crafts Alkylation and Direct C–H Arylation. Molecules. 2022; 27(23):8112. https://doi.org/10.3390/molecules27238112
Chicago/Turabian StyleOrtiz Villamizar, Marlyn C., Carlos E. Puerto Galvis, Silvia A. Pedraza Rodríguez, Fedor I. Zubkov, and Vladimir V. Kouznetsov. 2022. "Synthesis, In Silico and In Vivo Toxicity Assessment of Functionalized Pyridophenanthridinones via Sequential MW-Assisted Intramolecular Friedel-Crafts Alkylation and Direct C–H Arylation" Molecules 27, no. 23: 8112. https://doi.org/10.3390/molecules27238112
APA StyleOrtiz Villamizar, M. C., Puerto Galvis, C. E., Pedraza Rodríguez, S. A., Zubkov, F. I., & Kouznetsov, V. V. (2022). Synthesis, In Silico and In Vivo Toxicity Assessment of Functionalized Pyridophenanthridinones via Sequential MW-Assisted Intramolecular Friedel-Crafts Alkylation and Direct C–H Arylation. Molecules, 27(23), 8112. https://doi.org/10.3390/molecules27238112










