High Throughput Isolation and Data Independent Acquisition Mass Spectrometry (DIA-MS) of Urinary Extracellular Vesicles to Improve Prostate Cancer Diagnosis
Abstract
1. Introduction
2. Results
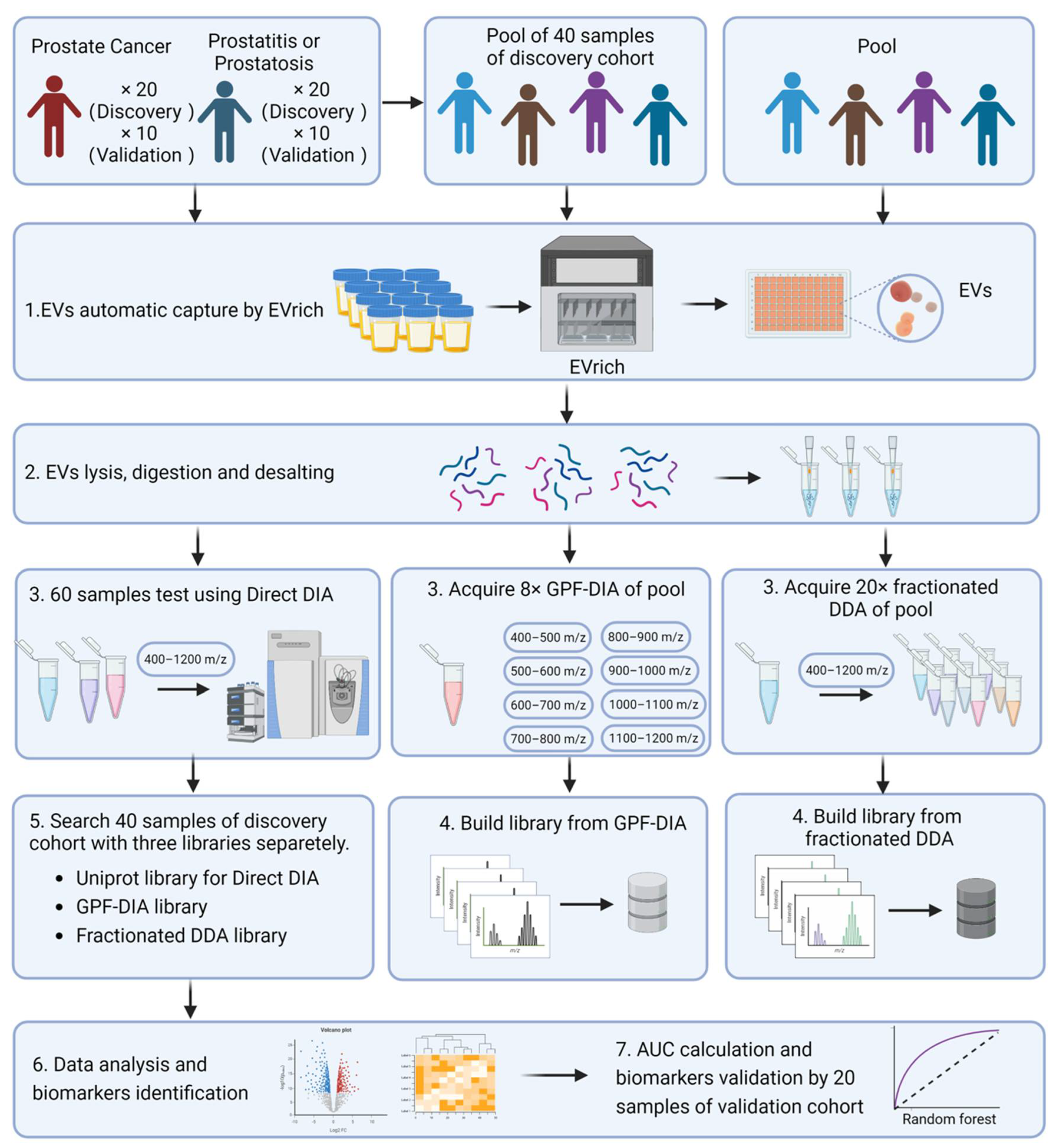
2.1. Automated Isolation and Characterization of Urinary EVs
2.2. Quantitative Measurement of Urinary EV Proteins through Integrated DIA-MS
2.3. EV Protein Profiling and Comparative Analyses for Prostate Cancer Outcome
2.4. Analysis of Identified EV Proteins in Classification of Prostate Cancer Cases and Controls
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Participants Recruitment and Demographic Characteristics Assessment
4.3. EVs Automatic Isolation by EVrich
4.4. Characterization of EVs by Transmission Electron Microscopy (TEM)
4.5. Nanoparticle Tracking Analysis
4.6. Western Blotting Analysis
4.7. Preparation of EV Samples for LC-MS
4.8. LC−MS/MS Analysis
4.9. Construction of Fractionated DDA, GPF, and Direct DIA Library
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu-Yao, G.L.; Greenberg, E.R. Changes in prostate cancer incidence and treatment in USA. Lancet 1994, 343, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W., Jr.; Kemper, A.R.; et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1901–1913. [Google Scholar] [PubMed]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Rontogianni, S.; Synadaki, E.; Li, B.; Liefaard, M.C.; Lips, E.H.; Wesseling, J.; Wu, W.; Altelaar, M. Proteomic profiling of extracellular vesicles allows for human breast cancer subtyping. Commun. Biol. 2019, 2, 325. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Skog, J.; Nordstrand, A.; Baranov, V.; Mincheva-Nilsson, L.; Breakefield, X.O.; Widmark, A. Prostate cancer-derived urine exosomes: A novel approach to biomarkers for prostate cancer. Br. J. Cancer 2009, 100, 1603–1607. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Sun, Y.; Huo, C.; Qiao, Z.; Shang, Z.; Uzzaman, A.; Liu, S.; Jiang, X.; Fan, L.Y.; Ji, L.; Guan, X.; et al. Comparative Proteomic Analysis of Exosomes and Microvesicles in Human Saliva for Lung Cancer. J. Proteome Res. 2018, 17, 1101–1107. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, Y.H.; Ding, Y.; Zhang, G.; Liu, Y.; Sun, J.; Yang, Y.; Zhan, Z.; Iliuk, A.; Gu, Z.; et al. Proteomics, Phosphoproteomics and Mirna Analysis of Circulating Extracellular Vesicles through Automated and High-Throughput Isolation. Cells 2022, 11, 2070. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, L.; Iliuk, A.; Tao, W.A. Highly Efficient Phosphoproteome Capture and Analysis from Urinary Extracellular Vesicles. J. Proteome Res. 2018, 17, 3308–3316. [Google Scholar] [CrossRef] [PubMed]
- Bandu, R.; Oh, J.W.; Kim, K.P. Mass spectrometry-based proteome profiling of extracellular vesicles and their roles in cancer biology. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.W.; Cho, J.Y. Exploring the key communicator role of exosomes in cancer microenvironment through proteomics. Proteome Sci. 2019, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Pino, L.K.; Just, S.C.; MacCoss, M.J.; Searle, B.C. Acquiring and Analyzing Data Independent Acquisition Proteomics Experiments without Spectrum Libraries. Mol. Cell. Proteom. 2020, 19, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Reubsaet, L.; Sweredoski, M.J.; Moradian, A. Data-Independent Acquisition for the Orbitrap Q Exactive HF: A Tutorial. J. Proteome Res. 2019, 18, 803–813. [Google Scholar] [CrossRef]
- Searle, B.C.; Swearingen, K.E.; Barnes, C.A.; Schmidt, T.; Gessulat, S.; Küster, B.; Wilhelm, M. Generating high quality libraries for DIA MS with empirically corrected peptide predictions. Nat. Commun. 2020, 11, 1548. [Google Scholar] [CrossRef]
- Bekker-Jensen, D.B.; Bernhardt, O.M.; Hogrebe, A.; Martinez-Val, A.; Verbeke, L.; Gandhi, T.; Kelstrup, C.D.; Reiter, L.; Olsen, J.V. Rapid and site-specific deep phosphoproteome profiling by data-independent acquisition without the need for spectral libraries. Nat. Commun. 2020, 11, 787. [Google Scholar] [CrossRef]
- Hadisurya, M.; Lee, Z.-C.; Luo, Z.; Zhang, G.; Ding, Y.; Zhang, H.; Iliuk, A.B.; Pili, R.; Boris, R.S.; Tao, W.A. Data-independent acquisition phosphoproteomics of urinary extracellular vesicles enables renal cell carcinoma grade differentiation. medRxiv 2022. [Google Scholar] [CrossRef]
- Iliuk, A.; Wu, X.; Li, L.; Sun, J.; Hadisurya, M.; Boris, R.S.; Tao, W.A. Plasma-Derived Extracellular Vesicle Phosphoproteomics through Chemical Affinity Purification. J. Proteome Res. 2020, 19, 2563–2574. [Google Scholar] [CrossRef]
- Mi, H.; Ebert, D.; Muruganujan, A.; Mills, C.; Albou, L.P.; Mushayamaha, T.; Thomas, P.D. PANTHER version 16: A revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res. 2021, 49, D394–D403. [Google Scholar] [CrossRef] [PubMed]
- Soman, A.; Asha Nair, S. Unfolding the cascade of SERPINA3: Inflammation to cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188760. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Li, S.; Liu, Z.; Zhang, C.; Bai, Z. CircSERPINA3 regulates SERPINA3-mediated apoptosis, autophagy and aerobic glycolysis of prostate cancer cells by competitively binding to MiR-653-5p and recruiting BUD13. J. Transl. Med. 2021, 19, 492. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.L.; Chen, F.S.; Mao, H. Clinical significance and role of up-regulation of SERPINA3 expression in endometrial cancer. World J. Clin. Cases 2019, 7, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Koivuluoma, S.; Tervasmäki, A.; Kauppila, S.; Winqvist, R.; Kumpula, T.; Kuismin, O.; Moilanen, J.; Pylkäs, K. Exome sequencing identifies a recurrent variant in SERPINA3 associating with hereditary susceptibility to breast cancer. Eur. J. Cancer 2021, 143, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.L.; Pei, X.F.; Qiao, X.; Yu, J.; Ye, H.; Xi, C.L.; Wang, P.Y.; Gong, Z.L. SERPINA3 Silencing Inhibits the Migration, Invasion, and Liver Metastasis of Colon Cancer Cells. Dig. Dis. Sci. 2018, 63, 2309–2319. [Google Scholar] [CrossRef]
- Péré-Brissaud, A.; Blanchet, X.; Delourme, D.; Pélissier, P.; Forestier, L.; Delavaud, A.; Duprat, N.; Picard, B.; Maftah, A.; Brémaud, L. Expression of SERPINA3s in cattle: Focus on bovSERPINA3-7 reveals specific involvement in skeletal muscle. Open Biol. 2015, 5, 150071. [Google Scholar] [CrossRef]
- Chelbi, S.T.; Wilson, M.L.; Veillard, A.C.; Ingles, S.A.; Zhang, J.; Mondon, F.; Gascoin-Lachambre, G.; Doridot, L.; Mignot, T.M.; Rebourcet, R.; et al. Genetic and epigenetic mechanisms collaborate to control SERPINA3 expression and its association with placental diseases. Hum. Mol. Genet 2012, 21, 1968–1978. [Google Scholar] [CrossRef]
- Rehman, I.; Evans, C.A.; Glen, A.; Cross, S.S.; Eaton, C.L.; Down, J.; Pesce, G.; Phillips, J.T.; Yen, O.S.; Thalmann, G.N.; et al. iTRAQ identification of candidate serum biomarkers associated with metastatic progression of human prostate cancer. PLoS ONE 2012, 7, e30885. [Google Scholar] [CrossRef]
- Murphy, K.; Murphy, B.T.; Boyce, S.; Flynn, L.; Gilgunn, S.; O’Rourke, C.J.; Rooney, C.; Stöckmann, H.; Walsh, A.L.; Finn, S.; et al. Integrating biomarkers across omic platforms: An approach to improve stratification of patients with indolent and aggressive prostate cancer. Mol. Oncol. 2018, 12, 1513–1525. [Google Scholar] [CrossRef]
- Meng, H.; Song, Y.; Zhu, J.; Liu, Q.; Lu, P.; Ye, N.; Zhang, Z.; Pang, Y.; Qi, J.; Wu, H. LRG1 promotes angiogenesis through upregulating the TGF-β1 pathway in ischemic rat brain. Mol. Med. Rep. 2016, 14, 5535–5543. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, J.; Zhang, X.; Wang, C.; Huang, Y.; Dai, K.; Zhang, X. TNF-α-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis. Cell Death Dis. 2017, 8, e2715. [Google Scholar] [CrossRef] [PubMed]
- Lind, G.E.; Skotheim, R.I.; Fraga, M.F.; Abeler, V.M.; Esteller, M.; Lothe, R.A. Novel epigenetically deregulated genes in testicular cancer include homeobox genes and SCGB3A1 (HIN-1). J. Pathol. 2006, 210, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Neal, D.E., Jr.; Clejan, S.; Sarma, D.; Moon, T.D. Prostate specific antigen and prostatitis. I. Effect of prostatitis on serum PSA in the human and nonhuman primate. Prostate 1992, 20, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Demory Beckler, M.; Higginbotham, J.N.; Franklin, J.L.; Ham, A.J.; Halvey, P.J.; Imasuen, I.E.; Whitwell, C.; Li, M.; Liebler, D.C.; Coffey, R.J. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol. Cell. Proteom. 2013, 12, 343–355. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, T.; Liu, R.; Bai, M.; Zhou, L.; Wang, X.; Li, S.; Wang, X.; Yang, H.; Li, J.; et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat. Commun. 2017, 8, 15016. [Google Scholar] [CrossRef]
- Maia, J.; Caja, S.; Strano Moraes, M.C.; Couto, N.; Costa-Silva, B. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front. Cell Dev. Biol. 2018, 6, 18. [Google Scholar] [CrossRef]
- Othman, N.; Jamal, R.; Abu, N. Cancer-Derived Exosomes as Effectors of Key Inflammation-Related Players. Front. Immunol. 2019, 10, 2103. [Google Scholar] [CrossRef]
- Shushkova, N.A.; Novikova, S.E.; Zgoda, V.G. Exosomes of malignant tumors: Prospects of omics diagnostics. Biomed Khim 2019, 65, 457–467. [Google Scholar] [CrossRef]
- Liu, Y.; Mikrani, R.; Xie, D.; Wazir, J.; Shrestha, S.; Ullah, R.; Baig, M.; Ahmed, A.; Srivastava, P.K.; Thapa, K.B.; et al. Chronic prostatitis/chronic pelvic pain syndrome and prostate cancer: Study of immune cells and cytokines. Fundam. Clin. Pharm. 2020, 34, 160–172. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Aus, G.; Abbou, C.; Bolla, M.; Heidenreich, A.; Schmid, H.-P.; Van Poppel, H.; Wolff, J.; Zattoni, F. EAU guidelines on prostate cancer. Eur. Urol. 2005, 48, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology Database. Available online: https://www.genome.jp/go/ (accessed on 16 August 2022).
- DrugBank Database. Available online: https://go.drugbank.com (accessed on 16 August 2022).
- STRING Database. Available online: https://string-db.org (accessed on 16 August 2022).
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, G.-Y.; Su, W.-C.; Chen, Y.-T.; Liu, Y.-F.; Wei, D.; Zhang, Y.-X.; Tang, Q.-Y.; Liu, Y.-X.; Wang, S.-Z.; et al. High Throughput Isolation and Data Independent Acquisition Mass Spectrometry (DIA-MS) of Urinary Extracellular Vesicles to Improve Prostate Cancer Diagnosis. Molecules 2022, 27, 8155. https://doi.org/10.3390/molecules27238155
Zhang H, Zhang G-Y, Su W-C, Chen Y-T, Liu Y-F, Wei D, Zhang Y-X, Tang Q-Y, Liu Y-X, Wang S-Z, et al. High Throughput Isolation and Data Independent Acquisition Mass Spectrometry (DIA-MS) of Urinary Extracellular Vesicles to Improve Prostate Cancer Diagnosis. Molecules. 2022; 27(23):8155. https://doi.org/10.3390/molecules27238155
Chicago/Turabian StyleZhang, Hao, Gui-Yuan Zhang, Wei-Chao Su, Ya-Ting Chen, Yu-Feng Liu, Dong Wei, Yan-Xi Zhang, Qiu-Yi Tang, Yu-Xiang Liu, Shi-Zhi Wang, and et al. 2022. "High Throughput Isolation and Data Independent Acquisition Mass Spectrometry (DIA-MS) of Urinary Extracellular Vesicles to Improve Prostate Cancer Diagnosis" Molecules 27, no. 23: 8155. https://doi.org/10.3390/molecules27238155
APA StyleZhang, H., Zhang, G.-Y., Su, W.-C., Chen, Y.-T., Liu, Y.-F., Wei, D., Zhang, Y.-X., Tang, Q.-Y., Liu, Y.-X., Wang, S.-Z., Li, W.-C., Wesselius, A., Zeegers, M. P., Zhang, Z.-Y., Gu, Y.-H., Tao, W. A., & Yu, E. Y.-W. (2022). High Throughput Isolation and Data Independent Acquisition Mass Spectrometry (DIA-MS) of Urinary Extracellular Vesicles to Improve Prostate Cancer Diagnosis. Molecules, 27(23), 8155. https://doi.org/10.3390/molecules27238155







