Luminous Self-Assembled Fibers of Azopyridines and Quantum Dots Enabled by Synergy of Halogen Bond and Alkyl Chain Interactions
Abstract
1. Introduction
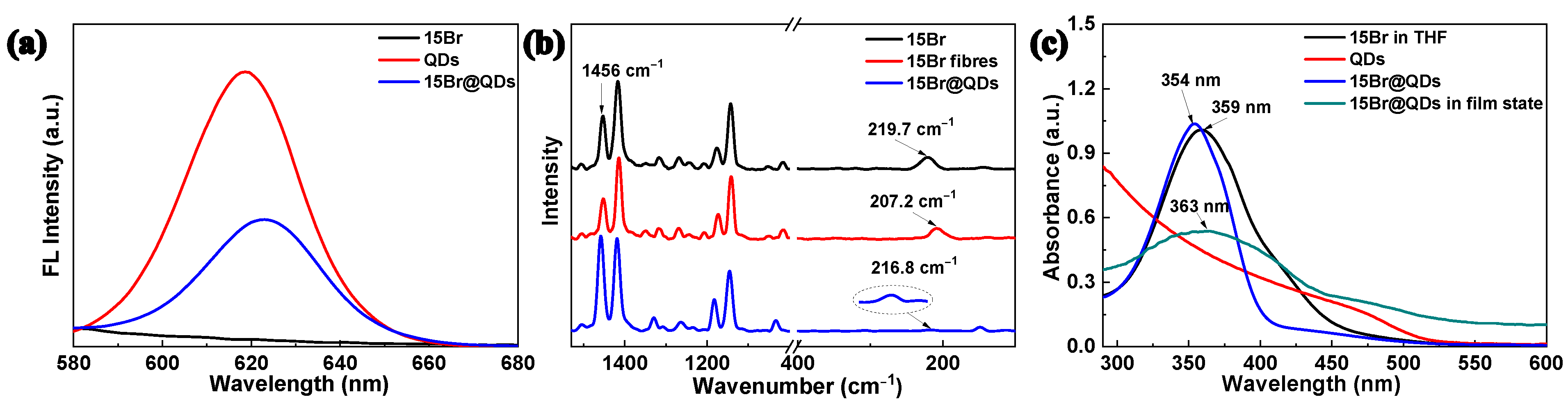
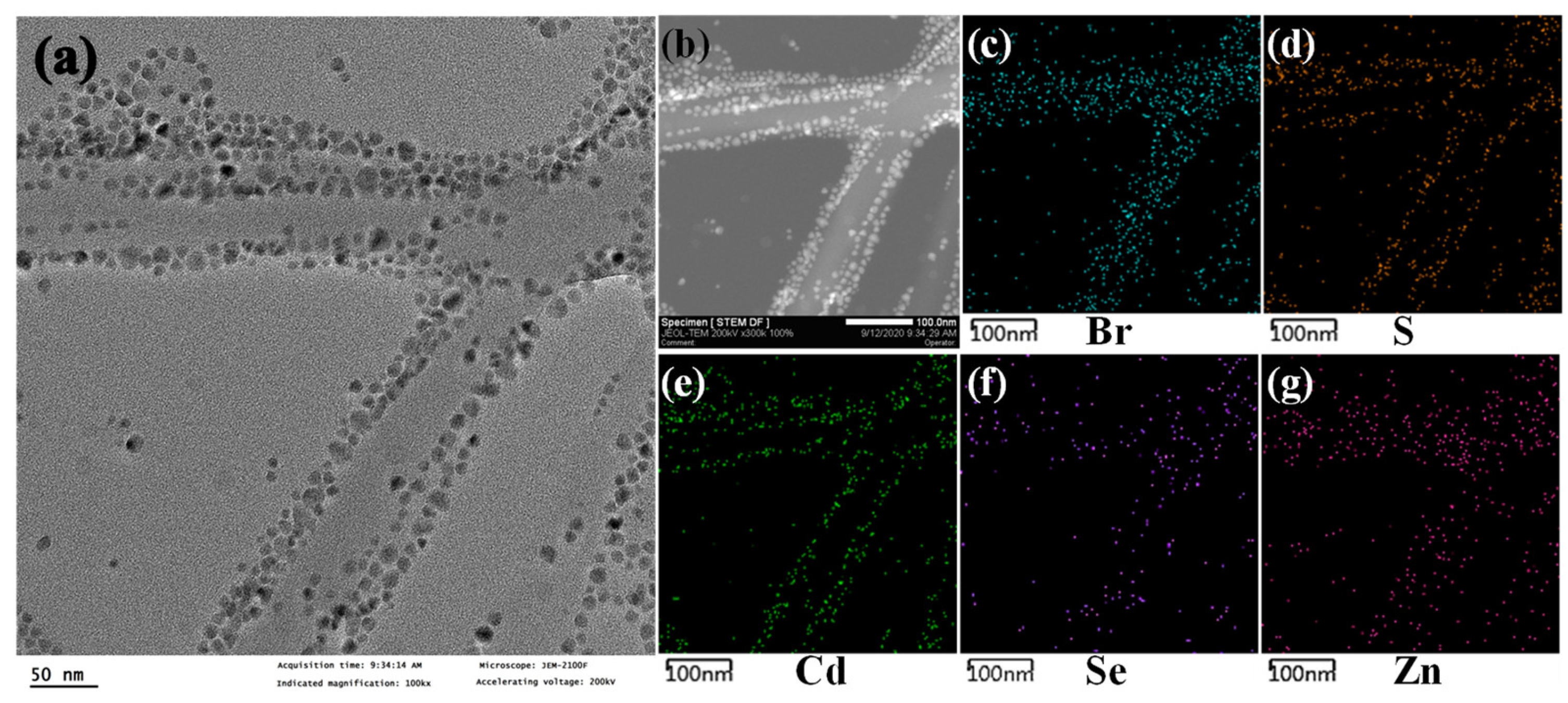
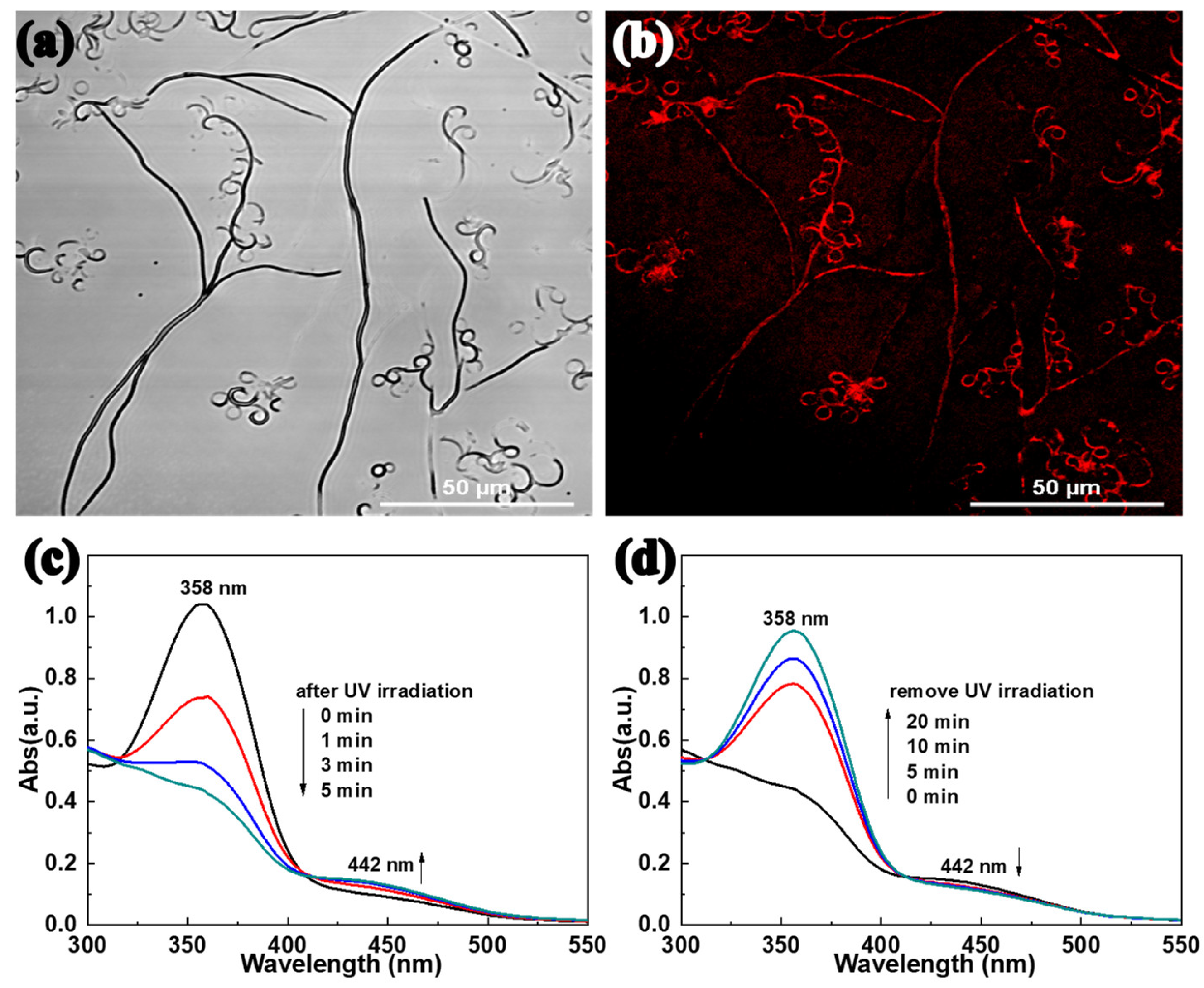
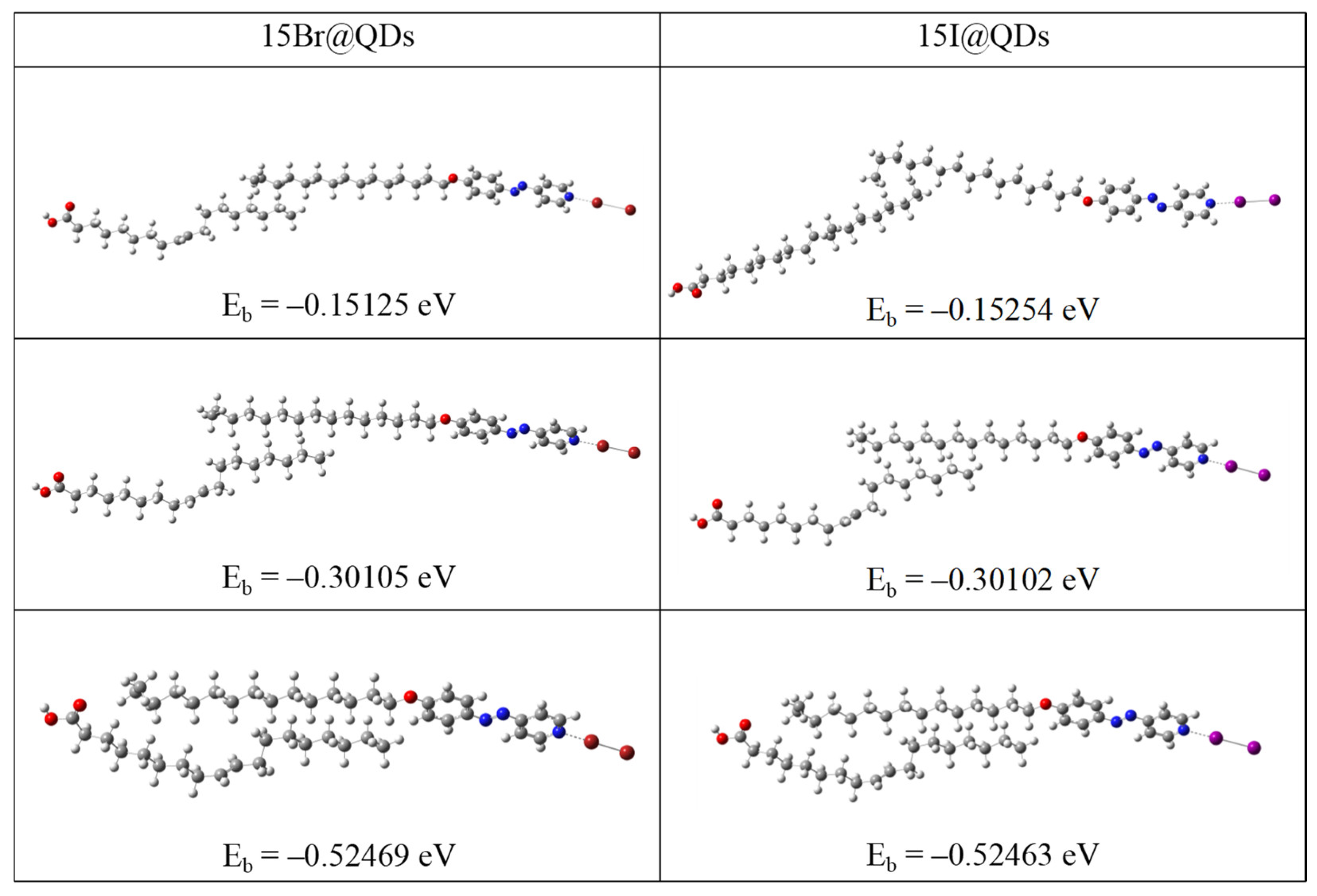
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Wang, C. Fabrication of PbS nanoparticles in polymer-fiber matrices by electrospinning. Adv. Mater. 2005, 17, 2485–2488. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Wang, C.; Wei, Y. Fabrication of Cds nanorods in PVP fiber matrices by electrospinning. Macromol. Rapid Commun. 2005, 26, 1325–1329. [Google Scholar] [CrossRef]
- Son, W.K.; Cho, D.; Park, W.H. Direct electrospinning of ultrafine titania fibres in the absence of polymer additives and formation of pure anatase titania fibres at low temperature. Nanotechnology. 2005, 17, 439–443. [Google Scholar] [CrossRef]
- Aoki, K.; Nakagawa, M.; Ichimura, K. Self-assembly of amphoteric azopyridine carboxylic acids: Organized structures and macroscopic organized morphology influenced by heat, pH change, and light. J. Am. Chem. Soc. 2000, 122, 10997–11004. [Google Scholar] [CrossRef]
- Nakagawa, M.; Ishii, D.; Aoki, K.; Seki, T.; Iyoda, T. Tubular and twisted Ni–P fibers molded from morphology-tunable and recyclable organic templates of hydrogen-bonded supramolecular assemblages. Adv. Mater. 2005, 17, 200–205. [Google Scholar] [CrossRef]
- Pasini, D.; Kraft, A. Supramolecular self-assembly of fibres. Curr. Opin. Solid State Mater. Sci. 2004, 8, 157–163. [Google Scholar] [CrossRef]
- Wu, Y.; Shah, D.U.; Liu, C.; Yu, Z.; Liu, J.; Ren, X.; Rowland, M.J.; Abell, C.; Ramage, M.H.; Scherman, O.A. Bioinspired supramolecular fibers drawn from a multiphase self-assembled hydrogel. Proc. Natl. Acad. Sci. USA 2017, 114, 8163–8168. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Zhang, L.; Yang, H.; Lu, Y. Photoresponsive liquid crystals based on halogen bonding of azopyridines. Chem. Commun. 2014, 50, 9647–9649. [Google Scholar] [CrossRef]
- Meazza, L.; Foster, J.A.; Fucke, K.; Metrangolo, P.; Resnati, G.; Steed, J.W. Halogen-bonding-triggered supramolecular gel formation. Nat. Chem. 2013, 5, 42–47. [Google Scholar] [CrossRef]
- Hu, H.; Qiu, Y.; Wang, J.; Zhao, D.; Wang, H.; Wang, Q.; Liao, Y.; Peng, H.; Xie, X. Photomodulated morphologies in halogen bond–driven assembly during gel–sol transition. Macromol. Rapid Commun. 2019, 40, 1800629. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Qiu, Y.; Zhao, X.; Xiong, B.; Liao, R.; Peng, H.; Liao, Y.; Xie, X. Visible light-triggered gel-to-sol transition in halogen-bond-based supramolecules. Soft Matter 2019, 15, 6411–6417. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, S.; Wang, T.; Yu, H. Enhanced ordering and efficient photoalignment of nanostructures in block copolymers enabled by halogen bond. Macromolecules 2020, 53, 1486–1493. [Google Scholar] [CrossRef]
- Li, X.; Ma, S.; Hu, J.; Ni, Y.; Lin, Z.; Yu, H. Photo-activated bimorph composites of kapton and liquid-crystalline polymer towards biomimetic circadian rhythms of albizia julibrissin leaves. J. Mater. Chem. C 2019, 7, 622–629. [Google Scholar] [CrossRef]
- Chen, Y.; Quan, M.; Yu, H.; Zhang, L.; Yang, H.; Lu, Y. Fabrication of nanofibres with azopyridine compounds in various acids and solvents. RSC Adv. 2015, 5, 31219–31225. [Google Scholar] [CrossRef]
- El Malah, T.; Nour, H.F. Click synthesis of shape-persistent azodendrimers and their orthogonal self-assembly to nanofibres. Aust. J. Chem. 2018, 71, 463–472. [Google Scholar] [CrossRef]
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulović, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 2013, 7, 13–23. [Google Scholar] [CrossRef]
- Jiang, C.; Zhong, Z.; Liu, B.; He, Z.; Zou, J.; Wang, L.; Wang, J.; Peng, J.; Cao, Y. Coffee-ring-free quantum dot thin film using inkjet printing from a mixed-solvent system on modified ZnO transport layer for light-emitting devices. ACS Appl. Mater. Interfaces 2016, 8, 26162–26168. [Google Scholar] [CrossRef] [PubMed]
- Anikeeva, P.O.; Halpert, J.E.; Bawendi, M.G.; Bulović, V. Quantum dot light-emitting devices with electroluminescence tunable over the entire visible spectrum. Nano Lett. 2009, 9, 2532–2536. [Google Scholar] [CrossRef]
- Pattantyus-Abraham, A.G.; Kramer, I.J.; Barkhouse, A.R.; Wang, X.; Konstantatos, G.; Debnath, R.; Levina, L.; Raabe, I.; Nazeeruddin, M.K.; Grätzel, M.; et al. Depleted-heterojunction colloidal quantum dot solar cells. ACS Nano. 2010, 4, 3374–3380. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Onses, M.S.; Lim, J.B.; Nam, S.; Oh, N.; Kim, H.; Yu, K.J.; Lee, J.W.; Kim, J.-H.; Kang, S.-K.; et al. High-resolution patterns of quantum dots formed by electrohydrodynamic jet printing for light-emitting diodes. Nano Lett. 2015, 15, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.Q.; Lam, W.Y.; Hatch, E.W.; Lidke, D.S. Quantum dots for quantitative imaging: From single molecules to tissue. Cell Tissue Res. 2015, 360, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; Kouhi, M.; Akbarzadeh, A.; Davaran, S. Quantum dots: Synthesis, bioapplications, and toxicity. Nanoscale Res. Lett. 2012, 7, 480. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-W.; Shi, H.-Q.; Li, W.-N.; Xiao, H.-M.; Fu, S.-Y.; Cao, X.-Z.; Li, Z.-X. Lanthanum-doped ZnO quantum dots with greatly enhanced fluorescent quantum yield. J. Mater. Chem. 2012, 22, 8221–8227. [Google Scholar] [CrossRef]
- Han, T.; Yuan, Y.; Liang, X.; Zhang, Y.; Xiong, C.; Dong, L. Colloidal stable quantum dots modified by dual functional group polymers for inkjet printing. J. Mater. Chem. C 2017, 5, 4629–4635. [Google Scholar] [CrossRef]
- Abitbol, T.; Wilson, J.T.; Gray, D.G. Electrospinning of fluorescent fibers from CdSe/ZnS quantum dots in cellulose triacetate. J. Appl. Polym. Sci. 2011, 119, 803–810. [Google Scholar] [CrossRef]
- He, X.; Tan, L.; Wu, X.; Yan, C.; Chen, D.; Meng, X.; Tang, F. Electrospun quantum dots/polymer composite porous fibers for turn-on fluorescent detection of lactate dehydrogenase. J. Mater. Chem. 2012, 22, 18471–18478. [Google Scholar] [CrossRef]
- Huo, S.; Duan, P.; Jiao, T.; Peng, Q.; Liu, M. Self-assembled luminescent quantum dots to generate full-color and white circularly polarized light. Angew. Chem. Int. Ed. 2017, 56, 12174–12178. [Google Scholar] [CrossRef]
- Duan, P.; Li, Y.; Li, L.; Deng, J.; Liu, M. Multiresponsive chiroptical switch of an azobenzene-containing lipid: Solvent, temperature, and photoregulated supramolecular chirality. J. Phys. Chem. B. 2011, 115, 3322–3329. [Google Scholar] [CrossRef]
- Draper, M.; Saez, I.M.; Cowling, S.J.; Gai, P.; Heinrich, B.; Donnio, B.; Guillon, D.; Goodby, J.W. Self-assembly and shape morphology of liquid crystalline gold metamaterials. Adv. Funct. Mater. 2011, 21, 1260–1278. [Google Scholar] [CrossRef]
- Duan, H.; Berggren, K.K. Directed self-assembly at the 10 nm scale by using capillary force-induced nanocohesion. Nano Lett. 2010, 10, 3710–3716. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, J.; Kang, S.M.; Jang, S.; Kang, D.; Moon, S.E.; Kim, H.N.; Yoon, H. Directional clustering of slanted nanopillars by elastocapillarity. Small 2016, 12, 3764–3769. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, Y.; Lu, W.; Liu, B. Capillary force driving directional 1D assembly of patchy colloidal discs. ACS Macro Lett. 2019, 8, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, K.; Li, Y.; Guan, G.; Li, H.; Luo, Y.; Zhao, F.; Zhang, Q.; Pei, Q.; Peng, H. A colour-tunable, weavable fibre-shaped polymer light-emitting electrochemical cell. Nat. Photonics 2015, 9, 233–238. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Zhang, T.; Huang, Z.; Yang, H.; Wang, J.; Jiang, K.; Fan, S.; Li, Q. Emission enhancement from CdSe/ZnS quantum dots induced by strong localized surface plasmonic resonances without damping. J. Phys. Chem. Lett. 2019, 10, 2113–2120. [Google Scholar] [CrossRef]
- Bhaskar, S.; Singh, A.K.; Das, P.; Jana, P.; Kanvah, S.; Bhaktha, S.; Ramamurthy, S.S. Superior resonant nanocavities engineering on the photonic crystal-coupled emission platform for the detection of femtomolar iodide and zeptomolar cortisol. ACS Appl. Mater. Interfaces 2020, 12, 34323–34336. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Broer, D.J. New insights into photoactivated volume generation boost surface morphing in liquid crystal coatings. Nat. commun. 2015, 6, 8334. [Google Scholar] [CrossRef]
- Cai, Z.; Huang, Z.; Li, Z.; Su, M.; Zhao, Z.; Qin, F.; Zhang, Z.; Yang, J.; Song, Y. Evaporation induced spontaneous micro-vortexes through engineering of the Marangoni flow. Angew. Chem. Int. Ed. 2020, 132, 23892–23897. [Google Scholar] [CrossRef]
- Erdélyi, M. Halogen bonding in solution. Chem. Soc. Rev. 2012, 41, 3547–3557. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Wang, H.; Zhao, X.R.; Jin, W.J. Anionic 3d cage networks self-assembled by iodine and v-shaped pentaiodides using dimeric oxoammonium cations produced in situ as templates. Dalton Trans. 2013, 42, 8788–8795. [Google Scholar] [CrossRef]
- Menzel, H.; Weichart, B.; Schmidt, A.; Paul, S.; Knoll, W.; Stumpe, J.; Fischer, T. Small-angle X-ray scattering and ultraviolet-visible spectroscopy studies on the structure and structural changes in langmuir-blodgett films of polyglutamates with azobenzene moieties tethered by alkyl spacers of different length. Langmuir 1994, 10, 1926–1933. [Google Scholar] [CrossRef]
- Ren, H.; Chen, D.; Shi, Y.; Yu, H.; Fu, Z. A carboxylic azo monomer and its homopolymer: Synthesis, self-organization and fluorescence behaviour in solution. Polym. Chem. 2015, 6, 270–277. [Google Scholar] [CrossRef]
- Kunitake, T. Synthetic bilayer membranes: Molecular design, self-organization, and application. Angew. Chem. Int. Ed. 1992, 31, 709–726. [Google Scholar] [CrossRef]
- Han, M.; Ichimura, K. In-plane and tilt reorientation of p-methoxyazobenzene side chains tethered to liquid crystalline polymethacrylates by irradiation with 365 nm light. Macromolecules 2001, 34, 90–98. [Google Scholar] [CrossRef]
- Tong, X.; Cui, L.; Zhao, Y. Confinement effects on photoalignment, photochemical phase transition, and thermochromic behavior of liquid crystalline azobenzene-containing diblock copolymers. Macromolecules 2004, 37, 3101–3112. [Google Scholar] [CrossRef]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Wu, S.; Niu, L.; Shen, J.; Zhang, Q.; Bubeck, C. Aggregation-induced reversible thermochromism of novel azo chromophore-functionalized polydiacetylene cylindrical micelles. Macromolecules 2009, 42, 362–367. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Horton, P.N.; Hursthouse, M.B.; Legon, A.C.; Bruce, W.D. Halogen bonding: A new interaction for liquid crystal formation. J. Am. Chem. Soc. 2004, 126, 16–17. [Google Scholar] [CrossRef]
- McAllister, L.J.; Präsang, C.; Wong, J.P.W.; Thatcher, R.J. Halogen-bonded liquid crystals of 4-alkoxystilbazoles with molecular iodine: A very short halogen bond and unusual mesophase stability. Chem. Commun. 2013, 49, 3946–3948. [Google Scholar] [CrossRef]
- Stammreich, H.; Forneris, R.; Tavares, Y. High-resolution Raman spectroscopy in the red and near infrared—II: Vibrational frequencies and molecular interactions of halogens and diatomic interhalogens. Spectrochim. Acta 1961, 17, 1173–1184. [Google Scholar] [CrossRef]
- Klaeboe, P. The Raman spectra of some iodine, bromine, and iodine monochloride charge-transfer coomplexes in solution. J. Am. Chem. Soc. 1967, 89, 3667–3676. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Guassian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules, 1st ed.; Oxford University Press, Inc.: New York, NY, USA, 1989. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Xue, L.; Chen, Y.; Hu, Y.; Sun, Z.; Mo, L.; Li, L.; Yu, H. Luminous Self-Assembled Fibers of Azopyridines and Quantum Dots Enabled by Synergy of Halogen Bond and Alkyl Chain Interactions. Molecules 2022, 27, 8165. https://doi.org/10.3390/molecules27238165
Pan Y, Xue L, Chen Y, Hu Y, Sun Z, Mo L, Li L, Yu H. Luminous Self-Assembled Fibers of Azopyridines and Quantum Dots Enabled by Synergy of Halogen Bond and Alkyl Chain Interactions. Molecules. 2022; 27(23):8165. https://doi.org/10.3390/molecules27238165
Chicago/Turabian StylePan, Ying, Lulu Xue, Yinjie Chen, Yingjie Hu, Zhicheng Sun, Lixin Mo, Luhai Li, and Haifeng Yu. 2022. "Luminous Self-Assembled Fibers of Azopyridines and Quantum Dots Enabled by Synergy of Halogen Bond and Alkyl Chain Interactions" Molecules 27, no. 23: 8165. https://doi.org/10.3390/molecules27238165
APA StylePan, Y., Xue, L., Chen, Y., Hu, Y., Sun, Z., Mo, L., Li, L., & Yu, H. (2022). Luminous Self-Assembled Fibers of Azopyridines and Quantum Dots Enabled by Synergy of Halogen Bond and Alkyl Chain Interactions. Molecules, 27(23), 8165. https://doi.org/10.3390/molecules27238165






