Design of an Internal/External Bicontinuous Conductive Network for High-Performance Asymmetrical Supercapacitors
Abstract
:1. Introduction
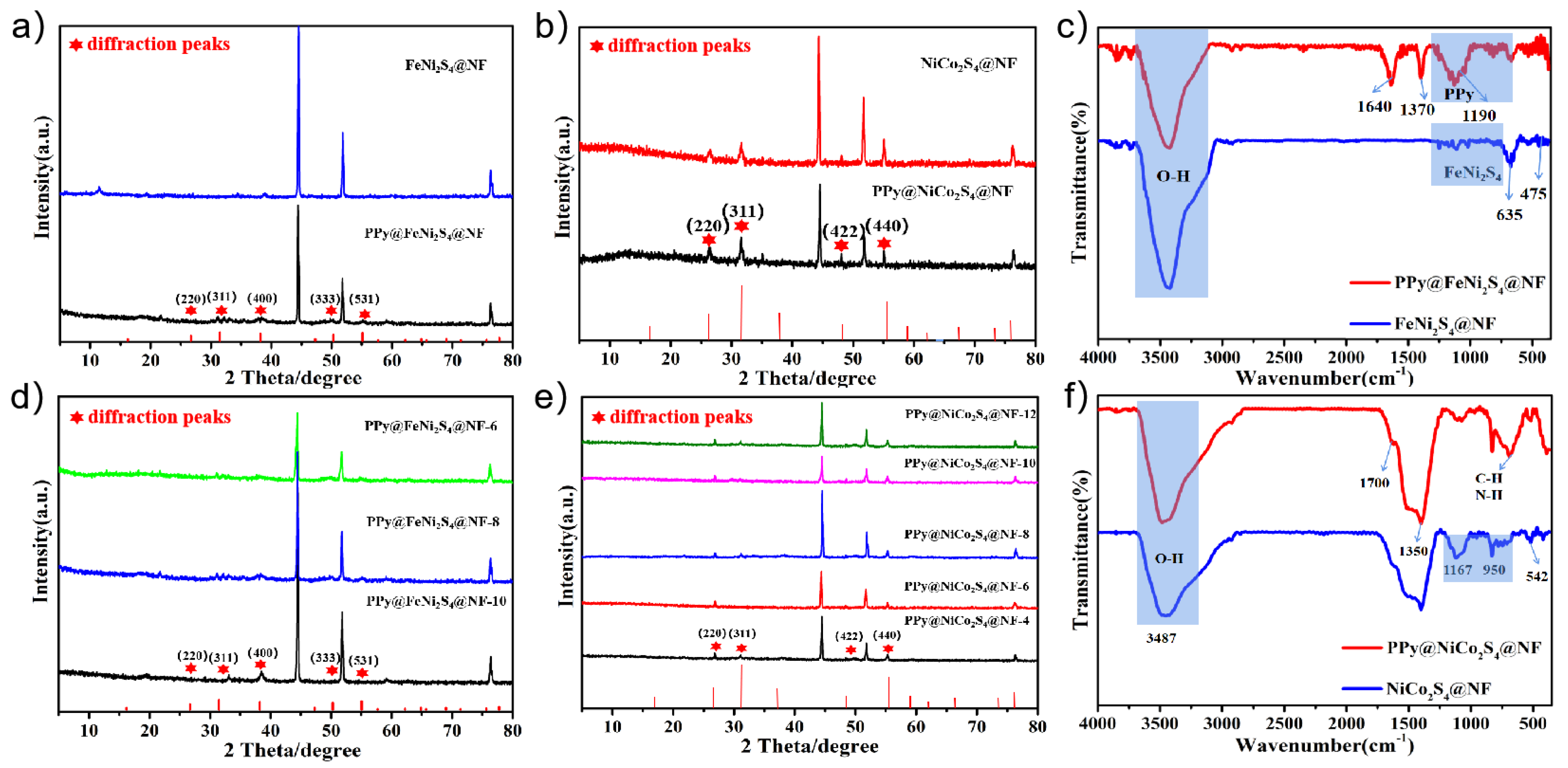
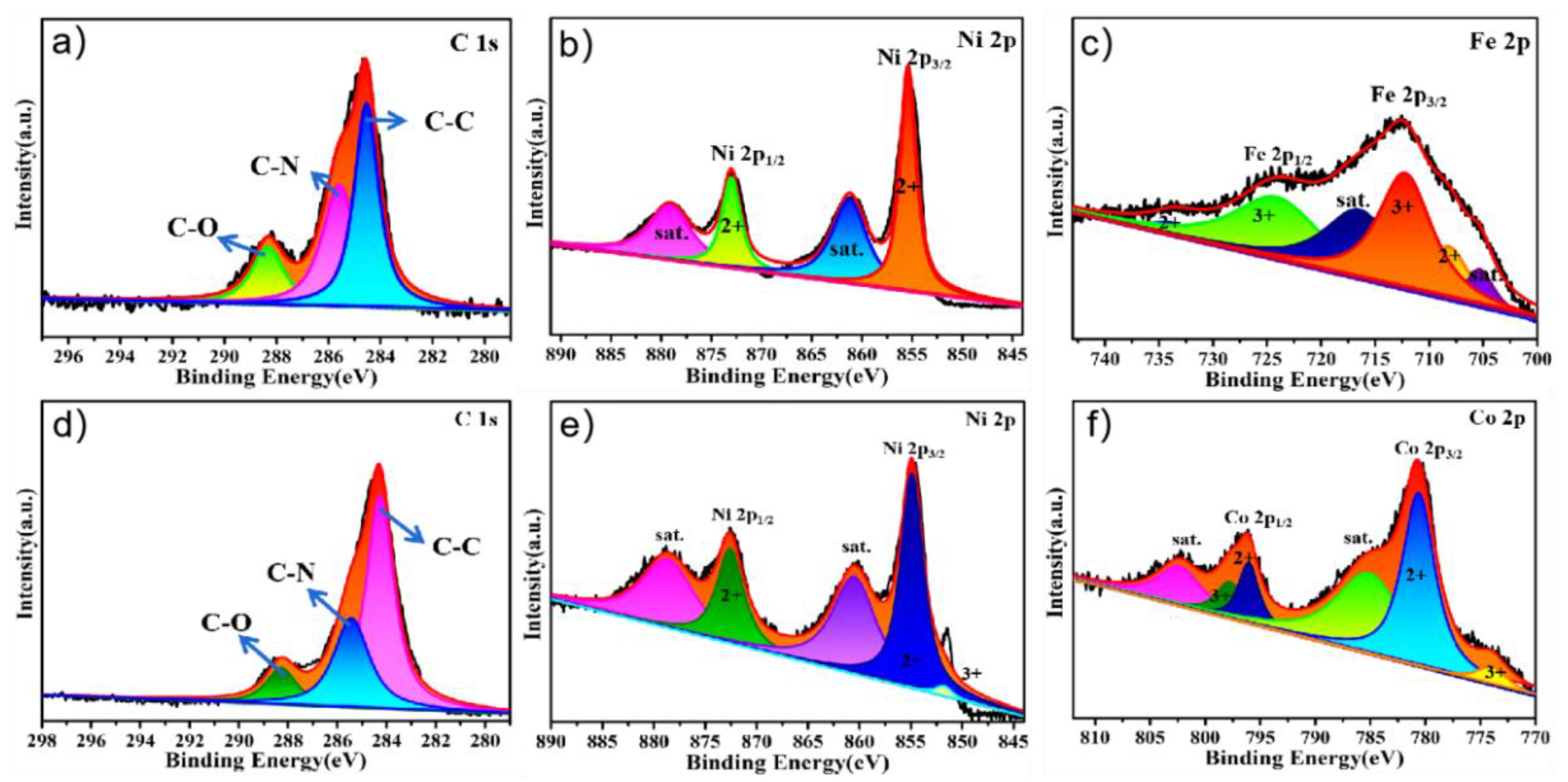
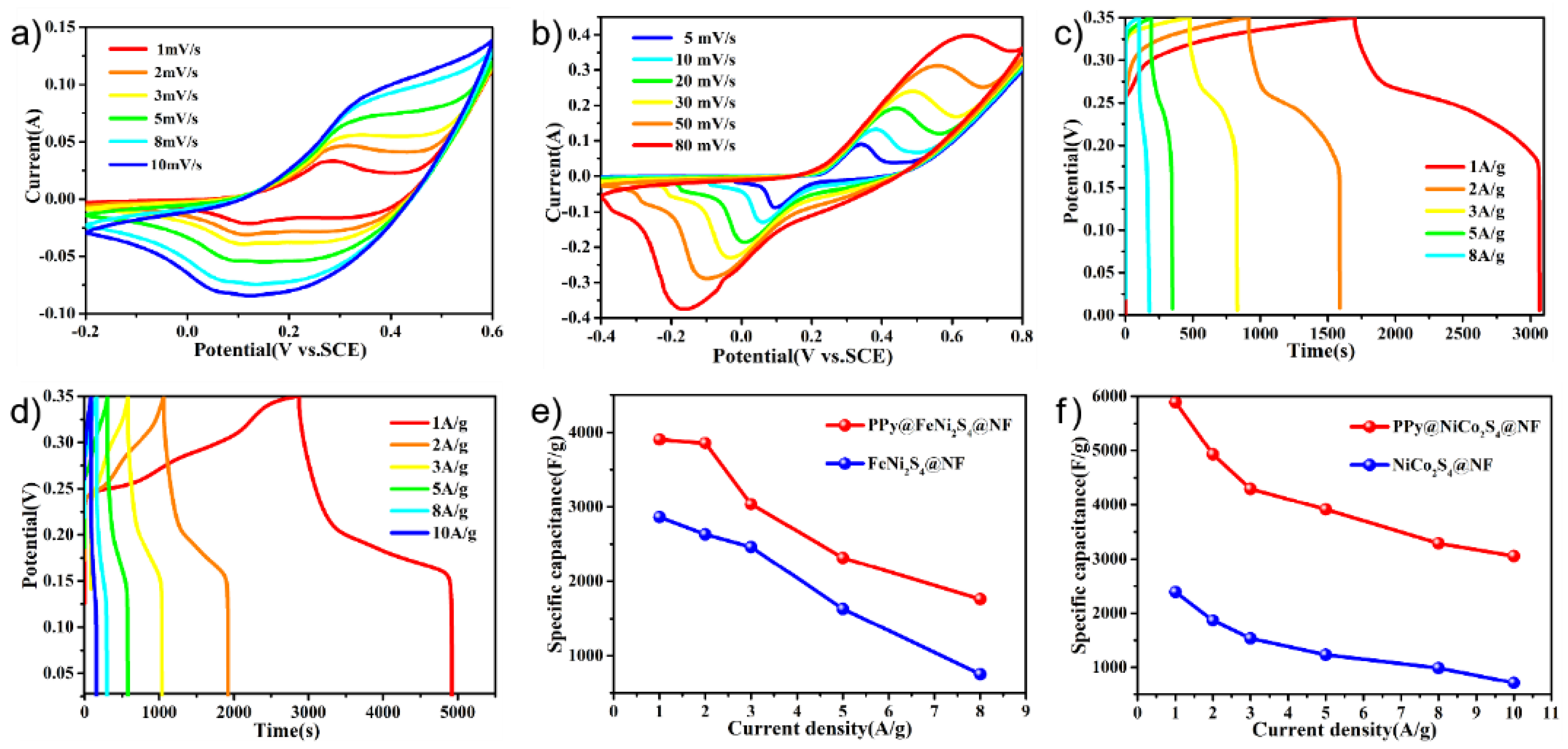
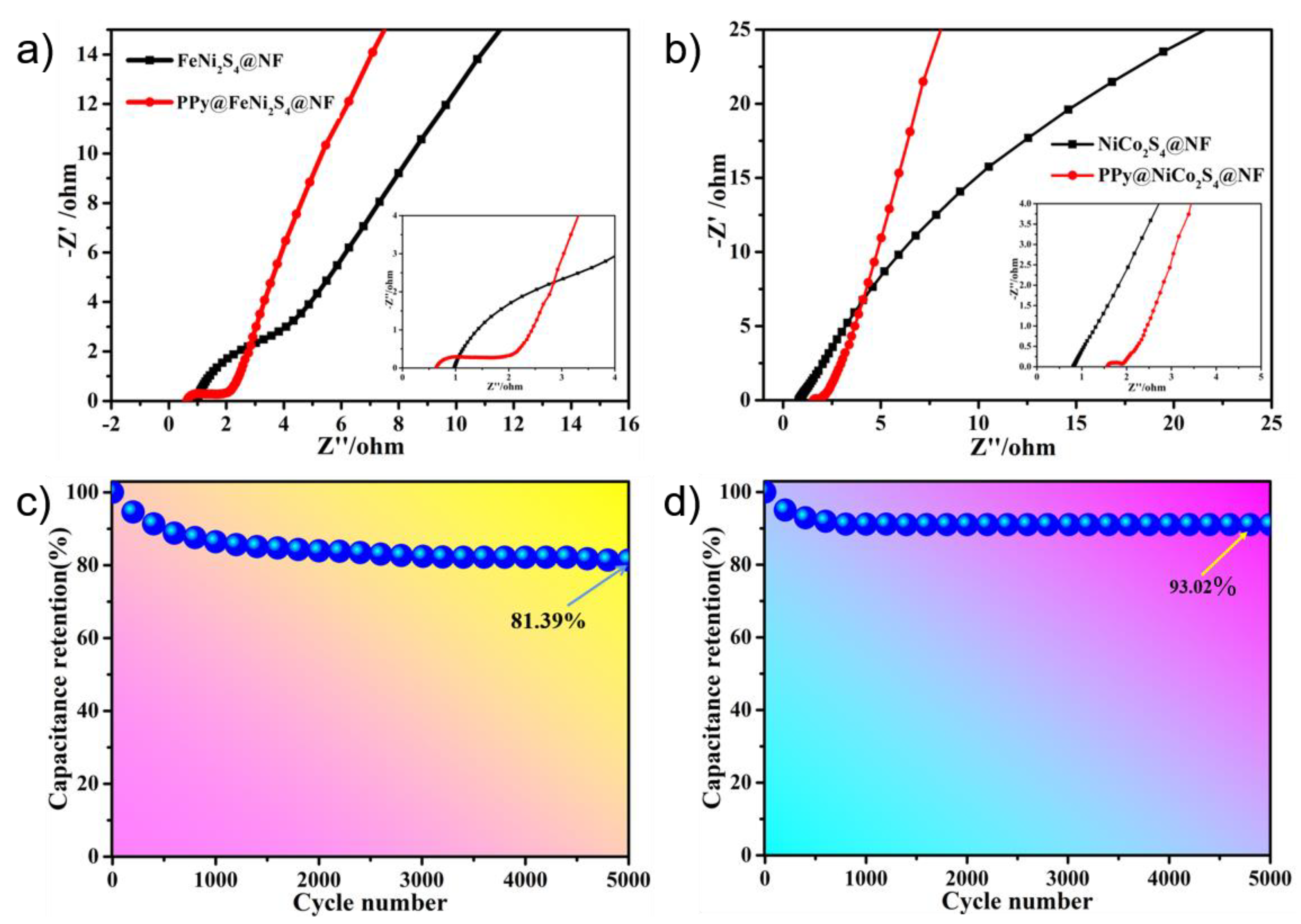
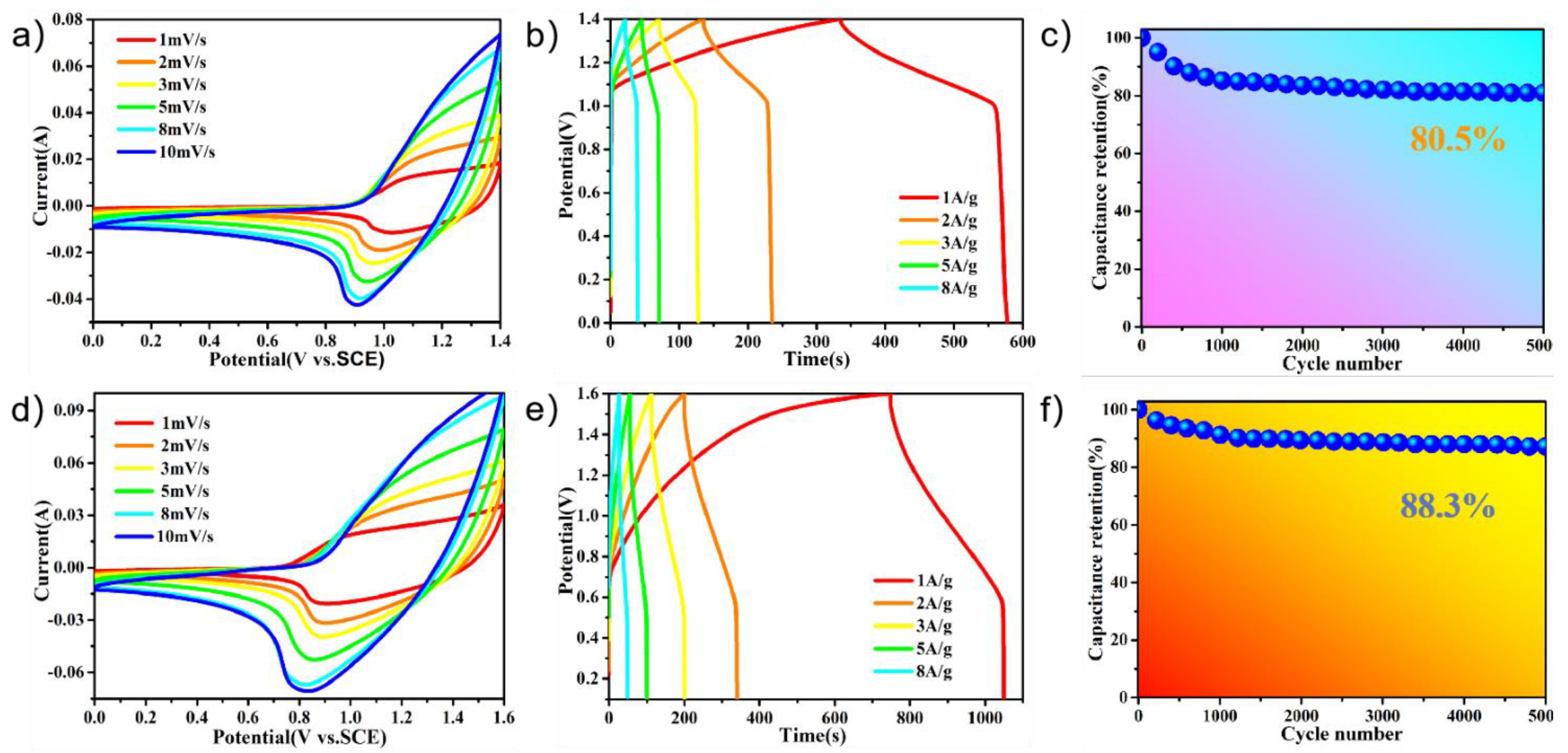
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Preparation of Materials
3.3. Materials Characterization
3.4. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, W.; Li, G.; Pei, A.; Li, Y.; Liao, L.; Wang, H.; Wan, J.; Liang, Z.; Chen, G.; Zhang, H. A manganese–hydrogen battery with potential for grid-scale energy storage. Nat. Energy 2018, 3, 428–435. [Google Scholar] [CrossRef]
- Montoto, E.C.; Nagarjuna, G.; Hui, J.; Burgess, M.; Sekerak, N.M.; Hernández-Burgos, K.; Wei, T.S.; Kneer, M.; Grolman, J.; Cheng, K.J.; et al. Redox Active Colloids as Discrete Energy Storage Carriers. J. Am. Chem. Soc. 2016, 49, 2649–2657. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. ChemInform Abstract: A Review of Electrode Materials for Electrochemical Supercapacitors. ChemInform 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.; Zeng, R.; Xiong, Y.; Chen, Y. Regulating Voltage Window and Energy Density of Aqueous Asymmetric Supercapacitors by Pinecone-like Hollow Fe2O3/MnO2 Nano-Heterostructure. Adv. Mater. Interfaces 2020, 7, 1901729. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Materials science. Electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [Green Version]
- Naoi, K.; Ishimoto, S.; Miyamoto, J.I.; Naoi, W. Second generation ‘nanohybrid supercapacitor’: Evolution of capacitive energy storage devices. Energy Environ. Sci. 2012, 5, 9363–9373. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Yan, C.; Li, K.; Zheng, Z. Wearable energy-dense and power-dense supercapacitor yarns enabled by scalable graphene-metallic textile composite electrodes. Nat. Commun. 2015, 6, 7260. [Google Scholar] [CrossRef] [Green Version]
- Owusu, K.A.; Qu, L.; Li, J.; Wang, Z.; Zhao, K.; Yang, C.; Hercule, K.M.; Lin, C.; Shi, C.; Wei, Q. Low-crystalline iron oxide hydroxide nanoparticle anode for high-performance supercapacitors. Nat. Commun. 2017, 8, 14264. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhang, X. Three-Dimensional Co3O4@NiCo2S4 Core/Shell Nanoflower Array with Enhanced Electrochemical Performance. Chemistryselect 2017, 2, 9537–9545. [Google Scholar] [CrossRef]
- Jinlong, L.; Tongxiang, L.; Meng, Y.; Ken, S.; Hideo, M. Performance comparison of NiCo2O4 and NiCo2S4 formed on Ni foam for supercapacitor. Compos. Part B Eng. 2017, 123, 28–33. [Google Scholar] [CrossRef]
- Chu, D.; Wei, X.; Song, X.; Zhang, Z.; Tan, L.; Ma, H.; Pang, H.; Wang, X.; Chen, Z. Controllable interfacial electron transfer induced by heterointerfaced sulfur-based catalysts with less electronegative anions for boosted hydrogen evolution reaction in the universal pH range. J. Mater. Chem. A 2022, 10, 21683–21689. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, M.; Yang, J.; Sun, C.; Dong, X. Shape-controlled synthesis of NiCo2S4 and their charge storage characteristics in supercapacitors. Nanoscale 2014, 6, 9824–9830. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, Z.; Zhang, A.; Jiang, D.; Liu, R. Hollow 3D Frame Structure Modified with NiCo2S4 Nanosheets and Spinous Fe2O3 Nanowires as Electrode Materials for High-Performance All-Solid-State Asymmetric Supercapacitors. Chem. A Eur. J. 2020, 26, 4790–4797. [Google Scholar]
- Liu, X.; Wu, Z.; Yin, Y. Hierarchical NiCo2S4@PANI core/shell nanowires grown on carbon fiber with enhanced electrochemical performance for hybrid supercapacitors. Chem. Eng. J. 2017, 323, 330–339. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, T.; Wang, Y.; Cui, J.; Wu, Y. 3D carbon coated NiCo2S4 nanowires doped with nitrogen for electrochemical energy storage and conversion. J. Colloid Interface Sci. 2019, 556, 449–457. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Z.; Niu, H.; Yang, Q.; Jia, H.; Shi, W. MOF-derived Co9S8 polyhedrons on NiCo2S4 nanowires for high-performance hybrid supercapacitors. Inorg. Chem. Front. 2020, 7, 4092–4100. [Google Scholar] [CrossRef]
- Zm, A.; Rong, Z.A.; Yu, L.; Yy, B.; Ws, A. Carbon nanotubes interpenetrating MOFs-derived Co-Ni-S composite spheres with interconnected architecture for high performance hybrid supercapacitor. J. Colloid Interface Sci. 2021, 602, 627–635. [Google Scholar]
- Cheng, X.Y.; Wang, D.; Ke, H.Z.; Li, Y.G.; Cai, Y.B.; Wei, Q.F. Hierarchical NiCo2S4/PANI/CNT nanostructures grown on graphene polyamide blend fiber as effective electrode for supercapacitors. Compos. Commun. 2022, 30, 101073. [Google Scholar] [CrossRef]
- Na, L.; Zp, A.; Xd, A.; Jie, Y.A.; Gx, A.; Ll, A.; Qi, W.; Ml, A.; Yza, E. In-situ growth of vertically aligned nickel cobalt sulfide nanowires on carbon nanotube fibers for high capacitance all-solid-state asymmetric fiber-supercapacitors. J. Energy Chem. 2020, 41, 209–215. [Google Scholar]
- Singh, A.; Ojha, S.K.; Singh, M.; Ojha, A.K. Controlled synthesis of NiCo2S4@NiCo2O4 Core@Shell nanostructured arrays decorated over the rGO sheets for high-performance asymmetric supercapacitor. Electrochim. Acta 2020, 349, 136349. [Google Scholar] [CrossRef]
- Wu, Y.; Ming, Y.; Lin, S.; Shi, W. Flexible yolk-shelled NiCo2S4 hollow spheres/RGO film electrodes for efficient supercapacitive energy storage. New J. Chem. 2018, 42, 16174–16182. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.; Li, F.; Wang, Z.; Alamusi; Hu, N.; Wen, Z.; Liu, Q. Merging of Kirkendall Growth and Ostwald Ripening: CuO@MnO2 Core-shell Architectures for Asymmetric Supercapacitors. Sci. Rep. 2014, 4, 4518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Gai, S.; He, F.; Niu, N.; Gao, P.; Chen, Y.; Yang, P. A sandwich-type three-dimensional layered double hydroxide nanosheet array/graphene composite: Fabrication and high supercapacitor performance. J. Mater. Chem. A 2013, 2, 1022–1031. [Google Scholar] [CrossRef]
- Hu, L.; Wu, L.; Liao, M.; Fa Ng, X. High-performance NiCo(2)O(4) nanofilm photodetectors fabricated by an interfacial self-assembly strategy. Adv. Mater. 2011, 23, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; An, C.; Wang, Y.; Jiao, L.; Yuan, H. Facile synthesis route of porous MnCo2O4 and CoMn2O4 nanowires and their excellent electrochemical properties in supercapacitors. J. Mater. Chem. A 2014, 2, 16480–16488. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.W.; Mathur, S.; Kim, K.H. Influence of Cycling Stability on Structure and Properties of MnCo2S4 Nanocomposite for High-Performance Supercapacitors. J. Alloys Compd. 2021, 868, 158850. [Google Scholar] [CrossRef]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef]
- Zha, D.; Fu, Y.; Zhang, L.; Zhu, J.; Wang, X. Design and fabrication of highly open nickel cobalt sulfide nanosheets on Ni foam for asymmetric supercapacitors with high energy density and long cycle-life. J. Power Sources 2018, 378, 31–39. [Google Scholar] [CrossRef]
- Syed, J.A.; Ma, J.; Zhu, J.B.; Tang, S.; Meng, X. Hierarchical Multicomponent Electrode with Interlaced Ni(OH)2 Nanoflakes Wrapped Zinc Cobalt Sulfide Nanotube Arrays for Sustainable High-Performance Supercapacitors. Adv. Energy Mater. 2017, 7, 1701228. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Chen, B.; Chang, Z.; Fu, L. Enhancing performance in sandwich-like cobalt sulfide and carbon for quasi-solid-state hybrid electrochemical capacitors. J. Mater. Chem. A 2017, 5, 8981–8988. [Google Scholar]
- Guo, C.; Liu, M.; Gao, G.K.; Tian, X.; Zhou, J.; Dong, L.Z.; Li, Q.; Chen, Y.; Li, S.L.; Lan, Y.Q. Anthraquinone Covalent Organic Framework Hollow Tubes as Binder Microadditives in LiS Batteries. Angew. Chem. Int. Ed. 2022, 61, 113315. [Google Scholar]
- Karimi, A.; Kazeminezhad, I.; Naderi, L.; Shahrokhian, S. Construction of a Ternary Nanocomposite, Polypyrrole/Fe–Co Sulfide-Reduced Graphene Oxide/Nickel Foam, as a Novel Binder-Free Electrode for High-Performance Asymmetric Supercapacitors. J. Phys. Chem. C 2020, 124, 4393–4407. [Google Scholar] [CrossRef]
- Wei, K.; Lu, C.; Wu, Z.; Pu, J.; Wang, Z. Homogeneous core-shell NiCo2S4 nanostructures supported on nickel foam for supercapacitors. J. Mater. Chem. A 2015, 3, 12452–12460. [Google Scholar]
- Zhang, Y.; Xu, J.; Zheng, Y.; Zhang, Y.; Hu, X.; Xu, T. NiCo2S4@NiMoO4 Core-Shell Heterostructure Nanotube Arrays Grown on Ni Foam as a Binder-Free Electrode Displayed High Electrochemical Performance with High Capacity. Nanoscale Res. Lett. 2017, 12, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Ji, X.; Wu, Z.; Yong, L. NiCo2S4 hollow microsphere decorated by acetylene black for high-performance asymmetric supercapacitor. Electrochim. Acta 2015, 186, 562–571. [Google Scholar] [CrossRef]
- Gao, G.K.; Wang, Y.R.; Wang, S.B.; Yang, R.X.; Chen, Y.; Zhang, Y.; Jiang, C.; Wei, M.J.; Ma, H.; Lan, Y.Q. Stepped Channels Integrated Lithium–Sulfur Separator via Photoinduced Multidimensional Fabrication of Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2021, 60, 10147–10154. [Google Scholar] [CrossRef]
- Yang, F.; Xu, K.; Hu, J. Construction of Co3O4@Fe2O3 core-shell nanowire arrays electrode for supercapacitors. J. Alloys Compd. 2017, 729, 1172–1176. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Zhang, X. Preparation and electrochemistry of one-dimensional nanostructured MnO2/PPy composite for electrochemical capacitor. Appl. Surf. Sci. 2010, 256, 4339–4343. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, R.-L.; Lu, M.; Wang, J.-H.; Jiang, C.; Gao, G.-K.; Dong, L.-Z.; Chen, Y.; Li, S.-L.; Lan, Y.-Q. Single Metal Site and Versatile Transfer Channel Merged into Covalent Organic Frameworks Facilitate High-Performance Li-CO2 Batteries. ACS Cent. Sci. 2021, 7, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Hu, L.; Zhao, S.; Wu, L. Preparation of MnCo2O4@Ni(OH)2 Core–Shell Flowers for Asymmetric Supercapacitor Materials with Ultrahigh Specific Capacitance. Adv. Funct. Mater. 2016, 26, 4085–4093. [Google Scholar]
- Mohamed, S.G.; Chen, C.J.; Chen, C.K.; Hu, S.F.; Liu, R.S. High-Performance Lithium-Ion Battery and Symmetric Supercapacitors Based on FeCo2O4 Nanoflakes Electrodes. Acs Appl. Mater. Interfaces 2014, 6, 22701–22708. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.C.; Lin, L.Y.; Xiao, B.C.; Chen, Y.S. Highly efficient supercapacitor electrode with two-dimensional tungsten disulfide and reduced graphene oxide hybrid nanosheets. J. Power Sources 2016, 320, 78–85. [Google Scholar] [CrossRef]
- Nrr, A.; Pmr, A.; Tkm, A.; Aky, B.; Sang, W. Architecture of superior hybrid electrode by the composition of Cu2O nanoflakes, novel cadmium ferrite (CdFe2O4) nanoparticles, and g-C3N4 sheets for symmetric and asymmetric supercapacitors. J. Energy Storage 2021, 43, 103302. [Google Scholar]
- Wang, L.; Yang, H.; Liu, X.; Zeng, R.; Li, M.; Huang, Y.; Hu, X. Constructing hierarchical tectorum-like a-Fe2O3/ppy nanoarrays on carbon cloth for solid-state asymmetric supercapacitors. Angew. Chem. Int. Ed. 2018, 129, 1125–1130. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhou, J.; Bian, L.; Zhu, E.; Hai, J.; Tang, J.; Tang, W. Graphene/polypyrrole intercalating nanocomposites as supercapacitors electrode. Electrochim. Acta 2013, 112, 44–52. [Google Scholar] [CrossRef]
- Yan, M.; Yao, Y.; Wen, J.; Lu, L.; Huang, Z. Construction of Hierarchical NiCo2S4@PPy Core-Shell Heterostructure Nanotubes Array on Ni Foam for High-Performance Asymmetric Supercapacitor. ACS Appl. Mater. Interfaces 1944, 8, 24525. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, J.; Zhang, L.; Wan, H.; Xia, D. Highly conductive NiCo2S4 urchin-like nanostructures for high-rate pseudocapacitors. Nanoscale 2013, 5, 8879–8883. [Google Scholar] [CrossRef]
- Tan, L.C.; Guo, D.X.; Chu, D.W.; Yu, J.; Zhang, L.L.; Yu, J.; Wang, J. Metal organic frameworks template-directed fabrication of hollow nickel cobalt selenides with pentagonal structure for high-performance supercapacitors. J. Electroanal. Chem. 2019, 851, 113469. [Google Scholar] [CrossRef]
- Chu, S.; Cui, F.; Pu, J. Preparation and Electrochemical Characterization of Hollow Hexagonal NiCo2S4 Nanoplates as Pseudocapacitor Materials. ACS Sustain. Chem. Eng. 2014, 2, 809–815. [Google Scholar]
- Chu, D.; Guo, D.; Xiao, B.; Tan, L.; Ma, H.; Pang, H.; Wang, X.; Jiang, Y. 3D Hollow Flower-like CoWO4 Derived from ZIF-67 Grown on Ni-foam for High-Performance Asymmetrical Supercapacitors. Chem. Asian J. 2020, 15, 1750–1755. [Google Scholar] [CrossRef]
- Xue, P.; Guo, C.; Li, L.; Li, H.; Luo, D.; Tan, L.; Chen, Z. A MOF-Derivative Decorated Hierarchical Porous Host Enabling Ultrahigh Rates and Superior Long-Term Cycling of Dendrite-Free Zn Metal Anodes. Adv. Mater. 2022, 34, 2110047. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, Y.; Zhang, M.; Ma, N.N.; Lan, Y.Q. Exfoliation of covalent organic frameworks into MnO2-loaded ultrathin nanosheets as efficient cathode catalysts for Li-CO2 batteries. Cell Rep. Phys. Sci. 2021, 2, 100392. [Google Scholar] [CrossRef]
- Movassagh-Alanagh, F.; Bordbar-Khiabani, A.; Ahangari-Asl, A. Fabrication of a ternary PANI@Fe3O4@CFs nanocomposite as a high performance electrode for solid-state supercapacitors—ScienceDirect. Int. J. Hydrogen Energy 2019, 44, 26794–26806. [Google Scholar] [CrossRef]
- Chu, D.W.; Zhao, X.; Xiao, B.X.; Libanori, A.; Zhou, Y.H.; Tan, L.C.; Ma, H.Y.; Pang, H.J.; Wang, X.M.; Jiang, Y.X.; et al. Nickel/Cobalt Molybdate Hollow Rods Induced by Structure and Defect Engineering as Exceptional Electrode Materials for Hybrid Supercapacitor. Chem. A Eur. J. 2021, 27, 8337–8343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Wang, S.; Wang, K.; Chen, Z.; Xu, Q. Facilely constructing 3D porous NiCo2S4 nanonetworks for high-performance supercapacitors. New J. Chem. 2014, 38, 4045–4048. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, Y.; Liu, M.; Gao, G.K.; Ji, W.; Jiang, C.; Huang, X.; Chen, Y.; Li, S.L.; Lan, Y.Q. Single-metal site-embedded conjugated macrocyclic hybrid catalysts enable boosted CO2 reduction and evolution kinetics in Li-CO2 batteries—ScienceDirect. Cell Rep. Phys. Sci. 2021, 2, 100583. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, X.; Wu, Z.; Song, W.; Banks, C.E. Spinel NiCo2O4 for use as a high-performance supercapacitor electrode material: Understanding of its electrochemical properties. J. Power Sources 2014, 267, 888–900. [Google Scholar] [CrossRef]
- Lan, Y.Q.; Dong, L.Z.; Zhang, Y.; Lu, Y.F.; Li, S.L. Well-Defined Dual Mn-Sites based Metal-Organic Framework to Promote CO2 Reduction/Evolution in Li-CO2 Batteries. Chem. Commun. 2021, 57, 8937–8940. [Google Scholar]
- Zheng, Y.; Xu, J.; Yang, X.; Zhang, Y.; Shang, Y.; Hu, X. Decoration NiCo2S4 nanoflakes onto Ppy nanotubes as core-shell heterostructure material for high-performance asymmetric supercapacitor. Chem. Eng. J. 2017, 333, 111–121. [Google Scholar] [CrossRef]
- Chu, D.W.; Li, F.B.; Song, X.M.; Ma, H.Y.; Tan, L.C.; Pang, H.J.; Wang, X.M.; Guo, D.X.; Xiao, B.X. A novel dual-tasking hollow cube NiFe2O4-NiCo-LDH@rGO hierarchical material for high preformance supercapacitor and glucose sensor. J. Colloid Interface Sci. 2020, 568, 130–138. [Google Scholar] [CrossRef]
- Pu, J.; Wang, Z.; Wu, K.; Yu, N.; Sheng, E. Co9S8 nanotube arrays supported on nickel foam for high-performance supercapacitors. Phys. Chem. Chem. Phys. PCCP 2013, 16, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zou, Y.; Li, B.; Xu, F.; Chu, H.; Qiu, S.; Zhang, J.; Sun, L.; Xiang, C. Polypyrrole-wrapped NiCo2S4 nanoneedles as an electrode material for supercapacitor applications. Ceram. Int. 2021, 47, 16562–16569. [Google Scholar] [CrossRef]
- Shi, Z.; Shen, X.; Zhang, Z.; Wang, X.; Gao, N.; Xu, Z.; Chen, X.; Liu, X. Hierarchically urchin-like hollow NiCo2S4 prepared by a facile template-free method for high-performance supercapacitors. J. Colloid Interface Sci. 2021, 604, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, H.; Lin, Y.; Chen, J.; Li, K.; Cheng, A. Design and construction of nickel-cobalt-sulfide nanoparticles in-situ grown on graphene with enhanced performance for asymmetric supercapacitors. Diam. Relat. Mater. 2020, 108, 107925. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Cheng, B.; You, W.; Yu], J. 0D/2D (Fe0.5Ni0.5)S2/rGO nanocomposite with enhanced supercapacitor and lithium ion battery performance. J. Power Sources 2019, 426, 266–274. [Google Scholar] [CrossRef]
- Xu, R.; Lin, J.; Wu, J.; Huang, M.; Fan, L.; He, X.; Wang, Y.; Xu, Z. A two-step hydrothermal synthesis approach to synthesize NiCo2S4/NiS hollow nanospheres for high-performance asymmetric supercapacitors. Appl. Surf. Sci. 2017, 422, 597–606. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, A.; Song, X.; Wei, L.; Ma, H.; Pang, H.; Li, W.; Liu, X.; Tan, L. Design of an Internal/External Bicontinuous Conductive Network for High-Performance Asymmetrical Supercapacitors. Molecules 2022, 27, 8168. https://doi.org/10.3390/molecules27238168
Shi A, Song X, Wei L, Ma H, Pang H, Li W, Liu X, Tan L. Design of an Internal/External Bicontinuous Conductive Network for High-Performance Asymmetrical Supercapacitors. Molecules. 2022; 27(23):8168. https://doi.org/10.3390/molecules27238168
Chicago/Turabian StyleShi, Anran, Xiumei Song, Lei Wei, Huiyuan Ma, Haijun Pang, Weiwei Li, Xiaowei Liu, and Lichao Tan. 2022. "Design of an Internal/External Bicontinuous Conductive Network for High-Performance Asymmetrical Supercapacitors" Molecules 27, no. 23: 8168. https://doi.org/10.3390/molecules27238168
APA StyleShi, A., Song, X., Wei, L., Ma, H., Pang, H., Li, W., Liu, X., & Tan, L. (2022). Design of an Internal/External Bicontinuous Conductive Network for High-Performance Asymmetrical Supercapacitors. Molecules, 27(23), 8168. https://doi.org/10.3390/molecules27238168






