Cerium Oxide Nanorods Synthesized by Dalbergia sissoo Extract for Antioxidant, Cytotoxicity, and Photocatalytic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
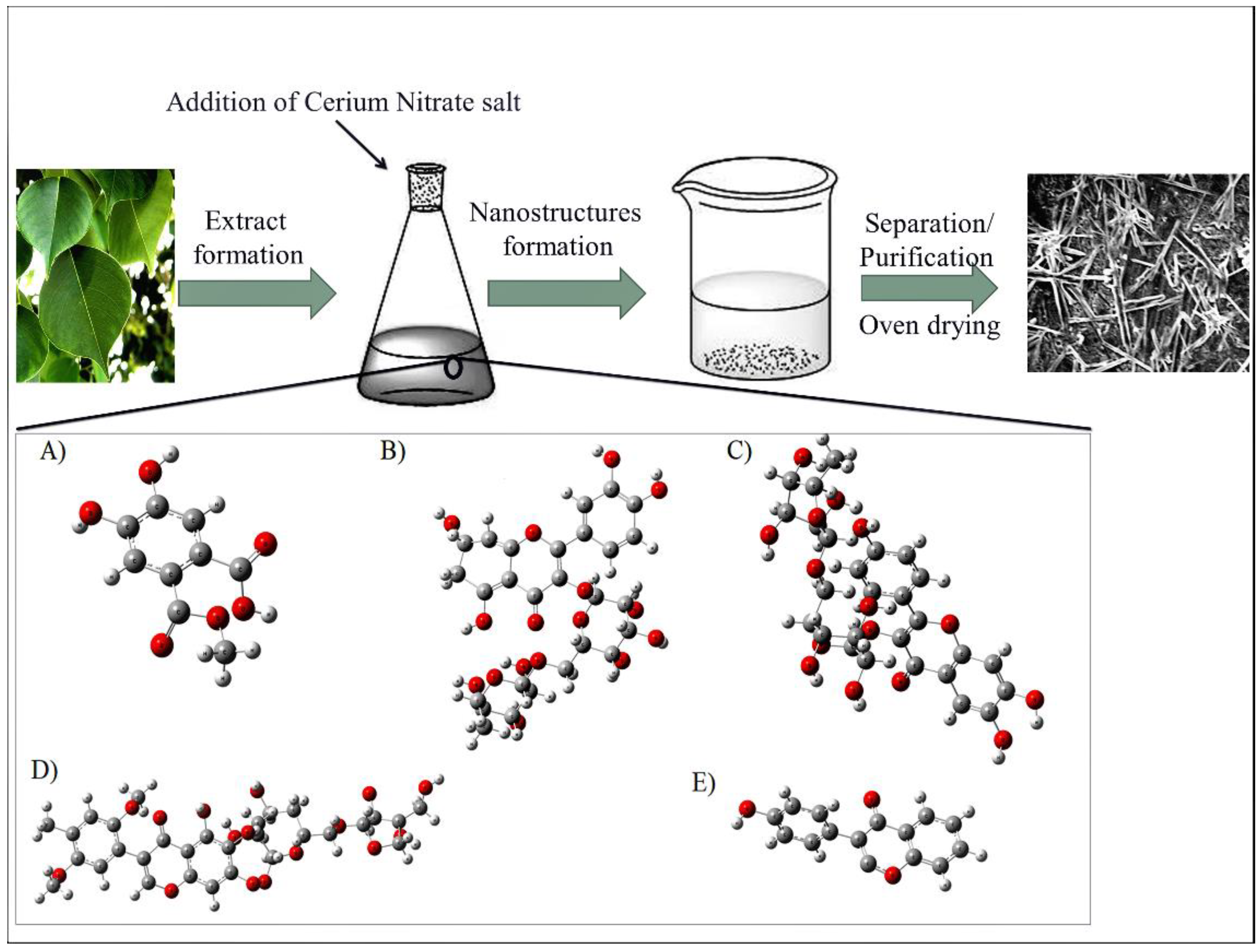
2.2. Preparation of D. sissoo-Stabilized CeO2-NRs
2.3. Characterization
2.4. DPPH Radical Scavenging Assay
2.5. Biochemical Assays for Human Lymphocytes Incubated with CeO2-NRs
2.6. Cytotoxicity of CeO2-NRs
2.7. Photocatalytic Efficacy of CeO2-NRs
3. Results and Discussion
3.1. Synthesis
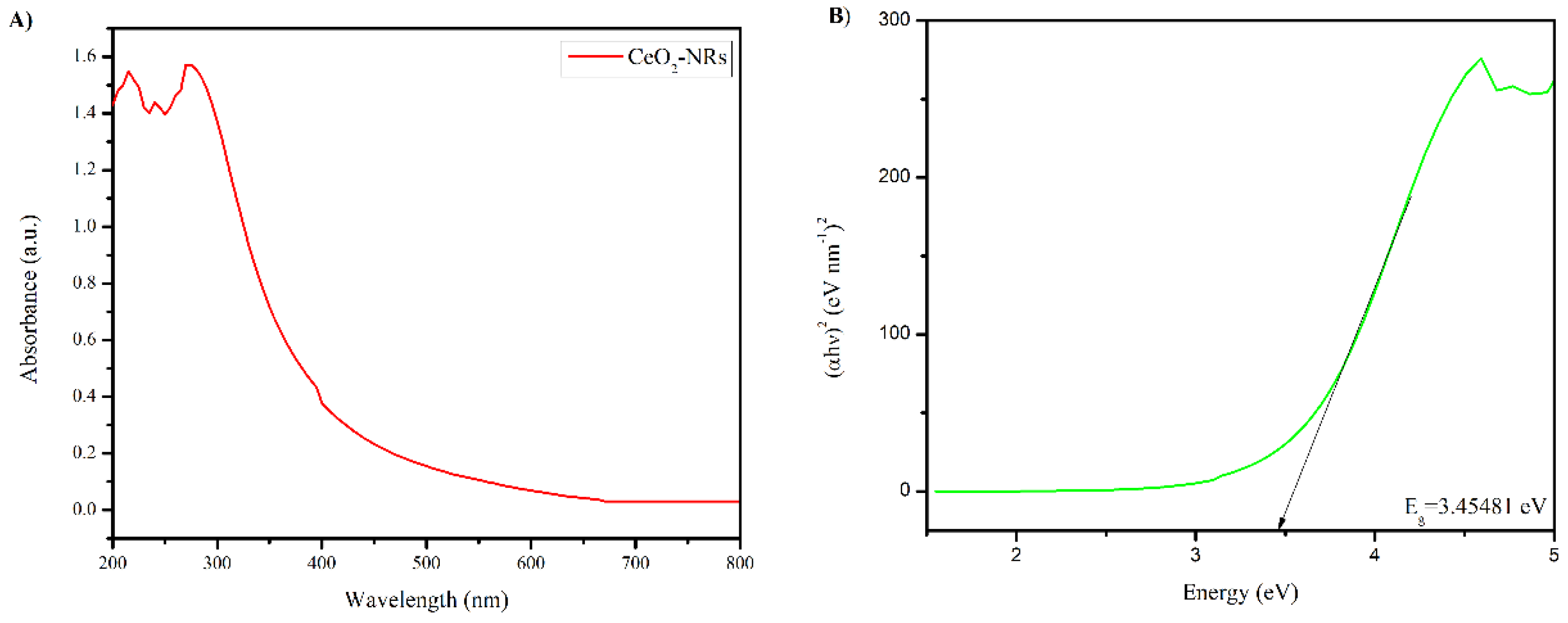
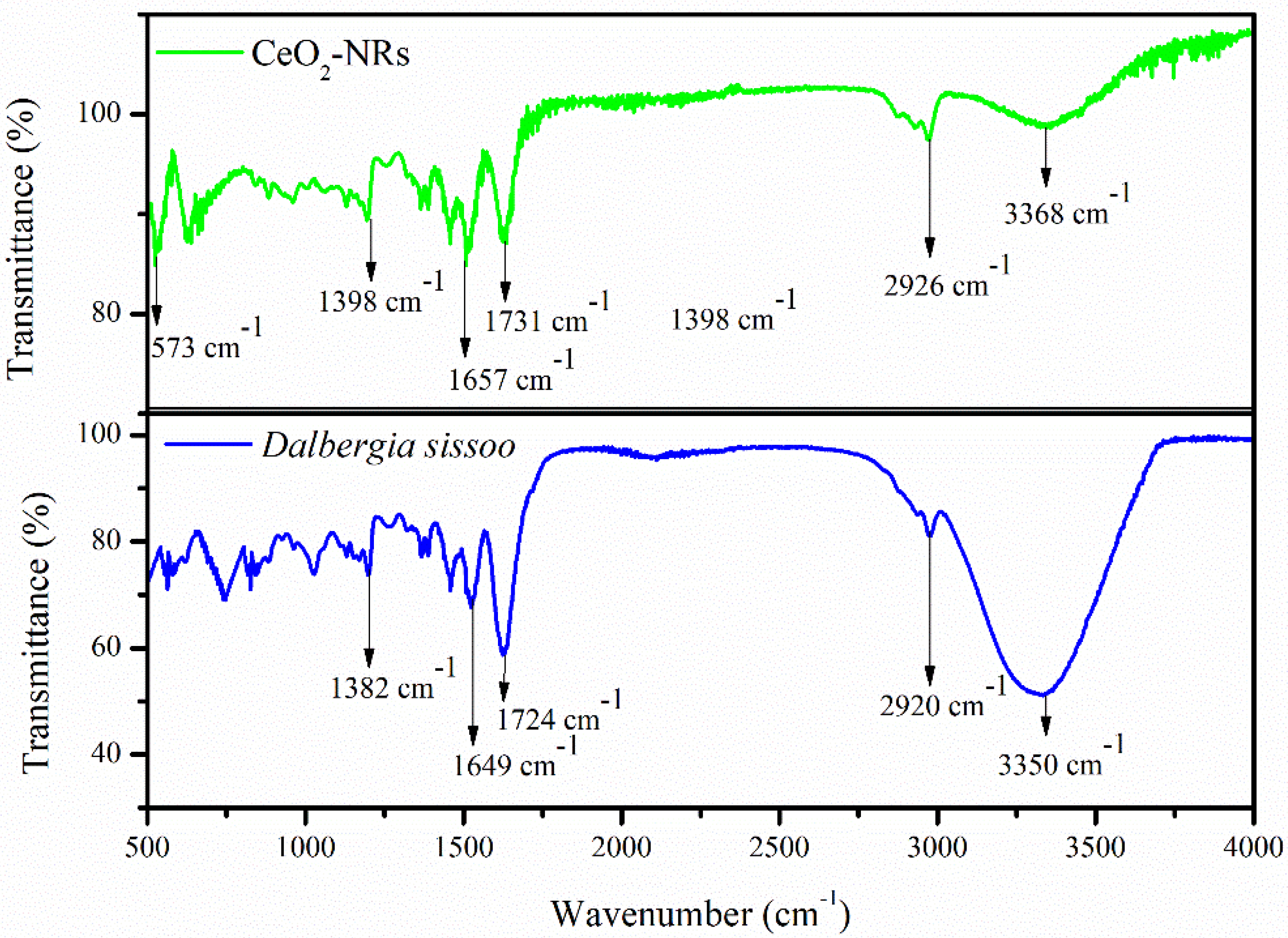
3.2. Characterization
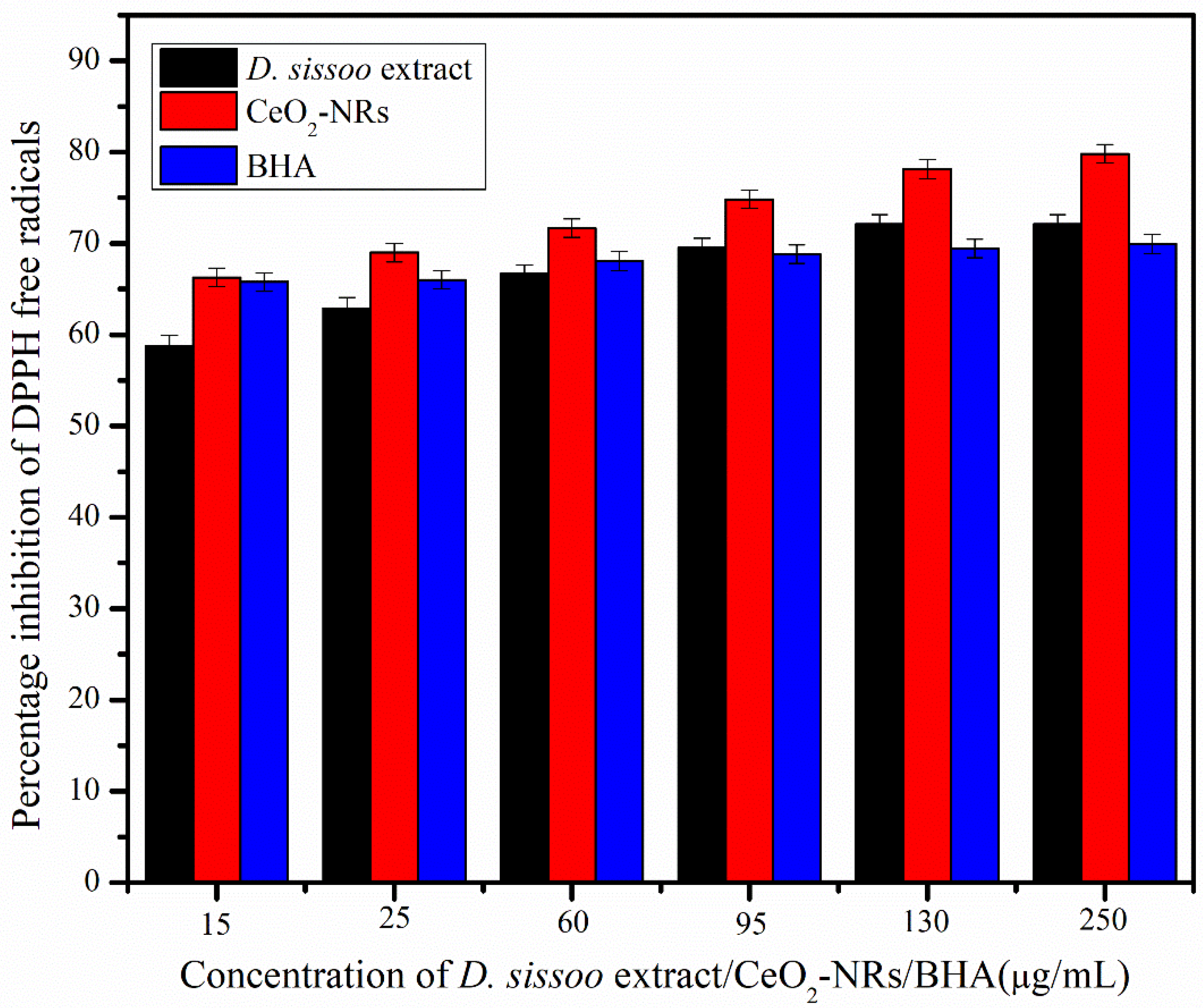
3.3. DPPH Assay for CeO2-NRs
3.4. Biochemical Assays for Human Lymphocytes Incubated with CeO2-NRs
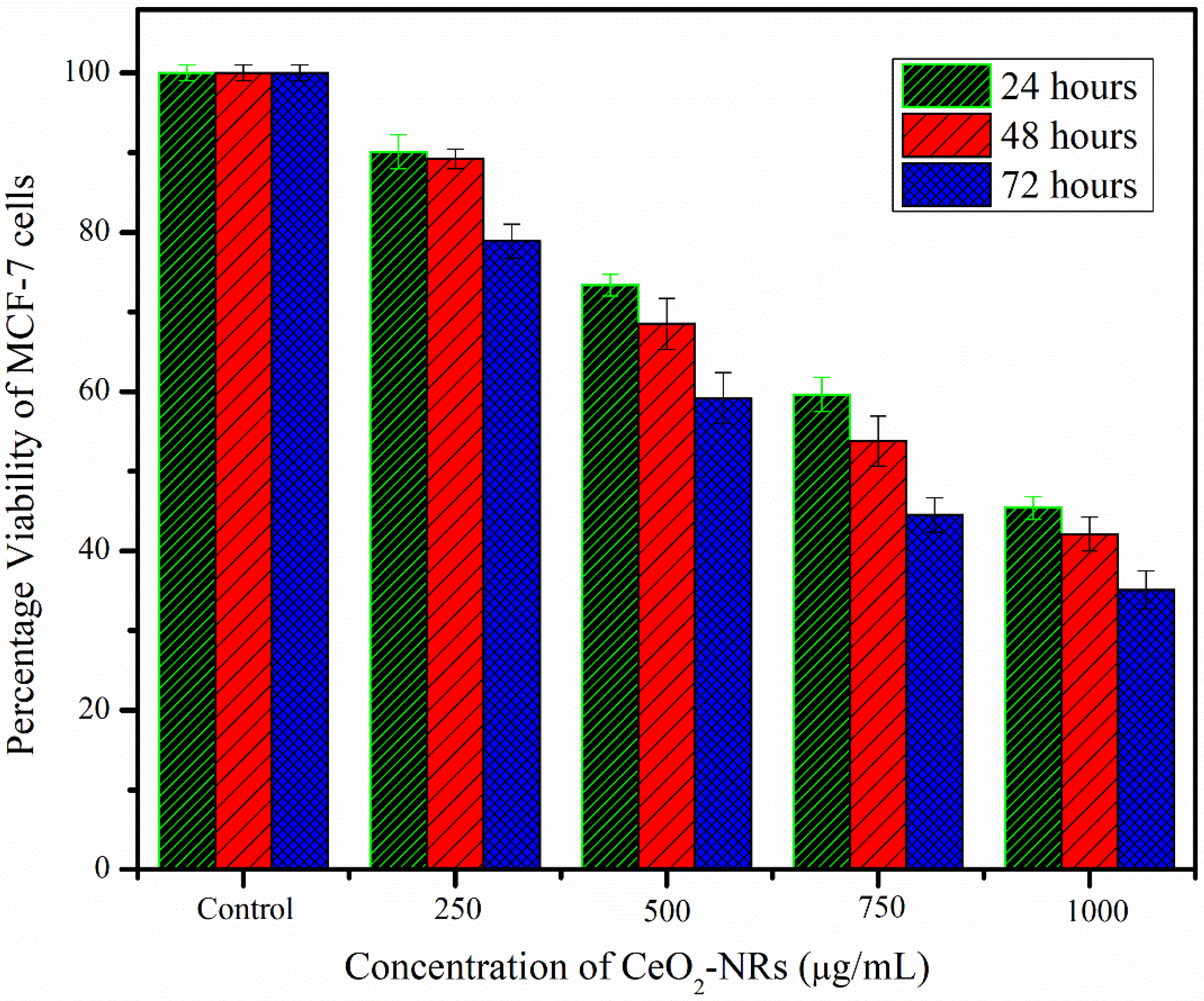
3.5. Cytotoxicity Effects of CeO2-NRs on the MCF-7 Cell Line
3.6. RSM-Based Optimization of Photocatalytic Activity of CeO2-NRs
3.6.1. Assortment of Predictive Model
3.6.2. ANOVA
3.6.3. Diagnostics Plots for the Selected Model
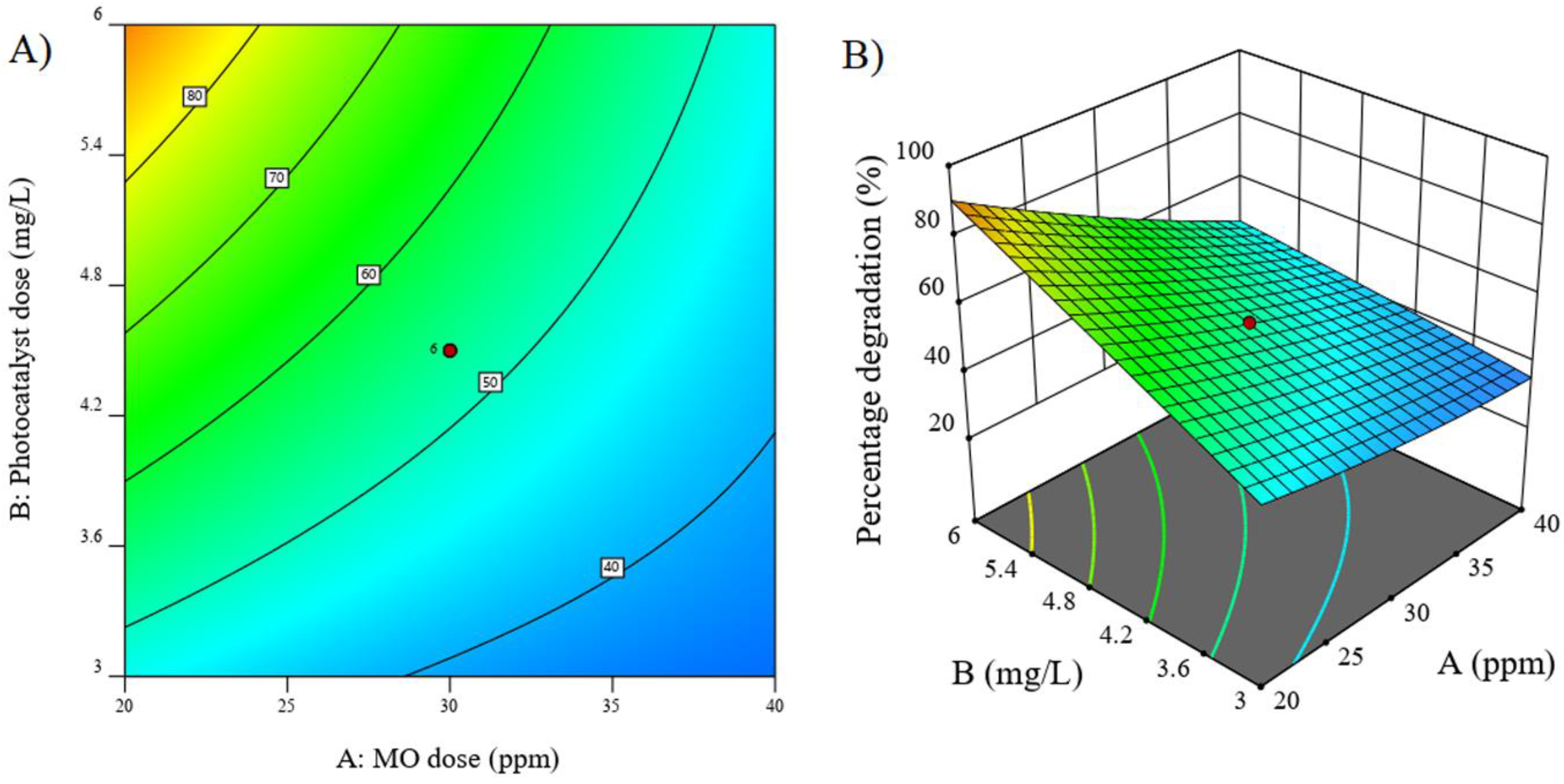
3.6.4. RSM-Based Three-Dimensional and Contour Plot
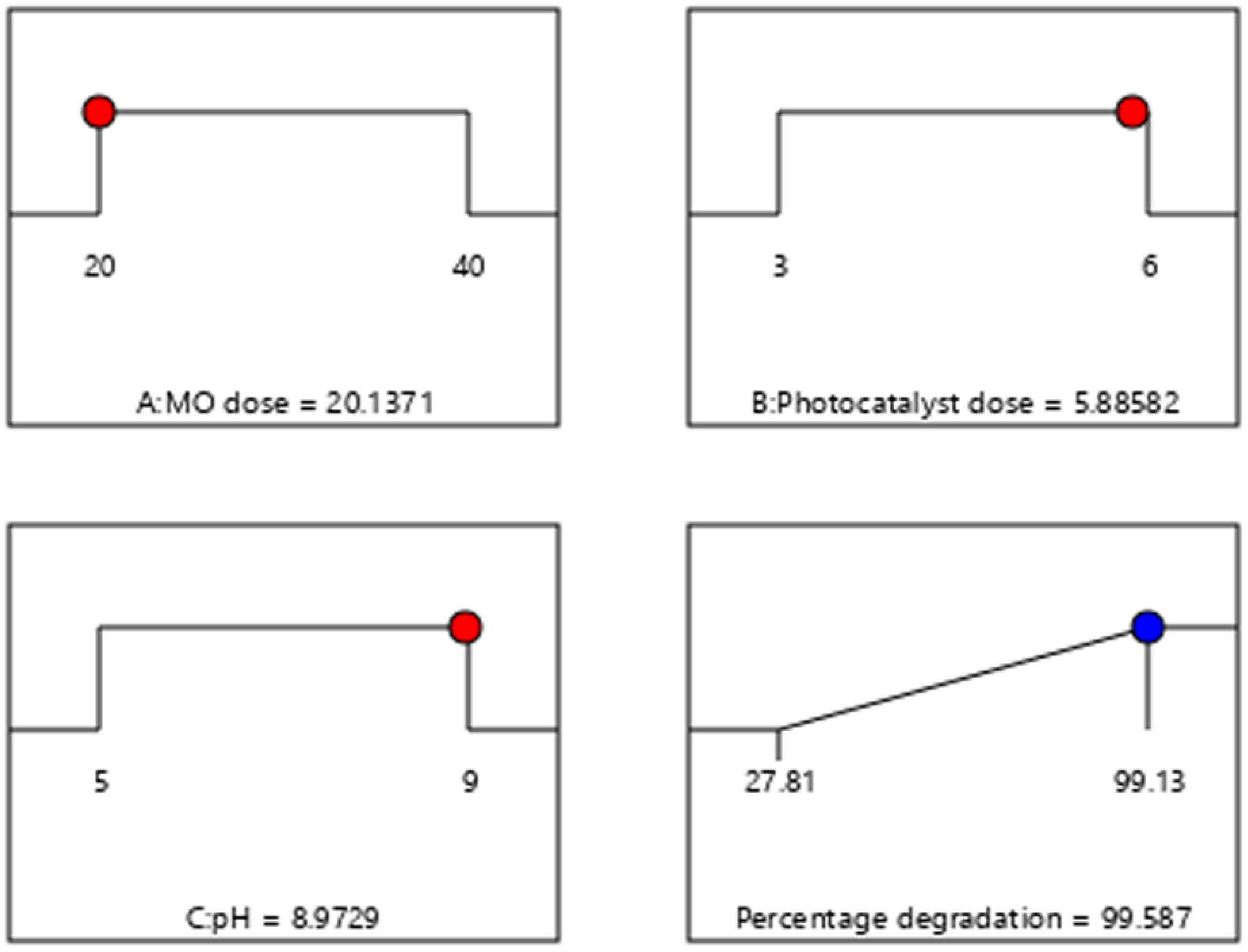
3.6.5. Optimization
4. Comparison with the Literature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.; Mutahir, S.; Wang, F.; Lei, W.; Xia, M.; Zhu, S. Facile one-step economical methodology of metal free g-C3N4 synthesis with remarkable photocatalytic performance under visible light to degrade trans-resveratrol. J. Hazard. Mater. 2019, 367, 293–303. [Google Scholar] [CrossRef]
- Angizi, S.; Alem, S.A.A.; Pakdel, A. Towards Integration of Two-Dimensional Hexagonal Boron Nitride (2D h-BN) in Energy Conversion and Storage Devices. Energies 2022, 15, 1162. [Google Scholar] [CrossRef]
- Chin, B.L.F.; Juwono, F.H.; Yong, K.S.C. Nanotechnology and Nanomaterials for Medical Applications. In Nanotechnology for Electronic Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 63–87. [Google Scholar]
- Prenzel, T.M.; Gehring, F.; Davis, Z.J.; Slack, N.; Katsavou, I.; Jakobsen, L.H.; Fito, C.; Hvid, N.; Serrano, J. Accelerating Sustainable Development and Production of Nanomaterials and Printed Electronics. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2022. [Google Scholar]
- Din, M.I.; Khalid, R.; Najeeb, J.; Hussain, Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies- a critical review. J. Clean. Prod. 2021, 298, 126567. [Google Scholar] [CrossRef]
- Azam, S.; Wei, Z.; Wang, R. Cerium oxide nanorods anchored on carbon nanofibers derived from cellulose paper as effective interlayer for lithium sulfur battery. J. Colloid Interface Sci. 2022, 615, 417–431. [Google Scholar] [CrossRef]
- Manjula, N. Electrocatalytic Determination of Isoprenaline Using well- shaped Cerium Oxide Nanorods Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2020, 15, 7359–7369. [Google Scholar] [CrossRef]
- Kim, N.-W.; Lee, D.-K.; Yu, H. Selective shape control of cerium oxide nanocrystals for photocatalytic and chemical sensing effect. RSC Adv. 2019, 9, 13829–13837. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Song, H. Synthesis of cerium oxide nanoparticles loaded on chitosan for enhanced auto-catalytic regenerative ability and biocompatibility for the spinal cord injury repair. J. Photochem. Photobiol. B Biol. 2019, 191, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Roudbaneh, S.Z.K.; Kahbasi, S.; Sohrabi, M.J.; Hasan, A.; Salihi, A.; Mirzaie, A.; Niyazmand, A.; Nanakali, N.M.Q.; Shekha, M.S.; Aziz, F.M.; et al. Albumin binding, antioxidant and antibacterial effects of cerium oxide nanoparticles. J. Mol. Liq. 2019, 296, 111839. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Naik, P. Synthesis and biomedical applications of Cerium oxide nanoparticles—A Review. Biotechnol. Rep. 2017, 17, 1–5. [Google Scholar] [CrossRef]
- Haider, S.; Khan, S.U.; Najeeb, J.; Naeem, S.; Rafique, H.; Munir, H.; Al-Masry, W.A.; Nazar, M.F. Synthesis of cadmium oxide nanostructures by using Dalbergia sissoo for response surface methodology based photocatalytic degradation of methylene blue. J. Clean. Prod. 2022, 365, 132822. [Google Scholar] [CrossRef]
- Munir, H.; Shahid, M.; Subhani, Z.; Afzal, M. Antimicrobial and antioxidant activities of hydrolysed and modified Gum Acacia modesta and Dalbergia sissoo. Oxid. Commun. 2015, 38, 1632–1644. [Google Scholar]
- Majeed, F.A.; Munir, H.; Rashid, R.; Zubair, M.T. Antimicrobial, cytotoxicity, mutagenicity and anti-epileptic potential of ethanol extracts of a multipurpose medicinal plant Dalbergia sissoo. Biocatal. Agric. Biotechnol. 2019, 19, 101155. [Google Scholar] [CrossRef]
- Khan, M.I.; Akhtar, M.N.; Ashraf, N.; Najeeb, J.; Munir, H.; Awan, T.I.; Tahir, M.B.; Kabli, M.R. Green synthesis of magnesium oxide nanoparticles using Dalbergia sissoo extract for photocatalytic activity and antibacterial efficacy. Appl. Nanosci. 2020, 10, 2351–2364. [Google Scholar] [CrossRef]
- Tabassam, N.; Mutahir, S.; Khan, M.A.; Khan, I.U.; Habiba, U.; Refat, M.S. Facile synthesis of cinnamic acid sensitized rice husk biochar for removal of organic dyes from wastewaters: Batch experimental and theoretical studies. Mater. Chem. Phys. 2022, 288, 126327. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Hussain, Z.; Hussain, T.; Mujahid, A.; Najeeb, J.; Izhar, F. Nanocatalytic Assemblies for Catalytic Reduction of Nitrophenols: A Critical Review. Crit. Rev. Anal. Chem. 2020, 50, 322–338. [Google Scholar] [CrossRef] [PubMed]
- Thill, A.S.; Figueiredo, W.T.; Lobato, F.O.; Vaz, M.O.; Fernandes, W.P.; Carvalho, V.E.; Soares, E.A.; Poletto, F.; Teixeira, S.R.; Bernardi, F. New horizons in photocatalysis: The importance of mesopores for cerium oxide. J. Mater. Chem. A 2020, 8, 24752–24762. [Google Scholar] [CrossRef]
- Miri, A.; Beiki, H.; Najafidoust, A.; Khatami, M.; Sarani, M. Cerium oxide nanoparticles: Green synthesis using Banana peel, cytotoxic effect, UV protection and their photocatalytic activity. Bioprocess Biosyst. Eng. 2021, 44, 1891–1899. [Google Scholar] [CrossRef]
- Soren, S.; Jena, S.R.; Samanta, L.; Parhi, P. Antioxidant Potential and Toxicity Study of the Cerium Oxide Nanoparticles Synthesized by Microwave-Mediated Synthesis. Appl. Biochem. Biotechnol. 2015, 177, 148–161. [Google Scholar] [CrossRef]
- Soltani, M.; Zarei, M.H.; Salimi, A.; Pourahmad, J. Mitochondrial protective and antioxidant agents protect toxicity induced by depleted uranium in isolated human lymphocytes. J. Environ. Radioact. 2019, 203, 112–116. [Google Scholar] [CrossRef]
- Alam, M.W.; Najeeb, J.; Naeem, S.; Usman, S.M.; Nahvi, I.; Alismail, F.; Abuzir, A.; Farhan, M.; Nawaz, A. Electrochemical Methodologies for Investigating the Antioxidant Potential of Plant and Fruit Extracts: A Review. Antioxidants 2022, 11, 1205. [Google Scholar] [CrossRef]
- Afsar, T.; Razak, S.; Almajwal, A. Effect of Acacia hydaspica R. Parker extract on lipid peroxidation, antioxidant status, liver function test and histopathology in doxorubicin treated rats. Lipids Heal. Dis. 2019, 18, 126. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, M.; Inaloz, H.S.; Baysal, V.; Delibas, N. The role of oxidants and antioxidants in psoriasis. J. Eur. Acad. Dermatol.Venereol. 2003, 17, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, Z.; Sabouri, M.; Amiri, M.S.; Khatami, M.; Darroudi, M. Plant-based synthesis of cerium oxide nanoparticles using Rheum turkestanicum extract and evaluation of their cytotoxicity and photocatalytic properties. Mater. Technol. 2022, 37, 555–568. [Google Scholar] [CrossRef]
- Chahal, S.; Phor, L.; Singh, S.; Singh, A.; Malik, J.; Goel, P.; Kumar, A.; Kumar, S.; Ankita; Kumar, P. An efficient and unique method for the growth of spindle shaped Mg-doped cerium oxide nanorods for photodegradation of p-Nitrophenol. Ceram. Int. 2022, 48, 28961–28968. [Google Scholar] [CrossRef]
- Putri, G.E.; Rilda, Y.; Syukri, S.; Labanni, A.; Arief, S. Highly antimicrobial activity of cerium oxide nanoparticles synthesized using Moringa oleifera leaf extract by a rapid green precipitation method. J. Mater. Res. Technol. 2021, 15, 2355–2364. [Google Scholar] [CrossRef]
- Zulfiqar, A.; Mumtaz, M.W.; Mukhtar, H.; Najeeb, J.; Irfan, A.; Akram, S.; Touqeer, T.; Nabi, G. Lipase-PDA-TiO2 NPs: An emphatic nano-biocatalyst for optimized biodiesel production from Jatropha curcas oil. Renew. Energy 2021, 169, 1026–1037. [Google Scholar] [CrossRef]
- Ashokkumar, R.; Ramaswamy, M. Phytochemical screening by FTIR spectroscopic analysis of leaf extracts of selected Indian medicinal plants. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 395–406. [Google Scholar]
- Dutta, D.; Mukherjee, R.; Patra, M.; Banik, M.; Dasgupta, R.; Mukherjee, M.; Basu, T. Green synthesized cerium oxide nanoparticle: A prospective drug against oxidative harm. Colloids Surf. B Biointerfaces 2016, 147, 45–53. [Google Scholar] [CrossRef]
- Sehra, S.; Sharma, J. Pharmacological Effects and Medicinal Importance of Dalbergia Sissoo—A Review. Int. J. Pharm. Chem. Biol. Sci. 2018, 8, 218673105. [Google Scholar]
- Tortolini, C.; Bollella, P.; Zumpano, R.; Favero, G.; Mazzei, F.; Antiochia, R. Metal Oxide Nanoparticle Based Electrochemical Sensor for Total Antioxidant Capacity (TAC) Detection in Wine Samples. Biosensors 2018, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Khaksar, M.R.; Rahimifard, M.; Baeeri, M.; Maqbool, F.; Navaei-Nigjeh, M.; Hassani, S.; Moeini-Nodeh, S.; Kebriaeezadeh, A.; Abdollahi, M. Protective effects of cerium oxide and yttrium oxide nanoparticles on reduction of oxidative stress induced by sub-acute exposure to diazinon in the rat pancreas. J. Trace Elem. Med. Biol. 2017, 41, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navada, M.K.; Karnikkar, N.G.; D’Souza, J.N.; Kouser, S.; Aroor, G.; Kudva, J.; Jayappa, M.D. Biosynthesis of phyto functionalized cerium oxide nanoparticles mediated from Scoparia dulsis L. for appraisal of anti-cancer potential against adenocarcinomic lung cancer cells and paracetamol sensing potentiality. Environ. Sci. Pollut. Res. 2022, 1–20. [Google Scholar] [CrossRef]
- Ratnam, M.V.; Karthikeyan, C.; Rao, K.N.; Meena, V. Magnesium oxide nanoparticles for effective photocatalytic degradation of methyl red dye in aqueous solutions: Optimization studies using response surface methodology. Mater. Today Proc. 2020, 26, 2308–2313. [Google Scholar] [CrossRef]
- Mortazavian, S.; Saber, A.; James, D.E. Optimization of photocatalytic degradation of acid blue 113 and acid red 88 textile dyes in a UV-C/TiO2 suspension system: Application of response surface methodology (RSM). Catalysts 2019, 9, 360. [Google Scholar] [CrossRef] [Green Version]
- Deriase, S.F.; El-Salamony, R.A.; Amdeha, E.; Al-Sabagh, A.M. Statistical optimization of photocatalytic degradation process of methylene blue dye by SnO–TiO2–AC composite using response surface methodology. Environ. Prog. Sustain. Energy 2021, 40, e13639. [Google Scholar] [CrossRef]
- Chaker, H.; Attar, A.E.; Djennas, M.; Fourmentin, S. A statistical modeling-optimization approach for efficiency photocatalytic degradation of textile azo dye using cerium-doped mesoporous ZnO: A central composite design in response surface methodology. Chem. Eng. Res. Des. 2021, 171, 198–212. [Google Scholar] [CrossRef]
- Kaur, J.; Bansal, S.; Singhal, S. Photocatalytic degradation of methyl orange using ZnO nanopowders synthesized via thermal decomposition of oxalate precursor method. Phys. B Condens. Matter 2013, 416, 33–38. [Google Scholar] [CrossRef]
- Sithole, R.; Machogo-Phao, L.F.E.; Moloto, M.J.; Gqoba, S.S.; Mubiayi, K.P.; Van Wyk, J.; Moloto, N. One-step synthesis of Cu3N, Cu2S and Cu9S5 and photocatalytic degradation of methyl orange and methylene blue. J. Photochem. Photobiol. A Chem. 2020, 397, 112577. [Google Scholar] [CrossRef]
- Khalilian, H.; Behpour, M.; Atouf, V.; Hosseini, S.N. Immobilization of S, N-codoped TiO2 nanoparticles on glass beads for photocatalytic degradation of methyl orange by fixed bed photoreactor under visible and sunlight irradiation. Sol. Energy 2015, 112, 239–245. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Mohana, V.; Manoharan, C. Green synthesis of cerium oxide nanoparticles using Calotropis procera flower extract and their photocatalytic degradation and antibacterial activity. Inorg. Chem. Commun. 2020, 119, 108086. [Google Scholar] [CrossRef]
- Jasim, S.A.; Patra, I.; Abdulhadi, A.M.; Al-Gazally, M.E.; Sharma, H.; Alawsi, T.; Mohammed, H.T.; Hussein, S.A.; Altimari, U.S.; Hammid, A.T.; et al. Magnetic CeO2/SrFe12O19 Nanocomposite: Synthesis, Characterization and Photocatalytic Degradation of Methyl Orange. Arab. J. Sci. Eng. 2022, 1–8. [Google Scholar] [CrossRef]
- Channei, D.; Nakaruk, A.; Jannoey, P.; Phanichphant, S. Preparation and characterization of Pd modified CeO2 nanoparticles for photocatalytic degradation of dye. Solid State Sci. 2019, 87, 9–14. [Google Scholar] [CrossRef]
- Phadi, B.M.; Oyewo, O.A.; Ramaila, S.; Mavuru, L.; Onwudiwe, D.C. Nanocomposite of CeVO4/BiVO4 loaded on reduced graphene oxide for the photocatalytic degradation of methyl orange. J. Clust. Sci. 2022, 33, 2707–2721. [Google Scholar] [CrossRef]




| CAT (units/mL) | |||
|---|---|---|---|
| 15 | 0.15 0.02 | 1.89 0.03 | 1.12 0.01 |
| 30 | 0.23 0.01 | 1.52 0.01 | 1.32 0.02 |
| 60 | 0.39 0.01 | 1.25 0.01 | 2.20 0.01 |
| 120 | 0.93 0.02 | 1.85 0.01 | 2.76 0.01 |
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | Degree of Significance |
| Model | 6513.65 | 9 | 723.74 | 149.51 | <0.0001 | Significant |
| MO Dose (A) | 2564.38 | 1 | 2564.38 | 529.74 | <0.0001 | |
| Photocatalyst Dose (B) | 2559.89 | 1 | 2559.89 | 528.81 | <0.0001 | |
| pH (C) | 541.83 | 1 | 541.83 | 111.93 | <0.0001 | |
| AB | 529.10 | 1 | 529.10 | 109.30 | <0.0001 | |
| AC | 80.14 | 1 | 80.14 | 16.55 | 0.0023 | |
| BC | 18.91 | 1 | 18.91 | 3.91 | 0.0763 | |
| A2 | 48.86 | 1 | 48.86 | 10.09 | 0.0099 | |
| B2 | 2.41 | 1 | 2.41 | 0.4983 | 0.4964 | |
| C2 | 175.23 | 1 | 175.23 | 36.20 | 0.0001 | |
| Residual | 48.41 | 10 | 4.84 | |||
| Lack of Fit | 47.62 | 5 | 9.52 | 60.50 | 0.0002 | Not Significant |
| Pure Error | 0.7872 | 5 | 0.1574 | |||
| Cor Total | 6562.06 | 19 | ||||
| Fit Statistics | ||||||
| Standard Deviation | 2.20 | |||||
| Square of Regression Coefficient (R2) | 0.9926 | |||||
| Adjusted R2 | 0.9860 | |||||
| Predicted R2 | 0.9429 | |||||
| Photocatalysts | Preparation Method | Irradiation Source | Degradation Percentage | Reference |
|---|---|---|---|---|
| Ce-ZnO | Hydrothermal | Fluorescent lamp | 94.06% | [16] |
| CeO2-NPs | Calotropis procera flower extract | Sunlight | 98% | [43] |
| CeO2/SrFe12O19 NCs | Co-precipitation | Xe lamp (UV radiations) | 88.38% | [44] |
| Pd-CeO2 | Precipitation and impregnation | Halogen lamp | 92% | [45] |
| CeVO4/BiVO4/rGO NCs | Solvothermal | Xe lamp | 98% | [46] |
| CeO2-NRs | Dalbergia sissoo extract | UV lamp | 99.317% | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.W.; Naeem, S.; Usman, S.M.; Kanwal, Q.; BaQais, A.; Aldughaylibi, F.S.; Nahvi, I.; Zaidi, N. Cerium Oxide Nanorods Synthesized by Dalbergia sissoo Extract for Antioxidant, Cytotoxicity, and Photocatalytic Applications. Molecules 2022, 27, 8188. https://doi.org/10.3390/molecules27238188
Alam MW, Naeem S, Usman SM, Kanwal Q, BaQais A, Aldughaylibi FS, Nahvi I, Zaidi N. Cerium Oxide Nanorods Synthesized by Dalbergia sissoo Extract for Antioxidant, Cytotoxicity, and Photocatalytic Applications. Molecules. 2022; 27(23):8188. https://doi.org/10.3390/molecules27238188
Chicago/Turabian StyleAlam, Mir Waqas, Sumaira Naeem, Sheikh Muhammad Usman, Qudsia Kanwal, Amal BaQais, Fatimah Saeed Aldughaylibi, Insha Nahvi, and Noushi Zaidi. 2022. "Cerium Oxide Nanorods Synthesized by Dalbergia sissoo Extract for Antioxidant, Cytotoxicity, and Photocatalytic Applications" Molecules 27, no. 23: 8188. https://doi.org/10.3390/molecules27238188
APA StyleAlam, M. W., Naeem, S., Usman, S. M., Kanwal, Q., BaQais, A., Aldughaylibi, F. S., Nahvi, I., & Zaidi, N. (2022). Cerium Oxide Nanorods Synthesized by Dalbergia sissoo Extract for Antioxidant, Cytotoxicity, and Photocatalytic Applications. Molecules, 27(23), 8188. https://doi.org/10.3390/molecules27238188








