Abstract
An electrochemical initiated tandem reaction of anilines with 2-formyl benzonitrile has been developed. Thus, unprecedented 3-N-aryl substituted isoindolinones have been conveniently achieved by constant current electrolysis in a divided cell using catalytic amount of electricity and supporting electrolyte and a Pt-cathode as working electrode. The origin of the electrochemical activation as well as the mechanism of the subsequent chemical cascade reactions have been investigated by DFT calculations.
1. Introduction
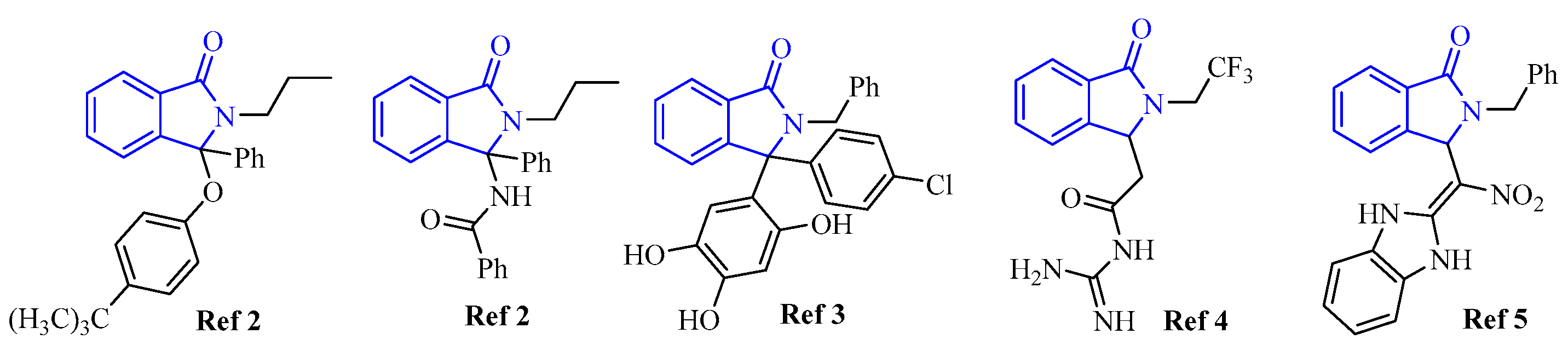
Among nitrogen-containing heterocycles [1], the class of isoindolinones has received considerable interest for decades due to their potential as bioactive ingredients in medicinal chemistry. By way of example, in 2005 an in silico screening of a first generation of isoindolinones highlighted their potential as inhibitors of the MDM2-p53 interaction [2]. Yet, subsequent studies have also shown that introducing other functional groups into the isoindolinone scaffold can significantly improve their pharmacological activity [3,4,5]. Therefore, structural modifications of the isoindolinone motif continue to be the subject of intense investigation for synthetic chemists who face the double challenge of creating new libraries of increasing structural complexity and, at the same time, proposing a sustainable synthesis (Figure 1).
Figure 1.
Representative isoindolinones tested for biological activity.
To this regard, we have been exploring for a decade tandem and sequential reactions of 2-formyl benzonitriles succeeding in developing convenient methodologies to access various isoindolinone-containing structures which include the ones with N and S moieties at the exocyclic position [6,7]. Our approaches complement several others that use strategies and synthons designed according to the distinctiveness of the extra functionalities and features of the desired products.
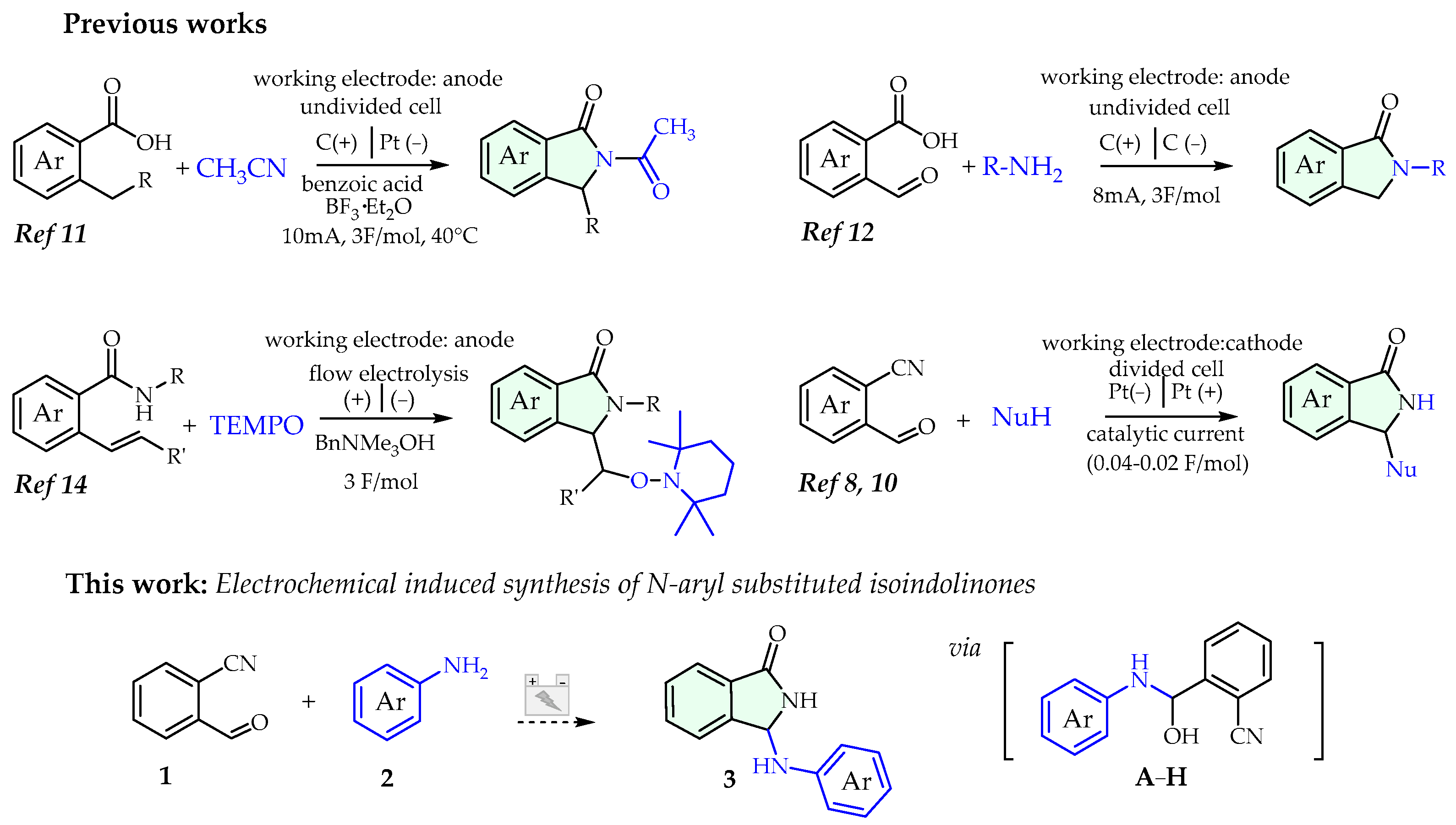
Besides purely chemical approaches, over the past decades, we [8,9,10] and others [11,12,13,14] also demonstrated the effectiveness of electrocatalysis to promote the synthesis of functionalized isoindolinones: these methods, framed in the picture of electro-organic chemistry renaissance [15,16,17,18], offer several benefits from a synthetic point of view, especially in terms of eco-friendly and waste minimization (Scheme 1).
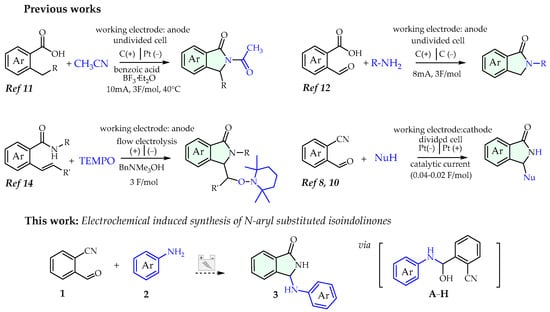
Scheme 1.
Electrosynthesis of isoindolinones (selected lit. of tandem approaches) and this work.
Based on our previous reports on this topic, we herein report an electrochemical induced tandem reaction of functionalized anilines with 2-formylbenzonitrile to install N-aryl substituents in the third position of the isoindolinone nucleus (Scheme 1).
Furthermore, to provide some more quantitative mechanistic insights, we herein explored the potential energy surface of the whole process by means of quantum-chemical calculations in the framework of density functional theory (DFT).
2. Results and Discussion
2.1. Optimization of Reaction Conditions
According to the Mayr’s scale, despite their low basicity, anilines still exhibit good nucleophilicity parameters toward reference electrophiles [19,20]; however, with respect to the carbonyl addition, aniline hemiaminals are rarely detected in organic solvent due to their marked tendency to release H2O yielding imines and, concurrently, because of the low global Keq of this reaction. Consistently, aniline itself proved to serve as nucleophilic catalyst in transimination reaction for oxime and hydrazone synthesis, via aniline Schiff base [21,22]. Indeed, imines derived from anilines are often the focus of various studies of dynamic covalent chemistry [23].
Said the above, to the extent that hamiaminals A–H are intended as crucial intermediates for the cascade reaction leading to isoindolinones 3 (via cyclization/rearrangement), anilines are quite challenging substrates with respect to alkyl or aliphatic amines in general.
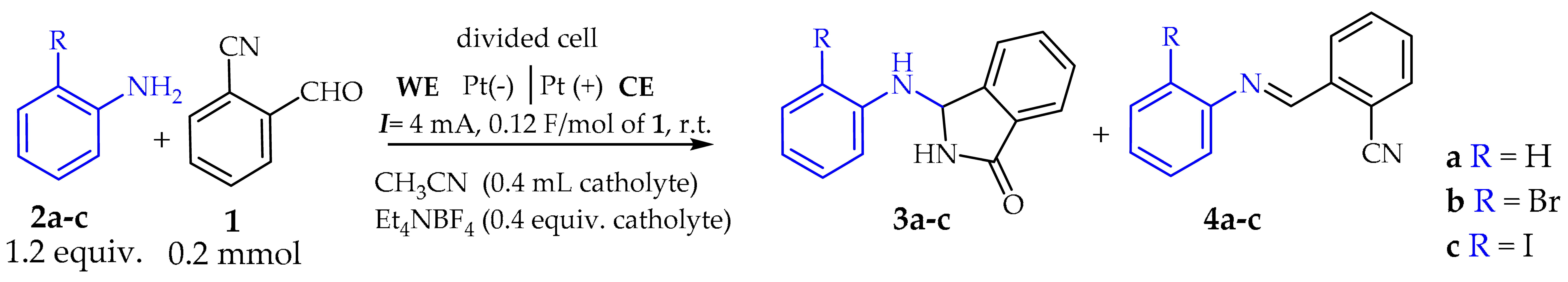
With the aim to attempt an electro-catalyzed process with aromatic amines as nucleophiles, we initiated our investigation by performing the reaction of the model compounds aniline (2a), 2-bromoaniline (2b) or 2-iodoaniline (2c) with the 2-formylbenzonitrile under a variety of electrochemical setup and conditions.
Standard conditions of Table 1 ensured a good 91% yield in the corresponding product 3 using 2a as nucleophile, while 80% and 57% yield were respectively obtained using the more challenging 2-halogenated anilines 2b and 2c which are known to be prone to cathodic dehalogenation [24].

Table 1.
Optimization of the reaction conditions (a).
Modifications of the standard reaction conditions such as current quantity/intensity (Table 1, entries 4, 9, 13), concentrations of the reagents (Table 1, entries 4, and 12), solvents (Table 1, entries 5, 6, and 7) etc., as well as variations of the electrochemical setup (divided vs. undivided cells, porosity of the glass separating septum, electrode materials), resulted in diminished yield for all the three products (see also supplementary conditions for further optimization details).
The data reported in Table 1 also show that starting material 1 might undergo extensive decomposition, even applying a current quantity as low as 0.06 F/mol of 1 (Table 1, entry 9). In fact, while under optimized conditions 3 is always observed as the most abundant product with 2a, 2b, and 2c, aldehyde 1 could not be recovered, regardless of whether the applied conditions were effective to yield isolable products. Conversely, a significant recovery of unreacted 2a–c anilines was ascertained in almost all the cases (except Table 1, entry 6). Yet, no better yields have been achieved using anilines 2 as limiting reagents (Table 1, entry 10).
2.2. Electrochemically Induced Synthesis of 3-N-Aryl Substituted Isoindolinones
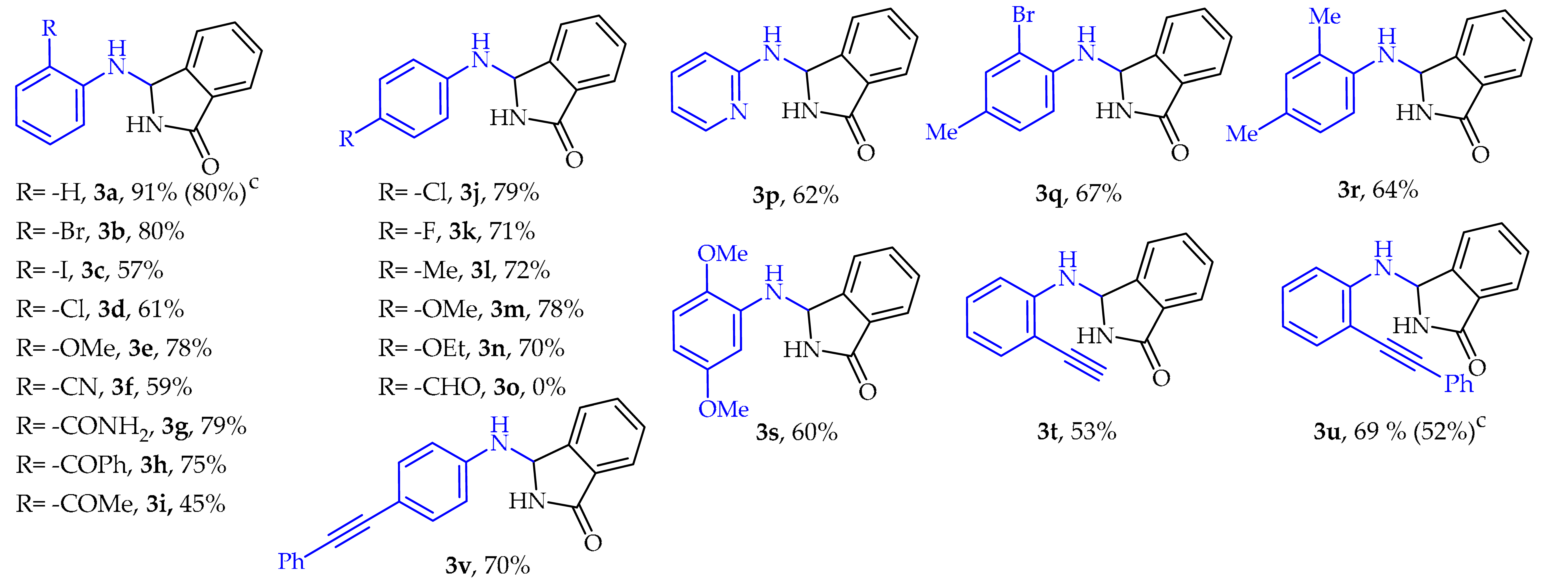
Having optimized the reaction conditions, we evaluated scope and limitation of the electrochemical method by testing the series of compounds reported in Table 2.

Table 2.
Synthesis of 3-N-aryl substituted isoindolinones (a,b).
As shown, a variety of substituent on the aniline molecule, such as alkyl (Me), alkoxy (OMe, OEt), halogens (Br, Cl, F), and/or functional groups such as alkynyl, cyano, amide, keto, formyl etc., were examined to altogether assess the influence of changes in electron density of the benzene ring, functional group tolerance, and steric hindrance effect.
Noteworthy, with respect to heterogeneous basic catalysis (3a and 3u, Table 2, data in parentheses), the electrochemical method emerges as superior, both in terms of efficiency and reaction times.
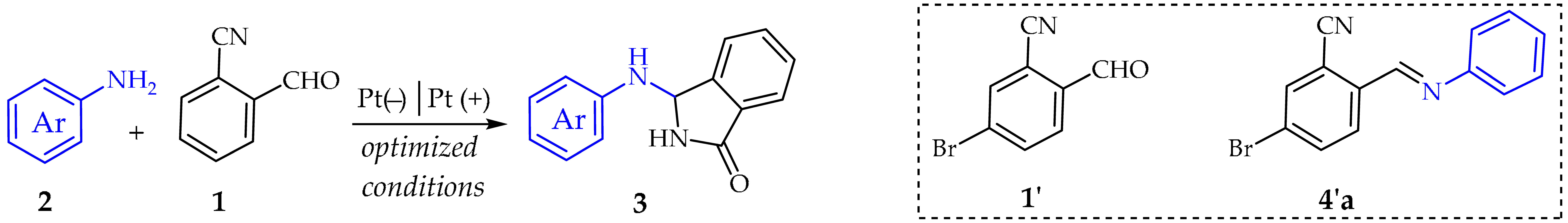
Though 2i and 2t furnished the corresponding products 3i and 3t with barely acceptable yields and no starting materials recovery, we were pleased to find that the electrochemical conditions were compatible with almost all the other anilines, including the ones having ortho- and para-alkynyl (2t–v) and ortho-benzoyl (2h) moieties. Moreover, 2-aminopyridine 2p also demonstrated a good reactivity under electrochemical conditions, leading to the corresponding hybrid pyridine-isoindolinone 3p with a 62% yield. It is worth noting that, with respect to the aryl amine, the selectivity is generally high (>85% based on recovered starting material), despite the moderate yields occasionally observed. Moreover, we want to remark the successful attainment of derivatives having sensible functionalities on the aniline moieties such as 3f (o-CN), 3g (o-CO2NH2), and 3i (o-COMe), useful for further diversification of the molecular structures. Conversely, p-aminobenzaldehyde 2o failed to yield any product, probably because of the low tolerance of the electrochemical ambient vs. the formyl group. Indeed, both 1 and 2o partially decomposed under the standard electrochemical conditions. Likewise, the attempt to use 5-bromo-2-formylbenzonitrile (1′) instead of 1 as a reagent with aniline 2a was unsuccessful. Indeed, extensive decomposition of 1′ occurred under standard electrochemical conditions, while only partial conversion to the corresponding imine 4′a was observed using heterogeneous basic catalysis (conditions reported in Table 2, note c).
2.3. Quantum-Chemical Calculations and Plausible Mechanism
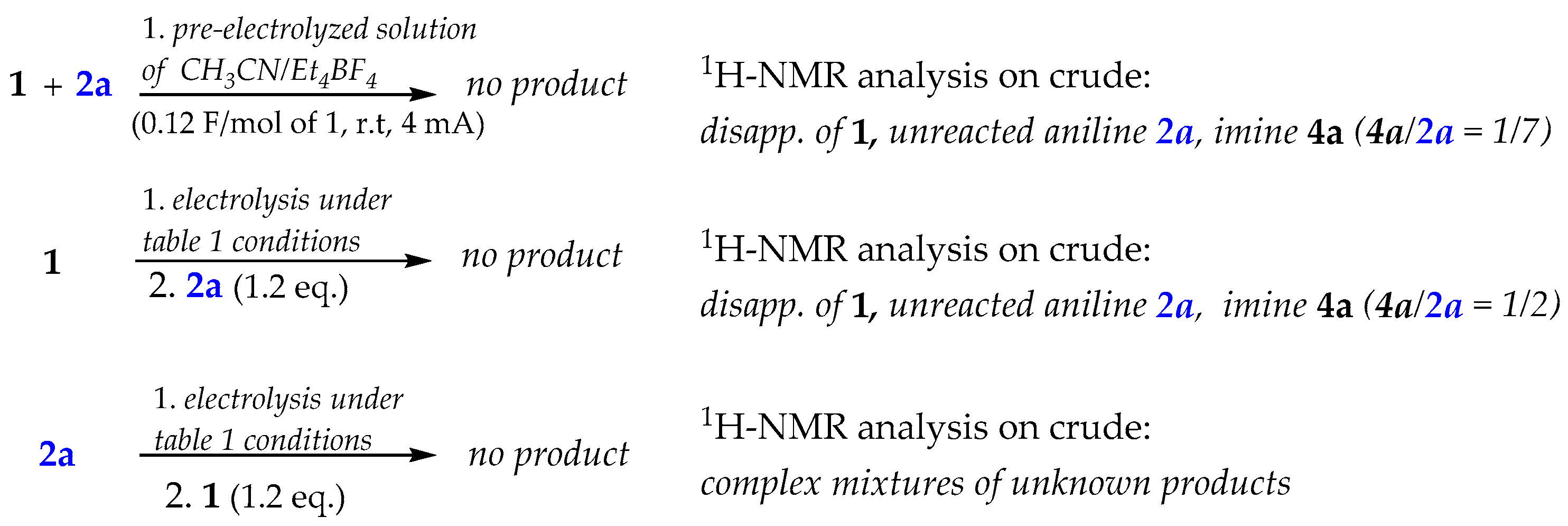
To gain an understanding of the electro-induced reaction pathway, we first performed some control experiments on the reaction model 2a + 1 under various conditions (Scheme 2).
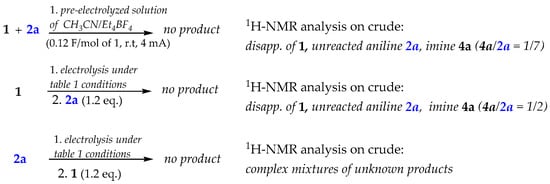
Scheme 2.
Control experiments of the reaction model 1 + 2a under different conditions.
1H-NMR on the crude mixtures clearly indicated that the presence of both the reagents (o-cyanobenzaldehyde and aniline) during the electricity supplying is a strict prerequisite to achieve the desired product 3.
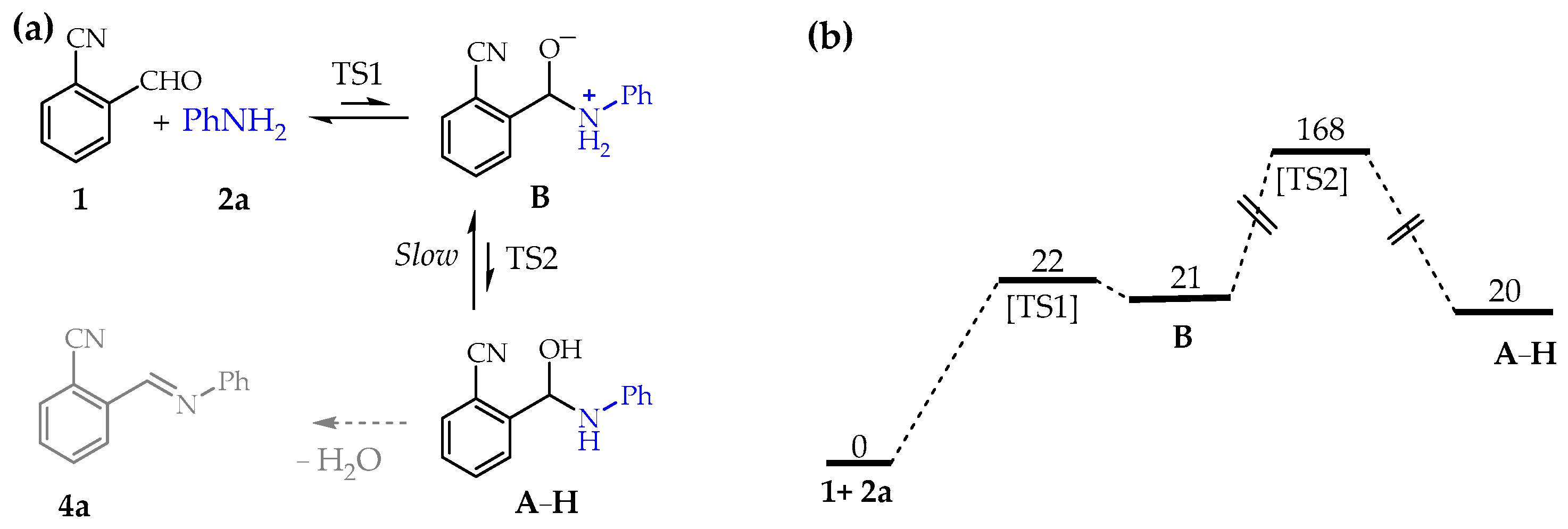
Thus, we opened our quantum-chemical investigations by analyzing the uncatalyzed nucleophilic addition of the aniline 2a to the aldehyde 1 in acetonitrile (Figure 2).
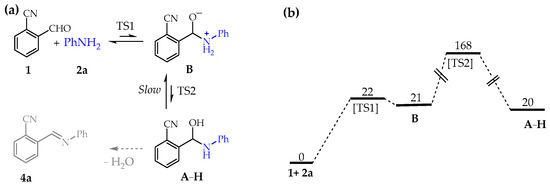
Figure 2.
Uncatalyzed hemiaminal formation in CH3CN. (a) Reaction pathway. (b) Standard free-energy values (kJ/mol in CH3CN at 298 K).
Not surprisingly, the uncatalyzed nucleophilic attack of 2a to 1 to produce the hemiaminal A–H is predicted as a disfavored process both kinetically and thermodynamically, with the zwitterion B present in very low concentration in pre-equilibrium with the reagents.
Consequently, to locate the origin of the electro-activation leading to 3a both the initial chemical species in the catholyte (i.e., 1, 2a, and the solvent CH3CN) and the fleeting intermediate B occurring during the uncatalyzed route to the imine 4a have been taken into account as potentially affected by the applied potential.
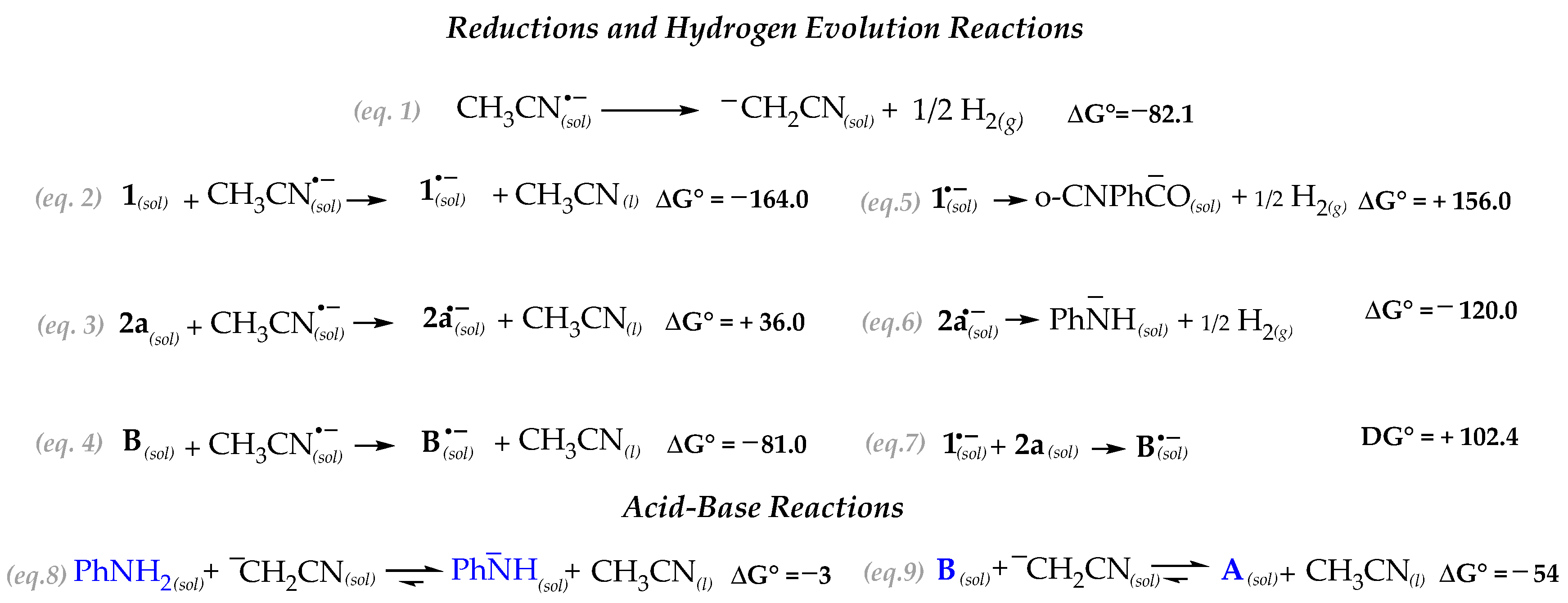
In Scheme 3 we report the energetic of the electro-reductive processes of all the species possibly involved in the reaction, conventionally referring the thermodynamics of the reactions as formally initiated by [CH3CN]− since, under constant current conditions, it is the species present in excess.
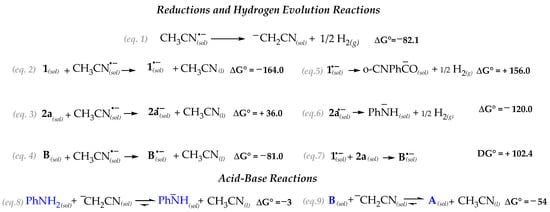
Scheme 3.
Standard free energy (kJ/mol) at 298 K of the plausible channels initiated by the Pt–electroreduction.
The ΔG° values clearly suggest that 2-formylbenzonitrile 1 has the highest oxidizing power. However, any process initiated by 1(sol)− (e.g., Equations (5) and (7)), is thermodynamically strongly disfavored. Zwitterionic intermediate B is likewise easily reduced (Equation (4)). However, this channel also reveals as totally ineffective due to the strongly thermodynamic driving force leading to B(sol)− dissociation (reverse of Equation (7)). Therefore, the data suggest that the electrochemical process acting as the reaction trigger is the formation of ¯CH2CN(sol) which follows the hydrogen evolution reaction (HER) (Equation (1)) [25]. The electrogenerated strong base ¯CH2CN(sol) might undergo to acid-base reaction with either the aniline 2a (to form the strong nucleophilic aryl amide anion) (Equation (8)) and, concurrently, with the zwitterion B (Equation (9)).
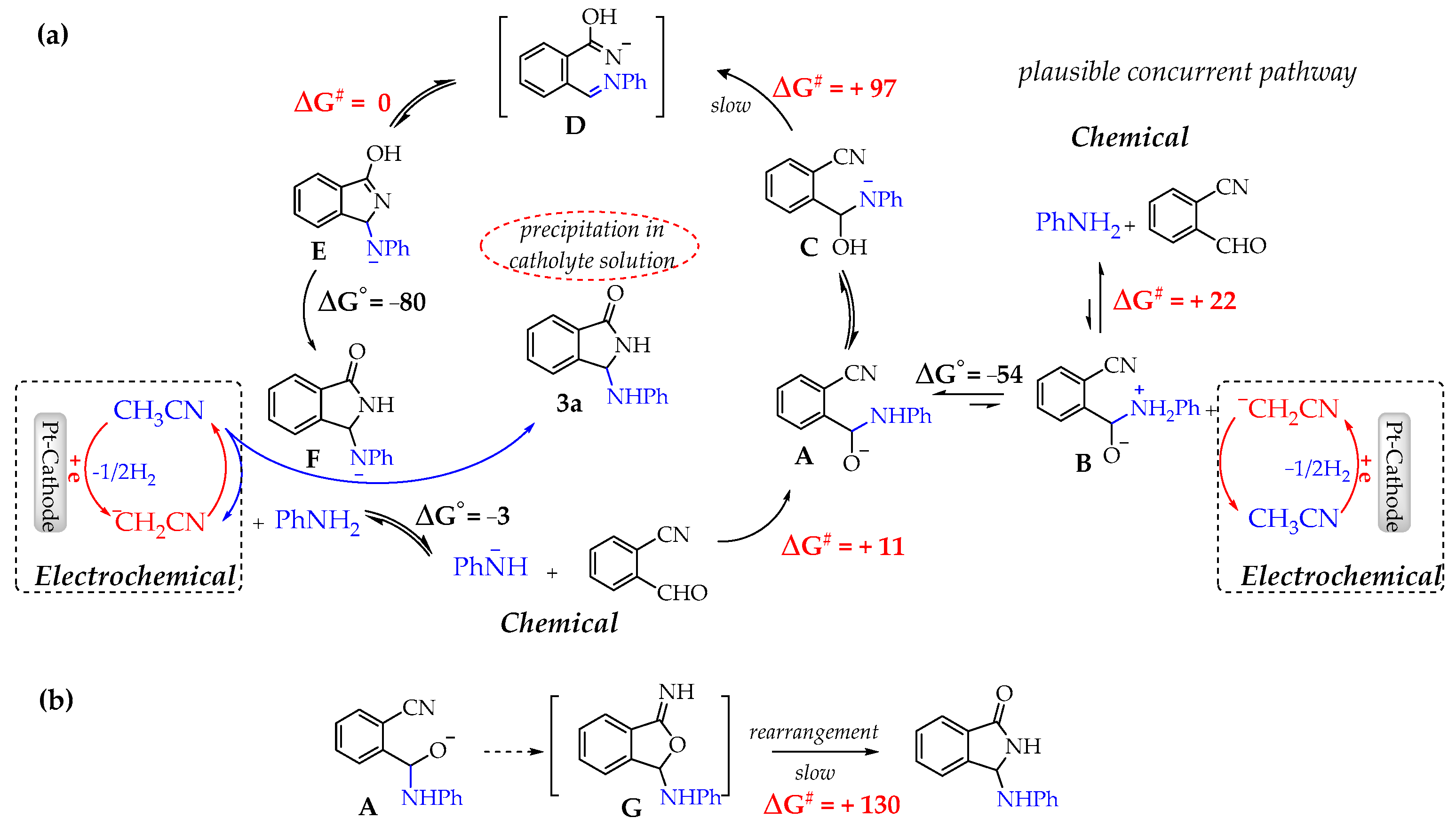
In Figure 3a is depicted the whole catalytic process which includes the electrochemical initiated cycle and the sequence of cascade reactions leading to the final product 3a.
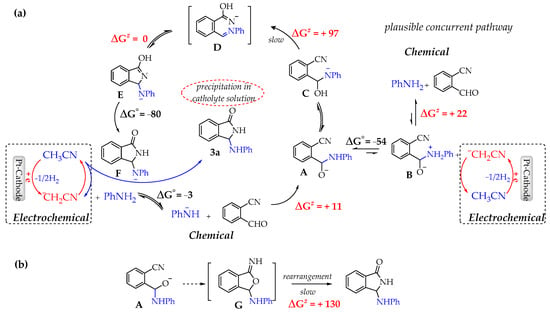
Figure 3.
(a) Proposed reaction pathways at 298 K in acetonitrile based on DFT calculations. (b) Alternative route. ΔG° and ΔG# values are given in kJ/mol.
As shown, after the electrochemically initiated process and the formation of the crucial anionic intermediate A, the sequence of unimolecular H- and HO- transfers leads to D which evolves without barrier to F, the conjugated base of the final product. After a thermodynamically strongly guided (ΔG° = −50 kJ/mol) acid–base reaction of F with CH3CN, 3a is formed and the base ¯CH2CN, able to re-initializing the catalytic cycle, restored. It is also equally reasonable that the acid–base reaction of electrogenerated base ¯CH2CN and zwitterion B contributes to the formation of the intermediate A.
Conversely, we want finally to remark that DFT calculations ruled out the possible alternative pathway involving A closure and rearrangement of the intermediate G. As shown in Figure 3b, the rearrangement step would imply higher activation energy.
3. Materials and Methods
3.1. General Information
Electrochemical reactions were conducted using Hewlett Packard DC Power Supply Mod. E3612A in constant current mode, in a U-divided glass cell separated through a porous G-3 glass plug. Platinum spirals (apparent area 1 cm2) were used as anode and cathode (distance between the electrodes 1 cm). Before using, Pt electrodes were treated with a Piranha solution (sulfuric acid/hydrogen peroxide 3:1) for 1 min, washed with double-distilled water and sonicated three times for 5 min with double-distilled water, acetone, and isopropanol. The reactions were monitored by thin layer chromatography (TLC) using Merck Silica Gel 60 F254 plates and were visualized by fluorescence quenching at 254 nm. Column chromatographic purification of products was carried out using silica gel 60 (70–230 mesh, Merck). The NMR spectra were recorded on Bruker Avance 400 spectrometers (400 MHz, 1H; 101 MHz, 13C). Spectra were referenced to residual CHCl3 (7.26 ppm, 1H; 77.00 ppm, 13C), MeOD (3.31 ppm, 1H; 49 ppm, 13C) or DMSO (2.50 ppm, 1H; 39.5 ppm, 13C) when indicated. Yields are given for isolated products showing one spot on a TLC plate and seldom impurities detectable in the NMR spectrum. High-resolution mass spectra (HRMS) were acquired using a Bruker SolariX XR Fourier transform ion cyclotron resonance mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) equipped with a 7 T refrigerated actively shielded superconducting magnet. The samples were ionized in positive ion mode using an electrospray (ESI) ionization source or the MALDI ion source.
3.2. Materials
All chemicals and solvents were obtained from commercial sources and were used without further purification. Pt electrodes (wires, wire, diam. 0.5 mm, 99.99% trace metals basis) were purchased from Sigma-Aldrich.
3.3. Procedure for Electrosynthesis of Compounds 3
A solution of 1 (0.2 mmol), 2 (0.24 mmol), and tetraethylammonium tetrafluoroborate (Et4NBF4) (0.08 mmol) in MeCN (0.4 mL) is added in the cathodic compartment of a U-divided cell equipped with platinum spirals (apparent area 1 cm2) as cathode (WE, working electrode) and anode (CE, counter electrode). Catholyte was constituted by a solution of Et4NBF4 (0.1 mmol) in MeCN (0.5 mL). Electrolysis was conducted under galvanostatic conditions (4 mA, 0.12 electrons/molecule of 1) at r.t. At the end of the electrolysis, TLC analysis showed disappearance of 1 and the reaction was in any case prolonged at r.t. under magnetic stirring for 6 h. The mixture was then concentrated in vacuum and directly purified by silica gel chromatography (Hexane: Ethyl Acetate from 4:1 to 3:2) to afford the desired products 3a–3v.
3-(phenylamino) isoindolin-1-one (3a) [26]: Prepared following general procedure using 1 (0.2 mmol, 26 mg) and aniline 2a (0.24 mmol, 22 mg). The crude was purified directly by flash chromatography to give a white solid 3a (41 mg, 91%). 1H-NMR (400 MHz, CDCl3) δ = 7.87 (d, J = 7.5 Hz, 1H, Ar), 7.62 (d, J = 4.1 Hz, 2H, Ar), 7.60–7.52 (m, 1H, Ar), 7.28 (d, J = 7.9 Hz, 2H, Ar), 6.89 (t, J = 7.4 Hz, 1H, Ar), 6.79 (d, J = 8.0 Hz, 2H, Ar), 6.60 (s, 1H, CONH), 6.19 (d, J = 10.7 Hz, 1H, CH), 4.11 (d, J = 10.7 Hz, 1H, NH). 13C-NMR (101 MHz, DMSO) δ = 168.8; 146.8; 145.7; 132.6; 131.8; 129.0; 128.8; 123.7; 122.5; 117.3; 113.5; 64.7. HR-MS (MALDI) m/z calcd for C14H13N2O [M + H+] 225.1022, found 225.1009, m/z calcd for C14H12N2ONa [M + Na+] 247.0841, found 247.0827.
3-((2-bromophenyl) amino) isoindolin-1-one (3b): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2-bromoaniline 2b (0.24 mmol, 41 mg). The crude was purified directly by flash chromatography to give a yellow solid 3b (48 mg, 80%). 1H-NMR (400 MHz, CDCl3) δ = 7.88 (d, J = 7.3 Hz, 1H, Ar); 7.67–7.53 (m, 3H, Ar); 7.49 (d, J = 8.0 Hz, 1H, Ar); 7.30–7.16 (m, 1H, Ar); 6.94 (s, 1H, CONH); 6.89 (d, J = 8.1 Hz, 1H, Ar), 6.73 (t, J = 7.7 Hz, 1H, Ar); 6.17 (d, J = 9.7 Hz, 1H, CH); 4.85 (d, J = 9.7 Hz, 1H, NH). 13C-NMR (101 MHz, CDCl3) δ = 169.6; 144.2; 142.6; 133.3; 132.8; 132.0; 129.9; 128.8; 124.0; 123.4; 120.5; 112.7; 111.2; 65.4. HR-MS (MALDI) m/z calcd for C14H12BrN2O [M + H+] 303.0127, found 303.0110.
3-((2-iodophenyl) amino) isoindolin-1-one (3c): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2-iodoaniline 2c (0.24 mmol, 52 mg). The crude was purified directly by flash chromatography to give a yellow solid 3c (40 mg, 57%). 1H-NMR (400 MHz, CDCl3) δ = 7.89 (dd, J = 7.4, 1.2 Hz, 1H, Ar); 7.74 (dd, J = 7.9, 1.5 Hz, 1H, Ar); 7.68–7.55 (m, 3H, Ar); 7.29 (d, J = 7.7 Hz, 1H, Ar); 6.83 (d, J = 8.1 Hz, 1H, Ar); 6.74 (s, 1H, CONH); 6.61 (t, J = 7.6 Hz, 1H, Ar); 6.17 (d, J = 9.6 Hz, 1H, CH); 4.69 (d, J = 9.6 Hz, 1H, NH). 13C-NMR (101 MHz, CDCl3) δ = 169.5; 145.0; 144.2; 139.9; 132.8; 131.9; 130.0; 129.8; 124.0; 123.4; 121.3; 112.1; 87.0; 65.8. HR-MS (MALDI) m/z calcd for C14H12IN2O [M + H+] 350.9988, found 350.9966.
3-((2-chlorophenyl) amino) isoindolin-1-one (3d): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2-chloroaniline 2d (0.24 mmol, 30 mg). Th crude was purified directly by flash chromatography to give a yellow solid 3c (31 mg, 61%). 1H-NMR (400 MHz, CDCl3) δ = 7.86 (dt, J = 7.5, 1.1 Hz, 1H, Ar); 7.66–7.51 (m, 3H, Ar); 7.49 (s, 1H, CONH); 7.33–7.22 (m, 1H, Ar); 7.23–7.13 (m, 1H, Ar); 6.92 (dd, J = 8.3, 1.4 Hz, 1H, Ar); 6.77 (td, J = 7.7, 1.4 Hz, 1H, Ar); 6.16 (d, J = 9.7 Hz, 1H, CH); 4.85 (d, J = 9.7 Hz, 1H, NH). 13C-NMR (101 MHz, CDCl3) δ = 169.6; 144.3; 141.6; 132.8; 132.0; 130.0; 129.9; 128.1; 124.0; 123.4; 120.7; 120.0; 112.6; 65.2. HR-MS (MALDI) m/z calcd for C14H12ClN2O [M + H+] 259.0632, found 259.0617.
3-((2-methoxyphenyl) amino) isoindolin-1-one (3e): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2-methoxyaniline 2e (0.24 mmol, 29 mg). The crude was purified directly by flash chromatography to give a yellow solid 3e (39 mg, 78%). 1H-NMR (400 MHz, CDCl3) δ = 7.87 (d, J = 7.4 Hz, 1H, Ar); 7.68–7.49 (m, 3H, Ar); 6.98–6.79 (m, 4H, Ar); 6.72 (s, 1H, CONH); 6.19 (d, J = 8.0 Hz, 1H, CH); 4.74 (d, J = 8.0 Hz, 1H, NH); 3.80 (s, 3H, OCH3). 13C-NMR (101 MHz, CDCl3) δ = 169.5; 147.7; 144.8; 135.2; 132.5; 132.0; 129.7; 123.9; 123.6; 121.4; 119.4; 111.4; 110.5; 65.4; 55.5. HR-MS (MALDI) m/z calcd for C15H15N2O2 [M + H+] 255.1128, found 255.1112.
2-((3-oxoisoindolin-1-yl) amino) benzonitrile (3f): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2-cyanoaniline 2f (0.24 mmol, 28 mg). The crude was purified directly by flash chromatography to give a yellow solid 3f (29 mg, 59%). 1H-NMR (400 MHz, CDCl3) δ = 7.88 (d, J = 7.3 Hz, 1H, Ar); 7.69–7.55 (m, 3H, Ar); 7.47 (m, 2H, Ar); 7.11 (s, 1H, CONH); 6.94–6.83 (m, 2H, Ar); 6.21 (d, J = 9.2 Hz, 1H, CH); 5.07 (d, J = 9.2 Hz, 1H, NH). 13C-NMR (101 MHz, CDCl3) δ = 169.7; 147.8; 143.5; 134.6; 133.4; 133.0; 131.8; 130.2; 124.2; 123.4; 119.3; 117.1; 112.0; 98.4; 64.8. HR-MS (MALDI) m/z calcd for C15H12N3O [M + H+] 250.0974, found 250.0962.
2-((3-oxoisoindolin-1-yl) amino) benzamide (3g): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2-aminobenzamide 2g (0.24 mmol, 32 mg). The crude was purified directly by flash chromatography to give a white solid 3g (42 mg, 79%). 1H-NMR (400 MHz, MeOD) δ = 7.79 (d, J = 7.5 Hz, 1H, Ar); 7.71–7.61 (m, 2H, Ar); 7.57 (t, J = 7.5 Hz, 1H, Ar); 7.45 (d, J = 7.6 Hz, 1H, Ar); 7.19 (t, J = 7.7 Hz, 1H, Ar); 6.76 (d, J = 8.3 Hz, 1H, Ar); 6.72 (s, 1H, CH); 6.57 (t, J = 7.6 Hz, 1H, Ar). 13C NMR (101 MHz, DMSO) δ 169.4; 168.9; 150.1; 145.4; 132.6; 132.2; 131.9; 128.9; 128.5; 123.5; 122.5; 116.4; 114.4; 113.4; 60.4. HR-MS (MALDI) m/z calcd for C15H14N3O2 [M + H+] 268.1080, found 268.1145
3-((2-benzoylphenyl) amino) isoindolin-1-one (3h): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2-aminobenzophenone 2h (0.24 mmol, 47 mg). The crude was purified directly by flash chromatography to give a yellow solid 3h (49 mg, 75%). 1H-NMR (400 MHz, CDCl3) δ = 8.81 (d, J = 8.4 Hz, 1H, NH); 7.88 (d, J = 7.4 Hz, 1H, Ar); 7.68–7.38 (m, 10H, Ar); 7.07 (s, 1H, CONH); 6.99 (d, J = 8.4 Hz, 1H, Ar); 6.75 (t, J = 7.6 Hz, 1H, Ar); 6.26 (d, J = 8.4 Hz, 1H, CH). 13C-NMR (101 MHz, CDCl3) δ = 199.4; 169.7; 149.2; 144.3; 139.7; 135.7; 135.1; 132.8; 131.9; 131.5; 129.9; 129.3; 128.2; 124.0; 123.4; 119.5; 116.6; 112.0; 64.3. HR-MS (MALDI) m/z calcd for C21H17N2O2 [M + H+] 329.1284, found 329.1264.
3-((2-acetylphenyl) amino) isoindolin-1-one (3i): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2-aminoacetophenone 2i (0.24 mmol, 32 mg). The crude was purified directly by flash chromatography to give a yellow solid 3i (24 mg, 45%). 1H-NMR (400 MHz, CDCl3) δ = 9.35 (d, J = 8.4 Hz, 1H, NH); 7.88 (d, J = 7.3 Hz, 1H, Ar); 7.84 (dd, J = 8.1, 1.5 Hz, 1H, Ar); 7.66–7.54 (m, 3H, Ar); 7.47–7.40 (m, 1H, Ar); 6.89 (d, J = 8.4 Hz, 1H, Ar); 6.84–6.77 (m, 1H, Ar); 6.72 (s, 1H, CONH); 6.22 (d, J = 8.4 Hz, 1H, CH); 2.59 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ = 201.3; 169.6; 148.7; 144.4; 135.3; 133.1; 132.8; 129.8, 124.0; 123.3; 119.4; 116.9; 111.9; 64.1; 28.1. HR-MS (MALDI) m/z calcd for C16H15N2O2 [M + H+] 267.1128, found 267.1111.
3-((4-chlorophenyl) amino) isoindolin-1-one (3j): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 4-chloroaniline 2j (0.24 mmol, 30 mg). The crude was purified directly by flash chromatography to give a white solid 3i (40 mg, 79%). 1H-NMR (400 MHz, MeOD) δ = 7.79 (d, J = 7.5 Hz, 1H, Ar); 7.70–7.60 (m, 2H, Ar); 7.57 (t, J = 7.3 Hz, 1H, Ar); 7.13 (d, J = 8.4 Hz, 2H, Ar); 6.77 (d, J = 8.4 Hz, 2H, Ar); 6.19 (s, 1H, CH). 13C-NMR (101 MHz, MeOD) δ = 172.5; 146.9; 146.8; 133.7; 133.4; 130.5; 130.0; 124.9; 124.2; 124.2; 116.5; 67.3. HR-MS (MALDI) m/z calcd for C14H12ClN2O [M + H+] 259.0632, found 259.0618.
3-((4-fluorophenyl) amino) isoindolin-1-one (3k): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 4-fluoroaniline 2k (0.24 mmol, 26 mg). The crude was purified directly by flash chromatography to give a yellow solid 3k (34 mg, 71%). 1H-NMR (400 MHz, MeOD) δ = 7.78 (d, J = 7.5 Hz, 1H, Ar); 7.65 (d, J = 6.4 Hz, 2H, Ar); 7.56 (t, J = 7.0 Hz, 1H, Ar); 6.90 (t, J = 8.6 Hz, 2H, Ar); 6.84–6.73 (m, 2H, Ar); 6.16 (s, 1H, CH). 13C-NMR (101 MHz, MeOD) δ = 178.4; 165.9; 163.6; 155.1; 152.9; 142.1; 141.4; 138.6; 133.3; 132.1; 124.9; 124.7; 124.3; 74.9. HR-MS (MALDI) m/z calcd for C14H12FN2O [M + H+] 243.0928, found 243.0913.
3-(p-tolylamino) isoindolin-1-one (3l): Prepared following general procedure A using 1 (0.2 mmol, 26 mg) and p-toluidine 2l (0.24 mmol, 26 mg). The crude was purified directly by flash chromatography to give a white solid 3l (34 mg, 72%). 1H-NMR (400 MHz, CDCl3) δ = 7.85 (d, J = 7.4 Hz, 1H, Ar); 7.65–7.51 (m, 3H, Ar); 7.07 (d, J = 7.8 Hz, 2H, Ar); 6.70 (d, J = 7.8 Hz, 2H, Ar); 6.64 (s, 1H, CONH); 6.14 (d, J = 8.6 Hz, 1H, CH); 3.96 (d, J = 8.6 Hz, 1H, NH); 2.28 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ = 169.5; 144.7; 143.0; 132.5; 132.0; 130.3; 129.8; 129.6; 123.9; 123.5; 114.7; 66.3; 20.5. HR-MS (MALDI) m/z calcd for C15H15N2O [M + H+] 239.1178, found 239.1166.
3-((4-methoxyphenyl) amino) isoindolin-1-one (3m): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 4-methoxyaniline 2m (0.24 mmol, 29 mg). The crude was purified directly by flash chromatography to give a brown solid 3m (39 mg, 78%). 1H-NMR (400 MHz, CDCl3) δ = 7.85 (d, J = 7.4 Hz, 1H, Ar); 7.65–7.49 (m, 3H, Ar); 6.84 (d, J = 8.7 Hz, 2H, Ar); 6.75 (d, J = 8.7 Hz, 2H, Ar); 6.67 (s, 1H, CONH); 6.06 (s, 1H, CH); 3.89–3.77 (m, 4H, OCH3 + NH). 13C-NMR (101 MHz, CDCl3) δ = 169.5; 154.1; 144.7; 139.0; 132.5; 131.9; 129.7; 123.9; 123.6; 116.6; 115.3; 67.2; 55.7. HR-MS (MALDI) m/z calcd for C15H15N2O2 [M + H+] 255.1128, found 255.1119.
3-((4-ethoxyphenyl) amino) isoindolin-1-one (3n): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 4-ethoxyaniline 2n (0.24 mmol, 33 mg). The crude was purified directly by flash chromatography to give a yellow solid 3n (37 mg, 70%). 1H-NMR (400 MHz, CDCl3) δ = 7.85 (d, J = 7.4 Hz, 1H, Ar); 7.64–7.48 (m, 3H, Ar); 6.86–6.81 (m, 2H, Ar); 6.77–6.71 (m, 2H, Ar); 6.65 (s, 1H, CONH); 6.07 (s, 1H, CH); 3.99 (q, J = 7.0 Hz, 2H, CH2); 3.81 (s, 1H, NH); 1.40 (t, J = 7.0 Hz, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ = 169.5; 153.4; 144.7; 138.9; 132.5; 131.9; 129.7; 123.9; 123.6; 116.6; 116.1; 67.2; 64.0. HR-MS (MALDI) m/z calcd for C16H17N2O2 [M + H+] 269.1284, found 269.1271.
3-(pyridin-2-ylamino) isoindolin-1-one (3p): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2-aminopyridine 2p (0.24 mmol, 22 mg). The crude was purified directly by flash chromatography to give a yellow solid 3p (28 mg, 62%). 1H-NMR (400 MHz, CDCl3) δ = 8.23–8.12 (m, 1H, Ar); 7.83 (d, J = 7.5 Hz, 1H, Ar); 7.60 (d, J = 4.1 Hz, 2H, Ar); 7.57–7.42 (m, 2H, Ar); 7.06 (s, 1H, CONH); 6.79–6.70 (m, 1H, Ar); 6.57 (d, J = 8.8 Hz, 1H, CH); 6.51 (d, J = 8.3 Hz, 1H, Ar); 4.93 (d, J = 8.8 Hz, 1H, NH). 13C-NMR (101 MHz, CDCl3) δ = 169.2; 157.1; 148.1; 144.7; 137.8; 132.5; 132.4; 129.7; 124.4; 123.9; 123.2; 115.1; 109.8; 63.4. HR-MS (MALDI) m/z calcd for C13H12N3O [M + H+] 226.0974, found 226.0971.
3-((2-bromo-4-methylphenyl) amino) isoindolin-1-one (3q): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2-bromo-4-methylaniline 2q (0.24 mmol, 44 mg). The crude was purified directly by flash chromatography to give a yellow solid 3q (42 mg, 67%). 1H-NMR (400 MHz, CDCl3) δ = 7.87 (d, J = 7.4 Hz, 1H, Ar); 7.61 (m, 2H, Ar); 7.57 (d, J = 7.4 Hz, 1H, Ar); 7.31 (s, 1H, Ar); 7.03 (d, J = 8.2 Hz, 1H, Ar); 6.94 (s, 1H, CONH); 6.79 (d, J = 8.2 Hz, 1H, Ar); 6.12 (d, J = 9.8 Hz, 1H, CH); 4.68 (d, J = 9.8 Hz, 1H, NH); 2.25 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ = 169.6; 144.4; 140.2; 133.5; 132.7; 131.9; 130.4; 129.9; 129.3; 123.9; 123.4; 113.0; 111.3; 65.8; 20.1. HR-MS (MALDI) m/z calcd for C15H14N2OBr [M + H+] 317.0284, found 317.0265.
3-((2,4-dimethylphenyl) amino) isoindolin-1-one (3r): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2,4-dimethylaniline 2r (0.24 mmol, 29 mg). The crude was purified directly by flash chromatography to give a yellow solid 3r (32 mg, 64%). 1H-NMR (400 MHz, CDCl3) δ = 7.87 (d, J = 7.4, 1H, Ar), 7.68–7.58 (m, 2H, Ar), 7.56 (m, 1H, Ar), 7.05–6.90 (m, 2H, Ar), 6.82 (m, 2H, Ar + CONH), 6.16 (s, 1H, CH), 3.86 (s, 1H, NH), 2.27 (s, 3H, CH3), 2.11 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ = 169.6; 145.1; 141.2; 132.7; 132.0; 129.9; 129.3; 127.8; 124.3; 124.0; 123.6; 112.1; 112.0; 66.2; 20.5; 17.6. HR-MS (MALDI) m/z calcd for C16H17N2O [M + H+] 253.1335, found 253.1325.
3-((2,5-dimethoxyphenyl) amino) isoindolin-1-one (3s): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2,5-dimethoxyaniline 2s (0.24 mmol, 36 mg). The crude was purified directly by flash chromatography to give a white solid 3s (34 mg, 61%). 1H-NMR (400 MHz, CDCl3) δ = 7.86 (d, J = 7.4 Hz, 1H, Ar); 7.67–7.48 (m, 3H, Ar); 6.80 (s, 1H, CONH); 6.73 (d, J = 8.8 Hz, 1H, Ar); 6.46 (d, J = 2.8 Hz, 1H, Ar); 6.31 (dd, J = 8.8, 2.8 Hz, 1H, Ar); 6.14 (d, J = 10.2 Hz, 1H, CH); 4.77 (d, J = 10.2 Hz, 1H, NH); 3.75 (d, J = 1.3 Hz, 6H, OCH3). 13C-NMR (101 MHz, CDCl3) δ = 169.5; 154.7; 144.7; 142.1; 136.2; 132.5; 132.1; 129.7; 123.9; 123.5; 111.1; 101.9; 99.6; 65.2; 56.0; 55.7. HR-MS (MALDI) m/z calcd for C16H17N2O3 [M + H+] 285.1233, found 285.1216.
3-((2-ethynylphenyl) amino) isoindolin-1-one (3t): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2-((trimethylsilyl)ethynyl)aniline 2t (0.24 mmol, 45 mg). The crude was purified directly by flash chromatography to give a yellow solid 3t (26 mg, 53%). 1H-NMR (400 MHz, CDCl3) δ = 7.89 (d, J = 7.3 Hz, 1H, Ar); 7.68–7.60 (m, 2H, Ar); 7.60–7.54 (m, 1H, Ar); 7.41 (dd, J = 7.7, 1.6 Hz, 1H, Ar); 7.28 (d, J = 1.6 Hz, 1H, Ar); 6.88–6.77 (m, 2H, Ar); 6.72 (s, 1H, CONH); 6.22 (d, J = 9.6 Hz, 1H, CH); 5.11 (d, J = 9.6 Hz, 1H, NH); 3.33 (s, 1H, CH). 13C-NMR (101 MHz, CDCl3) δ = 169.5, 146.9; 144.4; 133.4; 132.8; 131.9; 130.5; 129.9; 124.0; 123.4; 118.9; 110.8; 108.5; 83.7; 79.8; 65.1. HR-MS (MALDI) m/z calcd for C16H13N2O [M + H+] 249.1022, found 249.1008.
3-((2-(phenylethynyl) phenyl) amino) isoindolin-1-one (3u): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 2-(phenylethynyl) aniline 2u (0.24 mmol, 46 mg). The crude was purified directly by flash chromatography to give a yellow solid 3u (44 mg, 69%). 1H-NMR (400 MHz, CDCl3) δ = 7.89 (d, J = 7.4 Hz, 1H, Ar); 7.68–7.63 (m, 2H, Ar); 7.62–7.54 (m, 1H, Ar); 7.45 (d, J = 7.6 Hz, 1H, Ar); 7.40–7.35 (m, 2H, Ar); 7.32–7.26 (m, 4H, Ar); 6.88–6.80 (m, 3H, Ar + CONH); 6.23 (d, J = 9.5 Hz, 1H, CH); 5.13 (d, J = 9.5 Hz, 1H, NH). 13C-NMR (101 MHz, CDCl3) δ = 169.6; 146.1; 144.6; 132.9; 132.8; 132.0; 131.5; 130.1; 129.9; 128.5; 128.4; 128.4; 124.0; 123.3; 122.8; 119.1; 111.1; 109.8; 95.8; 85.1; 65.4. HR-MS (MALDI) m/z calcd for C22H17N2O [M + H+] 325.1335, found 325.1318.
3-((4-(phenylethynyl) phenyl) amino) isoindolin-1-one (3v): Prepared following general procedure using 1 (0.2 mmol, 26 mg) and 4-(phenylethynyl) aniline 2v (0.24 mmol, 46 mg). The crude was purified directly by flash chromatography to give a yellow solid 3v (45 mg, 70%). 1H-NMR (400 MHz, DMSO) δ = 9.06 (s, 1H, CONH); 7.70 (d, J = 7.4 Hz, 1H, Ar); 7.68–7.60 (m, 1H, Ar); 7.61–7.52 (m, 2H, Ar); 7.48 (d, J = 7.4 Hz, 2H, Ar); 7.38 (q, J = 8.2, 7.3 Hz, 3H, Ar); 7.31 (d, J = 8.2 Hz, 2H, Ar); 6.85 (d, J = 9.3 Hz, 1H, CH); 6.81 (d, J = 8.3 Hz, 2H, Ar); 6.24 (d, J = 9.3 Hz, 1H, NH). 13C NMR (101 MHz, DMSO) δ = 169.3; 147.9; 145.8; 133.0; 132.9; 132.5; 131.4; 129.7; 129.1; 128.4; 124.3; 123.7; 123.1; 113.9; 110.7; 91.2; 87.6; 64.7. HR-MS (MALDI) m/z calcd for C22H17N2O [M + H+] 325.1335, found 325.1314.
3.4. Computational Details
All the calculations were performed with the Gaussian 09 [27] program in the framework of the density functional theory (DFT) using the functional wB97XD functional using a version of Grimme’s D2 dispersion model [28] in conjunction with different basis sets: geometry optimizations were carried out with the 6–31G*. Energies were then refined through single point calculations adding a diffusion function with the 6–31 + G* basis set. All the structures were optimized in the gas phase and characterized through the calculation of the mass-weighted Hessian matrix, as minima (all positive eigenvalues of the Hessian matrix) or transition structures (1 negative eigenvalue of the Hessian matrix). The gas-phase Gibbs molar free energy (GX,gas) was then calculated, using the previous geometries and harmonic frequencies, for each species in the gas phase at 25 °C at the concentration of 1M using the standard statistical-mechanical relations. Finally, the solvation, i.e., excess, molar free energy (GX,solv) was calculated within the mean-field approximation in acetonitrile using the polarizable conductor calculation model [29]. Within this approximations the molar free energy (GX) for each species in solution corresponds to the usual equation
where [X] = 1.0 M for all the species in solution and [X] = DX:MWX (where D is the density of the species X) for the solvent, i.e., acetonitrile. For H2 the standard state corresponding to 1.0 bar of pressure was used. All the cartesian coordinates of the optimized geometries are collected in the Supplementary Information.
G°X = G°X,gas + GX,solv + RT ln [X]
4. Conclusions
In summary, performing constant current electrolysis with catalytic amount of electricity, we accessed unprecedented molecular architectures that encompass isoindolinone nucleus and functionalized anilines, two substructures playing relevant roles in the production of pharmaceuticals.
Mild conditions, catalytic loading of supporting electrolyte [30], short electrolysis/reaction time, as well as acceptable functional group tolerance are the major strong points of this synthetic approach.
Finally, the mechanistic insight offered by DFT computations, allowed us to provide a consistent picture of the effectiveness of the electrochemical activation to the generation of the highly nucleophilic aryl amide anion species that follows the HER of the solvent on Pt cathode.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238199/s1, Figure S1: The setup of electrochemical reaction; Table S1: Optimization studies; Figures S2–S10: 3a structure determination (2D-NMR Spectra). 1H, 13C NMR spectra of the products; DFT Data.
Author Contributions
V.M.: experiments, acquisition and analysis of the original data and tables, writing of the experimental section. T.C.: electrochemical set-up, cell design, analytical data. M.C.: structure determination, NMR experiments, manuscript revision. A.A.: project supervision, manuscript revision. M.A.: quantum-chemical calculations, mechanism elucidation. L.P.: research conceptualization, project supervision, writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
L.P. gratefully acknowledges DSFC, University of L’Aquila “Fondi Premiali” and CARISPAQ “Fondi Esercizio 2021”. M.A. wishes to thank Cineca (Italy) for an Iscra-C grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, I.R.; Ahmed, S.U.; Atkins, H.; Calvert, A.H.; Curtin, N.J.; Farnie, G.; Golding, B.T.; Griffin, R.J.; Guyenne, S.; Hutton, C.; et al. Isoindolinone-based inhibitors of the MDM2-p53 protein-protein interaction. Bioorg. Med. Chem. Lett. 2005, 15, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Riedinger, C.; Endicott, J.A.; Kemp, S.J.; Smyth, L.A.; Watson, A.; Valeur, E.; Golding, B.T.; Griffin, R.J.; Hardcastle, I.R.; Noble, M.E.; et al. Analysis of Chemical Shift Changes Reveals the Binding Modes of Isoindolinone Inhibitors of the MDM2-p53 Interaction. J. Am. Chem. Soc. 2008, 130, 16038–16044. [Google Scholar] [CrossRef] [PubMed]
- Schubert, G.; Rieke-Zapp, J.; Keil, J.; Kleemann, H.W.; Hanna, R.; Huang, B.G.; Wu, X.D.; Gouraud, Y. Process for Preparing (3-Oxo-2,3-dihydro-1H-isoindol-1-yl) Acetylguanidine Derivatives. U.S. Patent US20050124681, 9 June 2005. [Google Scholar]
- Peytam, F.; Adib, M.; Mahernia, S.; Rahmanian-Jazi, M.; Jahani, M.; Masoudi, B.; Mahdavi, M.; Amanlou, M. Isoindolin-1-one derivatives as urease inhibitors: Design, synthesis, biological evaluation, molecular docking and in-silico ADME evaluation. Bioorg. Chem. 2019, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, A.; Di Mola, A.; Intinoli, V.; Massa, A.; Roiser, L.; Waser, M.; Palombi, L. Palombi Asymmetric tandem hemiaminal-heterocyclization-aza-Mannich reaction of 2-formylbenzonitriles and amines using chiral phase transfer catalysis: An experimental and theoretical study. RSC Adv. 2016, 6, 31861–31870. [Google Scholar] [CrossRef]
- Serusi, L.; Massa, A.; Tedesco, C.; Capobianco, A.; Palombi, L. The First Highly Enantioselective Synthesis of 3-Sulfinyl-Substituted Isoindolinones Having Adjacent Carbon and Sulfur Stereocenters. J. Org. Chem. 2021, 86, 10630–10639. [Google Scholar] [CrossRef]
- Palombi, L.; Di Mola, A.; Massa, A. Quick and easy access to N-Mannich bases of 1-isoindolinones by catalytic electroactivation of primary and secondary amines and tandem reaction with 2-formyl benzonitriles. New J. Chem. 2015, 39, 81–84. [Google Scholar] [CrossRef]
- Palombi, L.; Vignes, C.; Di Mola, A.; Massa, A. Combined electrochemical/chemical methods for the synthesis and the molecular diversifying of isoindolinone-based heterocyclic scaffolds. Mol. Divers. 2014, 18, 323–333. [Google Scholar] [CrossRef]
- Antico, P.; Capaccio, V.; Di Mola, A.; Massa, A.; Palombi, L. Electrochemically Initiated Tandem and Sequential Conjugate Addition Processes: One-Pot Synthesis of Diverse Functionalized Isoindolinones. Adv. Synth. Catal. 2012, 354, 1717–1724. [Google Scholar] [CrossRef]
- Yu, M.; Gao, Y.; Zhang, L.; Zhang, Y.; Zhang, Y.; Yi, H.; Huang, Z.; Lei, A. Electrochemical-induced benzyl C-H amination towards the synthesis of isoindolinones via aroyloxy radical-mediated C-H activation. Green Chem. 2022, 24, 1445–1450. [Google Scholar] [CrossRef]
- Zou, Z.; Cai, G.; Chen, W.; Zou, C.; Li, Y.; Wu, H.; Chen, L.; Hu, J.; Li, Y.; Huang, Y. Metal-Free Cascade Formation of Intermolecular C–N Bonds Accessing Substituted Isoindolinones under Cathodic Reduction. J. Org. Chem. 2021, 86, 15777–15784. [Google Scholar] [CrossRef]
- Yi, X.; Hu, X. Formal Aza-Wacker Cyclization by Tandem Electrochemical Oxidation and Copper Catalysis. Angew. Chem. Int. Ed. 2021, 58, 4700–4704. [Google Scholar] [CrossRef]
- Folgueiras-Amador, A.A.; Philipps, K.; Guilbaud, S.; Poelakker, J.; Wirth, T. An Easy-to-Machine Electrochemical Flow Microreactor: Efficient Synthesis of Isoindolinone and Flow Functionalization. Angew. Chem. Int. Ed. 2017, 56, 15446–15450. [Google Scholar] [CrossRef]
- Little, R.D. A Perspective on Organic Electrochemistry. J. Org. Chem. 2020, 85, 13375–13390. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, K.; Zeng, C. Use of Electrochemistry in the Synthesis of Heterocyclic Structures. Chem. Rev. 2018, 118, 4485–4540. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef]
- Waldvogel, S.R.; Janza, B. Renaissance of Electrosynthetic Methods for the Construction of Complex Molecules. Angew. Chem. Int. Ed. 2014, 53, 7122–7123. [Google Scholar] [CrossRef] [PubMed]
- Brotzel, F.; Chu, Y.C.; Mayr, H. Nucleophilicities of Primary and Secondary Amines in Water. J. Org. Chem. 2007, 72, 3679–3688. [Google Scholar] [CrossRef]
- Appel, R.; Chelli, S.; Tokuyasu, T.; Troshin, K.; Mayr, H.J. Electrophilicities of benzaldehyde-derived iminium ions: Quantification of the electrophilic activation of aldehydes by iminium formation. J. Am. Chem. Soc. 2013, 135, 6579. [Google Scholar] [CrossRef]
- Dirksen, A.; Dirksen, S.; Hackeng, T.M.; Dawson, P.E. Nucleophilic Catalysis of Hydrazone Formation and Transimination: Implications for Dynamic Covalent Chemistry. J. Am. Chem. Soc. 2006, 128, 15602–15603. [Google Scholar] [CrossRef]
- Dirksen, A.; Hackeng, T.M.; Dawson, P.E. Nucleophilic Catalysis of Oxime Ligation. Angew. Chem. Int. Ed. 2006, 45, 7581–7584. [Google Scholar] [CrossRef] [PubMed]
- Ciaccia, M.; Pilati, S.; Cacciapaglia, R.; Mandolini, L.; Di Stefano, S. Effective catalysis of imine metathesis by means of fast transiminations between aromatic–aromatic or aromatic–aliphatic amines. Org. Biomol. Chem. 2014, 12, 3282–3287. [Google Scholar] [CrossRef]
- Formenti, D.; Ferretti, F.; Scharnagl, F.K.; Beller, M. Reduction of Nitro Compounds Using 3d-Non-Noble Metal Catalysts. Chem. Rev. 2019, 119, 2611–2680. [Google Scholar] [CrossRef] [PubMed]
- Palombi, L.; Feroci, M.; Orsini, M.; Inesi, A. Electrochemically-initiated Michael addition of chiral acetoacetic derivatives to methyl vinyl ketone: Stereocontrolled construction of quaternary carbon centers. Tetrahedron Asymmetry 2002, 13, 2311–2316. [Google Scholar] [CrossRef]
- Schmidt, R.R.; Schlipf, E. Synthese und Reaktionen von Pseudoisoindolon-(1)-und 2-Aza-phenalenon-Säureaddukten. Chem. Ber. 1970, 103, 3783–3790. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09 Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Caruso, T.; Feroci, M.; Inesi, A.; Orsini, M.; Scettri, A.; Palombi, L. Electrochemically Induced Addition Reactions in the Absence of Solvent and Supporting Electrolyte. Adv. Synth. Catal. 2006, 348, 1942–1947. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).