A GC-MS Protocol for the Identification of Polycyclic Aromatic Alkaloids from Annonaceae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Source of Analytes
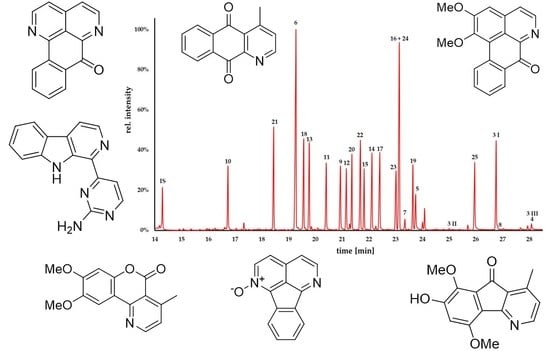
2.2. GC-MS Analysis
- = Kováts retention index
- = total retention time of the substance
- = total retention time of the n-alkane
- = number of the carbon atoms of the n-alkane
- = total retention time of the n-alkane + 1 carbon atom
- Equation 1. Determination of Kováts retention indices in case of temperature-programmed gas chromatography according to IUPAC (International Union of Pure and Applied Chemistry) [27].
3. Materials and Methods
3.1. Materials and Reagents
3.2. Instruments and Equipment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lúcio, A.S.S.C.; Almeida, J.R.G.D.S.; Da-Cunha, E.V.L.; Tavares, J.F.; Filho, J.M.B. Chapter Five—Alkaloids of the Annonaceae: Occurrence and a Compilation of Their Biological Activities. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 74, pp. 233–409. [Google Scholar] [CrossRef]
- Neske, A.; Hidalgo, J.R.; Cabedo, N.; Cortes, D. Acetogenins from Annonaceae family. Their potential biological applications. Phytochemistry 2020, 174, 112332. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W. Constituents of Eupomatia species. IX. NMR evidence for the structure of eupolauramine and hydroxyeupolauramine. Aust. J. Chem. 1984, 37, 1095–1104. [Google Scholar] [CrossRef]
- Tadić, D.; Cassels, B.K.; Leboeuf, M.; Cavé, A. Alkaloids of the Annonaceae. Part 69. Kinabaline and the aporphinoid biogenesis of azaanthracene and azafluorene alkaloids. Phytochemistry 1987, 26, 537–541. [Google Scholar] [CrossRef]
- Cavé, A.; Tadić, D.; Cassels, B.K. Spectral Properties of Ring-C-Oxygenated 4-Azafluorenes and 4-Azafluorenones. The structures of Natural Onychine Derivatives. Heterocycles 1988, 27, 407–421. [Google Scholar] [CrossRef]
- Tadić, D.; Cassels, B.K.; Cavé, A.; Goulart, M.O.; De Oliveira, A.B. Revised structures of the azafluorenone alkaloids from guatteria dielsiana. Phytochemistry 1987, 26, 1551–1552. [Google Scholar] [CrossRef]
- Zhang, J.; El-Shabrawy, A.-R.O.; El-Shanawany, M.A.; Schiff, P.L.S., Jr.; Slatkin, D.J. New Azafluorene Alkaloids from Oxandra xylopioides. J. Nat. Prod. 1987, 50, 800–806. [Google Scholar] [CrossRef]
- Irie, H.; Koyama, J.; Sugita, T.; Suzuta, Y. Synthesis of an Alkaloid Onychine and Related Compounds: Revised Structure of Onychine. Heterocycles 1979, 12, 1017–1019. [Google Scholar] [CrossRef]
- Bracher, F. Polycyclic aromatic alkaloids. VII. Studies on the structure of dielsine. Arch. Pharm. 1992, 325, 645–648. [Google Scholar] [CrossRef]
- De Almeida, M.L.; Braz-F, R.; Von Bülow, V.; Gottlieb, O.R.; Maia, J.S. Onychine, an alkaloid from Onychopetalum amazonicum. Phytochemistry 1976, 15, 1186–1187. [Google Scholar] [CrossRef]
- Goulart, M.O.; Santana, A.E.G.; De Oliveira, A.B.; De Oliveira, G.G.; Maia, J.G.S. Azafluorenones and azaanthraquinone from Guatteria dielsiana. Phytochemistry 1986, 25, 1691–1695. [Google Scholar] [CrossRef]
- Lu, Z.-M.; Zhang, Q.-J.; Chen, R.-Y.; Yu, D.-Q. Four new alkaloids from Polyalthia nemoralis (Annonaceae). J. Asian Nat. Prod. Res. 2008, 10, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Leboeuf, M.; Andre, C.; Forgacs, P.; Provost, J.; Chiaroni, A.; Riche, C. Alkaloids of the Annonaceae. Part 33. Annomontine and methoxyannomontine, two new pyrimidine-β-carboline-type alkaloids from Annona montana. J. Chem. Soc. Perkin Trans. 1982, 1, 1205–1208. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chang, F.-R.; Pan, W.-B.; Wu, Y.-C. Four alkaloids from Annona cherimola. Phytochemistry 2001, 56, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Pudjiastuti, P.; Mukhtar, M.R.; Hadi, A.H.A.; Saidi, N.; Morita, H.; Litaudon, M.; Awang, K. (6,7-dsimethoxy-4-methylisoquinolinyl)-(4’-methoxyphenyl)-methanone, a New Benzylisoquinoline Alkaloid from Beilschmiedia brevipes. Molecules 2010, 15, 2339–2346. [Google Scholar] [CrossRef]
- Katsui, N.; Sato, K.; Tobinaga, S.; Takeuchi, N. Alkaloids of lysichiton camtschatcense schott var. japonicum makino. Tetrahedron Lett. 1966, 7, 6257–6261. [Google Scholar] [CrossRef]
- Rao, J.U.M.; Giri, G.S.; Hanumaiah, T.; Rao, K.V.J. Sampangine, a New Alkaloid from Cananga odorata. J. Nat. Prod. 1986, 49, 346–347. [Google Scholar] [CrossRef]
- Bowden, B.; Picker, K.; Ritchie, E.; Taylor, W. Constituents of Eupomatia species. IV. Structure and synthesis of eupolauridine (EL base-1) and some observations on the structures of eupolauramine (EL base-2) and hydroxyeupolauramine (EL base-3). Aust. J. Chem. 1975, 28, 2681–2701. [Google Scholar] [CrossRef]
- Waterman, P.G.; Muhammad, I. Chemistry of the Annonaceae. Part 14. Sesquiterpenes and alkaloids from Cleistopholis patens. Phytochemistry 1985, 24, 523–527. [Google Scholar] [CrossRef]
- Mérienne, C.; Arango, G.J.; Cortes, D.; Cassels, B.K.; Cavé, A. Alkaloids of the Annonaceae. Part 80. Azafluorenones from Oxandra cf. major and biogenetic considerations. Phytochemistry 1987, 26, 2093–2098. [Google Scholar] [CrossRef]
- El-Shanawany, M.A.; Slatkin, D.J.; Schiff, P.L.; El-Shabrawy, A. A new alkaloid from oxandra xylopioides diels. Bull. Pharm. Sci. Assiut 1985, 8, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarty, M.; Patra, A. 6,7-Dimethoxyonychine and Other Alkaloids of Polyalthia longifolia. Indian J. Chem. Sect. B 1990, 21, 394–395. [Google Scholar] [CrossRef]
- Chen, B.; Ye, Q.; Li, B.; Zhang, G. A new azafluorene alkaloid from Miliusa balansae. Indian J. Heterocycl. Chem. 2002, 12, 81–82. [Google Scholar]
- Yoshida, N.C.; De Siqueira, J.M.; Rodrigues, R.P.; Correia, R.P.; Garcez, W.S. An Azafluorenone Alkaloid and a Megastigmane from Unonopsis lindmanii (Annonaceae). J. Braz. Chem. Soc. 2013, 24, 529–533. [Google Scholar] [CrossRef]
- Chaves, M.H.; Santos, L.D.A.; Lago, J.H.G.; Roque, N.F. Alkaloids from Porcelia macrocarpa. J. Nat. Prod. 2001, 64, 240–242. [Google Scholar] [CrossRef]
- Talip, M.A.; Azziz, S.S.S.A.; Wong, C.F.; Awang, K.; Naz, H.; Bakri, Y.M.; Ahmad, M.S.; Litaudon, M. New Azafluorenone Derivative and Antibacterial Activities of Alphonsea cylindrica Barks. Nat. Prod. Sci. 2017, 23, 151–156. [Google Scholar] [CrossRef] [Green Version]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; (The “Gold Book”); McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997; Online version (2019-) created by S.J. Chalk; Available online: https://goldbook.iupac.org/terms/view/R05360 (accessed on 27 October 2022).
- NIST/EPA/NIH Mass Spectral Database (NIST17). Available online: https://chemdata.nist.gov/ (accessed on 27 October 2022).
- Jourjine, I.A.P.; Bracher, F. Department of Pharmacy—Center for Drug Research, Ludwig Maximilians University Munich, Munich, Germany. 2023; manuscript in preparation.
- Bracher, F. Polycyclic Aromatic Alkaloids. Part 2. Synthesis of Onychine and Eupolauridine. Arch. Pharm. 1989, 20, 293–294. [Google Scholar] [CrossRef]
- Bracher, F. A regioselective Synthesis of Azafluorenone Alkaloids. Synlett 1991, 1991, 95–96. [Google Scholar] [CrossRef]
- Bracher, F. Polycyclische aromatische Alkaloide, 10. Mitt.: Annonaceen-Alkaloide mit antimykotischer Aktivität. Arch. Pharm. 1994, 327, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Bracher, F. Polycyclische aromatische Alkaloide, I. Synthese von Cleistopholin und Sampangin. Liebigs Ann. Chem. 1989, 1989, 87–88. [Google Scholar] [CrossRef]
- Plodek, A.; König, M.; Bracher, F. Synthesis of the Azaoxoaporphine Alkaloid Sampangine and Ascididemin-Type Pyridoacridines through TMPMgCl·LiCl-Mediated Ring Closure. Eur. J. Org. Chem. 2015, 2015, 1302–1308. [Google Scholar] [CrossRef]
- Bracher, F. Polycyclic aromatic alkaloids. Part 9: Total synthesis and anticandidal activity of diazafluoroanthene alkaloids. Pharmazie 1993, 48, 521–523. [Google Scholar] [PubMed]
- Melzer, B.; Bracher, F. A divergent approach to benzylisoquinoline-type and oxoaporphine alkaloids via regioselective direct ring metalation of alkoxy isoquinolines. Org. Biomol. Chem. 2015, 13, 7664–7672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puzik, A.; Bracher, F. A convenient approach to the canthin-4-one ring system: Total synthesis of the alkaloids tuboflavine and norisotuboflavine. J. Heterocycl. Chem. 2009, 46, 770–773. [Google Scholar] [CrossRef]
- Bracher, F.; Hildebrand, D. β-Carbolin-Alkaloide, II Tributyl(1-ethoxyvinyl)stannan als C2-Baustein für die Synthese von β-Carbolin-Alkaloiden. Liebigs Ann. Chem. 1993, 1993, 837–839. [Google Scholar] [CrossRef]
- Hetzler, B.E.; Trauner, D.; Lawrence, A.L. Natural product anticipation through synthesis. Nat. Rev. Chem. 2022, 6, 170–181. [Google Scholar] [CrossRef]
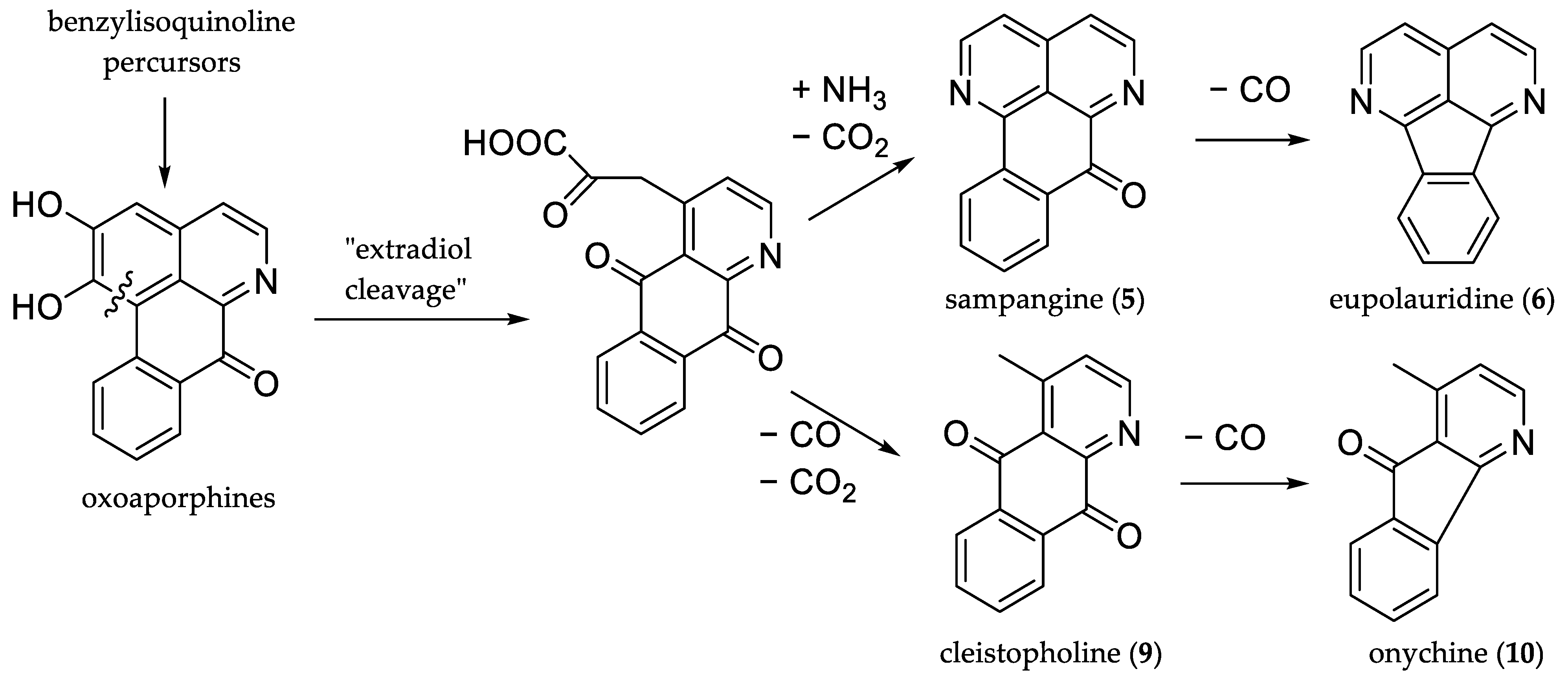
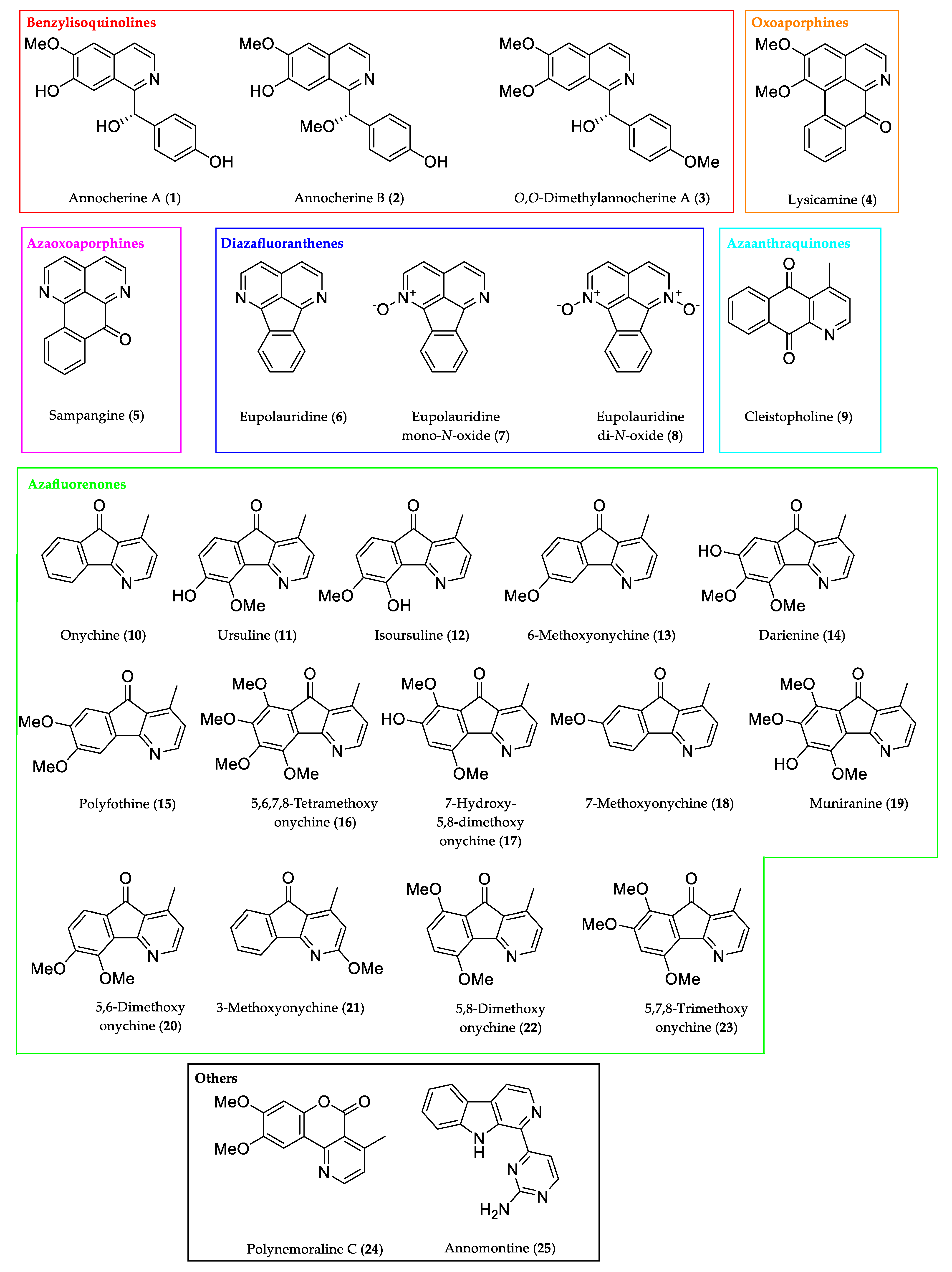
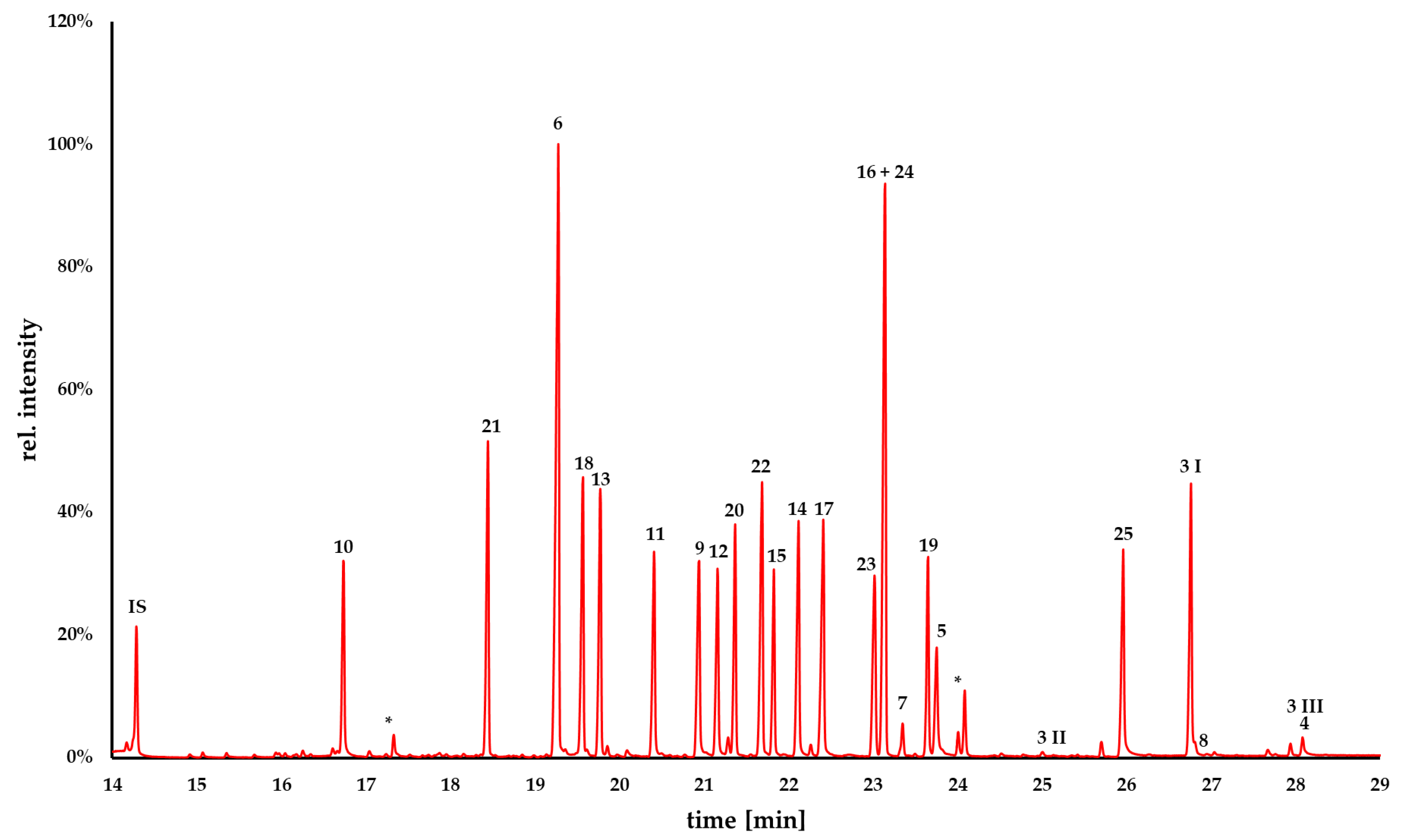
| Code | Trivial Name [Biological Source] | CAS 1 Number | Chemical Formula | M [g/mol] | Characteristic Ions [m/z] (Base Peak in Bold) | RRT 2 (Fluorene) | Kováts Index 3 |
|---|---|---|---|---|---|---|---|
| 1 | Annocherine A [14] | 344928-12-7 | C17H15NO4 | 297.10 | (I) 280, 265, 220 | 1.818 | 2925 |
| (II) 279, 264, 236 | 1.985 | 3295 | |||||
| 2 | Annocherine B [14] | 344928-13-8 | C18H17NO4 | 311.12 | (I) 280, 265, 220 | 1.818 | 2925 |
| (II) 279, 264, 236 | 1.985 | 3295 | |||||
| 3 | O,O-Dimethylannocherine A ** [15] | 1268489-61-7 | C19H19NO4 | 325.13 | (I) 322, 308, 292 | 1.872 | 3045 |
| (II) 308, 294, 278 | 1.750 | 2784 | |||||
| (III) 307, 292, 248 | 1.937 | 3190 | |||||
| 4 | Lysicamine [16] | 15444-20-9 | C18H13NO3 | 291.09 | 291, 248, 177 | 1.964 | 3258 |
| 5 | Sampangine [17] | 116664-93-8 | C15H8N2O | 232.06 | 232, 204, 151 | 1.663 | 2614 |
| 6 | Eupolauridine [18] | 58786-39-3 | C14H8N2 | 204.06 | 204, 177, 150 | 1.346 | 2074 |
| 7 | Eupolauridine mono-N-oxide [19] | 96889-95-1 | C14H8N2O | 220.06 | 220, 204, 165 | 1.630 | 2552 |
| 8 | Eupolauridine di-N-oxide [19] | 96889-96-2 | C14H8N2O2 | 236.06 | 236, 220, 204 | 1.885 | 3072 |
| 9 | Cleistopholine [19] | 96889-94-0 | C14H9NO2 | 223.06 | 223, 195, 167 | 1.467 | 2267 |
| 10 | Onychine [10] | 58787-04-5 | C13H9NO | 195.07 | 195, 166, 139 | 1.171 | 1823 |
| 11 | Ursuline [20] | 111316-34-8 | C14H11NO3 | 241.07 | 241, 223, 183 | 1.430 | 2204 |
| 12 | Isoursuline [21] | 112368-57-7 | C14H11NO3 | 241.07 | 241, 212, 198 | 1.481 | 2290 |
| 13 | 6-Methoxyonychine [11] | 105418-67-5 | C14H11NO2 | 225.08 | 225, 182, 154 | 1.386 | 2135 |
| 14 | Darienine [20] | 111316-27-9 | C15H13NO4 | 271.08 | 271, 256, 225 | 1.546 | 2400 |
| 15 | Polyfothine [22] | 122908-91-2 | C15H13NO3 | 255.09 | 255, 212, 169 | 1.529 | 2371 |
| 16 | 5,6,7,8-Tetramethoxyonychine [23] | N/A | C17H17NO5 | 315.11 | 315, 300, 239 | 1.621 | 2536 |
| 17 | 7-Hydroxy-5,8-dimethoxyonychine [24] | N/A | C15H13NO4 | 271.08 | 271, 242, 172 | 1.566 | 2436 |
| 18 | 7-Methoxyonychine [25] | 117719-70-7 | C14H11NO2 | 225.08 | 255, 210, 154 | 1.369 | 2108 |
| 19 | Muniranine [26] | N/A | C16H15NO5 | 301.10 | 301, 283, 200 | 1.653 | 2596 |
| 20 | 5,6-Dimethoxyonychine * | 112368-58-8 | C15H13NO3 | 255.09 | 255, 254, 226 | 1.494 | 2312 |
| 21 | 3-Methoxyonychine * | 145013-64-5 | C14H11NO2 | 225.08 | 225, 224, 196 | 1.290 | 1989 |
| 22 | 5,8-Dimethoxyonychine * | 112368-60-2 | C15H13NO3 | 255.09 | 255, 254, 226 | 1.516 | 2349 |
| 23 | 5,7,8-Trimethoxyonychine * | N/A | C16H15NO4 | 285.10 | 285, 270, 256 | 1.612 | 2519 |
| 24 | Polynemoraline C [12] | 1129491-73-1 | C15H13NO4 | 271.09 | 271, 228, 185 | 1.617 | 2528 |
| 25 | Annomontine [13] | 82504-00-5 | C15H11N5 | 261.10 | 261, 245, 220 | 1.816 | 2920 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jourjine, I.A.P.; Bauernschmidt, C.; Müller, C.; Bracher, F. A GC-MS Protocol for the Identification of Polycyclic Aromatic Alkaloids from Annonaceae. Molecules 2022, 27, 8217. https://doi.org/10.3390/molecules27238217
Jourjine IAP, Bauernschmidt C, Müller C, Bracher F. A GC-MS Protocol for the Identification of Polycyclic Aromatic Alkaloids from Annonaceae. Molecules. 2022; 27(23):8217. https://doi.org/10.3390/molecules27238217
Chicago/Turabian StyleJourjine, Ilya A. P., Carolin Bauernschmidt, Christoph Müller, and Franz Bracher. 2022. "A GC-MS Protocol for the Identification of Polycyclic Aromatic Alkaloids from Annonaceae" Molecules 27, no. 23: 8217. https://doi.org/10.3390/molecules27238217
APA StyleJourjine, I. A. P., Bauernschmidt, C., Müller, C., & Bracher, F. (2022). A GC-MS Protocol for the Identification of Polycyclic Aromatic Alkaloids from Annonaceae. Molecules, 27(23), 8217. https://doi.org/10.3390/molecules27238217