Autofluorescence-Based Investigation of Spatial Distribution of Phenolic Compounds in Soybeans Using Confocal Laser Microscopy and a High-Resolution Mass Spectrometric Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals and Reagents
2.3. Fractional Maceration
2.4. Optical Microscopy
2.5. Liquid Chromatography
2.6. Mass Spectrometry
3. Results and Discussion
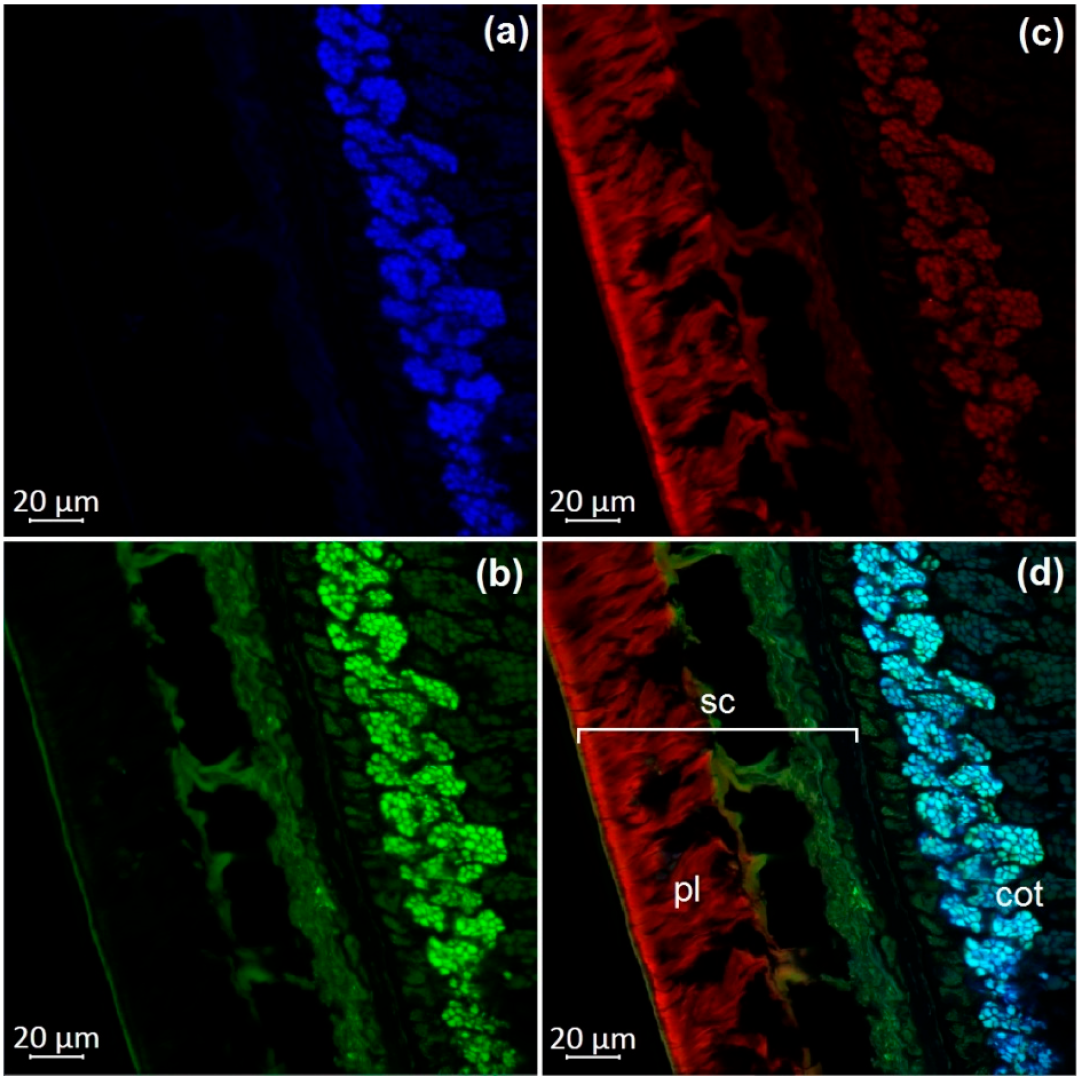
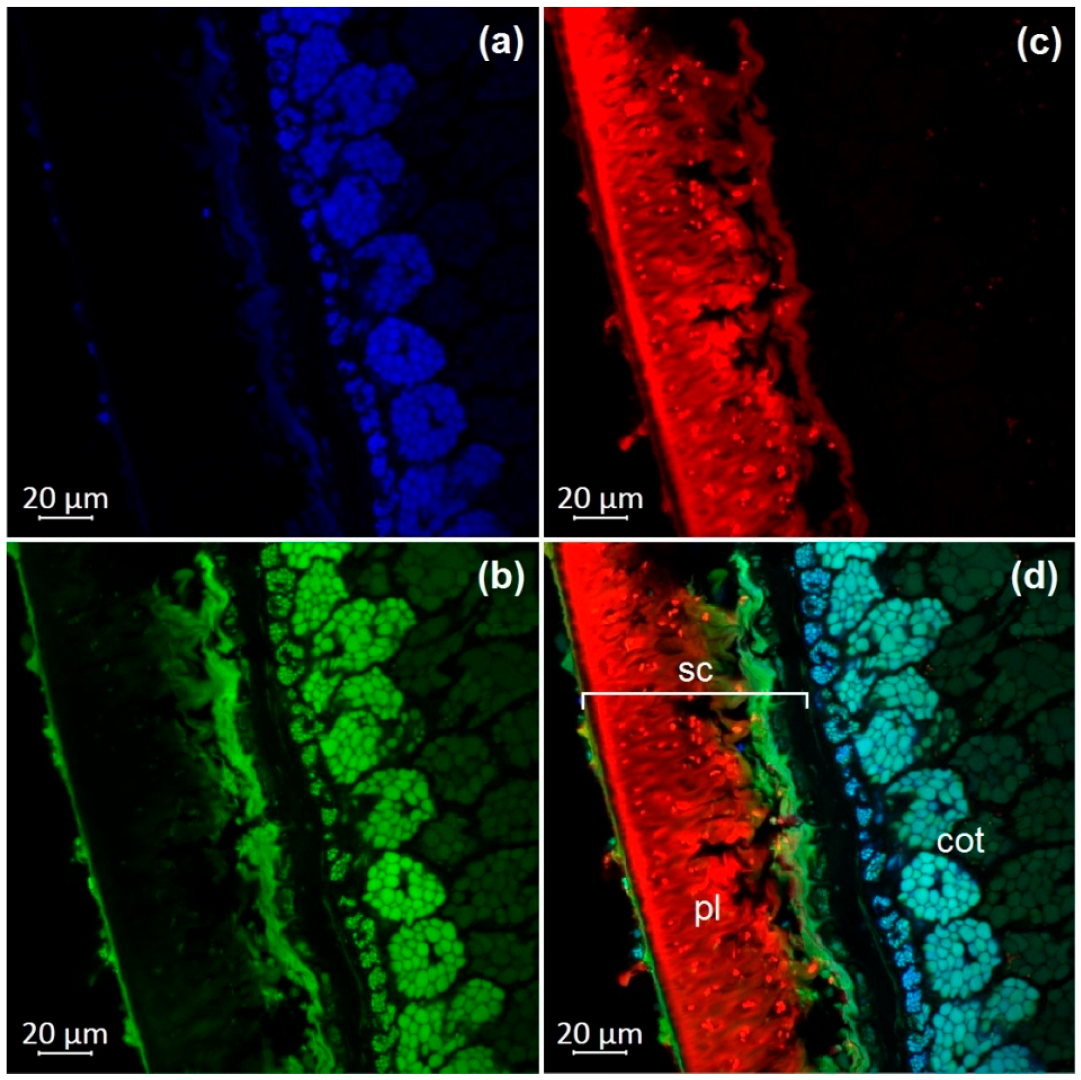
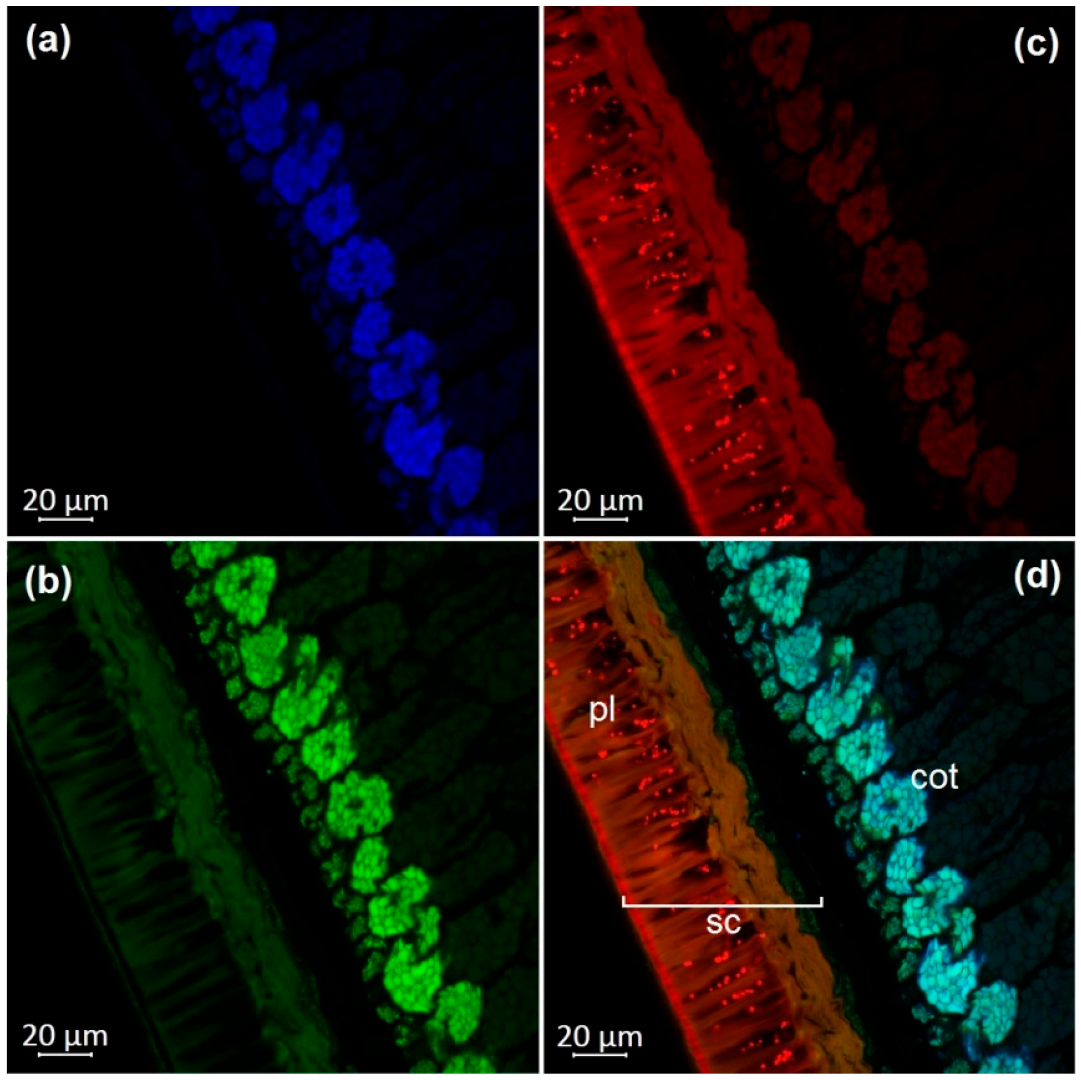
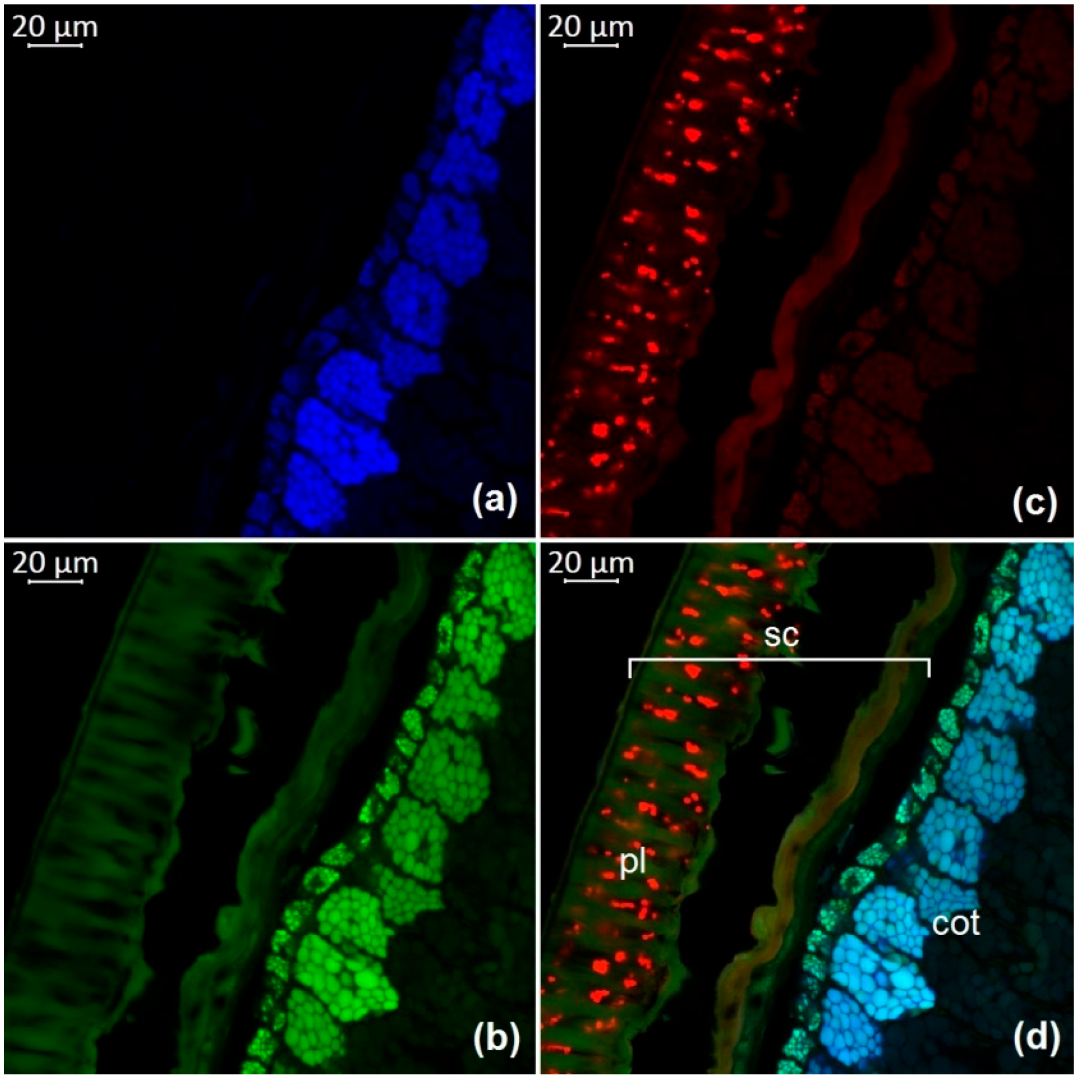
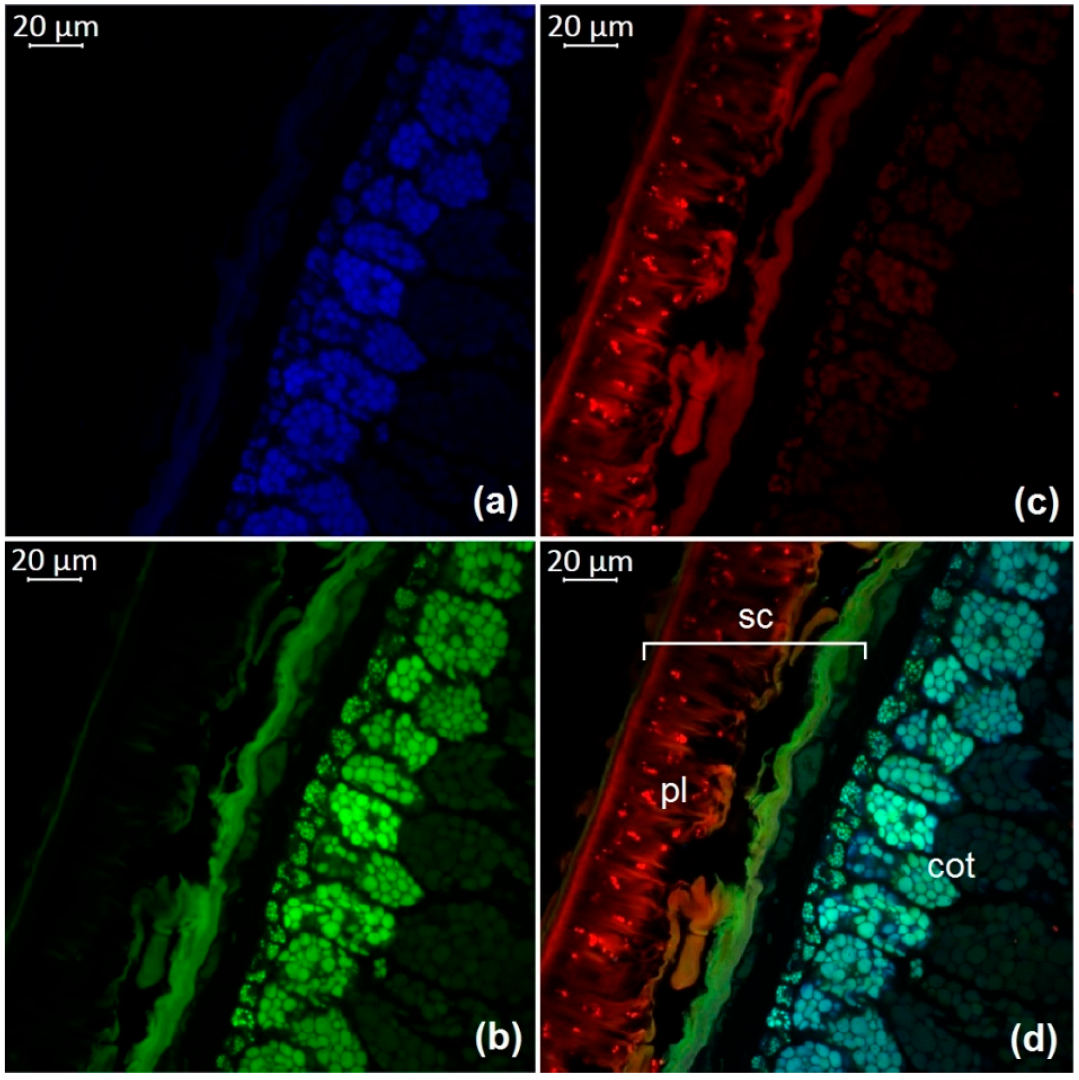
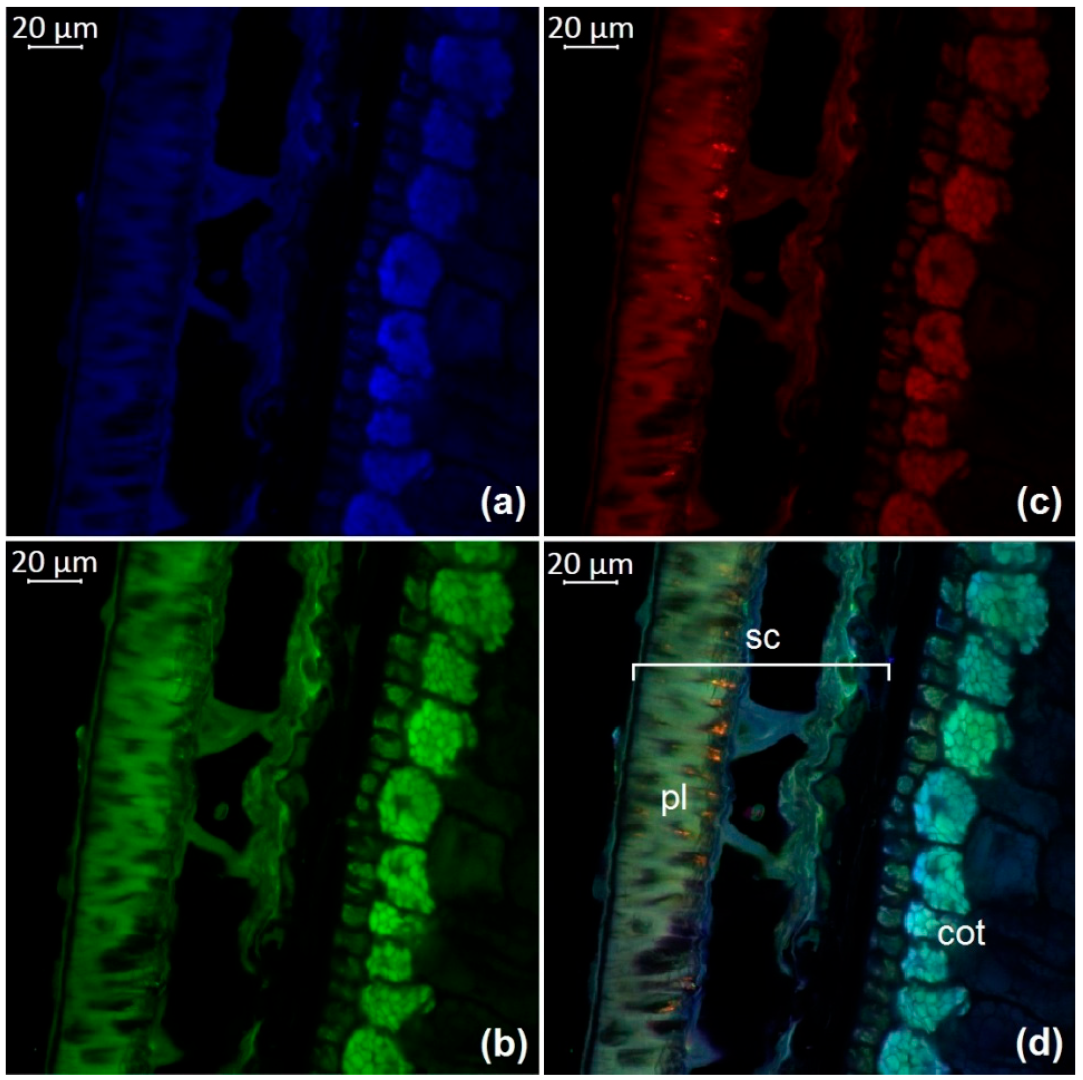
3.1. Optical Microscopy of Soybean Components
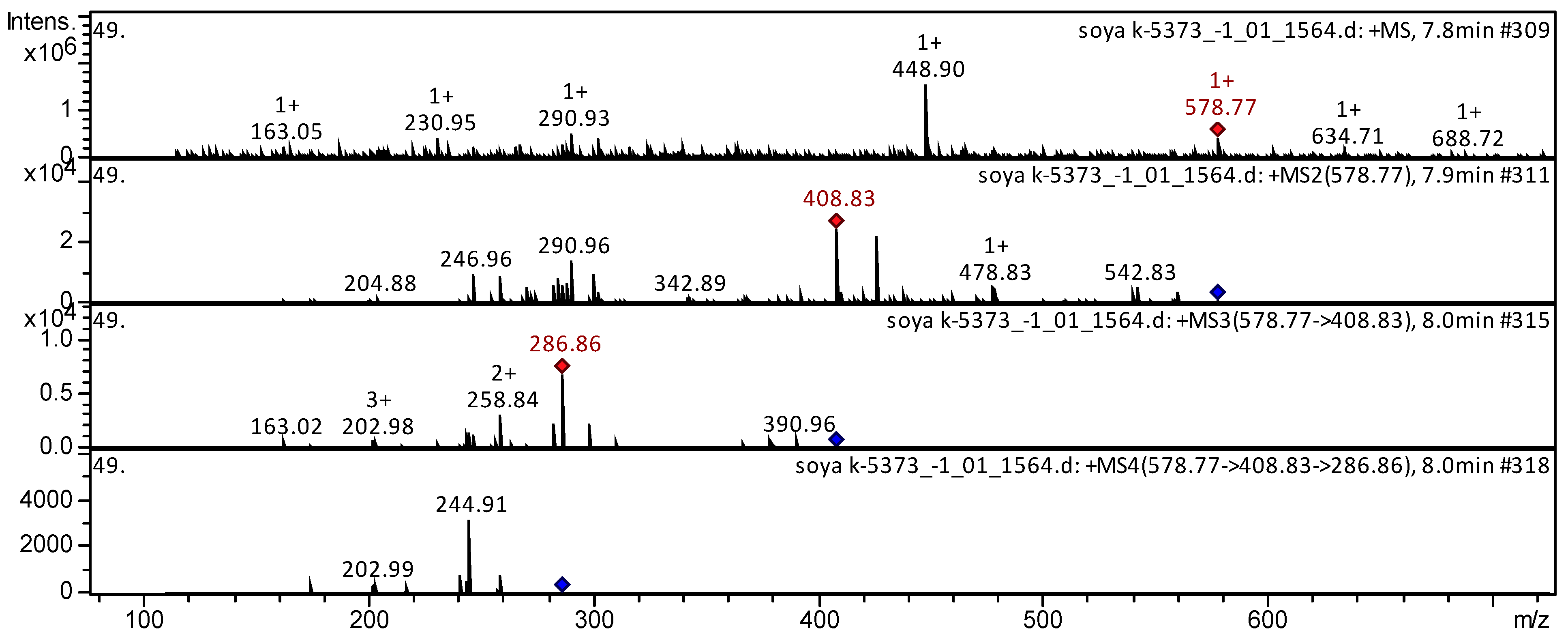
3.2. Tandem Mass Spectrometric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hymowitz, T. On the domestication of the soybean. Econ. Bot. 1970, 24, 408–421. [Google Scholar] [CrossRef]
- Dixit, A.K.; Antony, J.; Sharma, N.K.; Tiwari, R.K. 12. Soybean constituents and their functional benefits. Res. Singpost 2011, 37, 661. [Google Scholar]
- Pratap, A.; Gupta, S.K.; Kumar, J.; Solanki, R. Soybean. In Technological Innovations in Major World Oil Crops; Springer: New York, NY, USA, 2012; Volume 1. [Google Scholar]
- Sinegovskii, M.; Yuan, S.; Sinegovskaya, V.; Han, T. Current status of the soybean industry and research in the Russian Federation. Soybean Sci. 2018, 37, 1–7. (In Russian) [Google Scholar]
- Omoni, A.O.; Aluko, R.E. Soybean foods and their benefits: Potential mechanisms of action. Nutr. Rev. 2005, 63, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Duodu, K.G. Bioactive polyphenols and peptides in cowpea (Vigna unguiculata) and their health promoting properties: A review. J. Func. Foods 2017, 38, 686–697. [Google Scholar] [CrossRef]
- Sudo, E.; Teranishi, M.; Hidema, J.; Taniuchi, T. Visualization of flavonol distribution in the abaxial epidermis of onion scales via detection of its autofluorescence in the absence of chemical processes. Biosci. Biotechnol. Biochem. 2009, 73, 2107–2109. [Google Scholar] [CrossRef]
- Salunkhe, D.K.; Jadhav, S.J.; Kadam, S.S.; Chavan, J.K. Chemical, biochemical, and biological significance of polyphenols in cereals and legumes. Crit. Rev. Food Sci. Nutr. 1982, 17, 277–305. [Google Scholar] [CrossRef]
- Benitez, E.R.; Funatsuki, H.; Kaneko, Y.; Matsuzawa, Y.; Bang, S.W.; Takahashi, R. Soybean maturity gene effects on seed coat pigmentation and cracking in response to low temperatures. Crop Sci. 2004, 44, 2038–2042. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zhang, F.X.; Zhang, M.W.; Wei, Z.C.; Yang, C.Y.; Zhang, Y.; Tang, X.J.; Deng, Y.Y.; Chi, J.W. Phenolic composition and antioxidant activity in seed coats of 60 Chinese black soybean (Glycine max L. Merr.) varieties. J. Agric. Food Chem. 2011, 59, 5935–5944. [Google Scholar] [CrossRef]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R.; Veit, M.; Weissenböck, G.; Schnitzler, J.-P. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J. Exp. Bot. 1998, 49, 953–965. [Google Scholar] [CrossRef]
- Razgonova, M.; Zinchenko, Y.; Pikula, K.; Tekutyeva, L.; Son, O.; Zakharenko, A.; Kalenik, T.; Golokhvast, K. Spatial Distribution of Polyphenolic Compounds in Corn Grains (Zea mays L. var. Pioneer) Studied by Laser Confocal Microscopy and High-Resolution Mass Spectrometry. Plants 2022, 11, 630. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miyashita, K.; Shimizu, H.; Sugiyama, J. Three-dimensional internal structure of a soybean seed by observation of autofluorescence of sequential sections. J. Jpn. Soc. Food Sci. Technol. 2003, 50, 213–217. [Google Scholar] [CrossRef][Green Version]
- Pegg, T.J.; Gladish, D.K.; Baker, R.L. Algae to angiosperms: Autofluorescence for rapid visualization of plant anatomy among diverse taxa. Appl. Plant Sci. 2021, 9, e11437. [Google Scholar] [CrossRef]
- Slattery, R.A.; Grennan, A.K.; Sivaguru, M.; Sozzani, R.; Ort, D.R. Light sheet microscopy reveals more gradual light attenuation in light-green versus dark-green soybean leaves. J. Exp. Bot. 2016, 67, 4697–4709. [Google Scholar] [CrossRef]
- Wang, Z.; Amirkhani, M.; Avelar, S.A.G.; Yang, D.; Taylor, A.G. Systemic Uptake of Fluorescent Tracers by Soybean (Glycine max (L.) Merr.) Seed and Seedlings. Agriculture 2020, 10, 248. [Google Scholar] [CrossRef]
- Krishnan, H.B.; Jurkevich, A. Confocal Fluorescence Microscopy Investigation for the Existence of Subdomains within Protein Storage Vacuoles in Soybean Cotyledons. Int. J. Mol. Sci. 2022, 23, 3664. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Corcel, M.; Devaux, M.-F.; Guillon, F.; Barron, C. Identification of tissular origin of particles based on autofluorescence multispectral image analysis at the macroscopic scale. In Proceedings of the EPJ Web of Conferences, Crete, Greece, 17–29 August 2017; p. 05012. [Google Scholar]
- Lichtenthaler, H.K.; Schweiger, J. Cell wall bound ferulic acid, the major substance of the blue-green fluorescence emission of plants. J. Plant Physiol. 1998, 152, 272–282. [Google Scholar] [CrossRef]
- Donaldson, L. Softwood and hardwood lignin fluorescence spectra of wood cell walls in different mounting media. IAWA J. 2013, 34, 3–19. [Google Scholar] [CrossRef]
- Brillouet, J.M.; Riochet, D. Cell wall polysaccharides and lignin in cotyledons and hulls of seeds from various lupin (Lupinus L.) species. J. Sci. Food Agric. 1983, 34, 861–868. [Google Scholar] [CrossRef]
- Krzyzanowski, F.C.; Franca Neto, J.D.B.; Mandarino, J.M.G.; Kaster, M. Evaluation of lignin content of soybean seed coat stored in a controlled environment. Rev. Bras. De Sementes 2008, 30, 220–223. [Google Scholar] [CrossRef]
- Brillouet, J.M.; Carré, B. Composition of cell walls from cotyledons of Pisum sativum, Vicia faba and Glycine max. Phytochemistry 1983, 22, 841–847. [Google Scholar] [CrossRef]
- Monago-Maraña, O.; Durán-Merás, I.; Galeano-Díaz, T.; de la Peña, A.M. Fluorescence properties of flavonoid compounds. Quantification in paprika samples using spectrofluorimetry coupled to second order chemometric tools. Food Chem. 2016, 196, 1058–1065. [Google Scholar] [CrossRef]
- Roshchina, V.V.; Kuchin, A.V.; Yashin, V.A. Application of Autofluorescence for Analysis of Medicinal Plants. Spectrosc. Int. J. 2017, 2017, 7159609. [Google Scholar] [CrossRef]
- Talamond, P.; Verdeil, J.-L.; Conéjéro, G. Secondary metabolite localization by autofluorescence in living plant cells. Molecules 2015, 20, 5024–5037. [Google Scholar] [CrossRef] [PubMed]
- Collings, D.A. Anthocyanin in the vacuole of red onion epidermal cells quenches other fluorescent molecules. Plants 2019, 8, 596. [Google Scholar] [CrossRef]
- Troszynska, A.; Ciska, E. Phenolic compounds of seed coats of white and coloured varieties of pea (Pisumsativum L.) and their total antioxidant activity. Czech J. Food Sci. 2002, 20, 15–22. [Google Scholar] [CrossRef]
- Jo, H.; Lee, J.Y.; Cho, H.; Choi, H.J.; Son, C.K.; Bae, J.S.; Bilyeu, K.; Song, J.T.; Lee, J.D. Genetic diversity of soybeans (Glycine max (L.) merr.) with black seed coats and green cotyledons in Korean germplasm. Agronomy 2021, 11, 581. [Google Scholar] [CrossRef]
- Moïse, J.A.; Han, S.; Gudynaitę-Savitch, L.; Johnson, D.A.; Miki, B.L. Seed coats: Structure, development, composition, and biotechnology. Vitr. Cell. Dev. Biol. Plant 2005, 41, 620–644. [Google Scholar] [CrossRef]
- Jeng, T.L.; Shih, Y.J.; Wu, M.T.; Sung, J.M. Comparisons of flavonoids and anti-oxidative activities in seed coat, embryonic axis and cotyledon of black soybeans. Food Chem. 2010, 123, 1112–1116. [Google Scholar] [CrossRef]
- Tsamo, A.T.; Mohammed, H.; Mohammed, M.; Papoh Ndibewu, P.; Dapare Dakora, F. Seed coat metabolite profiling of cowpea (Vigna unguiculata L. Walp.) accessions from Ghana using UPLC-PDA-QTOF-MS and chemometrics. Nat. Prod. Res. 2020, 34, 1158–1162. [Google Scholar] [CrossRef]
- Rodriguez-Perez, C.; Gomez-Caravaca, A.M.; Guerra-Hernandez, E.; Cerretani, L.; Garcia-Villanova, B.; Verardo, V. Comprehensive metabolite profiling of Solanum tuberosum L. (potato) leaves T by HPLC-ESI-QTOF-MS. Food Res. Int. 2018, 112, 390–399. [Google Scholar] [CrossRef]
- Perchuk, I.; Shelenga, T.; Gurkina, M.; Miroshnichenko, E.; Burlyaeva, M. Composition of Primary and Secondary Metabolite Compounds in Seeds and Pods of Asparagus Bean (Vigna unguiculata (L.) Walp.) from China. Molecules 2020, 25, 3778. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Zou, L.; Liu, X.; Chen, J.; Tan, M.; Mei, Y.; Wei, L. Comparison of Multiple Bioactive Constituents in the Flower and the Caulis of Lonicera japonica Based on UFLC-QTRAP-MS/MS Combined with Multivariate Statistical Analysis. Molecules 2019, 24, 1936. [Google Scholar] [CrossRef]
- Qin, D.; Wang, Q.; Li, H.; Jiang, X.; Fang, K.; Wang, Q.; Li, B.; Pan, C.; Wu, H. Identification of key metabolites based on non-targeted metabolomics and chemometrics analyses provides insights into bitterness in Kucha [Camellia kucha (Chang et Wang) Chang]. Food Res. Int. 2020, 138, 109789. [Google Scholar] [CrossRef]
- Oertel, A.; Matros, A.; Hartmann, A.; Arapitsas, P.; Dehmer, K.J.; Martens, S.; Mock, H.P. Metabolite profiling of red and blue potatoes revealed cultivar and tissue specific patterns for anthocyanins and other polyphenols. Planta 2017, 246, 281–297. [Google Scholar] [CrossRef]
- Deuber, H.; Guignard, C.; Hoffmann, L.; Evers, D. Polyphenol and glycoalkaloid contents in potato cultivars grown in Luxembourg. Food Chem. 2012, 135, 2814–2824. [Google Scholar]
- Sharma, M.; Sandhir, R.; Singh, A.; Kumar, P.; Mishra, A.; Jachak, S.; Singh, S.P.; Singh, J.; Roy, J. Comparison analysis of phenolic compound characterization and their biosynthesis genes between two diverse bread wheat (Triticum aestivum) varieties differing for chapatti (unleavened flat bread) quality. Front. Plant. Sci. 2016, 7, 1870. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSn. J. Funct. Foods 2011, 3, 144–158. [Google Scholar] [CrossRef]
- Papazian, S.; Parrot, D.; Buryskova, F.; Tasdemir, D. Surface chemical defence of the eelgrass Zostera marina against microbial foulers. Sci. Rep. 2019, 9, 3323. [Google Scholar] [CrossRef]
- Aita, S.E.; Capriotti, A.L.; Cavaliere, C.; Cerrato, A.; Giannelli Moneta, B.; Montone, C.M.; Piovesana, S.; Laganà, A. Andean Blueberry of the Genus Disterigma: A High-Resolution Mass Spectrometric Approach for the Comprehensive Characterization of Phenolic Compounds. Separations 2021, 8, 58. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Jauregui, O.; Medina-Remon, A.; Lamuela-Raventos, R.M. Evaluation of a Method to Characterize the Phenolic Profile of Organic and Conventional Tomatoes. Agricult. Food Chem. 2012, 60, 3373–3380. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yao, P.; Leung, K.; Wang, H.; Kong, X.P.; Wang, L.; Dong, T.T.; Chen, Y.; Tsim, K.W.K. The Yin-Yang Property of Chinese Medicinal Herbs Relates to Chemical Composition but Not Anti-Oxidative Activity: An Illustration Using Spleen-Meridian Herbs. Front. Pharmacol. 2018, 9, 1304. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A.; Abbas, F.A.; Refaat, S.; El-Shafae, A.M.; Fikry, E. UPLC-ESI-MS/MS Profile of The Ethyl Acetate Fraction of Aerial Parts of Bougainvillea ‘Scarlett O’Hara’ Cultivated in Egypt. Egypt J. Chem. 2021, 64, 22. [Google Scholar] [CrossRef]
- Zhou, X.J.; Yan, L.L.; Yin, P.P.; Shi, L.L.; Zhang, J.H.; Liu, Y.J.; Ma, C. Structural characterisation and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chem. 2014, 164, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Ozarowski, M.; Piasecka, A.; Paszel-Jaworska, A.; de Chaves, D.S.A.; Romaniuk, A.; Rybczynska, M.; Gryszczynska, A.; Sawikowska, A.; Kachlicki, P.; Mikolajczak, P.L.; et al. Comparison of bioactive compounds content in leaf extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro cytotoxic potential on leukemia cell lines. Braz. J. Pharmacol. 2018, 28, 179–191. [Google Scholar] [CrossRef]
- Ojwang, L.O.; Yang, L.; Dykes, L.; Awika, J. Proanthocyanidin profile of cowpea (Vigna unguiculata) reveals catechin-O- glucoside as the dominant compound. Food Chem. 2013, 139, 35–43. [Google Scholar] [CrossRef]
- Vijayan, K.P.R.; Raghu, A.V. Tentative characterization of phenolic compounds in three species of the genus Embelia by liquid chromatography coupled with mass spectrometry analysis. Spectrosc. Lett. 2019, 52, 653–670. [Google Scholar] [CrossRef]
- Sun, J.; Liang, F.; Bin, Y.; Li, P.; Duan, C. Screening Non-colored Phenolics in Red Wines using Liquid Chromatography/Ultraviolet and Mass Spectrometry/Mass Spectrometry Libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef]
- Fuchs, C.; Bakuradze, T.; Steinke, R.; Grewal, R.; Eckert, G.P.; Richling, E. Polyphenolic composition of extracts from winery by-products and effects on cellular cytotoxicity and mitochondrial functions in HepG2 cells. J. Funct. Foods. 2020, 70, 103988. [Google Scholar] [CrossRef]
- Hamed, A.R.; El-Hawary, S.S.; Ibrahim, R.M.; Abdelmohsen, U.R.; El-Halawany, A.M. dentification of Chemopreventive Components from Halophytes Belonging to Aizoaceae and Cactaceae Through LC/MS–Bioassay Guided Approach. J. Chrom. Sci. 2021, 59, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.W.; Li, J.; Gao, X.M.; Amponsem, E.; Kang, L.Y.; Hu, L.M.; Zhang, B.L.; Chang, Y.X. Simultaneous determination of stilbenes, phenolic acids, flavonoids and anthraquinones in Radix polygoni multiflori by LC-MS/MS. J. Pharm. Biomed. Anal. 2012, 62, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, P.; Liu, B.; Wei, L.; Xu, Y. Simultaneous determination of fourteen compounds of Hedyotis diffusa Willd extract in rats by UHPLC-MS/MS method: Application to pharmacokinetics and tissue distribution study. J. Pharm. Biomed. Anal. 2018, 159, 490–512. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, D.; Vlase, L.; Olah, N. Lc/MS analysis of isoflavones from Fabaceae species extracts. Farmacia 2010, 58, 177–183. [Google Scholar]
- Yin, Y.; Zhang, K.; Wei, L.; Chen, D.; Chen, Q.; Jiao, M.; Li, X.; Huang, J.; Gong, Z.; Kang, N.; et al. The Molecular Mechanism of Antioxidation of Huolisu Oral Liquid Based on Serum Analysis and Network Analysis. Front. Pharmacol. 2021, 12, 710976. [Google Scholar] [CrossRef]
- Wojakowska, A.; Piasecka, A.; García-López, P.M.; Zamora-Natera, F.; Krajewski, P.; Marczak, Ł.; Kachlicki, P.; Stobiecki, M. Structural analysis and profiling of phenolic secondary metabolites of Mexican lupine species using LC-MS techniques. Phytochemistry 2013, 92, 71–86. [Google Scholar] [CrossRef]
- Teles, Y.C.E.; Rebello Horta, C.C.; de Fatima Agra, M.; Siheri, W.; Boyd, M.; Igoli, J.O.; Gray, A.I.; de Fatima Vanderlei de Souza, M. New Sulphated Flavonoids from Wissadula periplocifolia (L.) C. Presl (Malvaceae). Molecules 2015, 20, 20161–20172. [Google Scholar] [CrossRef]
- Vera de Rosso, V.; Hillebrand, S.; Cuevas Montilla, E.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of anthocyanins from acerola (Malpighia emarginata DC.) and ac-ai (Euterpe oleracea Mart.) by HPLC–PDA–MS/MS. J. Food Compos. Anal. 2008, 21, 291–299. [Google Scholar] [CrossRef]
- Marcia Fuentes, J.A.; Lopez-Salas, L.; Borras-Linares, I.; Navarro-Alarcon, M.; Segura-Carretero, A.; Lozano-Sanchez, J. Development of an Innovative Pressurized Liquid Extraction Procedure by Response Surface Methodology to Recover Bioactive Compounds from Carao Tree Seeds. Foods 2021, 10, 398. [Google Scholar] [CrossRef]
- Thomford, N.E.; Dzobo, K.; Chopera, D.; Wonkam, A.; Maroyi, A.; Blackhurst, D.; Dandara, C. In vitro reversible and time-dependent CYP450 inhibition profiles of medicinal herbal plant extracts Newbouldia laevis and Cassia abbreviata: Implications for herb-drug interactions. Molecules 2016, 21, 891. [Google Scholar] [CrossRef]
- Yang, D.; Du, X.; Liang, X.; Han, R.; Liang, Z.; Liu, Y.; Liu, F.; Zhao, J. Different roles of the mevalonate and methylerythritol phosphate pathways in cell growth and tanshinone production of Salvia miltiorrhiza hairy roots. PLoS ONE 2012, 7, e46797. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC-DAD-ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant. 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Ekeberg, D.; Flate, P.-O.; Eikenes, M.; Fongen, M.; Naess-Andresen, C.F. Qualitative and quantitative determination of extractives in heartwood of Scots pine (Pinus sylvestris L.) by gas chromatography. J. Chromatogr. A 2006, 1109, 267–272. [Google Scholar] [CrossRef]
- Kim, S.; Oh, S.; Noh, H.B.; Ji, S.; Lee, S.H.; Koo, J.M.; Choi, C.W.; Jhun, H.P. In Vitro Antioxidant and Anti-Propionibacterium acnes Activities of Cold Water, Hot Water, and Methanol Extracts, and Their Respective Ethyl Acetate Fractions, from Sanguisorba officinalis L. Roots. Molecules 2018, 23, 3001. [Google Scholar] [CrossRef]
- Xu, L.L.; Xu, J.J.; Zhong, K.R.; Shang, Z.P.; Wang, F.; Wang, R.F.; Zhang, L.; Zhang, J.Y.; Liu, B. Analysis of Non-Volatile Chemical Constituents of Menthae Haplocalycis Herba by Ultra-High Performance Liquid Chromatography-High Resolution Mass Spectrometry. Molecules 2017, 22, 1756. [Google Scholar] [CrossRef]
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K.A.; Nieman, K.M.; Dall’Asta, C.; Del Rio, D. Phenolic and Volatile Composition of a Dry Spearmint (Mentha spicata L.) Extract. Molecules 2016, 21, 1007. [Google Scholar] [CrossRef]
- Olennikov, D.O.; Chirikova, N.K.; Okhlopkova, Z.M.; Zulfugarov, I.S. Chemical Composition and Antioxidant Activity of Tánara Ótó (Dracocephalum palmatum Stephan), a Medicinal Plant Used by the North-Yakutian Nomads. Molecules 2013, 18, 14106. [Google Scholar] [CrossRef]
- Yin, N.W.; Wang, S.X.; Jia, L.D.; Zhu, M.C.; Yang, J.; Zhou, B.J.; Yin, J.M.; Lu, K.; Wang, R.; Li, J.N.; et al. Identification and Characterization of Major Constituents in Different-Colored Rapeseed Petals by UPLC-HESI-MS/MS. J. Agric. Food Chem. 2019, 67, 11053–11065. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Ultra-high performance liquid chromatography coupled to mass spectrometry applied to the identification of valuable phenolic compounds from Eucalyptus wood. J. Chromatogr. B 2013, 938, 65–74. [Google Scholar] [CrossRef]
- Abeywickrama, G.; Debnath, S.C.; Ambigaipalan, P.; Shahidi, F. Phenolics of Selected Cranberry Genotypes (Vaccinium macrocarpon Ait.) and Their Antioxidant Efficacy. J. Agric. Food Chem. 2016, 64, 9342–9351. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, X.; Yang, T.; Slovin, J.; Chen, P. Profiling polyphenols of two diploid strawberry (Fragaria vesca) inbred lines using UHPLC-HRMSn. Food Chem. 2014, 146, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Paudel, L.; Wyzgoski, F.J.; Scheerens, J.C.; Chanon, A.M.; Reese, R.N.; Smiljanic, D.; Wesdemiotis, C.; Blakeslee, J.J.; Riedl, K.M.; Rinaldi, P.L. Nonanthocyanin secondary metabolites of black raspberry (Rubus occidentalis L.) fruits: Identification by HPLC-DAD, NMR, HPLC-ESI-MS, and ESI-MS/MS analyses. J. Agr. Food Chem. 2013, 61, 12032–12043. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Martin, M.L.; Fuguet, E.; Jimenes-Ardon, A.; Herrero-Urbe, L.; Tamayo-Castillo, G.; Lluis Torres, J. Identification of polyphenols from antiviral Chamaecrista nictitans extract using high-resolution LC–ESI–MS/MS. Anal. Bioanal. Chem. 2014, 406, 5501–5506. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; Garcia-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and Comprehensive Evaluation of (Poly)phenolic Compounds in Pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef]
- Leveques, A.; Actis-Goretta, L.; Rein, M.J.; Williamson, G.; Dionisi, F.; Giuffrida, F. UPLC–MS/MS quantification of total hesperetin and hesperetin enantiomers in biological matrices. J. Pharmaceut. Biomed. Anal. 2012, 57, 1–6. [Google Scholar] [CrossRef]
- Bodalska, A.; Kowalczyk, A.; Włodarczyk, M.; Fecka, I. Analysis of Polyphenolic Composition of a Herbal Medicinal Product-Peppermint Tincture. Molecules 2019, 25, 69. [Google Scholar] [CrossRef]
- Fathoni, A.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.C.; Haib, J. Identification of Nonvolatile Compounds in Clove (Syzygium aromaticum) from Manado. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016. [Google Scholar] [CrossRef]
- Navarro, M.; Arnaez, E.; Moreira, I.; Quesada, S.; Azofeifa, G.; Wilhelm, K.; Vargas, F.; Chen, P. Polyphenolic Characterization, Antioxidant, and Cytotoxic Activities of Mangifera indica Cultivars from Costa Rica. Foods 2019, 8, 384. [Google Scholar] [CrossRef]
- Gordon, A.; Schadow, B.; Quijano, C.E.; Marx, F. Chemical characterization and antioxidant capacity of berries from Clidemia rubra (Aubl.) Mart. (Melastomataceae). Food Res. Int. 2011, 44, 2120–2127. [Google Scholar] [CrossRef]
- Thomas, M.C.; Dunn, S.R.; Altvater, J.; Dove, S.G.; Nette, G.W. Rapid Identification of Long-Chain Polyunsaturated Fatty Acids in a Marine Extract by HPLC-MS Using Data-Dependent Acquisition. Analyt. Chem. 2012, 84, 5976–5983. [Google Scholar] [CrossRef]
- Marzouk, M.M.; Hussein, S.R.; Elkhateeb, A.; El-shabrawy, M.; Abdel-Hameed, E.-S.S.; Kawashty, S.A. Comparative study of Mentha species growing wild in Egypt: LC-ESI-MS analysis and chemosystematic significance. J. Appl. Pharm. Sci. 2018, 8, 116–122. [Google Scholar]
- Pandey, B.P.; Pradhan, S.P.; Adhikari, K. LC-ESI-QTOF-MS for the Profiling of the Metabolites and in Vitro Enzymes Inhibition Activity of Bryophyllum pinnatum and Oxalis corniculate Collected from Ramechhap District of Nepal. Chem. Biodivers. 2020, 17, e2000155. [Google Scholar]
- Razgonova, M.; Zakharenko, A.; Pikula, K.; Manakov, Y.; Ercisli, S.; Derbush, I.; Kislin, E.; Seryodkin, I.; Sabitov, A.; Kalenik, T.; et al. LC-MS/MS Screening of Phenolic Compounds in Wild and Cultivated Grapes Vitis amurensis Rupr. Molecules 2021, 26, 360. [Google Scholar] [CrossRef]
- Zakharenko, A.M.; Razgonova, M.P.; Pikula, K.S.; Golokhvast, K.S. Simultaneous determination of 78 compounds of Rhodiola rosea extract using supercritical CO2-extraction and HPLC-ESI-MS/MS spectrometry. HINDAWY. Biochem. Res. Int. 2021, 2021, 9957490. [Google Scholar] [CrossRef]
- Bonzanini, F.; Bruni, R.; Palla., G.; Serlataite., N.; Caligiani, A. Identification and Distribution of Lignans in Punica granatum L. Fruit Endocarp, Pulp, Seeds, Wood Knots and Commercial Juices by GC–MS. Food Chem. 2009, 117, 745–749. [Google Scholar] [CrossRef]
- Eklund, P.C.; Backman, M.J.; Kronberg, L.A.; Smeds, A.I.; Sjoholm, R.E. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 2008, 43, 97–107. [Google Scholar] [CrossRef]
- Suarez Montenegro, Z.J.; Alvarez-Rivera, G.; Mendiola, J.A.; Ibanez, E.; Cifuentes, A. Extraction and Mass Spectrometric Characterization of Terpenes Recovered from Olive Leaves Using a New Adsorbent-Assisted Supercritical CO2 Process. Foods 2021, 10, 1301. [Google Scholar] [CrossRef]
- Bakir, D.; Akdeniz, M.; Ertas, A.; Yilmaz, M.A.; Yener, I.; First, M.; Kolak, U. A GC-MS method validation for quantitative investigation of some chemical markers in Salvia hypargeia Fisch. & C.A. Mey. of Turkey: Enzyme inhibitory potential of ferruginol. J. Food Biochem. 2020, 44, e13350. [Google Scholar] [CrossRef]
- Li, W.-H.; Chang, S.-T.; Chang, S.-C.; Chang, H.-T. Isolation of antibacterial diterpenoids from Cryptomeria japonica bark. Nat. Prod. Res. 2008, 22, 1085–1093. [Google Scholar] [CrossRef]
- Salih, E.Y.A.; Julkunen-Tiitto, R.; Lampi, A.-M.; Kanninen, M.; Luukkanen, A.; Sipi, M.; Lehtonen, M.; Vuorela, H.; Fyhrquist, P. Terminalia laxiflora and Terminalia brownii contain a broad spectrum of antimycobacterial compounds including ellagitannins, ellagic acid derivatives, triterpenes, fatty acids and fatty alcohols. J. Ethnopharmacol. 2018, 227, 82–96. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, S.; Xuan, Z.; Ge, D.; Chen, X.; Zhang, J.; Wang, Q.; Wu, Y.; Liu, B. The Phenolic Fraction of Mentha haplocalyx and Its Constituent Linarin Ameliorate Inflammatory Response through Inactivation of NF-κB and MAPKs in Lipopolysaccharide-Induced RAW264.7 Cells. Molecules 2017, 22, 811. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, T.C.W.; Woo, K.-S.; Fung, K.-P. Chemistry and Biological Activities of Caffeic Acid Derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueñas, M.; Carvalho, A.M.; Ferreira, I.C.; Santos-Buelga, C. Characterization of phenolic compounds in flowers of wild medicinal plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Primo da Silva, L.; Pereira, E.; Pires, T.C.S.P.; Alves, M.J.; Pereira, O.R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott fruits: A detailed study of its nutritional, chemical and bioactive properties. Food Res. Int. 2019, 119, 34–43. [Google Scholar] [CrossRef] [PubMed]
- De Rosso, M.; Panighel, A.; Vedota, A.D.; Gardiman, M.; Flamini, R. Characterization of Non-Anthocyanic Flavonoids in Some Hybrid Red Grape Extracts Potentially Interesting for Industrial Uses. Molecules 2015, 20, 18095–18106. [Google Scholar] [CrossRef] [PubMed]
- Kafle, B.; Baak, J.; Brede, C. Quantification by LC–MS/MS of astragaloside IV and isoflavones in Astragali radix can be more accurate by using standard addition. Phytochem. Anal. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Liu, R.; Ma, R.; Yu, C.; Bi, C.W.; Yin, Y.; Xu, H.; Shang, H.; Bi, K.; Li, Q. Quantitation of eleven active compounds of Aidi injection in rat plasma and its application to comparative pharmacokinetic study. J. Chromatogr. B. 2016, 1026, 105–113. [Google Scholar] [CrossRef]
- Garg, M.; Chawla, M.; Chunduri, V.; Kumar, R.; Sharma, S.; Sharma, N.K.; Kaur, N.; Kumar, A.; Mundey, J.K.; Saini, M.K.; et al. Transfer of grain colors to elite wheat cultivars and their characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Chang, Q.; Wong, Y.-S. Identification of Flavonoids in Hakmeitau Beans (Vigna sinensis) by High-Performance Liquid Chromatography–Electrospray Mass Spectrometry (LC-ESI/MS). Agric. Food Chem. 2004, 52, 6694–6699. [Google Scholar] [CrossRef]
- Lago-Vanzela, E.S.; Da-Silva, R.; Gomes, E.; Garcia-Romero, E.; Hermosin-Gutierres, E. Phenolic Composition of the Edible Parts (Flesh and Skin) of Bordô Grape (dersswq) Using HPLC–DAD–ESI-MS/MS. Agric. Food Chem. 2011, 59, 13136–13146. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; He, Y.; Shi, M.; Han, X.; Li, W.; Zhang, X.; Wen, X. Metabolic Profiling of Pitaya (Hylocereus polyrhizus) during Fruit Development and Maturation. Molecules 2019, 24, 1114. [Google Scholar] [CrossRef]
- Diretto, G.; Jin, H.; Capell, T.; Zhu, C.; Gomez-Gomez, L. Differential accumulation of pelargonidin glycosides in petals at three different developmental stages of the orange-flowered gentian (Gentiana lutea L. var. aurantiaca). PLoS ONE 2019, 14, e0212062. [Google Scholar] [CrossRef]
- Kajdzanoska, M.; Gjamovski, V.; Stefova, M. HPLC-DAD-ESI-MSn identification of phenolic compounds in cultivated strawberries from Macedonia. Maced. J. Chem. Chem. Eng. 2010, 29, 181–194. [Google Scholar] [CrossRef]
- Costa de Camargo, A.; Regitano-d’Arce, M.A.B.; Telles Biasoto, A.C.; Shahidi, F. Low Molecular Weight Phenolics of Grape Juice and Winemaking Byproducts: Antioxidant Activities and Inhibition of Oxidation of Human Low-Density Lipoprotein Cholesterol and DNA Strand Breakage. J. Agricult. Food Chem. 2014, 62, 12159–12171. [Google Scholar] [CrossRef]
- Sun, L.; Tao, S.; Zhang, S. Characterization and Quantification of Polyphenols and Triterpenoids in Thinned Young Fruits of Ten Pear Varieties by UPLC-Q TRAP-MS/MS. Molecules 2019, 24, 159. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Hasan, R.A.; Cheng, H.; El-Shazly, A.M.; Wink, M. Senna singueana: Antioxidant, Hepatoprotective, Antiapoptotic Properties and Phytochemical Profiling of a Methanol Bark Extract. Molecules 2017, 22, 1502. [Google Scholar] [CrossRef]
- Ölschläger, C.; Regos, I.; Zeller, F.J.; Treutter, D. Identification of galloylated propelargonidins and procyanidins in buckwheat grain and quantification of rutin and flavanols from homostylous hybrids originating from F. esculentum x F. homotropicum. Phytochemistry 2008, 69, 1389–1397. [Google Scholar] [CrossRef]
- Ayoub, M.; de Camargo, A.C.; Shahidi, F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef]
- Deng, Y.; He, M.; Feng, F.; Feng, X.; Zhang, Y.; Zhang, F. The distribution and changes of glycoalkaloids in potato tubers under different storage time based on MALDI-TOF mass spectrometry imaging. Talanta 2021, 221, 121453. [Google Scholar] [CrossRef]
- Shakya, R.; Navarre, D.A. LC-MS Analysis of Solanidane Glycoalkaloid Diversity among Tubers of Four Wild Potato Species and Three Cultivars (Solanum tuberosum). J. Agric. Food Chem. 2008, 56, 6949–6958. [Google Scholar] [CrossRef]
- Hossain, M.B.; Brunton, N.P.; Rai, D.K. Effect of Drying Methods on the Steroidal Alkaloid Content of Potato Peels, Shoots and Berries. Molecules 2016, 21, 403. [Google Scholar] [CrossRef] [PubMed]



| № | Class of Compound | Identified Compound | Formula | Mass | Molecular Ion [M − H]− | Molecular Ion [M + H]+ | 2 Fragmentation MS/MS | 3 Fragmentation MS/MS | 4 Fragmentation MS/MS | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Amino acid | L-Leucine [(S)-2-Amino-Methylpentanoic acid] | C6H13NO2 | 131.1729 | 132 | 114 | Potato leaves [34]; Vigna unguiculata [35]; Lonicera japonica [36]; Camellia kucha [37] | |||
| 2 | Benzaldehyde | Vanillin | C8H8O3 | 152.15 | 153 | 151 | 136 | Potato [38,39]; Triticum [40]; millet grains [41] | ||
| 3 | Trans-cinnamic acid | Ferulic acid | C10H10O4 | 194.184 | 195 | 177; 141 | 126 | Lonicera japonica [36]; Potato [38,39]; Zostera marina [42]; Andean blueberry [43]; Tomato [44]; Codonopsis Radix [45]; Bougainvillea [46] | ||
| 4 | Amino acid | L-Tryptophan [Tryptophan; (S)-Tryptophan] | C11H12N2O2 | 204.2252 | 205 | 188 | 144 | 118 | Vigna unguiculata [35]; Camellia kucha [37]; Perilla frutescens [47]; Passiflora incarnata [48]; Vigna inguiculata [49]; | |
| 5 | Stilbene | Resveratrol [trans-Resveratrol; 3,4′,5-Trihydroxystilbene; Stilbentriol] | C14H12O3 | 228.2433 | 229 | 210 | 141; 169 | 123 | Embelia [50]; Red wines [51]; vinery products [52]; A. cordifolia; F. glaucescens; F. herrerae [53]; Radix polygoni multiflori [54] | |
| 6 | Isoflavone | Daidzein [4′,7 -Dihydroxyisoflavone; Daidzeol] | C15H10O4 | 254.2375 | 255 | 227; 199; 137 | 181 | Hedyotis diffusa [55]; Isoflavones [56] | ||
| 7 | Ribonucleoside composite of adenine (purine) | Adenosine | C10H13N5O4 | 267.2413 | 268 | 136 | Lonicera japonica [36]; Huolisu Oral Liquid [57] | |||
| 8 | 7-hydroxyisoflavone | Formononetin [Biochanin B; Formononetol] | C16H12O4 | 268.2641 | 269 | 254; 159; 118 | 237; 181; 118 | 237; 181 | Astragali Radix [45]; Isoflavones [56]; Huolisu Oral Liquid [57]; | |
| 9 | Flavone | Apigenin [5,7-Dixydroxy-2-(40Hydroxyphenyl)-4H-Chromen-4-One] | C15H10O5 | 270.2369 | 271 | 153; 215 | 111 | Lonicera japonica [36]; millet grains [41]; Andean blueberry [43]; Hedyotis diffusa [55]; Mexican lupine species [58]; Wissadula periplocifolia [59] | ||
| 10 | Anthocyanin | Pelargonidin [Pelargonidol chloride] | C15H11O5+ | 271.2493 | 271 | 215; 197; 153 | 197; 169; 141 | 169 | acerola [60] | |
| 11 | Flavan-3-ol | Epiafzelechin [(epi)Afzelechin] | C15H14O5 | 274.2687 | 275 | 247; 193; 147 | 193; 175 | A. cordifolia; F. glaucescens; F. herrerae [53]; Cassia granidis [61]; Cassia abbreviata [62] | ||
| 12 | Omega-3 fatty acid | Stearidonic acid [6,9,12,15-Octadecatetraenoic acid; Moroctic acid] | C18H28O2 | 276.4137 | 277 | 217 | 190 | G. linguiforme [53]; Salviae Miltiorrhizae [63]; Rhus coriaria [64] | ||
| 13 | Sceletium alkaloid | 4′-O-desmethyl mesembranol | C16H23NO3 | 277.3587 | 276 | 234 | 218 | 218 | A. cordifolia [53] | |
| 14 | Omega-3 fatty acid | Linolenic acid (Alpha-Linolenic acid; Linolenate) | C18H30O2 | 278.4296 | Salviae [63]; rice [65]; Pinus sylvestris [66] | |||||
| 15 | Octadec-9-enoic acid | Oleic acid (Cis-9-Octadecenoic acid; Cis-Oleic acid) | C18H34O2 | 282.4614 | 283 | 209; 153 | Zostera marina [42]; Sanguisorba officinalis [67]; Pinus sylvestris [66] | |||
| 16 | Flavone | Acacetin [Linarigenin; Buddleoflavonol] | C16H12O5 | 284.2635 | 285 | 270; 224 | 241 | Mexican lupine species [58]; Wissadula periplocifolia [59]; Mentha [68,69]; Dracocephalum palmatum [70] | ||
| 17 | Flavone | 6,7-Dihydroxy-4′-methoxyisoflavone | C16H12O5 | 284.2635 | 285 | 270; 229; 145 | 242; 152 | Mentha [68] | ||
| 18 | Flavonol | Kaempferol [3,5,7-Trihydroxy-2-(4-hydro- xyphenyl)-4H-chromen-4-one] | C15H10O6 | 286.2363 | 285 | 257; 184; 117 | 117 | Potato leaves [34]; Lonicera japonica [36]; Potato [38]; Andean blueberry [43]; Rhus coriaria [64]; Rapeseed petals [71] | ||
| 19 | Flavan-3-ol | Catechin | C15H14O6 | 290.2681 | 291 | 243; 189 | 215; 197 | Potato [39]; Triticum [40]; millet grains [41]; Eucalyptus [72]; Vaccinium macrocarpon [73] | ||
| 20 | Flavan-3-ol | (epi)catechin | C15H14O6 | 290.2681 | 291 | 273; 117 | 255; 145 | millet grains [41]; C. edulis [53]; Radix polygoni multiflori [54]; Camellia kucha [37] | ||
| 21 | Flavone | Chrysoeriol [Chryseriol] | C16H12O6 | 300.2629 | 301 | 299; 253; 152 | 226 | Dracocephalum palmatum [70]; Rhus coriaria [64]; Rice [65]; Mentha [68]; Mexican lupine species [58] | ||
| 22 | Hydroxybenzoic acid | Ellagic acid [Benzoaric acid; Elagostasine; Lagistase; Eleagic acid] | C14H6O8 | 302.1926 | 303 | 275; 202 | 157 | 139 | Rhus coriaria [64]; strawberry [74]; Rubus occidentalis [75]; vinery products [52]; Chamaecrista nictitans [76]; Punica granatum [77] | |
| 23 | Flavonol | Quercetin | C15H10O7 | 302.2357 | 303 | 244; 202; 184 | 175; 156 | 129 | Potato leaves [34]; Triticum [40]; Tomato [44]; millet grains [41]; Red wines [51]; vinery products [52]; Rhus coriaria [64]; Eucalyptus [72]; Vaccinium macrocarpon [73] | |
| 24 | Flavanone | Hesperitin [Hesperetin] | C16H14O6 | 302.2788 | 303 | 202; 257; 185 | 156 | Andean blueberry [43]; [78]; Red wines [51]; Mentha [79] | ||
| 25 | Diterpenoid | Tanshinone IIB [(S)-6-(Hydroxymethyl)-1,6-Dimethyl-6,7,8,9-Tetrahydrophenanthro [1,2-B]Furan-10,11-Dione] | C19H18O4 | 310.3438 | 311 | 292; 189; 135 | 217; 135 | Salviae miltiorrhiza [63] | ||
| 26 | Flavone | 5,7-Dimethoxyluteolin | C17H14O6 | 314.2895 | 313 | 212; 185; 113 | 113 | Syzygium aromaticum [80] | ||
| 27 | Omega-hydroxy-long-chain fatty acid | 19-Hydroxynonadecanoic acid | C19H38O3 | 314.5032 | 315 | 287; 241; 187 | 241; 187 | 169; 124 | A. cordifolia [53] | |
| 28 | Flavonol | Rhamnetin I [beta-Rhamnocitrin; Quercetin 7-Methyl ether] | C16H12O7 | 316.2623 | 317 | 299; 243; 189;165; 123 | 147; 123 | Rhus coriaria L. (Sumac) [64]; Mangifera indica [81] | ||
| 29 | Flavonol | Isorhamnetin [Isorhamnetol; Quercetin 3′-Methyl ether; 3-Methylquercetin] | C16H12O7 | 316.2623 | 317 | 288; 243; 189 | 260; 242; 187 | Andean blueberry [43]; Eucalyptus [72]; Astragali Radix [45]; Embelia [50]; Rapeseed petals [71]; Syzygium aromaticum [80] | ||
| 30 | Flavonol | Myricetin | C15H10O8 | 318.2351 | 319 | 271; 217 | 243; 189; 171 | 171 | millet grains [41]; Red wines [51]; Andean blueberry [43]; Sanguisorba officinalis [67]; F. glaucescens [53]; Clidemia rubra [82] | |
| 31 | Hydroxycoumarin | Umbelliferone hexoside | C15H16O8 | 324.2827 | 325 | 306; 289;225; 163 | 145 | G. linguiforme [53] | ||
| 32 | Long-Chain Polyunsaturated Fatty Acid | Docosahexaenoic acid [Doconexent; Cervonic acid] | C22H32O2 | 328.4883 | 329 | 327; 281; 181; 115 | 199 | Marine extracts [83] | ||
| 33 | Trihydroxyflavone | Jaceosidin [5,7,4′-trihydroxy-6′,5′-dimetoxyflavone] | C17H14O7 | 330.2889 | 331 | 329; 285; 231; 191; 163 | 328; 286; 216 | Mentha [68,84] | ||
| 34 | Trihydroxyflavone | 5,7-Dimethoxy-3,3′,4′-trihydroxyflavone | C17H14O7 | 330.2889 | 331 | 303; 185 | 157 | Oxalis corniculata [85] | ||
| 35 | Flavonol | Myricetin 5-Methyl ether [5-O-Methylmyricetin] | C16H12O8 | 332.2617 | 333 | 287; 241; 205; 177 | 177; 149 | 149; 123 | Vitis amurensis [86]; Rhodiola rosea [87] | |
| 36 | Alpha, omega-dicarboxylic acid | Eicosatetraenedioic acid | C20H30O4 | 334.4498 | 335 | 307; 289; 233 | 277; 246; 207 | G. linguiforme [53] | ||
| 37 | Flavone | Syringetin | C17H14O8 | 346.2883 | 347 | 317; 290; 219; 169 | 289; 272; 219 | 261; 173 | C. edulis [53] | |
| 38 | Lignan | Matairesinol [(−)-Matairesinol; Artigenin Congener] | C20H22O6 | 358.3851 | 359 | 325; 289; 258; 198 | 143 | 127 | Punica granatum [88]; Lignans [89] | |
| 39 | Flavone | 5,6-Dihydroxy-7,8,3′,4′- tetramethoxyflavone | C19H18O8 | 374.3414 | 375 | 346; 219; 173 | 319; 273; 219; 173 | 273; 219; 173 | Mentha [68] | |
| 40 | Hydroxycinnamic acid | Caffeic acid derivative | C16H18O9Na | 377.2985 | 377 | 341; 215 | 179 | Bougainvillea [46]; Embelia [50] | ||
| 41 | Sterol | Campesterol [Dihydrobrassicasterol] | C28H48O | 400.6801 | 401 | 381; 304; 225; 171 | 363; 345; 279; 225; 169 | 345; 261; 202 | A. cordifolia; C. edulis [53] | |
| 42 | Sterol | Stigmasterol [Stigmasterin; Beta-Stigmasterol] | C29H48O | 412.6908 | 413 | 301; 279; 189 | 171 | Hedyotis diffusa [55]; A. cordifolia; F. pottsii [53]; Olive leaves [90]; Salvia [91] | ||
| 43 | Sterol | Beta-Sitostenone [Stigmast-4-En-3-One; Sitostenone] | C29H48O | 412.6908 | 413 | 395; 345; 301; 171 | 189; 171 | F. herrerae [53]; Cryptomeria japonica bark [92]; Terminalia laxiflora [93] | ||
| 44 | Hydroxybenzoic acid | Salvianolic acid D | C20H18O10 | 418.3509 | 419 | 373; 293; 212; 127 | 329; 271; 192; 127 | Mentha [69,94]; Salvia multiorrizae [95] | ||
| 45 | Iridoid monoterpenoid | Dihydroisovaltrate | C22H32O8 | 424.4847 | 425 | 365; 327; 281; 207 | 309; 253 | 235 | Rhus coriaria [64] | |
| 46 | Flavone | Apigenin-7-O-glucoside [Apigetrin; Cosmosiin] | C21H20O10 | 432.3775 | 433 | 271 | 153; 214 | Tomato [44]; Grataegi fructus [45]; Mexican lupine species [58]; Dracocephalum palmatum [70]; Mentha [84]; Malva sylvestris [96] | ||
| 47 | Hydroxybenzoic acid | Ellagic acid pentoside [Ellagic acid 4-O-xylopyranoside] | C19H14O12 | 434.3073 | 433 | 257 | 227; 157 | 199; 127 | Strawberry [74]; Chamaecrista nictitans [76]; Punica granatum [77]; Rubus ulmifolius [97] | |
| 48 | Flavonol | Dihydrokaempferol-3-O-rhamnoside | C21H22O10 | 434.3934 | 433 | 259 | 258; 229 | 199 | Vitis vinifera [98] | |
| 49 | Dihydroflavonol | Aromadendrin 7-O-rhamnoside | C21H22O10 | 434.3934 | 435 | 261; 243 | 243; 165 | 215; 161 | Eucalyptus [72] | |
| 59 | Flavone | Calycosin-7-O-beta-D-glucoside | C22H22O10 | 446.4041 | 447 | 285 | 270; 225; 145 | 242; 152 | Astragali radix [99]; [100]; Huolisu Oral Liquid [57]; | |
| 51 | Flavone | AcacetinO-glucoside | C22H22O10 | 446.4041 | 447 | 285 | 269; 227; 145 | 241 | Mexican lupine species [58] | |
| 52 | Flavonol | Kaempferol-3-O-hexoside | C21H20O11 | 448.3769 | 449 | 329; 203 | 303; 257; 203; 185; 157 | Andean blueberry [43]; vinery products [52]; F. glaucescens [53]; Rhus coriaria [64]; Punica granatum [77]; Cytisus multiflorus; Malva sylvestris [96] | ||
| 53 | Anthocyanin | Cyanidin-3-O-glucoside [Cyanidin 3-O-beta-D-Glucoside; Kuromarin] | C21H21O11+ | 449.3848 | 449 | 287 | 213; 175 | 213; 185; 141 | Triticum [40,101]; acerola [60]; Rice [65]; Clidemia rubra [82]; Rapeseed petals [71]; Vigna sinensis [102]; Vitis labrusca [103] | |
| 54 | Anabolic steroid | Vebonol | C30H44O3 | 452.6686 | 453 | 444; 389; 340; 276 | 435; 395; 336; 259 | 417; 331; 268 | Rhus coriaria [64]; Hylocereus polyrhizus [104] | |
| 55 | Anthocyanin | Pelargonidin 3-O-(6-O-malonyl-beta-D-glucoside) | C24H23O13 | 519.4388 | 519 | 271 | 215; 153 | 197 | Gentiana lutea [105]; Wheat [101]; Strawberry [106] | |
| 56 | Indole sesquiterpene alkaloid | Sespendole | C33H45NO4 | 519.7147 | 520 | 184; 502 | 166 | Rhus coriaria [64]; Hylocereus polyrhizus [104] | ||
| 57 | Flavonol | Kaempferol diacetyl hexoside | C25H24O13 | 532.4503 | 533 | 285 | 270; 229; 145 | 242; 224; 152 | A. cordifolia [53] | |
| 58 | Flavone | AcacetinO-glucoside malonylated | C25H24O13 | 532.4503 | 533 | 285 | 269; 228; 145 | 196; 152 | Mexican lupine species [58] | |
| 59 | Condensed tannin | Procyanidin A-type dimer | C30H24O12 | 576.501 | 577 | 547; 493; 425; 245; 181 | 217 | 189; 161 | Vaccinium macrocarpon [73]; grape juice [107]; pear [108] | |
| 60 | Condensed tannin | Proanthocyanidin B1 [Procyanidin B1; Procyanidin Dimer B1; (−)-epicatechin-(4beta->8)-(+)-catechin; Epicatechin-(4beta->8)-ent-epicatechin] | C30H26O12 | 578.5202 | 579 | 409; 343; 291; 247; 205 | 287; 259; 203; 163 | 245 | Camellia kucha [37]; millet grains [41]; Vigna inguiculata [49]; vinery products [52]; Andean blueberry [43]; Vaccinium macrocarpon [73]; strawberry [74]; grape juice [107]; pear [108]; Senna singueana [109] | |
| 61 | Condensed tannin | Procyanidin B2 [Epicatechin-(4beta->8)-epicatechin] | C30H26O12 | 578.5202 | 579 | 427; 291; 247; 211 | 408; 327; 227; 139 | 379; 287; 257; 163 | millet grains [41]; F. esculentum [110]; Red wines [51]; blackberry [111] | |
| 62 | Steroidal alkaloid | Alpha-chaconine | C45H73NO14 | 852.0594 | 852 | 706 | 560 | 398 | Potato [39,112,113,114] | |
| 63 | Steroidal alkaloid | Solanidadiene solatriose | C45H73NO15 | 868.9588 | 868 | 706; 661; 560; 477 | 560; 398 | 382; 327 | Potato [113] |
| № | Class of Compound | Identified Compound | Formula | k-569 (China) | k-5586 (Sweden) | k-5367 (China) | k-5373 (China) | k-11538 (Russia) | k-11559 (Russia) | Primorskaya-86 (Russia) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Isoflavone | Daidzein [4′,7 -Dihydroxyisoflavone; Daidzeol] | C15H10O4 | |||||||
| 2 | 7-hydroxyisoflavone | Formononetin [Biochanin B; Formononetol] | C16H12O4 | |||||||
| 3 | Flavone | Apigenin | C15H10O5 | |||||||
| 4 | 7-hydroxyisoflavone | Formononetin [Biochanin B; Formononetol] | C16H12O4 | |||||||
| 5 | Flavone | Apigenin | C15H10O5 | |||||||
| 6 | Flavone | Acacetin [Linarigenin; Buddleoflavonol] | C16H12O5 | |||||||
| 7 | Flavone | 6,7-Dihydroxy-4′-methoxyisoflavone | C16H12O5 | |||||||
| 8 | Flavone | Chrysoeriol [Chryseriol] | C16H12O6 | |||||||
| 9 | Flavone | 5,7-Dimethoxyluteolin | C17H14O6 | |||||||
| 10 | Trihydroxyflavone | Jaceosidin | C17H14O7 | |||||||
| 11 | Trihydroxyflavone | 5,7-Dimethoxy-3,3′,4′-trihydroxyflavone | C17H14O7 | |||||||
| 12 | Flavone | Syringetin | C17H14O8 | |||||||
| 13 | Flavone | 5,6-Dihydroxy-7,8,3′,4′-tetramethoxyflavone | C19H18O8 | |||||||
| 14 | Flavone | Apigenin-7-O-glucoside | C21H20O10 | |||||||
| 15 | Flavone | Calycosin-7-O-beta-D-glucoside | C22H22O10 | |||||||
| 16 | Flavone | Acacetin O-glucoside | C22H22O10 | |||||||
| 17 | Flavone | Acacetin O-glucoside malonylated | C25H24O13 | |||||||
| 18 | Flavonol | Kaempferol | C15H10O6 | |||||||
| 19 | Flavonol | Quercetin | C15H10O7 | |||||||
| 20 | Flavonol | Rhamnetin I | C16H12O7 | |||||||
| 21 | Flavonol | Isorhamnetin | C16H12O7 | |||||||
| 22 | Flavonol | Myricetin | C15H10O8 | |||||||
| 23 | Flavonol | Myricetin 5-Methyl ether [5-O-Methylmyricetin] | C16H12O8 | |||||||
| 24 | Flavonol | Dihydrokaempferol-3-O-rhamnoside | C21H22O10 | |||||||
| 25 | Dihydroflavonol | Aromadendrin 7-O-rhamnoside | C21H22O10 | |||||||
| 26 | Flavonol | Kaempferol-3-O-hexoside | C21H20O11 | |||||||
| 27 | Flavonol | Kaempferol diacetyl hexoside | C25H24O13 | |||||||
| 28 | Flavan-3-ol | Epiafzelechin [(epi)Afzelechin] | C15H14O5 | |||||||
| 29 | Flavan-3-ol | Catechin | C15H14O6 | |||||||
| 30 | Flavan-3-ol | (epi)catechin | C15H14O6 | |||||||
| 31 | Flavanone | Hesperitin [Hesperetin] | C16H14O6 | |||||||
| 32 | Anthocyanin | Pelargonidin [Pelargonidol chloride] | C15H11O5+ | |||||||
| 33 | Anthocyanin | Cyanidin-3-O-glucoside | C21H21O11+ | |||||||
| 34 | Anthocyanin | Pelargonidin 3-O-(6-O-malonyl-beta-D-glucoside) | C24H23O13 | |||||||
| 35 | Condensed tannin | Procyanidin A-type dimer | C30H24O12 | |||||||
| 36 | Condensed tannin | Proanthocyanidin B1 | C30H26O12 | |||||||
| 37 | Condensed tannin | Proanthocyanidin B2 | C30H26O12 | |||||||
| 38 | Phenolic acid | Ferulic acid | C10H10O4 | |||||||
| 39 | Phenolic acid | Ellagic acid | C14H6O8 | |||||||
| 40 | Phenolic acid | Caffeic acid derivative | C16H18O9Na | |||||||
| 41 | Phenolic acid | Salvianolic acid D | C20H18O10 | |||||||
| 42 | Phenolic acid | Ellagic acid pentoside | C19H14O12 | |||||||
| 43 | Stilbene | Resveratrol | C14H12O3 | |||||||
| 44 | Hydroxycoumarin | Umbelliferone hexoside | C15H16O8 | |||||||
| 45 | Lignan | Matairesinol | C20H22O6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razgonova, M.P.; Zinchenko, Y.N.; Kozak, D.K.; Kuznetsova, V.A.; Zakharenko, A.M.; Ercisli, S.; Golokhvast, K.S. Autofluorescence-Based Investigation of Spatial Distribution of Phenolic Compounds in Soybeans Using Confocal Laser Microscopy and a High-Resolution Mass Spectrometric Approach. Molecules 2022, 27, 8228. https://doi.org/10.3390/molecules27238228
Razgonova MP, Zinchenko YN, Kozak DK, Kuznetsova VA, Zakharenko AM, Ercisli S, Golokhvast KS. Autofluorescence-Based Investigation of Spatial Distribution of Phenolic Compounds in Soybeans Using Confocal Laser Microscopy and a High-Resolution Mass Spectrometric Approach. Molecules. 2022; 27(23):8228. https://doi.org/10.3390/molecules27238228
Chicago/Turabian StyleRazgonova, Mayya P., Yulia N. Zinchenko, Darya K. Kozak, Victoria A. Kuznetsova, Alexander M. Zakharenko, Sezai Ercisli, and Kirill S. Golokhvast. 2022. "Autofluorescence-Based Investigation of Spatial Distribution of Phenolic Compounds in Soybeans Using Confocal Laser Microscopy and a High-Resolution Mass Spectrometric Approach" Molecules 27, no. 23: 8228. https://doi.org/10.3390/molecules27238228
APA StyleRazgonova, M. P., Zinchenko, Y. N., Kozak, D. K., Kuznetsova, V. A., Zakharenko, A. M., Ercisli, S., & Golokhvast, K. S. (2022). Autofluorescence-Based Investigation of Spatial Distribution of Phenolic Compounds in Soybeans Using Confocal Laser Microscopy and a High-Resolution Mass Spectrometric Approach. Molecules, 27(23), 8228. https://doi.org/10.3390/molecules27238228











