Shikonin as a WT1 Inhibitor Promotes Promyeloid Leukemia Cell Differentiation
Abstract
1. Introduction
2. Results
2.1. WT1 Overexpression Promoted HL-60 Cell Resistance to H2O2
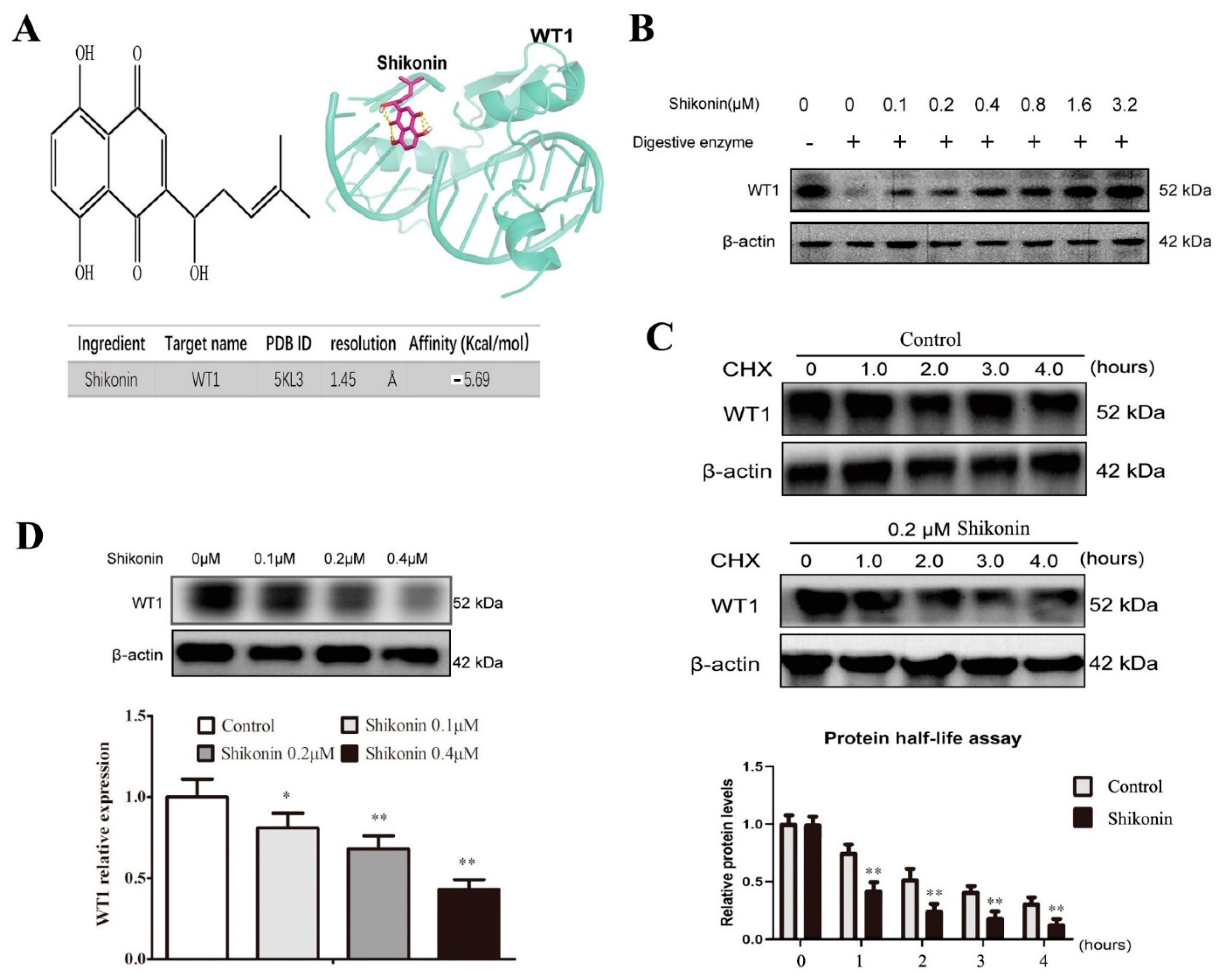
2.2. Shikonin Is Screened as a WT1 Inhibitor
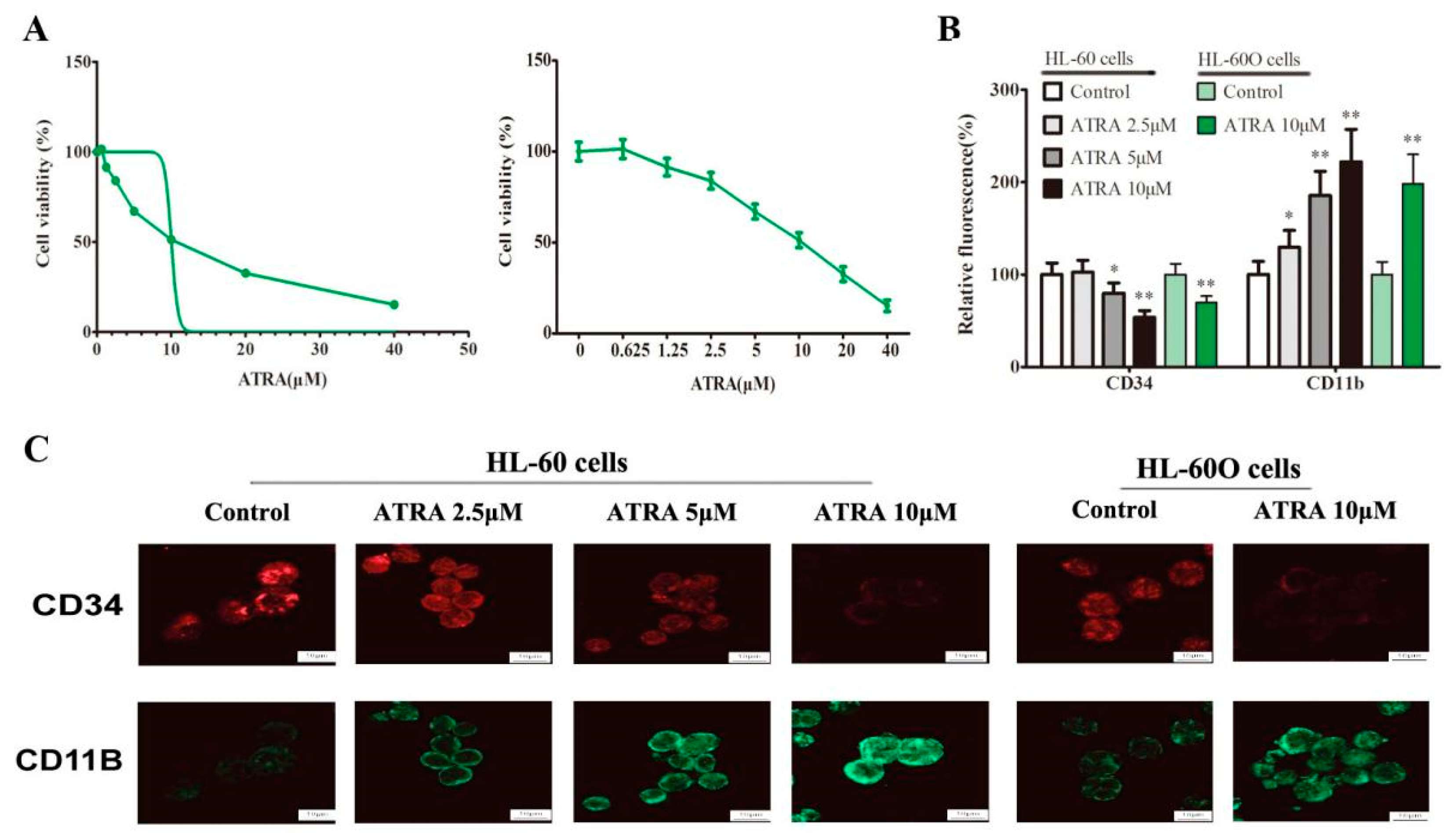
2.3. Shikonin Promotes HL-60 Cell Differentiation
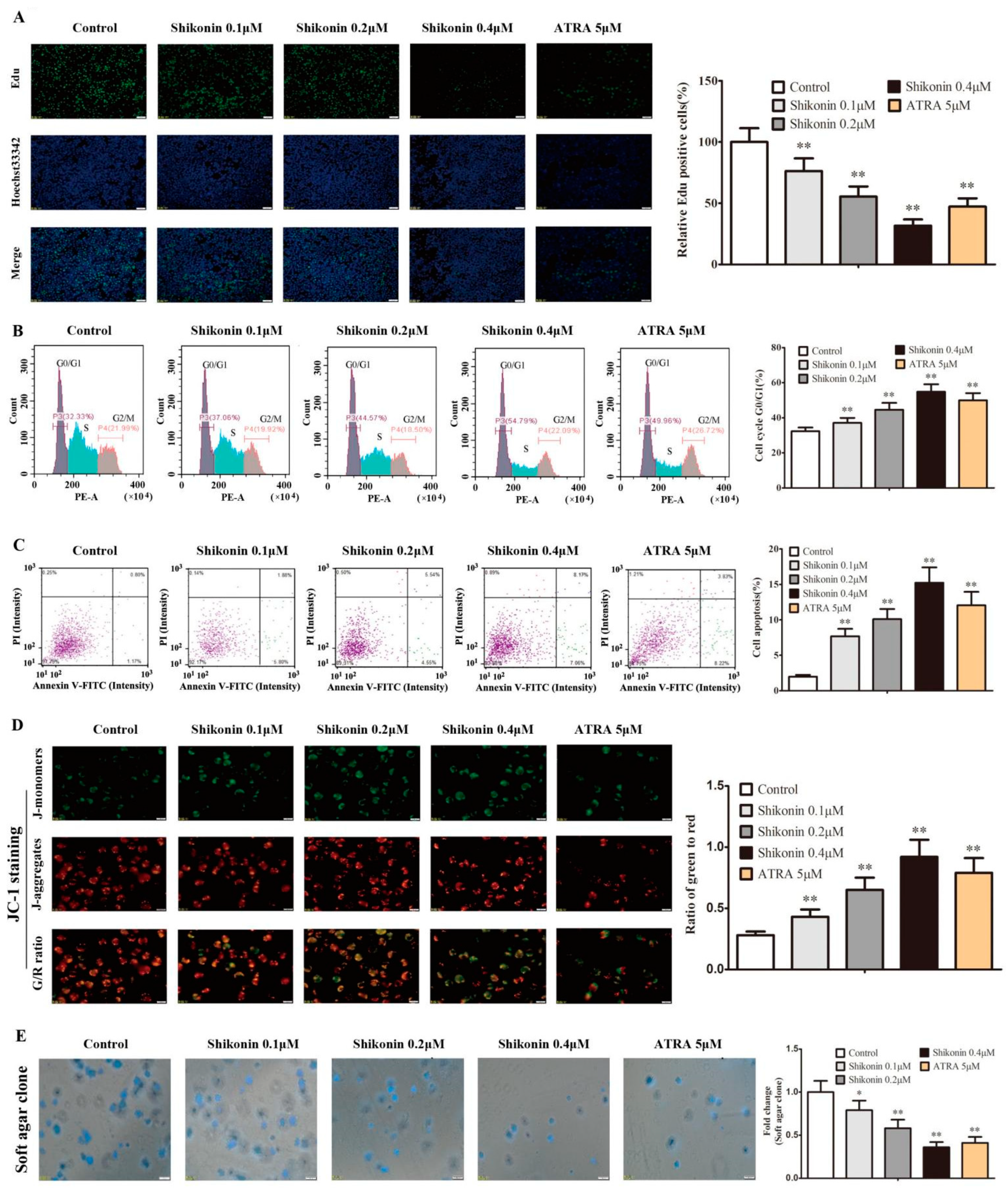
2.4. Shikonin-Differentiated HL-60 Cells Gradually Lose the Ability of Cell Proliferation and Self-Renewal
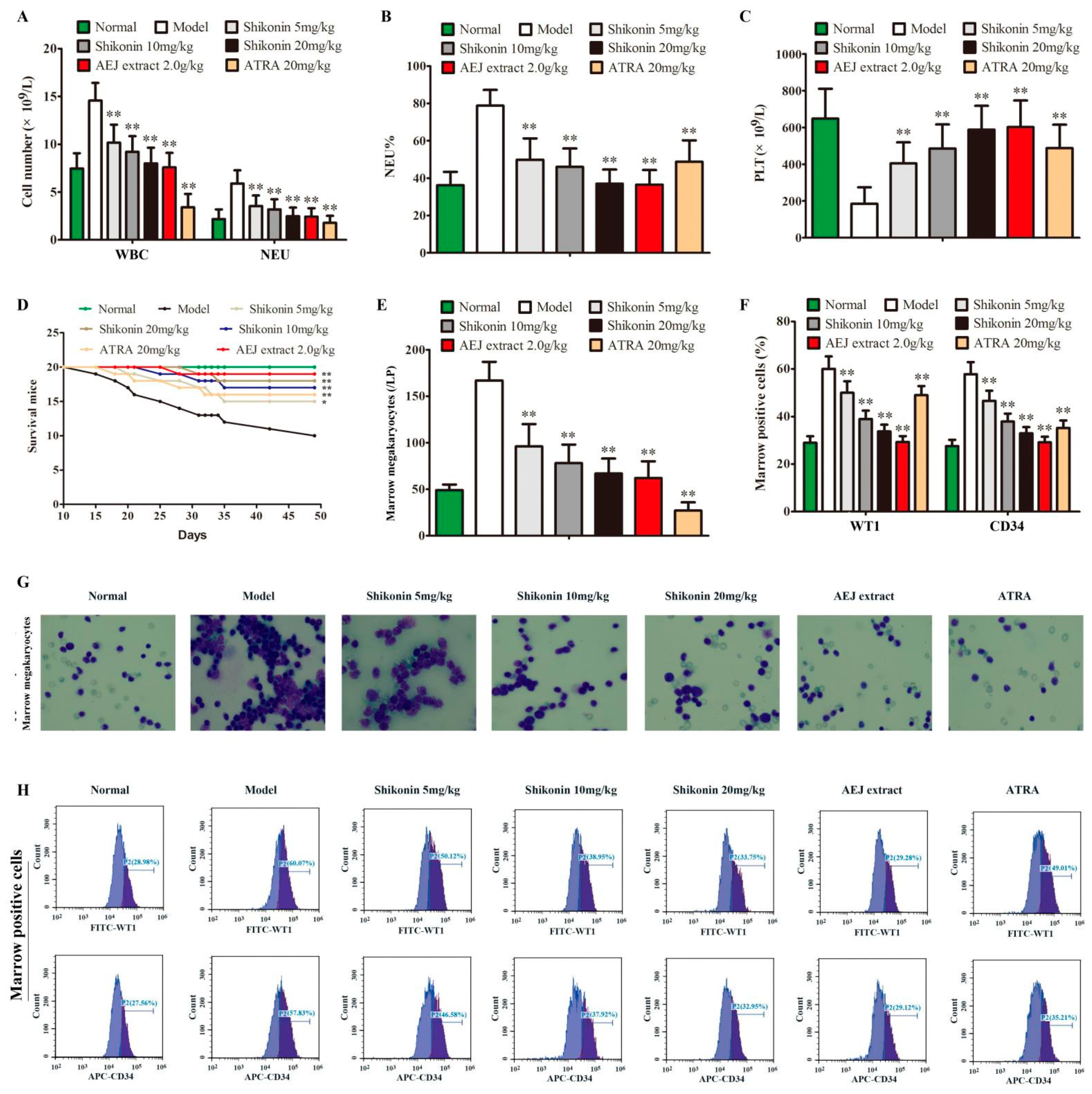
2.5. Shikonin Has Anti-Leukemia Activity In Vivo
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Lentivirus Transfection
4.4. Western Blot
4.5. DNA Injury
4.6. The Assays of Cell Viability, Morphology, and Differentiation Markers
4.7. The Interactions of CD34 Protein–WT1 Protein and Shikonin–WT1 Protein
4.8. Co-Immunoprecipitation
4.9. Drug Affinity Responsive Target Stability (DARTS)
4.10. Protein Half-Life Assay
4.11. Cell Proliferation and Mitochondrial Membrane Potential (MMP) Assays
4.12. Cell Cycle and Apoptosis Assays
4.13. Cell Self-Renewal Assay
4.14. Mouse Leukemia Model
4.15. Data Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Yang, X.; Wang, J.X. Precision therapy for acute myeloid leukemia. J. Hematol. Oncol. 2018, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.M.; Dobson, S.M.; Voisin, V.; McLeod, J.; Kennedy, J.A.; Mitchell, A.; Jin, L.; Eppert, K.; Bader, G.; Minden, M.D.; et al. CD200 expression marks leukemia stem cells in human AML. Blood Adv. 2020, 4, 5402–5413. [Google Scholar] [CrossRef]
- Morgan, G.; Ward, R.; Barton, M. The contribution of cytotoxic chemotherapy to 5-year survival in adult malignancies. Clin. Oncol. (R Coll. Radiol.) 2004, 16, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Chen, L.; Wang, M.; Xi, J.; Liu, X.; Xie, M.; Li, D.; Gulati, E.S.; Gong, S.; et al. Arsenic trioxide and all-trans retinoic acid (ATRA) treatment for acute promyelocytic leukemia in all risk groups: Study protocol for a randomized controlled trial. Trials 2018, 19, 476. [Google Scholar] [CrossRef] [PubMed]
- Buschner, G.; Feuerecker, B.; Spinner, S.; Seidl, C.; Essler, M. Differentiation of acute myeloid leukemia (AML) cells with ATRA reduces (18)F-FDG uptake and increases sensitivity towards ABT-737-induced apoptosis. Leuk. Lymphoma 2021, 62, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ueda, M.; Stetson, L.; Ignatz-Hoover, J.; Moreton, S.; Chakrabarti, A.; Xia, Z.; Karan, G.; de Lima, M.; Agrawal, M.K.; et al. A Novel Glycogen Synthase Kinase-3 Inhibitor Optimized for Acute Myeloid Leukemia Differentiation Activity. Mol. Cancer Ther. 2016, 15, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Stubbins, R.J.; Karsan, A. Differentiation therapy for myeloid malignancies: Beyond cytotoxicity. Blood Cancer J. 2021, 11, 193. [Google Scholar] [CrossRef]
- Dejjuy, D.; Dechsukhum, C.; Pattanapanyasat, K.; Noulsri, E.; Dissen, G.A.; Leeanansaksiri, W. Novel WT1 target genes: IL-2, IL-2RB, and IL-2RG discovered during WT1 silencing using lentiviral-based RNAi in myeloid leukemia Cells. Biomed. Res. Int. 2020, 2020, 7851414. [Google Scholar] [CrossRef]
- Lambert, J.; Lambert, J.; Thomas, X.; Marceau-Renaut, A.; Micol, J.B.; Renneville, A.; Clappier, E.; Hayette, S.; Récher, C.; Raffoux, E.; et al. Early detection of WT1 measurable residual disease identifies high-risk patients, independent of transplantation in AML. Blood Adv. 2021, 5, 5258–5268. [Google Scholar] [CrossRef]
- Yoshitomi, H.; Lee, K.Y.; Yao, K.; Shin, S.H.; Zhang, T.; Wang, Q.; Paul, S.; Roh, E.; Ryu, J.; Chen, H.; et al. GSK3β-Mediated Expression of CUG-Translated WT1 Is Critical for Tumor Progression. Cancer Res. 2021, 81, 945–955. [Google Scholar] [CrossRef]
- Rein, L.A.; Chao, N.J. WT1 vaccination in acute myeloid leukemia: New methods of implementing adoptive immunotherapy. Expert. Opin. Investig. Drugs 2014, 23, 417–426. [Google Scholar] [CrossRef]
- Yuen, K.Y.; Lin, X.Y.; Zhou, Y.Z.; Luo, H.; Liu, Y.; Xu, L.H. Optimal time-points for detecting expression levels of BAALC, EVI1, and WT1 genes in patients with acute myeloid leukemia: A meta-analysis. Hematology 2021, 26, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Narita, M.; Yamahira, A.; Taniguchi, T.; Furukawa, T.; Yoshida, T.; Miyazawa, T.; Nashimoto, M.; Takahashi, M. Induction of apoptosis of leukemic cells by TRUE gene silencing using small guide RNAs targeting the WT1 mRNA. Leuk. Res. 2013, 37, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chai, X.; Gong, C.; Zou, L. WT1 inhibits AML cell proliferation in a p53-dependent manner. Cell Cycle 2021, 20, 1552–1560. [Google Scholar] [CrossRef]
- Rampal, R.; Alkalin, A.; Madzo, J.; Vasanthakumar, A.; Pronier, E.; Patel, J.; Li, Y.; Ahn, J.; Abdel-Wahab, O.; Shih, A.; et al. DNA hydroxymethylation profiling reveals that WT1 mutations result in loss of TET2 function in acute myeloid leukemia. Cell Rep. 2014, 9, 1841–1855. [Google Scholar] [CrossRef]
- Yamasaki, A.E.; Warshaw, J.N.; Kyalwazi, B.L.; Matsui, H.; Jepsen, K.; Panopoulos, A.D. An iPSC line derived from a human acute myeloid leukemia cell line (HL-60-iPSC) retains leukemic abnormalities and displays myeloid differentiation defects. Stem. Cell. Res. 2020, 49, 102096. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, H.W.; Yang, T.; Qin, Y.H.; Li, X.H. Alteration of the expression ratio between WT1 gene and its isomers during all-trans retinoic acid-induced differentiation of HL-60 cells. Chin. J. Pathol. 2010, 39, 183–186. [Google Scholar]
- Sekiya, M.; Adachi, M.; Hinoda, Y.; Imai, K.; Yachi, A. Downregulation of Wilms’ tumor gene (wt1) during myelomonocytic differentiation in HL60 cells. Blood 1994, 83, 1876–1882. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, J.H.; Meng, Q.Q.; Li, S.S.; Zhou, W.; Xiao, S. Advance in Anti-tumor Mechanisms of Shikonin, Alkannin and their Derivatives. Mini. Rev. Med. Chem. 2018, 18, 164–172. [Google Scholar] [CrossRef]
- Su, M.L.; He, Y.; Li, Q.S.; Zhu, B.H. Efficacy of Acetylshikonin in Preventing Obesity and Hepatic Steatosis in db/db Mice. Molecules 2016, 21, 976. [Google Scholar] [CrossRef]
- Wang, F.; Mayca Pozo, F.; Tian, D.; Geng, X.; Yao, X.; Zhang, Y.; Tang, J. Shikonin inhibits cancer through P21 upregulation and apoptosis induction. Front. Pharmacol. 2020, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hong, Z.; Peng, Z.; Zhao, Y.; Shao, R. Acetylshikonin from Zicao ameliorates renal dysfunction and fibrosis in diabetic mice by inhibiting TGF-β1/Smad pathway. Hum. Cell 2018, 31, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; He, L.; Wang, J.; Wang, Q.; Sun, C.; Li, Y.; Jia, K.; Wang, J.; Xu, T.; Ming, R.; et al. Anti-angiogenic effect of Shikonin in rheumatoid arthritis by downregulating PI3K/AKT and MAPKs signaling pathways. J. Ethnopharmacol. 2020, 260, 113039. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Müller, G.A.; Rathore, M.S.; Mishra, D.P.; Dihazi, H. Anti-Leukemic Activity of Shikonin: Role of ERP57 in Shikonin Induced Apoptosis in Acute Myeloid Leukemia. Cell Physiol. Biochem. 2016, 39, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, Z.; Wang, J.; Li, W.; Liu, G.; Zhang, B.; Tang, Y. Insights into the mechanism of Arnebia euchroma on leukemia via network pharmacology approach. BMC Complement. Med. Ther. 2020, 20, 322. [Google Scholar] [CrossRef]
- Wagner, N.; Ninkov, M.; Vukolic, A.; Cubukcuoglu, D.G.; Rassoulzadegan, M.; Michiels, J.F.; Wagner, K.D. Implications of the wilms’ tumor suppressor wt1 in cardiomyocyte differentiation. Int. J. Mol. Sci. 2021, 22, 4346. [Google Scholar] [CrossRef]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem. Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef]
- Guo, C.J.; He, J.L.; Song, X.M.T.; Tan, L.; Wang, M.; Jiang, P.D.; Li, Y.Z.; Cao, Z.X.; Peng, C. Pharmacological properties and derivatives of shikonin-A review in recent years. Pharmacol. Res. 2019, 149, 104463. [Google Scholar] [CrossRef]
- Morceau, F.; Chateauvieux, S.; Orsini, M.; Trécul, A.; Dicato, M.; Diederich, M. Natural compounds and pharmaceuticals reprogram leukemia cell differentiation pathways. Biotechnol. Adv. 2015, 33, 785–797. [Google Scholar] [CrossRef]
- Algar, E.M.; Khromykh, T.; Smith, S.I.; Blackburn, D.M.; Bryson, G.J.; Smith, P.J. A WT1 antisense oligonucleotide inhibits proliferation and induces apoptosis in myeloid leukaemia cell lines. Oncogene 1996, 12, 1005–1014. [Google Scholar]
- Deuel, T.F.; Guan, L.S.; Wang, Z.Y. Wilms’ tumor gene product WT1 arrests macrophage differentiation of HL-60 cells through its zinc-finger domain. Biochem. Biophys. Res. Commun. 1999, 254, 192–196. [Google Scholar]
- Cilloni, D.; Gottardi, E.; De Micheli, D.; Serra, A.; Volpe, G.; Messa, F.; Rege-Cambrin, G.; Guerrasio, A.; Divona, M.; Lo Coco, F.; et al. Quantitative assessment of WT1 expression by real time quantitative PCR may be a useful tool for monitoring minimal residual disease in acute leukemia patients. Leukemia 2002, 16, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Fackler, M.J.; Krause, D.S.; Smith, O.M.; Civin, C.I.; May, W.S. Full-length but not truncated CD34 inhibits hematopoietic cell differentiation of M1 cells. Blood 1995, 85, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Scharnhorst, V.; Dekker, P.; van der Eb, A.J.; Jochemsen, A.G. Physical interaction between Wilms tumor 1 and p73 proteins modulates their functions. J. Biol. Chem. 2000, 275, 10202–10211. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, Y.; Osada, T.; Woo, C.Y.; Clay, T.M.; Lyerly, H.K.; Morse, M.A. Immunotherapeutic targeting of Wilms’ tumor protein. Curr. Opin. Mol. Ther. 2007, 9, 62–69. [Google Scholar] [PubMed]
- Opydo-Chanek, M.; Cichoń, I.; Rak, A.; Kołaczkowska, E.; Mazur, L. The pan-Bcl-2 inhibitor obatoclax promotes differentiation and apoptosis of acute myeloid leukemia cells. Investig. New Drugs 2020, 38, 1664–1676. [Google Scholar] [CrossRef] [PubMed]
- Surka, C.; Jin, L.; Mbong, N.; Lu, C.C.; Jang, I.S.; Rychak, E.; Mendy, D.; Clayton, T.; Tindall, E.; Hsu, C.; et al. CC-90009, a novel cereblon E3 ligase modulator, targets acute myeloid leukemia blasts and leukemia stem cells. Blood 2021, 137, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Xu, H.Z.; Shan, H.Z.; Liu, M.; Lu, Y.; Fang, Z.X.; Jin, J.; Jing, B.; Xiao, X.H.; Gao, S.M.; et al. Targeting USP47 overcomes tyrosine kinase inhibitor resistance and eradicates leukemia stem/progenitor cells in chronic myelogenous leukemia. Nat. Commun. 2021, 12, 51. [Google Scholar] [CrossRef]
- Agarwal, A.; Bolosky, W.J.; Wilson, D.B.; Eide, C.A.; Olson, S.B.; Fan, G.; Druker, B.J. Differentiation of leukemic blasts is not completely blocked in acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2019, 116, 24593–24599. [Google Scholar] [CrossRef]
- Carter, J.L.; Hege, K.; Yang, J.; Kalpage, H.A.; Su, Y.; Edwards, H.; Hüttemann, M.; Taub, J.W.; Ge, Y. Targeting multiple signaling pathways: The new approach to acute myeloid leukemia therapy. Signal. Transduct. Target. Ther. 2020, 5, 288. [Google Scholar] [CrossRef]
- Xu, Y.C.; Lin, Y.S.; Zhang, L.; Lu, Y.; Sun, Y.L.; Fang, Z.G.; Li, Z.Y.; Fan, R.F. MicroRNAs of bone marrow mesenchymal stem cell-derived exosomes regulate acute myeloid leukemia cell proliferation and apoptosis. Chin. Med. J. 2020, 133, 2829–2839. [Google Scholar]
- Paris, J.; Morgan, M.; Campos, J.; Spencer, G.J.; Shmakova, A.; Ivanova, I.; Mapperley, C.; Lawson, H.; Wotherspoon, D.A.; Sepulveda, C.; et al. Targeting the RNA m 6 A reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell. Stem. Cell 2019, 25, 137–148.e6. [Google Scholar] [CrossRef]
- Trusler, O.; Huang, Z.; Goodwin, J.; Laslett, A.L. Cell surface markers for the identification and study of human naive pluripotent stem cells. Stem. Cell. Res. 2018, 26, 36. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.H.; Alicea, C.; Owens, J.W.; Eigsti, C.L.; Malech, H.L. Characterization of an early dendritic cell precursor derived from murine lineage-negative hematopoietic progenitor cells. Exp. Hematol. 2002, 30, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Boulos, J.C.; Rahama, M.; Hegazy, M.E.F.; Efferth, T. Shikonin derivatives for cancer prevention and therapy. Cancer Lett. 2019, 459, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Logsdon, N.J.; Shin, Y.J.; Palumbo, S.; Knox, A.; Irish, J.D.; Rounseville, S.P.; Rummel, S.R.; Mohamed, M.; Ahmad, K.; et al. Impaired Myofibroblast Dedifferentiation Contributes to Nonresolving Fibrosis in Aging. Am. J. Respir. Cell. Mol. Biol. 2020, 62, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M. Isothermal Titration Calorimetry. Methods Mol. Biol. 2021, 2263, 135–159. [Google Scholar]
- Jerabek-Willemsen, M.; Wienken, C.J.; Braun, D.; Baaske, P.; Duhr, S. Molecular interaction studies using microscale thermophoresis. Assay Drug. Dev. Technol. 2011, 9, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Bahoush, G.; Vafapour, M.; Kariminejad, R. New translocation in acute myeloid leukemia M4 eos. Leuk. Res. Rep. 2020, 14, 100209. [Google Scholar] [CrossRef] [PubMed]
- Chen Chen, T.; Yu, S.C.; Hsu, C.M.; Tsai, F.J.; Tsai, Y. A water-based topical Chinese traditional medicine (Zicao) for wound healing developed using 2-hydroxypropyl-β-cyclodextrin. Colloids Surf B Biointerfaces 2018, 165, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yao, X.; Zhang, Y.; Tang, J. Synthesis, biological function and evaluation of Shikonin in cancer therapy. Fitoterapia 2019, 134, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Peng, F.; Wang, Y.; Yang, L.; Wu, F.; Wang, X.; Ye, C.; Han, B.; He, G. Shikonin induces colorectal carcinoma cells apoptosis and autophagy by targeting galectin-1/JNK signaling axis. Int. J. Biol. Sci. 2020, 16, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.M.; Guo, Z.Z.; Zhu, Y.F.; Kong, M.Y.; Zhang, R.R.; Lu, L.L.; Wu, F.C.; Liu, Z.Q.; Wu, J.J. Houttuynia cordata Thunb. and its bioactive compound 2-undecanone significantly suppress benzo(a)pyrene-induced lung tumorigenesis by activating the Nrf2-HO-1/NQO-1 signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 242. [Google Scholar] [CrossRef] [PubMed]
- Athanasios, A.; Charalampos, V.; Vasileios, T.; Ashraf, G.M. Protein-protein interaction (PPI) network: Recent advances in drug discovery. Curr. Drug. Metab. 2017, 18, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Lomenick, B.; Hao, R.; Jonai, N.; Chin, R.M.; Aghajan, M.; Warburton, S.; Wang, J.; Wu, R.P.; Gomez, F.; Loo, J.A.; et al. Target identification using drug affinity responsive target stability (DARTS). Proc. Natl. Acad. Sci. USA 2009, 106, 21984–21989. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.Y.; Wang, C.Q.; He, H.; Yu, Z.Y.; Tong, Y.; Liu, G.; Yang, Y.Q.; Li, L.; Pang, L.; Qi, H.Y. Ethyl acetate extract of Caesalpinia sappan L. inhibited acute myeloid leukemia via ROS-mediated apoptosis and differentiation. Phytomedicine 2020, 68, 153142. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Sun, L.; Xia, H.; Tian, S.; Liu, M.; Hou, J.; Li, J.; Lin, H.; Du, G. Shikonin as a WT1 Inhibitor Promotes Promyeloid Leukemia Cell Differentiation. Molecules 2022, 27, 8264. https://doi.org/10.3390/molecules27238264
Guo Z, Sun L, Xia H, Tian S, Liu M, Hou J, Li J, Lin H, Du G. Shikonin as a WT1 Inhibitor Promotes Promyeloid Leukemia Cell Differentiation. Molecules. 2022; 27(23):8264. https://doi.org/10.3390/molecules27238264
Chicago/Turabian StyleGuo, Zhenzhen, Luyao Sun, Haojie Xia, Shibin Tian, Mengyue Liu, Jiejie Hou, Jiahuan Li, Haihong Lin, and Gangjun Du. 2022. "Shikonin as a WT1 Inhibitor Promotes Promyeloid Leukemia Cell Differentiation" Molecules 27, no. 23: 8264. https://doi.org/10.3390/molecules27238264
APA StyleGuo, Z., Sun, L., Xia, H., Tian, S., Liu, M., Hou, J., Li, J., Lin, H., & Du, G. (2022). Shikonin as a WT1 Inhibitor Promotes Promyeloid Leukemia Cell Differentiation. Molecules, 27(23), 8264. https://doi.org/10.3390/molecules27238264




