Uptake, Translocation, and Fate of Carcinogenic Aristolochic Acid in Typical Vegetables in Soil−Plant Systems
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
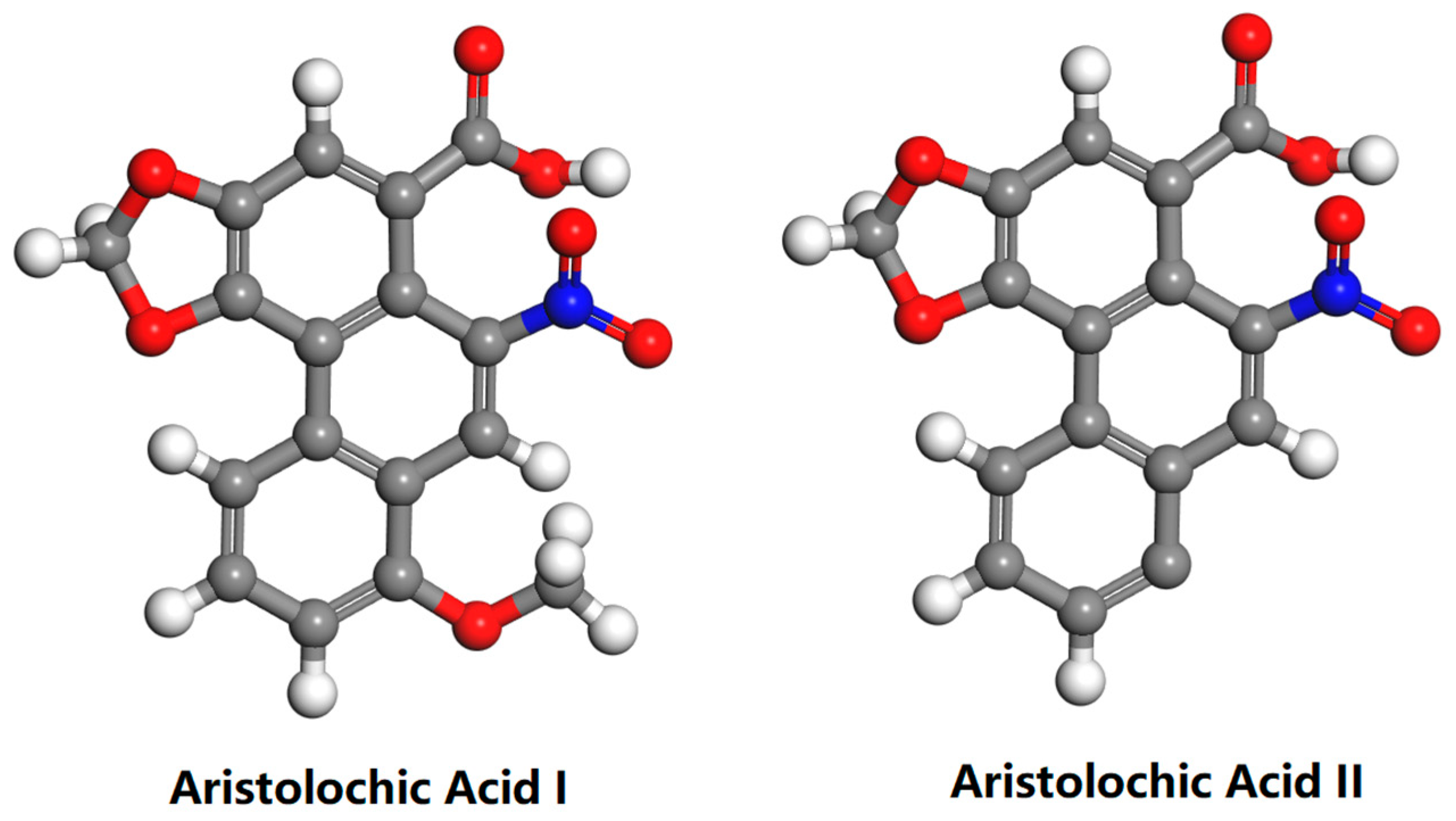
3.1. Chemicals
3.2. Preparation of Herbal Extracts
3.3. Cultivation of Lettuce, Celery, and Tomato in AA-Contaminated Soil
3.4. Sample Preparation
3.5. HPLC-MS-MS Analysis
3.6. Calibration and Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ang, L.P.; Yen, L.L.; Kalusalingam, A.; Khan, A.; Kotra, V.; Rahman Sarker, M.M.; Liew, K.B.; Long, C.M. Determination of Aristolochic Acid Using Isocratic RP-HPLC Method. Prog. Drug Discov. Biomed. Sci. 2021, 4, 1–14. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Zhao, X.-H.; Sun, Z.-H.; Li, G.-C.; Liu, G.-C.; Sun, L.-R.; Hou, J.-Q.; Zhou, W. Recognition of the toxicity of aristolochic acid. J. Clin. Pharm. Ther. 2019, 44, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liu, Y.; Wu, Y.; Dai, Z.; Ma, S. Rapid Analysis of Aristolochic Acid Analogues in Traditional Chinese Patent Medicine by LC-MS/MS. J. Anal. Methods Chem. 2020, 2020, 8823596. [Google Scholar] [CrossRef]
- Li, Y.-L.; Tian, M.; Yu, J.; Shang, M.-Y.; Cai, S.-Q. Studies on morphology and aristolochic acid analogue constituents of Asarum campaniflorum and a comparison with two official species of Asari Radix et Rhizoma. J. Nat. Med. 2010, 64, 442–451. [Google Scholar] [CrossRef]
- Xiong, H.; Fan, Y.; Mao, X.; Guo, L.; Yan, A.; Guo, X.; Wan, Y.; Wan, H. Thermosensitive and magnetic molecularly imprinted polymers for selective recognition and extraction of aristolochic acid I. Food Chem. 2022, 372, 131250. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Sun, J.; Zhou, G.; Jiang, X.; Wang, X. QuEChERS pretreatment combined with high-performance liquid chromatography-tandem mass spectrometry for determination of aristolochic acids I and II in Chinese herbal patent medicines. RSC Adv. 2020, 10, 25319–25324. [Google Scholar] [CrossRef]
- Youl, E.N.H.; Husson, C.; El Khattabi, C.; El Mere, S.; Declèves, A.-E.; Pochet, S.; Nortier, J.; Antoine, M.-H. Characterization of cytotoxic effects of aristolochic acids on the vascular endothelium. Toxicol. Vitr. 2020, 65, 104811. [Google Scholar] [CrossRef] [PubMed]
- Au, C.-K.; Chan, C.-K.; Tung, K.-K.; Zhang, J.; Chan, W. Quantitation of DNA Adducts of Aristolochic Acids in Repair-Deficient Cells: A Mechanistic Study of the DNA Repair Mechanism. Chem. Res. Toxicol. 2020, 33, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Dickman, K.G.; Moriya, M.; Zavadil, J.; Sidorenko, V.S.; Edwards, K.L.; Gnatenko, D.V.; Wu, L.; Turesky, R.J.; Wu, X.-R.; et al. Aristolochic acid-associated urothelial cancer in Taiwan. Proc. Natl. Acad. Sci. USA 2012, 109, 8241–8246. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.-K.; Chan, K.-K.J.; Liu, N.; Chan, W. Quantitation of Protein Adducts of Aristolochic Acid I by Liquid Chromatography–Tandem Mass Spectrometry: A Novel Method for Biomonitoring Aristolochic Acid Exposure. Chem. Res. Toxicol. 2021, 34, 144–153. [Google Scholar] [CrossRef]
- Jadot, I.; Declèves, A.-E.; Nortier, J.; Caron, N. An Integrated View of Aristolochic Acid Nephropathy: Update of the Literature. Int. J. Mol. Sci. 2017, 18, 297. [Google Scholar] [CrossRef] [Green Version]
- Debelle, F.D.; Vanherweghem, J.-L.; Nortier, J.L. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008, 74, 158–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlović, N.M.; Maksimović, V.; Maksimović, J.D.; Orem, W.H.; Tatu, C.A.; Lerch, H.E.; Bunnell, J.E.; Kostić, E.N.; Szilagyi, D.N.; Paunescu, V. Possible health impacts of naturally occurring uptake of aristolochic acids by maize and cucumber roots: Links to the etiology of endemic (Balkan) nephropathy. Environ. Geochem. Health 2013, 35, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-J.; Li, J.-Y.; Wu, S.-F.; Wu, W.-Y.; Yao, C.-L.; Yao, S.; Zhang, J.-Q.; Guo, D.-A. Two New Aristolochic Acid Analogues from the Roots of Aristolochia contorta with Significant Cytotoxic Activity. Molecules 2021, 26, 44. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Pavlović, N.M.; Chan, W. Development of a novel liquid chromatography-tandem mass spectrometric method for aristolochic acids detection: Application in food and agricultural soil analyses. Food Chem. 2019, 289, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liang, S.; Cui, Y.; Rong, Y.; Song, J.; Lv, D. Study on environmental behaviour of fluopyram in different banana planting soil. Sci. Rep. 2021, 11, 15346. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Guo, X.; Wang, F.; Huang, J.; Sun, B.; Wang, X. Sourdough improves the quality of whole-wheat flour products: Mechanisms and challenges—A review. Food Chem. 2021, 360, 130038. [Google Scholar] [CrossRef]
- Wu, C.; Dong, F.; Mei, X.; Ning, J.; She, D. Distribution, Dissipation, and Metabolism of Neonicotinoid Insecticides in the Cotton Ecosystem under Foliar Spray and Root Irrigation. J. Agric. Food Chem. 2019, 67, 12374–12381. [Google Scholar] [CrossRef]
- Chan, C.-K.; Liu, Y.; Pavlović, N.M.; Chan, W. Etiology of Balkan Endemic Nephropathy: An Update on Aristolochic Acids Exposure Mechanisms. Chem. Res. Toxicol. 2018, 31, 1109–1110. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chan, C.-K.; Liu, Y.; Yao, J.; Mitić, B.; Kostić, E.N.; Milosavljević, B.; Davinić, I.; Orem, W.H.; Tatu, C.A.; et al. Aristolochic Acids as Persistent Soil Pollutants: Determination of Risk for Human Exposure and Nephropathy from Plant Uptake. J. Agric. Food Chem. 2018, 66, 11468–11476. [Google Scholar] [CrossRef]
- Lukinich-Gruia, A.T.; Nortier, J.; Pavlović, N.M.; Milovanović, D.; Popović, M.; Drăghia, L.P.; Păunescu, V.; Tatu, C.A. Aristolochic acid I as an emerging biogenic contaminant involved in chronic kidney diseases: A comprehensive review on exposure pathways, environmental health issues and future challenges. Chemosphere 2022, 297, 134111. [Google Scholar] [CrossRef]
- Jelaković, B.; Dika, Ž.; Arlt, V.M.; Stiborova, M.; Pavlović, N.M.; Nikolić, J.; Colet, J.-M.; Vanherweghem, J.-L.; Nortier, J.L. Balkan Endemic Nephropathy and the causative role of Aristolochic Acid. Semin. Nephrol. 2019, 39, 284–296. [Google Scholar] [CrossRef]
- Stiborová, M.; Arlt, V.M.; Schmeiser, H.H. Balkan endemic nephropathy: An update on its aetiology. Arch. Toxicol. 2016, 90, 2595–2615. [Google Scholar] [CrossRef] [Green Version]
- Tung, K.-K.; Chan, C.-K.; Zhao, Y.; Chan, K.-K.J.; Liu, G.; Pavlović, N.M.; Chan, W. Occurrence and Environmental Stability of Aristolochic Acids in Groundwater Collected from Serbia: Links to Human Exposure and Balkan Endemic Nephropathy. Environ. Sci. Technol. 2020, 54, 1554–1561. [Google Scholar] [CrossRef]
- Chan, W.; Pavlovic, N.M.; Li, W.; Chan, C.K.; Liu, J.; Deng, K.; Wang, Y.; Milosavljevic, B.; Kostic, E.N. Quantitation of Aristolochic Acids in Corn, Wheat Grain, and Soil Samples Collected in Serbia: Identifying a Novel Exposure Pathway in the Etiology of Balkan Endemic Nephropathy. J. Agric. Food Chem. 2016, 64, 5928–5934. [Google Scholar] [CrossRef]
- Li, W.; Hu, Q.; Chan, W. Uptake and Accumulation of Nephrotoxic and Carcinogenic Aristolochic Acids in Food Crops Grown in Aristolochia clematitis-Contaminated Soil and Water. J. Agric. Food Chem. 2016, 64, 107–112. [Google Scholar] [CrossRef]
- Drăghia, L.P.; Lukinich-Gruia, A.T.; Oprean, C.; Pavlović, N.M.; Păunescu, V.; Tatu, C.A. Aristolochic acid I: An investigation into the role of food crops contamination, as a potential natural exposure pathway. Environ. Geochem. Health 2021, 43, 4163–4178. [Google Scholar] [CrossRef]
- Ca, P.; Cc, P.; Sb, C.; Na, D. Evaluation of vermiculite application rates on Growth and Yield of Brassica Napus (RAPE). Sci. Herit. J. 2020, 4, 46–50. [Google Scholar]
- Xu, T.; Chen, W.; Zhou, J.; Dai, J.; Li, Y.; Zhao, Y. Computational Analysis of Naturally Occurring Aristolochic Acid Analogues and Their Biological Sources. Biomolecules 2021, 11, 1344. [Google Scholar] [CrossRef]
- Chen, P.; Li, X.; Yan, X.; Tian, M. Solid-Phase Extraction of Aristolochic Acid I from Natural Plant Using Dual Ionic Liquid-Immobilized ZIF-67 as Sorbent. Separations 2021, 8, 22. [Google Scholar] [CrossRef]
- Wang, Y.; Chan, W. Determination of aristolochic acids by high-performance liquid chromatography with fluorescence detection. J. Agric. Food Chem. 2014, 62, 5859–5864. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Pan, G.; Chan, W. Analysis of aristolochic acids in Houttuynia cordata by liquid chromatography–tandem mass spectrometry. J. Mass Spectrom. 2021, 56, e4652. [Google Scholar] [CrossRef] [PubMed]
- Gruia, A.T.; Oprean, C.; Ivan, A.; Cean, A.; Cristea, M.; Draghia, L.; Damiescu, R.; Pavlovic, N.M.; Paunescu, V.; Tatu, C.A. Balkan endemic nephropathy and aristolochic acid I: An investigation into the role of soil and soil organic matter contamination, as a potential natural exposure pathway. Env. Geochem. Health 2018, 40, 1437–1448. [Google Scholar] [CrossRef] [PubMed]





| Precision | Accuracy | LOD | LOQ | ||||
|---|---|---|---|---|---|---|---|
| Concn Added (ng/g) | Intraday a (%RSD) | Interday b (%RSD) | Concn Found c (ng/g) | Error | |||
| AA I | 50 | 8.7% | 15.1% | 46.7 ± 6.9 | −6.7% | 2.5 | 8.5 |
| 500 | 5.3% | 7.8% | 473.4 ± 30.0 | −5.3% | |||
| 1000 | 5.0% | 8.1% | 989.8 ± 33.3 | −1.0% | |||
| AA II | 50 | 8.9% | 13.4% | 47.8 ± 4.3 | −4.5% | 10.7 | 35.8 |
| 500 | 4.6% | 13.7% | 480.4 ± 57.3 | −3.9% | |||
| 1000 | 9.6% | 12.2% | 970.9 ± 80.9 | −2.9% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, Y.; Wang, C.; Li, K.; Tang, W.; Sun, J.; Wang, X. Uptake, Translocation, and Fate of Carcinogenic Aristolochic Acid in Typical Vegetables in Soil−Plant Systems. Molecules 2022, 27, 8271. https://doi.org/10.3390/molecules27238271
Zhang J, Wang Y, Wang C, Li K, Tang W, Sun J, Wang X. Uptake, Translocation, and Fate of Carcinogenic Aristolochic Acid in Typical Vegetables in Soil−Plant Systems. Molecules. 2022; 27(23):8271. https://doi.org/10.3390/molecules27238271
Chicago/Turabian StyleZhang, Jinghe, Yinan Wang, Changhong Wang, Kan Li, Weifang Tang, Jing Sun, and Xikui Wang. 2022. "Uptake, Translocation, and Fate of Carcinogenic Aristolochic Acid in Typical Vegetables in Soil−Plant Systems" Molecules 27, no. 23: 8271. https://doi.org/10.3390/molecules27238271
APA StyleZhang, J., Wang, Y., Wang, C., Li, K., Tang, W., Sun, J., & Wang, X. (2022). Uptake, Translocation, and Fate of Carcinogenic Aristolochic Acid in Typical Vegetables in Soil−Plant Systems. Molecules, 27(23), 8271. https://doi.org/10.3390/molecules27238271






