Quantum Dot Nanobeads as Multicolor Labels for Simultaneous Multiplex Immunochromatographic Detection of Four Nitrofuran Metabolites in Aquatic Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. Principle of the QB-Based Multiplex Fluorescent Lateral Flow Immunoassay
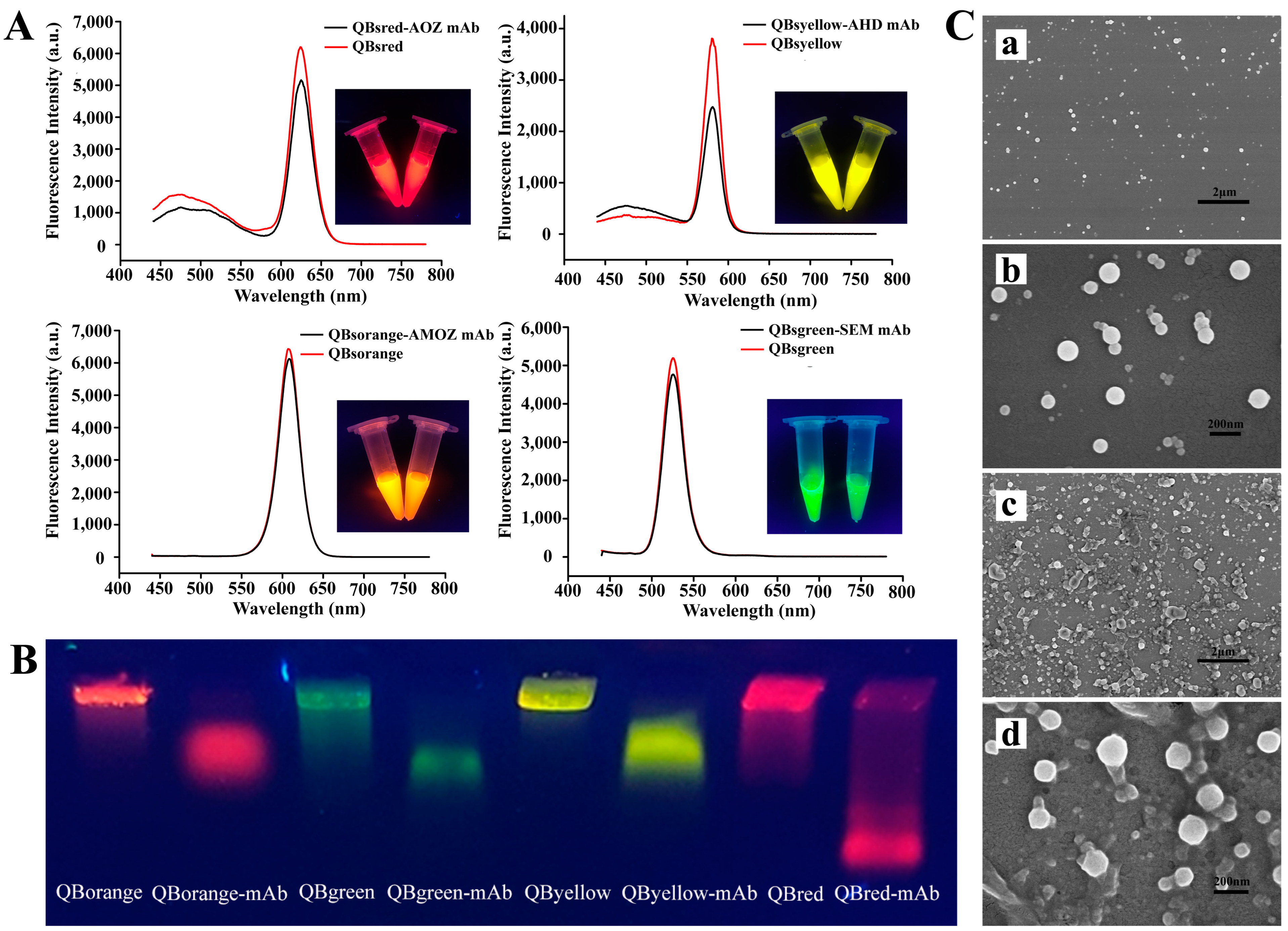
2.2. Characterization of QBs and QB-mAb Probes
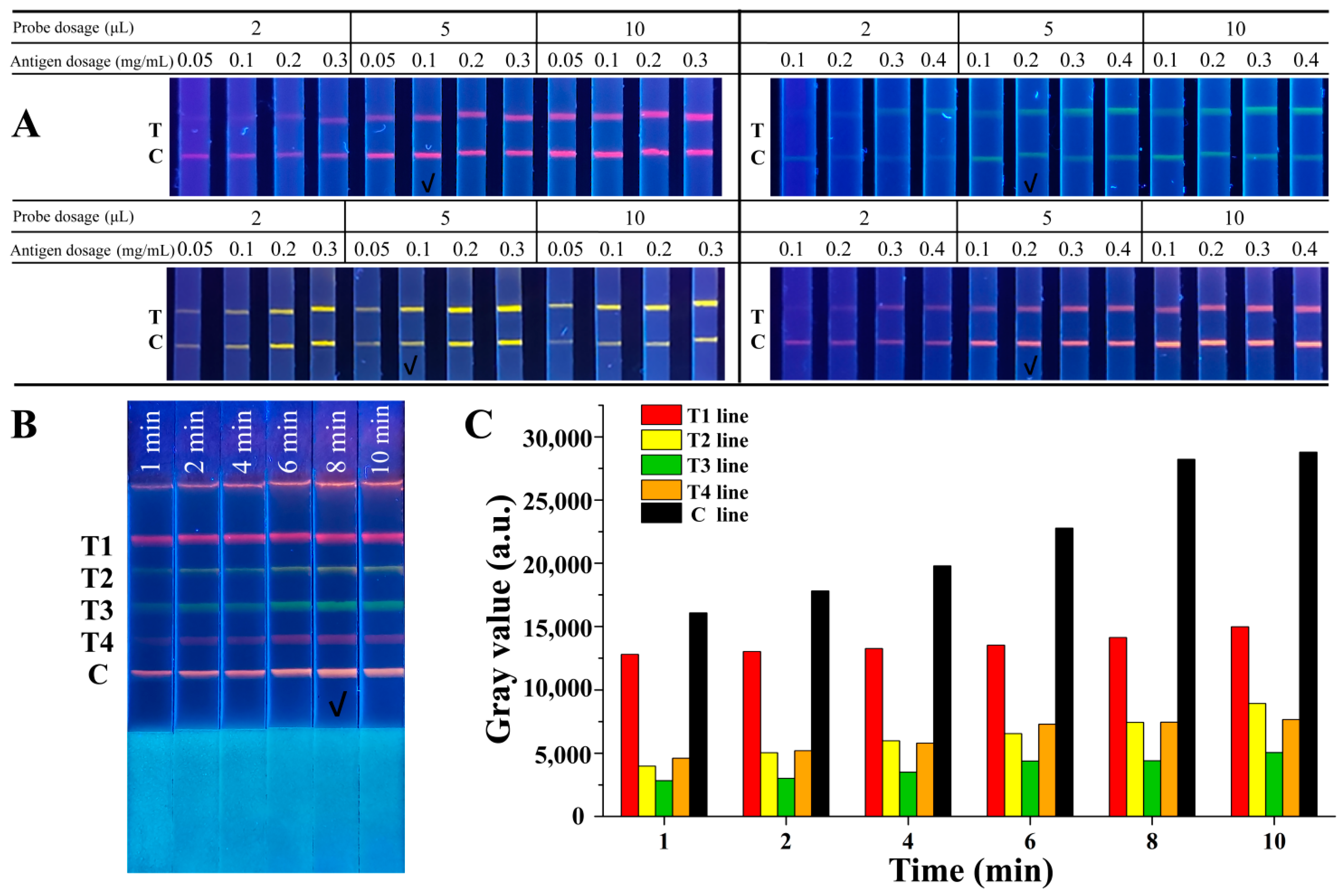
2.3. Optimization of the QB-Based mICA Strip
2.4. Performance Evaluation of Multicolor ICA
2.4.1. Sensitivity
2.4.2. Cross-Reaction and Specificity
2.4.3. Accuracy and Precision
2.5. Application in Real Samples
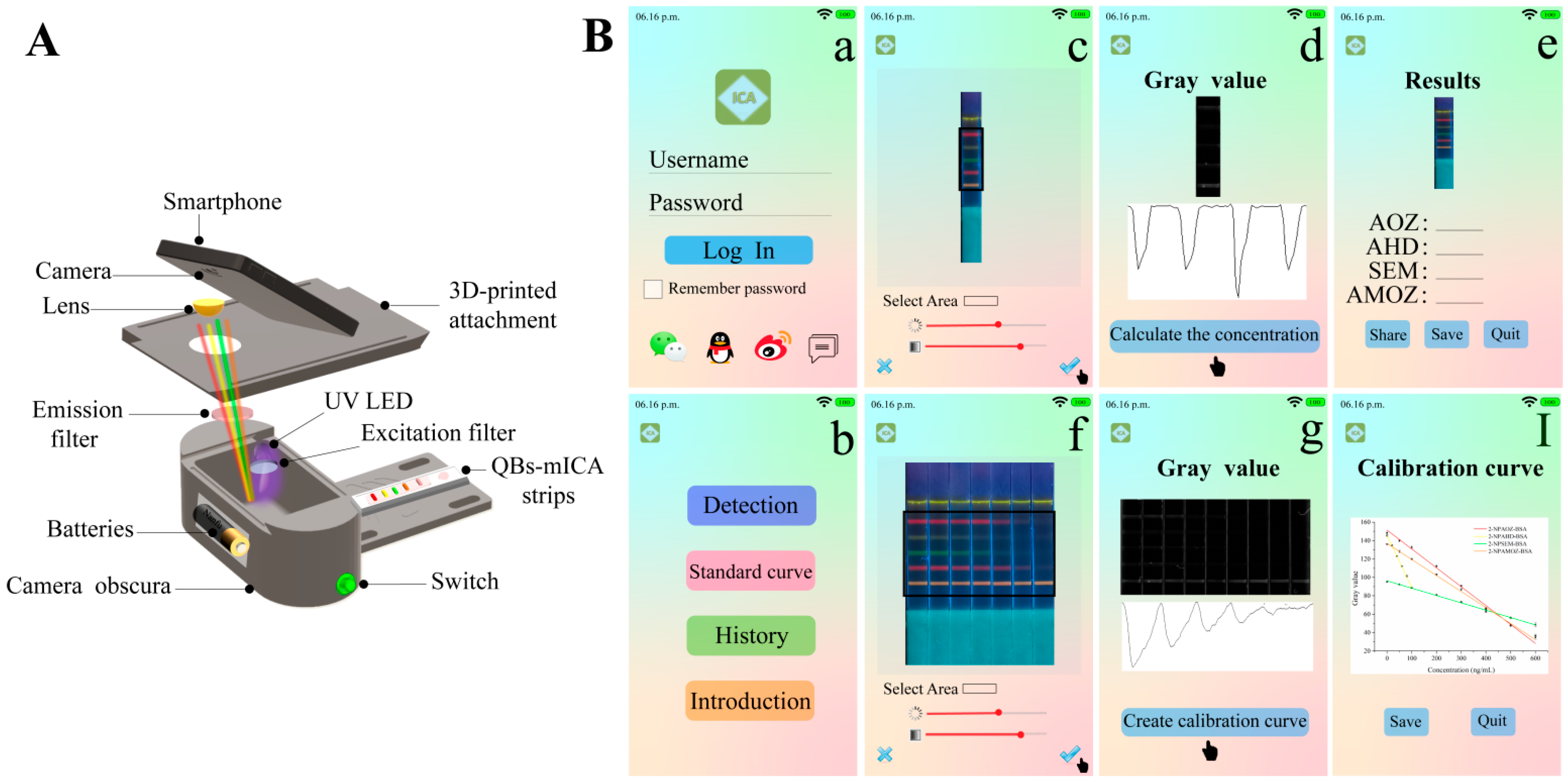
2.6. Conceptual Products for On-Site Detection
3. Materials and Methods
3.1. Materials
3.2. Preparation of QB-mAb Probes
3.3. Characterization of QB-mAb Probes
3.4. Preparation of the QB-Based mICA Strip
3.5. Simultaneous Detection of AOZ, AHD, SEM, and AMOZ
3.6. Performance Evaluation of mICA Strip
3.7. Real Sample Analysis
3.8. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kaufmann, A.; Butcher, P.; Maden, K.; Walker, S.; Widmer, M. Determination of nitrofuran and chloramphenicol residues by high resolution mass spectrometry versus tandem quadrupole mass spectrometry. Anal. Chim. Acta 2015, 862, 41–52. [Google Scholar] [CrossRef]
- Alam, M.M.; Haque, M.M. Presence of antibacterial substances, nitrofuran metabolites and other chemicals in farmed pangasius and tilapia in Bangladesh: Probabilistic health risk assessment. Toxicol. Rep. 2021, 8, 248–257. [Google Scholar] [CrossRef]
- Wu, W.; Yang, S.; Liu, J.; Mi, J.; Dou, L.; Pan, Y.; Mari, G.M.; Wang, Z. Progress in immunoassays for nitrofurans detection. Food Agr. Immunol. 2020, 31, 907–926. [Google Scholar] [CrossRef]
- Wang, K.; Kou, Y.; Wang, M.; Ma, X.; Wang, J. Determination of Nitrofuran Metabolites in Fish by Ultraperformance Liquid Chromatography-Photodiode Array Detection with Thermostatic Ultrasound-Assisted Derivatization. ACS Omega 2020, 5, 18887–18893. [Google Scholar] [CrossRef]
- Luo, X.; Yu, Y.; Kong, X.; Wang, X.; Ji, Z.; Sun, Z.; You, J. Rapid microwave assisted derivatization of nitrofuran metabolites for analysis in shrimp by high performance liquid chromatography-fluorescence detector. Microchem. J. 2019, 150, 104189. [Google Scholar] [CrossRef]
- Melekhin, A.O.; Tolmacheva, V.V.; Shubina, E.G.; Dmitrienko, S.G.; Apyari, V.V.; Grudev, A.I. Determination of nitrofuran metabolites in honey using a new derivatization reagent, magnetic solid-phase extraction and LC-MS/MS. Talanta 2021, 230, 122310. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, F.; Sun, Y.; Mi, T.; Wang, L.; Li, Q.; Li, J.; Ma, W.; Liu, W.; Zuo, J.; et al. Development of a highly sensitive lateral flow immunoassay based on receptor-antibody-amorphous carbon nanoparticles to detect 22 β-lactams in milk. Sens. Actuat. B Chem. 2020, 321, 128458. [Google Scholar] [CrossRef]
- Wang, Z.; Xing, K.; Ding, N.; Wang, S.; Zhang, G.; Lai, W. Lateral flow immunoassay based on dual spectral-overlapped fluorescence quenching of polydopamine nanospheres for sensitive detection of sulfamethazine. J. Hazard. Mater. 2022, 423, 127204. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Y.; Leng, Y.; Lai, W.; Huang, X.; Xiong, Y. Emerging design strategies for constructing multiplex lateral flow test strip sensors. Biosens. Bioelectron. 2020, 157, 112168. [Google Scholar] [CrossRef]
- Lu, Q.; Song, S.; Wu, X.; Kuang, H.; Liu, L. Development of a gold immunochromatographic strip for the rapid detection of 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ) in catfish. Food Agr. Immunol. 2020, 31, 751–763. [Google Scholar] [CrossRef]
- Nguyen, A.V.T.; Dao, T.D.; Trinh, T.T.T.; Choi, D.Y.; Yu, S.T.; Park, H.; Yeo, S.J. Sensitive detection of influenza a virus based on a CdSe/CdS/ZnS quantum dot-linked rapid fluorescent immunochromatographic test. Biosens. Bioelectron. 2020, 155, 112090. [Google Scholar] [CrossRef]
- Chen, D.; Delmas, J.M.; Hurtaud-Pessel, D.; Verdon, E. Development of a multi-class method to determine nitroimidazoles, nitrofurans, pharmacologically active dyes and chloramphenicol in aquaculture products by liquid chromatography-tandem mass spectrometry. Food Chem. 2020, 311, 125924. [Google Scholar] [CrossRef]
- Zou, R.; Chang, Y.; Zhang, T.; Si, F.; Liu, Y.; Zhao, Y.; Liu, Y.; Zhang, M.; Yu, X.; Qiao, X.; et al. Up-Converting Nanoparticle-Based Immunochromatographic Strip for Multi-Residue Detection of Three Organophosphorus Pesticides in Food. Front. Chem. 2019, 7, 18. [Google Scholar] [CrossRef]
- Lou, D.; Fan, L.; Cui, Y.; Zhu, Y.; Gu, N.; Zhang, Y. Fluorescent Nanoprobes with Oriented Modified Antibodies to Improve Lateral Flow Immunoassay of Cardiac Troponin, I. Anal. Chem. 2018, 90, 6502–6508. [Google Scholar] [CrossRef]
- Rong, Z.; Bai, Z.; Li, J.; Tang, H.; Shen, T.; Wang, Q.; Wang, C.; Xiao, R.; Wang, S. Dual-color magnetic-quantum dot nanobeads as versatile fluorescent probes in test strip for simultaneous point-of-care detection of free and complexed prostate-specific antigen. Biosens. Bioelectron. 2019, 145, 111719. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Lin, H.; Siddanakoppalu, P.N.; Zhou, J.; Chen, G.; Yu, Z. Quantum-dot-based lateral flow immunoassay for the rapid detection of crustacean major allergen tropomyosin. Food Control 2019, 106, 106714. [Google Scholar] [CrossRef]
- Duan, H.; Li, Y.; Shao, Y.; Huang, X.; Xiong, Y. Multicolor quantum dot nanobeads for simultaneous multiplex immunochromatographic detection of mycotoxins in maize. Sens. Actuat. B Chem. 2019, 291, 411–417. [Google Scholar] [CrossRef]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y. Quantum dot nanobead-based fluorescent immunochromatographic assay for simultaneous quantitative detection of fumonisin B1, dexyonivalenol, and zearalenone in grains. Food Control 2020, 117, 107331. [Google Scholar] [CrossRef]
- Yin, X.; Dou, L.; Yao, X.; Liu, S.; Zhang, L.; Zhao, M.; Su, L.; Sun, J.; Wang, J.; Zhang, D. Controllable assembly metal-organic frameworks and gold nanoparticles composites for sensitive immunochromatographic assay. Food Chem. 2022, 367, 130737. [Google Scholar] [CrossRef]
- Zhang, G.G.; Xu, S.L.; Xiong, Y.H.; Duan, H.; Chen, W.Y.; Li, X.M.; Yuan, M.F.; Lai, W.H. Ultrabright fluorescent microsphere and its novel application for improving the sensitivity of immunochromatographic assay. Biosens. Bioelectron. 2019, 135, 173–180. [Google Scholar] [CrossRef]
- Rong, Z.; Wang, Q.; Sun, N.; Jia, X.; Wang, K.; Xiao, R.; Wang, S. Smartphone-based fluorescent lateral flow immunoassay platform for highly sensitive point-of-care detection of Zika virus nonstructural protein 1. Anal. Chim. Acta 2019, 1055, 140–147. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, C.; Jin, J.; Huang, L.; Yu, W.; Su, B.; Hu, J. Ratiometric Fluorescent Lateral Flow Immunoassay for Point-of-Care Testing of Acute Myocardial Infarction. Angew. Chem. 2021, 133, 13152–13159. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing nanoparticles with cancer-targeting antibodies: A comparison of strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef]
- Yan, J.X.; Hu, W.J.; You, K.H.; Ma, Z.E.; Xu, Y.; Li, Y.P.; He, Q.H. Biosynthetic Mycotoxin Conjugate Mimetics-Mediated Green Strategy for Multiplex Mycotoxin Immunochromatographic Assay. J. Agric. Food Chem. 2020, 68, 2193–2200. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Cheng, Y.; Guan, B.; Xia, F.; Fan, L.; Gao, X.; Sun, X.; Li, X.; Zhu, L. Quantum Dot Nanobeads as Multicolor Labels for Simultaneous Multiplex Immunochromatographic Detection of Four Nitrofuran Metabolites in Aquatic Products. Molecules 2022, 27, 8324. https://doi.org/10.3390/molecules27238324
Liu X, Cheng Y, Guan B, Xia F, Fan L, Gao X, Sun X, Li X, Zhu L. Quantum Dot Nanobeads as Multicolor Labels for Simultaneous Multiplex Immunochromatographic Detection of Four Nitrofuran Metabolites in Aquatic Products. Molecules. 2022; 27(23):8324. https://doi.org/10.3390/molecules27238324
Chicago/Turabian StyleLiu, Xiuying, Yuanyuan Cheng, Binbin Guan, Fei Xia, Ling Fan, Xue Gao, Xiaofei Sun, Xuepeng Li, and Lijie Zhu. 2022. "Quantum Dot Nanobeads as Multicolor Labels for Simultaneous Multiplex Immunochromatographic Detection of Four Nitrofuran Metabolites in Aquatic Products" Molecules 27, no. 23: 8324. https://doi.org/10.3390/molecules27238324
APA StyleLiu, X., Cheng, Y., Guan, B., Xia, F., Fan, L., Gao, X., Sun, X., Li, X., & Zhu, L. (2022). Quantum Dot Nanobeads as Multicolor Labels for Simultaneous Multiplex Immunochromatographic Detection of Four Nitrofuran Metabolites in Aquatic Products. Molecules, 27(23), 8324. https://doi.org/10.3390/molecules27238324







