Highly Selective Methodology for Entrapment and Subsequent Removal of Cobalt (II) Ions under Optimized Conditions by Micellar-Enhanced Ultrafiltration
Abstract
1. Introduction
2. Results and Discussions
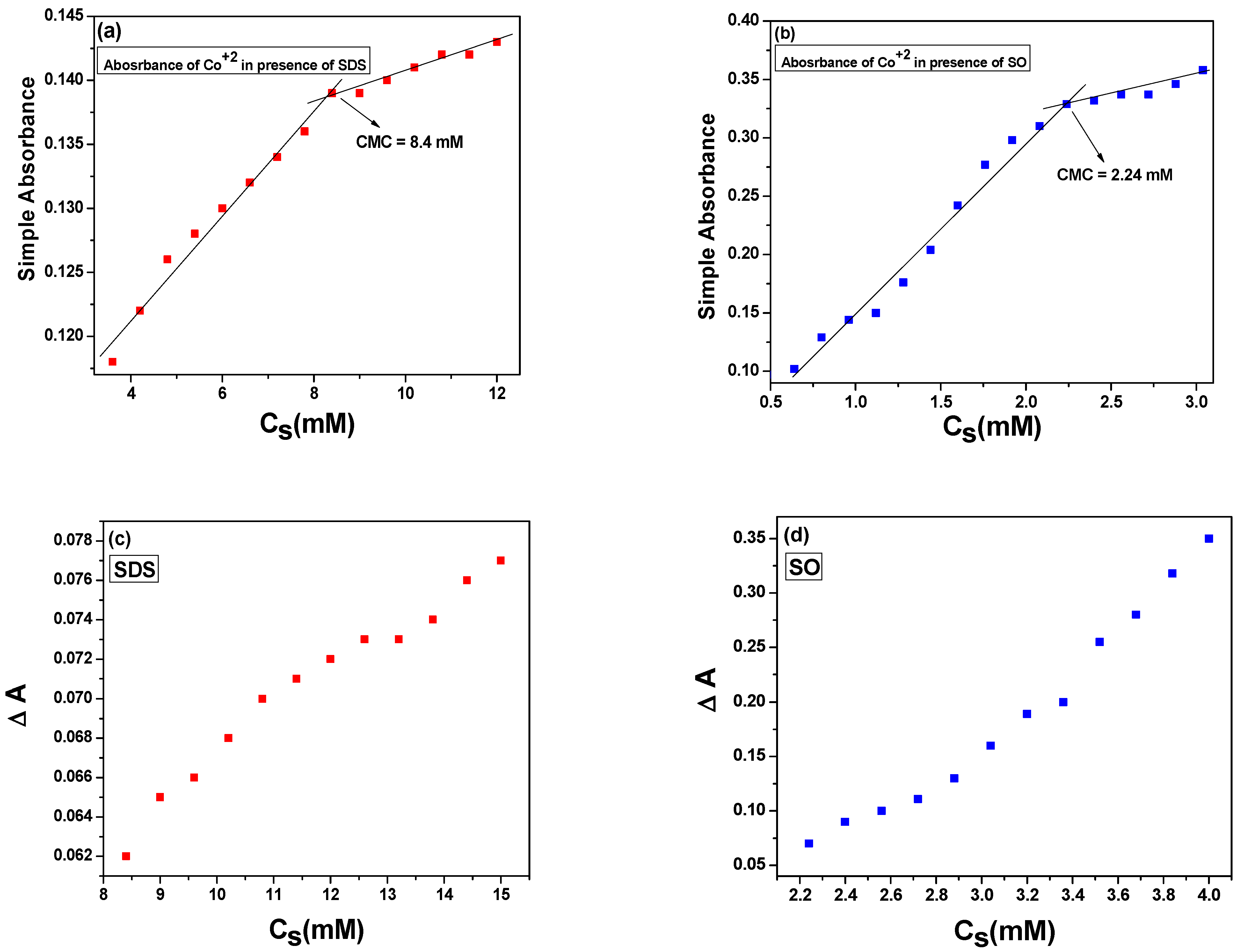
2.1. UV-Visible Spectroscopy
2.1.1. Simple and Differential Absorption Spectra
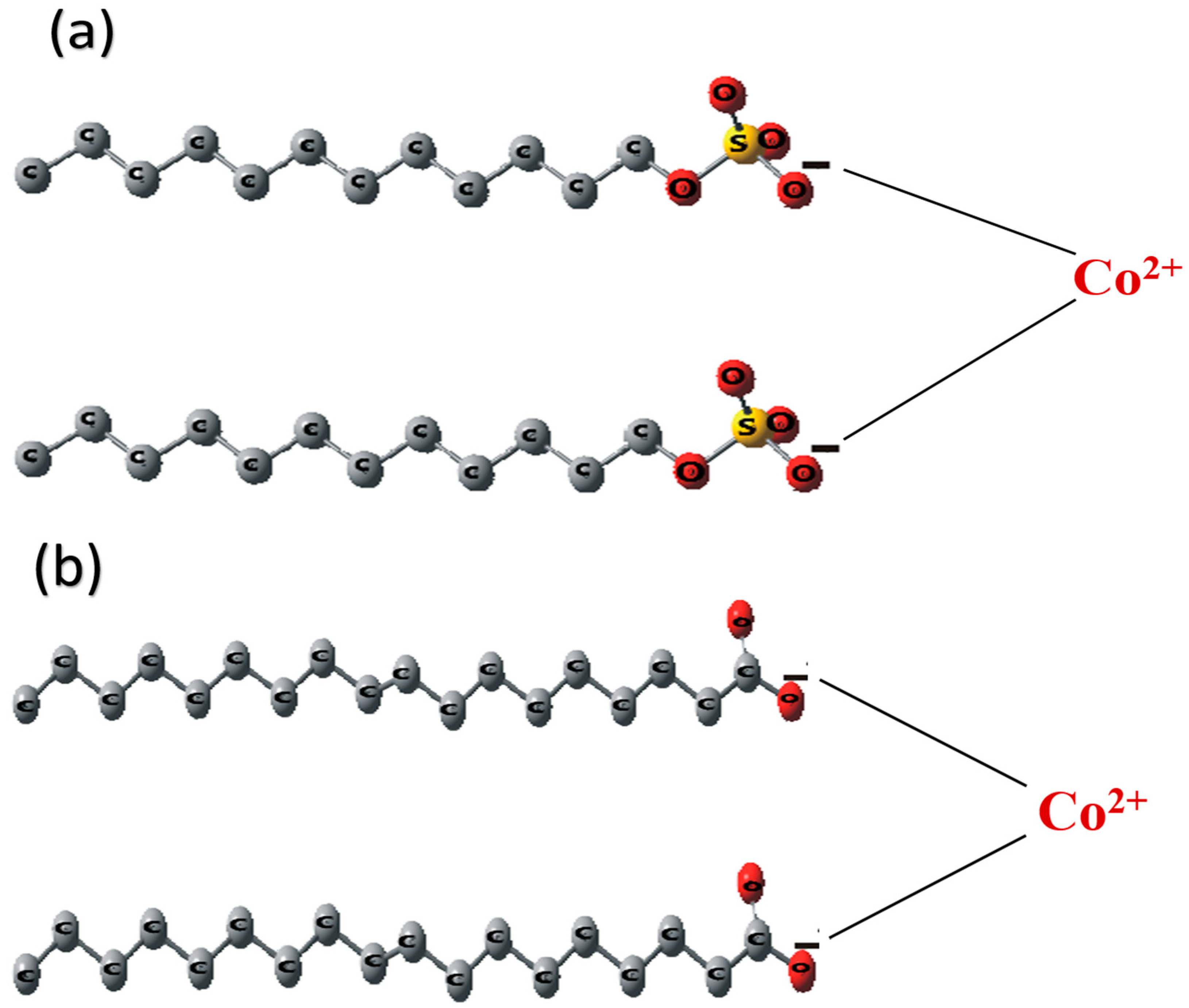
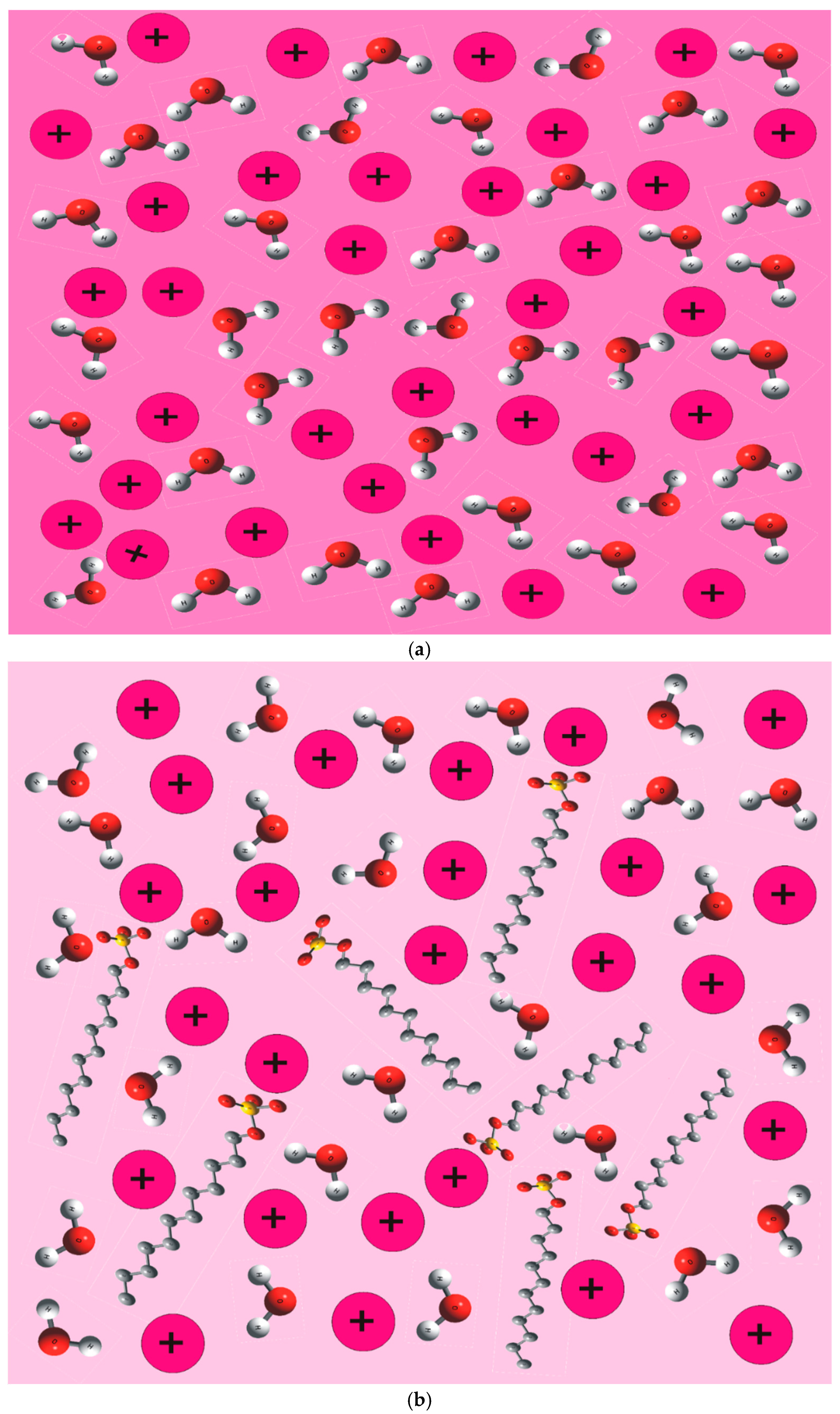
2.1.2. Interaction of Co2+ in SDS and SO Systems
2.1.3. Interaction of Co2+ in SDS/SO System
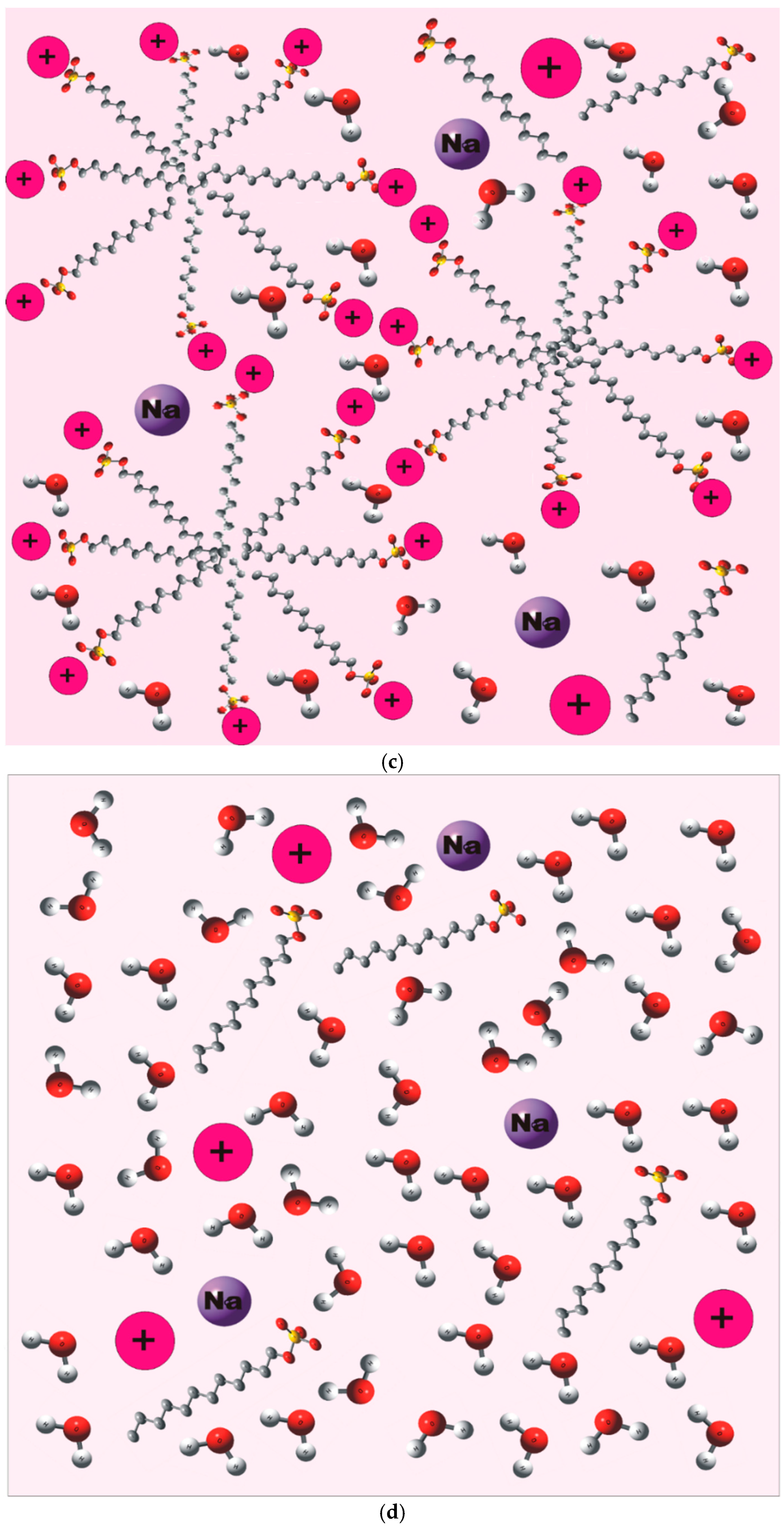
2.2. MEUF for the Removal of Single Metal Ion (Co2+)
2.2.1. Rejection Percentage
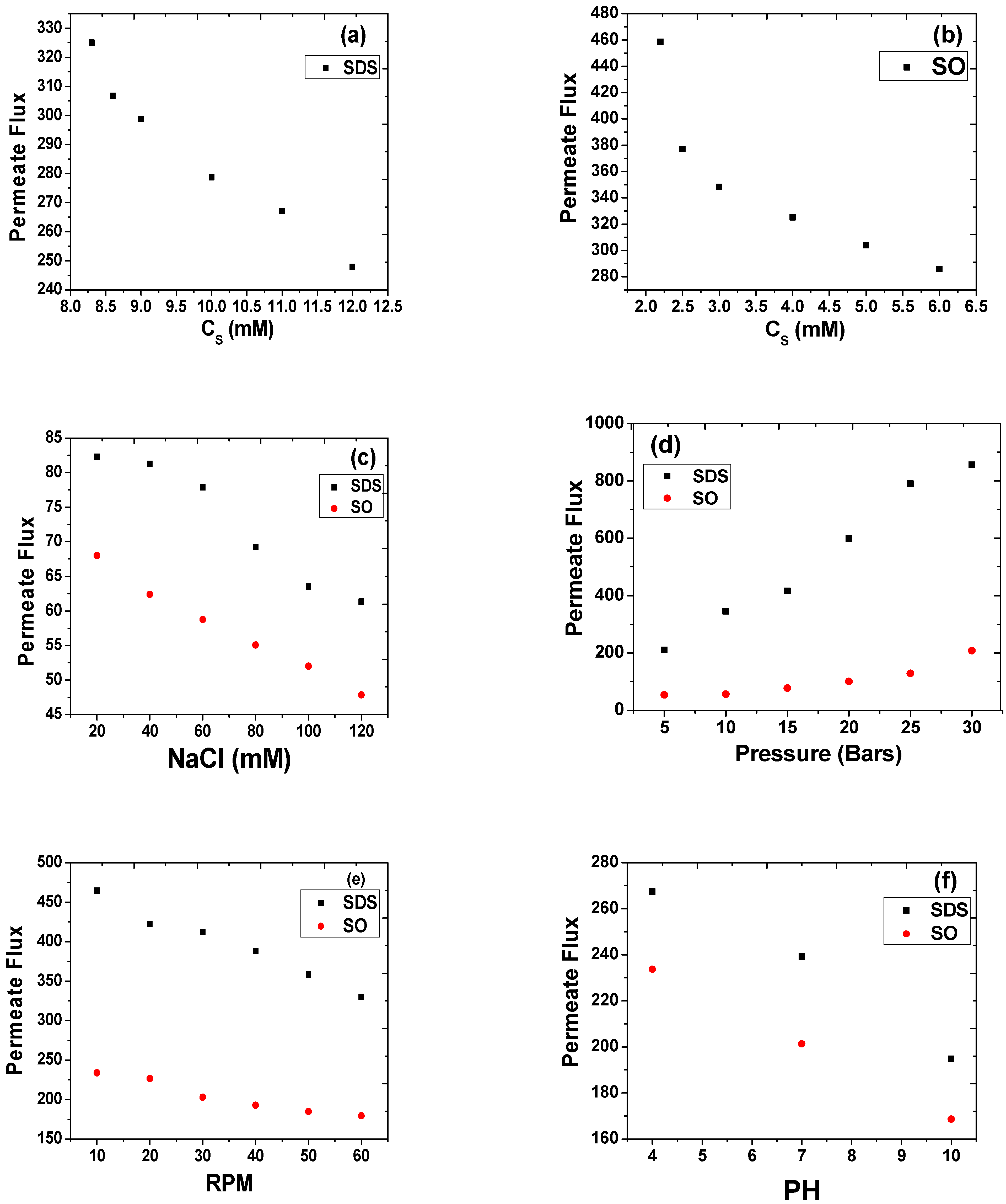
Surfactant Concentration
Concentration of Electrolyte
Effect of Transmembrane Pressure
RPM
pH
2.2.2. Permeate Flux
Concentration of Surfactants
Concentration of Electrolyte
Effect of Transmembrane Pressure
Effect of RPM
Effect of pH
3. Materials and Methods
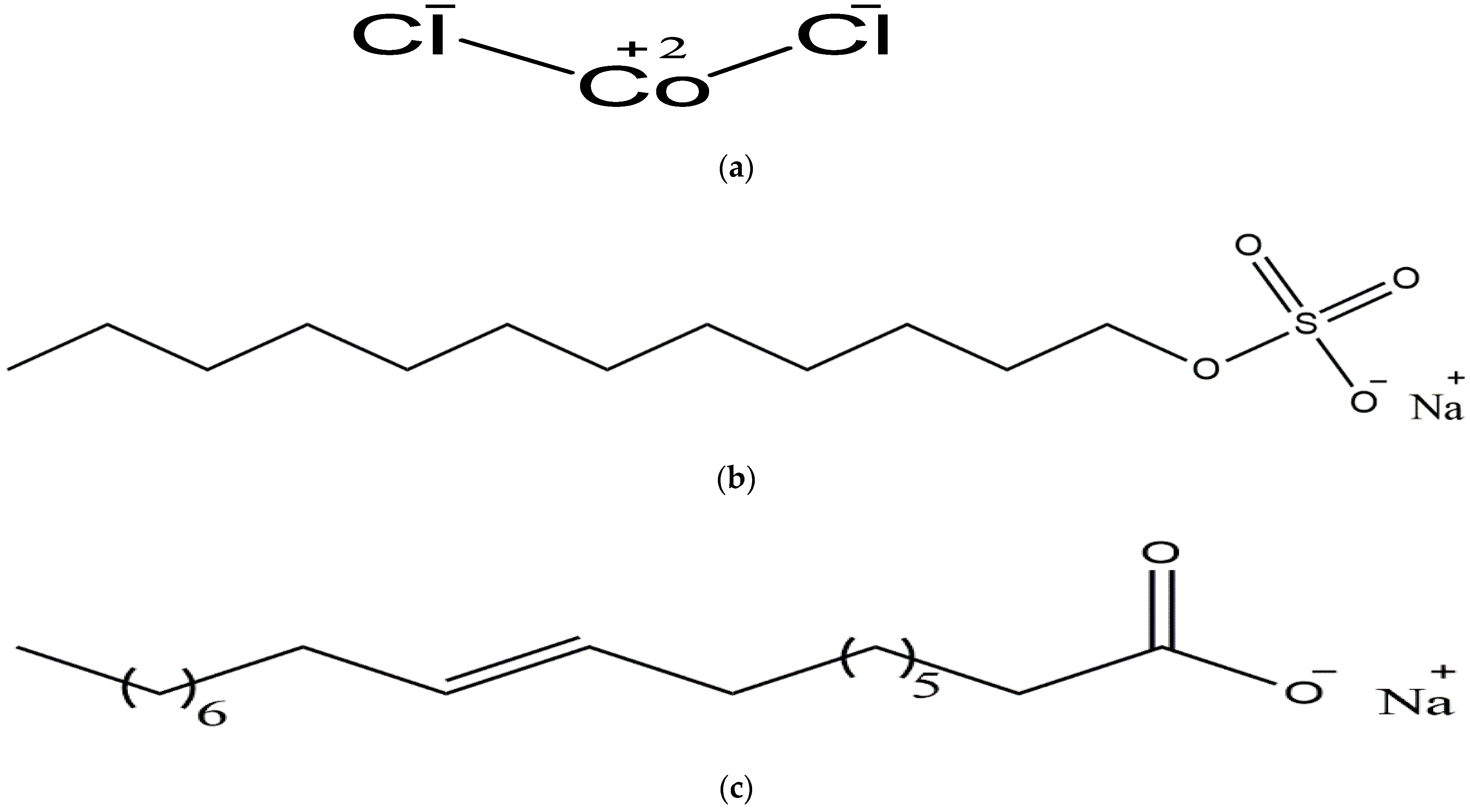
3.1. Materials
3.2. Parameter Calculated
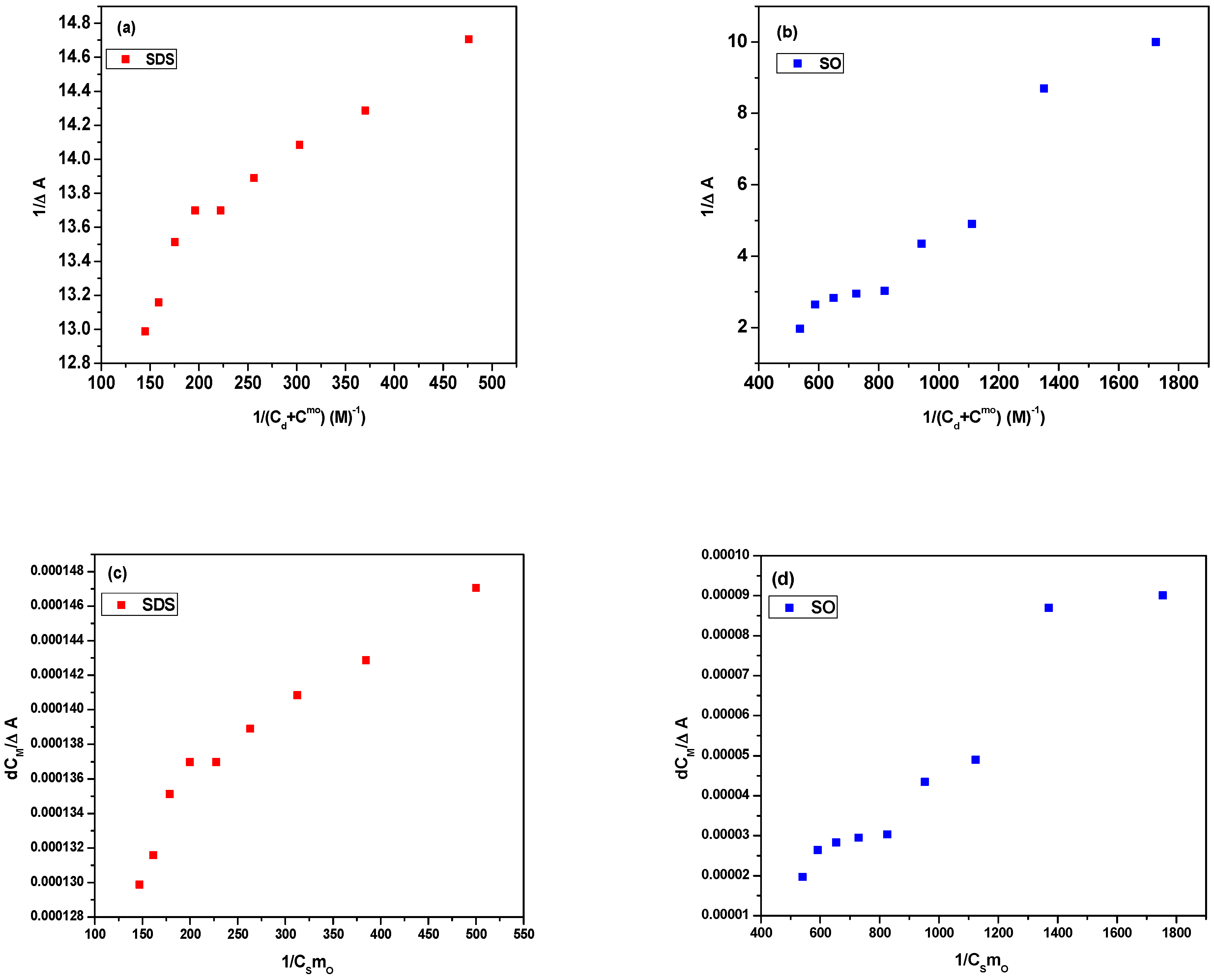
3.2.1. Partition Constant and Gibbs Energy of Partition
3.2.2. Binding Constant and Gibbs Energy of Binding
3.2.3. Rejection Coefficient (R%)
3.2.4. Permeate Flux (J)
4. Experimental Methods
4.1. Solution Preparation
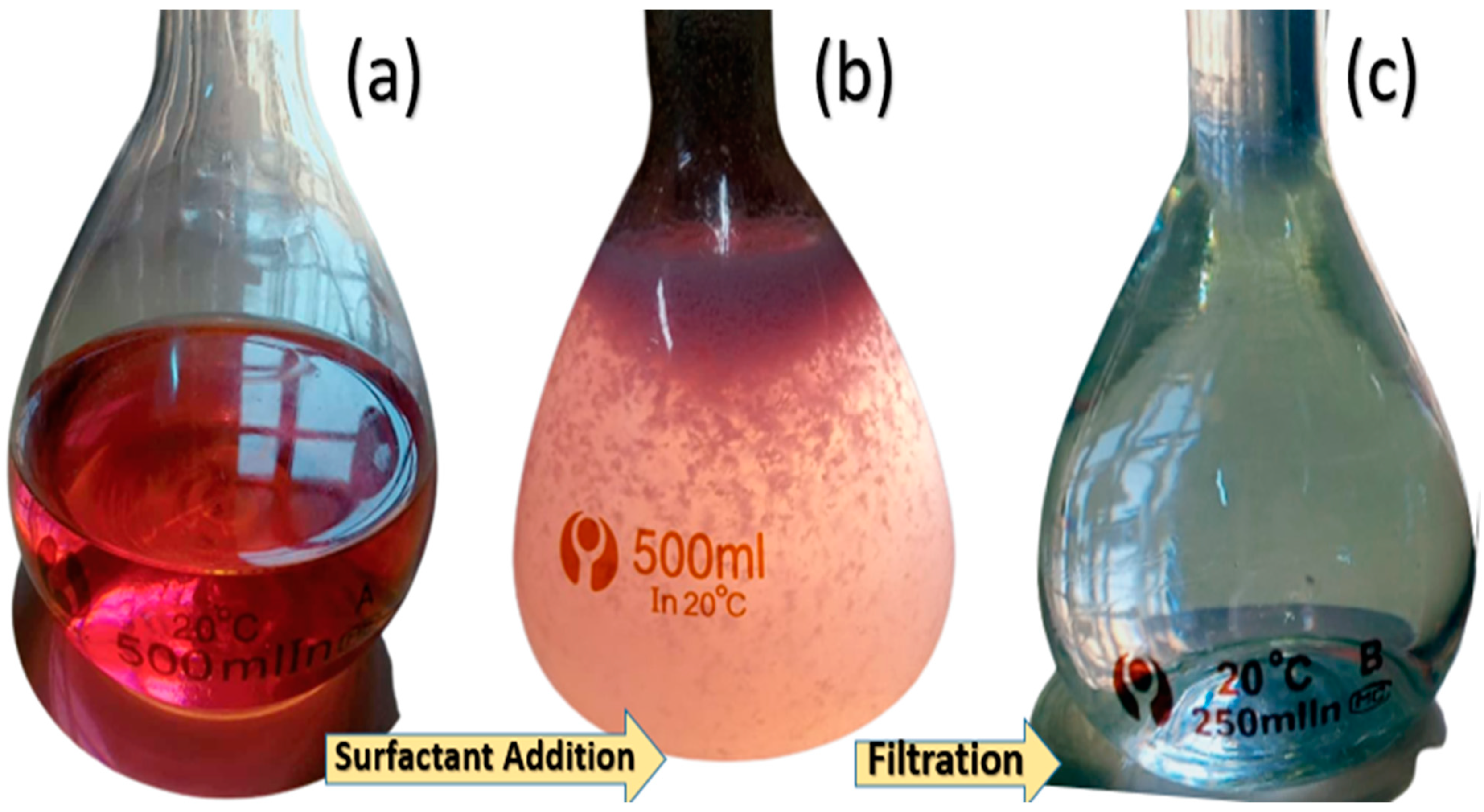
4.2. Micellar Enhanced Ultrafiltration (MEUF)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Abbasi-Garravand, E.; Mulligan, C.N. Removal of trivalent chromium from water by micellar enhanced ultrafiltration technique. Gen. Conf. 2013, 206, 9. [Google Scholar]
- Vibhandik, A.D.; Marathe, K.V. Removal of Ni(II) ions from wastewater by micellar enhanced ultrafiltration using mixed surfactants. Front. Chem. Sci. Eng. 2014, 8, 79–86. [Google Scholar] [CrossRef]
- Li, F.; Li, X.; Zhang, J.D.; Peng, L.; Liu, C.Y. Removal of organic matter and heavy metals of low concentration from wastewater via micellar-enhanced ultrafiltration: An overview. Earth Environ. Sci. 2017, 52, 1–7. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Jana, D.K.; Roy, K.; Dey, S. Comparative assessment on lead removal using micellar-enhanced ultrafiltration (MEUF) based on a type-2 fuzzy logic and response surface methodology. Sep. Purif. Technol. 2018, 207, 28–41. [Google Scholar] [CrossRef]
- Malik, A. Metal bioremediation through growing cells. Environ. Int. 2004, 30, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Soylu, M.; Gökkuş, Ö.; Özyonar, F. Foam separation for effective removal of disperse and reactive dyes from aqueous solutions. Sep. Purif. Technol. 2020, 247, 116985. [Google Scholar] [CrossRef]
- Qi, Y.; Zhu, L.; Shen, X.; Sotto, A.; Gao, C.; Shen, J. Polythyleneimine-modified original positive charged nanofiltration membrane: Removal of heavy metal ions and dyes. Sep. Purif. Technol. 2019, 222, 117–124. [Google Scholar] [CrossRef]
- Yusaf, A.; Adeel, S.; Usman, M.; Mansha, A.; Ahmad, M. Removal of Heavy Metal Ions from Wastewater Using Micellar-Enhanced Ultrafiltration Technique (MEUF): A Brief Review; Springer: Berlin/Heidelberg, Germany, 2019; pp. 297–315. [Google Scholar]
- Shi, Y.; Huang, L.; Mahmud, S.; Zhang, G.; Li, H.; Wang, Y.; Xiao, T.; Zeng, Q.; Liu, Z.; Yu, H.; et al. High-efficiently capturing trace thallium (Ι) from wastewater via the prussian blue@polytetrafluoroethylene hybrid membranes. Chem. Eng. J. 2023, 451, 138712. [Google Scholar] [CrossRef]
- Abbas, K.H.A.N.; Muhammad, A.S.I.M.; Usman, M.; Farooqi, Z.H.; Zaman, K.; Abdur, R.A.U.F.; Amir, Z.A.D.A. The Interactions of Co-Solvent, Co-Solute and Amphiphilic Anionic Dye with Aqueous Solutions of Sodium Dodecyl Sulfate. Walailak J. Sci. Technol. 2015, 12, 1107–1119. [Google Scholar]
- Rosen, M.J.; Joy, T. Kunjappu, Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 1–455. [Google Scholar]
- Alam, M.S.; Siddiq, A.M.; Mandal, A.B. Effect of (chloride salt) electrolytes on the mixed micellization of (equimolar) a cationic gemini (dimeric) surfactant and a cationic conventional (monomeric) surfactant. J. Dispers. Sci. Technol. 2016, 38, 303–308. [Google Scholar] [CrossRef]
- Burdíková, J.; Mravec, F.; Pekař, M. The formation of mixed micelles of sugar surfactants and phospholipids and their interactions with hyaluronan. Colloid Polym. Sci. 2016, 294, 823–831. [Google Scholar] [CrossRef]
- Khan, S.A.; Asiri, A.M. Fluorescence quenching of environmentally benign highly fluorescence donor (D)-π-acceptor (A)-π-donor (D) quinoline dye by silver nanoparticles and anionic surfactant in liquid stage. J. Mol. Liq. 2016, 221, 381–385. [Google Scholar] [CrossRef]
- Alam, M.S.; Ragupathy, R.; Mandal, A.B. The Self-Association and Mixed Micellization of an Anionic Surfactant, Sodium Dodecyl Sulfate, and a Cationic Surfactant, Cetyltrimethylammonium Bromide: Conductometric, Dye Solubilization, and Surface Tension Studies. J. Dispers. Sci. Technol. 2015, 37, 1645–1654. [Google Scholar] [CrossRef]
- Petcu, A.R.; Rogozea, E.A.; Lazar, C.A.; Olteanu, N.L.; Meghea, A.; Mihaly, M. Specific interactions within micelle microenvironment in different charged dye/surfactant systems. Arab. J. Chem. 2016, 9, 9–17. [Google Scholar] [CrossRef]
- Mandal, A.B.; Nair, B.U.; Ramaswamy, D. Determination of the critical micelle concentration of surfactants and the partition coefficient of an electrochemical probe by using cyclic voltammetry. Langmuir ACS J. Surf. Colloids 1988, 4, 736–739. [Google Scholar] [CrossRef]
- Mandal, A.B. Self-diffusion studies on various micelles using ferrocene as electrochemical probe. Langmuir ACS J. Surf. Colloids 1993, 9, 1932–1933. [Google Scholar] [CrossRef]
- Geetha, B.; Mandal, A.B. Self-diffusion studies on. omega.-methoxy polyethylene glycol macromonomer micelles by using cyclic voltammetric and fourier transform pulsed gradient spin-echo nuclear magnetic resonance techniques. Langmuir 1995, 11, 1464–1467. [Google Scholar] [CrossRef]
- Geetha, B.; Mandal, A.B. Determination of the critical micelle concentration of the methoxy polyethylene glycol based macromonomer and partition coefficient of a new electrochemical probe using a cyclic voltammetric technique. Langmuir ACS J. Surf. Colloids 1997, 13, 2410–2413. [Google Scholar] [CrossRef]
- Yusaf, A.; Usman, M.; Siddiq, M.; Bakhtiar, M.; Mansha, A.; Shaukat, S.; Rehman, H.F. Mixed Micellar Solubilization of Naphthol Green B Followed by Its Removal from Synthetic Effluent by Micellar-Enhanced Ultrafiltration under Optimized Conditions. Molecules 2022, 27, 6436. [Google Scholar] [CrossRef]
- Mandal, A.B.; Nair, B.U. Cyclic voltammetric technique for the determination of the critical micelle concentration of surfactants, self-diffusion coefficient of micelles, and partition coefficient of an electrochemical probe. J. Phys. Chem. 1991, 95, 9008–9013. [Google Scholar] [CrossRef]
- BaranáMandal, A.; UnniáNair, B. Cyclic voltammetric studies on the ternary system decaglycerol dioleate–heptane–water. J. Chem. Soc. Faraday Trans. 1991, 87, 133–136. [Google Scholar]
- Li, T.; Liu, L.; Zhang, Z.; Han, Z. Preparation of nanofibrous metal-organic framework filter for rapid adsorption and selective separation of cationic dye from aqueous solution. Sep. Purif. Technol. 2020, 237, 116360. [Google Scholar] [CrossRef]
- Yusaf, A.; Usman, M.; Mansha, A.; Saeed, M.; Ahmad, M.; Siddiq, M. Micellar-enhanced ultrafiltration (MEUF) for removal of rhodamine B (RhB) from aqueous system. J. Dispers. Sci. Technol. 2020, 43, 1–13. [Google Scholar] [CrossRef]
- Krishnan, R.S.G.; Thennarasu, S.; Mandal, A.B. Self-assembling characteristics of a new nonionic gemini surfactant. J. Phys. Chem. B 2004, 108, 8806–8816. [Google Scholar] [CrossRef]
- Huang, J.; Li, H.; Zeng, G.; Shi, L.; Gu, Y.; Shi, Y.; Tang, B.; Li, X. Removal of Cd(II) by MEUF-FF with anionic-nonionic mixture at low concentration. Sep. Purif. Technol. 2018, 207, 199–205. [Google Scholar] [CrossRef]
- Schwarze, M.; Schaefer, L.; Chiappisi, L.; Gradzielski, M. Micellar enhanced ultrafiltration (MEUF) of methylene blue with carboxylate surfactants. Sep. Purif. Technol. 2018, 199, 20–26. [Google Scholar] [CrossRef]
- Rafique, R.F.; Lee, S. Micellar Enhanced Ultrafiltration (MEUF) and Activated Carbon Fiber (ACF) Hybrid Processes for the Removal of Cadmium from an Aqueous Solution. Korean Chem. Eng. Res. 2014, 52, 775–780. [Google Scholar] [CrossRef]
- Jafari, A.; Rezaee, R.; Nasseri, S.; Mahvi, A.H.; Maleki, A.; Safari, M.; Shahmoradi, B.; Daraei, H. Application of micellar enhanced ultrafiltration (MEUF) for arsenic (v) removal from aqueous solutions and process optimization. J. Dispers. Sci. Technol. 2017, 38, 1588–1593. [Google Scholar] [CrossRef]
- Gohain, B.; Sarma, S.; Dutta, R.K. Protonated dye-surfactant ion pair formation between neutral red and anionic surfactants in aqueous submicellar solutions. J. Mol. Liq. 2008, 142, 130–135. [Google Scholar] [CrossRef]
- Panda, M.; Kabir-ud-Din. Solubilization of polycyclic aromatic hydrocarbons by gemini–conventional mixed surfactant systems. J. Mol. Liq. 2013, 187, 106–113. [Google Scholar] [CrossRef]
- Noor, S.; Taj, M.B.; Ashar, A. Solubilization of cationic dye in single and mixed micellar media. J. Mol. Liq. 2021, 330, 115613. [Google Scholar] [CrossRef]
- Akhter, M.S. Effect of acetamide on the critical micelle concentration of aqueous solutions of some surfactants. Colloids Surf. A Physicochem. Eng. Asp. 1997, 121, 103–109. [Google Scholar] [CrossRef]
- Haq, N.U.; Usman, M.; Mansha, A.; Rashid, M.A.; Munir, M.; Rana, U.A. Solubilization of reactive blue 19 by the micelles of cationic surfactant Cetyltrimethyl ammonium bromide (CTAB). J. Mol. Liq. 2014, 196, 264–269. [Google Scholar] [CrossRef]
- Wang, J.; Lin, C.Y.; Moore, C.; Jhunjhunwala, A.; Jokerst, J.V. Switchable Photoacoustic Intensity of Methylene Blue via Sodium Dodecyl Sulfate Micellization. Langmuir ACS J. Surf. Colloids 2018, 34, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Zeng, G.M.; Zhou, C.F.; Li, X.; Shi, L.J.; He, S.B. Adsorption of surfactant micelles and Cd2+/Zn2+ in micellar-enhanced ultrafiltration. J. Hazard. Mater. 2010, 183, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Taj Muhammad, M.; Khan, M.N. Study of electrolytic effect on the interaction between anionic surfactant and methylene blue using spectrophotometric and conductivity methods. J. Mol. Liq. 2017, 234, 309–314. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, S.H. Heavy metals removal from aqueous solution through micellar enhanced ultrafiltration: A review. Environ. Eng. Res. 2018, 24, 363–375. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 1–15. [Google Scholar] [CrossRef]
- Muntaha, S.T.; Khan, M.N. Study of changes in conductivity and spectral behaviour before and after micelle formation in the dye-surfactant system. J. Mol. Liq. 2014, 197, 191–196. [Google Scholar] [CrossRef]
- Safavi, A.; Abdollahi, H.; Maleki, N.; Zeinali, S. Interaction of anionic dyes and cationic surfactants with ionic liquid character. J. Colloid Interface Sci. 2008, 322, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Saeed, R.; Usman, M.; Rasool, N.; Ahmad, M.; Khan, Z.A.; Farooqi, Z.H.; Siddiq, M.; Zahoor, A.F. Partitioning of thiophene derivatives between solvent and micellar media of cationic surfactant, cetyl trimethyl ammonium bromide. J. Mol. Liq. 2017, 240, 389–394. [Google Scholar] [CrossRef]
- Mungray, A.; Kulkarni, S.; Mungray, A. Removal of heavy metals from wastewater using micellar enhanced ultrafiltration technique: A review. Open Chem. 2012, 10, 27–46. [Google Scholar] [CrossRef]
- Schwarze, M. Micellar-enhanced ultrafiltration (MEUF)–state of the art. Environ. Sci. Water Res. Technol. 2017, 3, 598–624. [Google Scholar] [CrossRef]
- Koyuncu, I.; Topacik, D.; Wiesner, M.R. Factors influencing flux decline during nanofiltration of solutions containing dyes and salts. Water Res. 2004, 38, 432–440. [Google Scholar] [CrossRef]
- Hassani, A.H.; Mirzayee, R.; Nasseri, S.; Borghei, M.; Gholami, M.; Torabifar, B. Nanofiltration process on dye removal from simulated textile wastewater. Int. J. Environ. Sci. Technol. 2008, 5, 401–408. [Google Scholar] [CrossRef]
- Zaghbani, N.; Hafiane, A.; Dhahbi, M. Removal of Eriochrome Blue Black R from wastewater using micellar-enhanced ultrafiltration. J. Hazard. Mater. 2009, 168, 1417–1421. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Puasa, S.W. Reactive dyes decolourization from an aqueous solution by combined coagulation/micellar-enhanced ultrafiltration process. Chem. Eng. J. 2007, 132, 257–265. [Google Scholar] [CrossRef]
- Tanhaei, B.; Pourafshari Chenar, M.; Saghatoleslami, N.; Hesampour, M.; Laakso, T.; Kallioinen, M.; Sillanpää, M.; Mänttäri, M. Simultaneous removal of aniline and nickel from water by micellar-enhanced ultrafiltration with different molecular weight cut-off membranes. Sep. Purif. Technol. 2014, 124, 26–35. [Google Scholar] [CrossRef]
- Daniş, Ü.; Keskinler, B. Chromate removal from wastewater using micellar enhanced crossflow filtration: Effect of transmembrane pressure and crossflow velocity. Desalination 2009, 249, 1356–1364. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Puasa, S.W.; Zulkali, M.M.D. Micellar-enhanced ultrafiltration for removal of reactive dyes from an aqueous solution. Desalination 2006, 191, 153–161. [Google Scholar] [CrossRef]
- Xu, K.; Zeng, G.-m.; Huang, J.-h.; Wu, J.-y.; Fang, Y.-y.; Huang, G.; Li, J.; Xi, B.; Liu, H. Removal of Cd2+ from synthetic wastewater using micellar-enhanced ultrafiltration with hollow fiber membrane. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 140–146. [Google Scholar] [CrossRef]
- Zaghbani, N.; Hafiane, A.; Dhahbi, M. Separation of methylene blue from aqueous solution by micellar enhanced ultrafiltration. Sep. Purif. Technol. 2007, 55, 117–124. [Google Scholar] [CrossRef]
- Kumar, S.; Nandi, B.K.; Guria, C.; Mandal, A. Oil Removal from Produced Water by Ultrafiltration using Polysulfone Membrane. Braz. J. Chem. Eng. 2017, 34, 583–596. [Google Scholar] [CrossRef]
- Son, G.; Lee, S. Application of micellar enhanced ultrafiltration and activated carbon fiber hybrid processes for lead removal from an aqueous solution. Korean J. Chem. Eng. 2011, 28, 793–799. [Google Scholar] [CrossRef]
- Abedi, S.; Nekouei, F. Removal of direct yellow 12 from water samples by cloud point extraction using Triton X-100 as nonionic surfactant. E-J. Chem. 2011, 8, 1588–1595. [Google Scholar] [CrossRef]
- Yusaf, A.; Usman, M.; Mansha, A.; Siddiq, M.; Amjad, Z.; Irshad, S.; Sultana, H. Self-organised surfactant assemblies as nanostructured dye carriers: A mixed micellar comparative approach for enhanced dye solubilisation. Int. J. Environ. Anal. Chem. 2022, 1–12. [Google Scholar] [CrossRef]
- Hanif, S.; Usman, M.; Hussain, A.; Rasool, N.; Zubair, M.; Rana, U.A. Solubilization of Benzothiazole (BNZ) by micellar media of Sodium dodecyl sulphate and Cetyl trimethylammonium bromide. J. Mol. Liq. 2015, 211, 7–14. [Google Scholar] [CrossRef]
- Banipal, T.S.; Kaur, R.; Banipal, P.K. Effect of sodium chloride on the interactions of ciprofloxacin hydrochloride with sodium dodecyl sulfate and hexadecyl trimethylammonium bromide: Conductometric and spectroscopic approach. J. Mol. Liq. 2018, 255, 113–121. [Google Scholar] [CrossRef]
- Ali, A.; Uzair, S.; Malik, N.A.; Ali, M. Study of interaction between cationic surfactants and cresol red dye by electrical conductivity and spectroscopy methods. J. Mol. Liq. 2014, 196, 395–403. [Google Scholar] [CrossRef]
- Rehman, A.; Nisa, M.U.; Usman, M.; Ahmad, Z.; Bokhari, T.H.; Rahman, H.M.A.U.; Rasheed, A.; Kiran, L. Application of cationic-nonionic surfactant based nanostructured dye carriers: Mixed micellar solubilization. J. Mol. Liq. 2021, 326, 115345. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K. Solubilization of Hydrophobic Dyes in Surfactant Solutions. Materials 2013, 6, 580–608. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, S.; Sohrabi, B.; Tehrani-Bagha, A.R. The study of Sunset Yellow anionic dye interaction with gemini and conventional cationic surfactants in aqueous solution. Dyes Pigments 2012, 95, 768–775. [Google Scholar] [CrossRef]
- Saeed, R.; Usman, M.; Mansha, A.; Rasool, N.; Naqvi, S.A.R.; Zahoor, A.F.; Rahman, H.M.A.; Rana, U.A.; Al-Zahrani, E. Partitioning of structurally related thiophene derivatives between solvent and micellar media of anionic surfactant sodium dodecyl sulphate. Colloids Surf. A Physicochem. Eng. Asp. 2017, 512, 51–60. [Google Scholar] [CrossRef]
- Rehman, A.; Usman, M.; Bokhari, T.H.; Haq, A.u.; Saeed, M.; Rahman, H.M.A.U.; Siddiq, M.; Rasheed, A.; Nisa, M.U. The application of cationic-nonionic mixed micellar media for enhanced solubilization of Direct Brown 2 dye. J. Mol. Liq. 2020, 301, 112408. [Google Scholar] [CrossRef]


| Surfactants | Kc × 10−4 (dm3mol−1) | Kb × 10−5 (dm3mol−1) | Kx × 10−5 | ΔGb (kJmol−1) | ΔGp (kJmol−1) |
|---|---|---|---|---|---|
| SDS | 0.268 | 2.50 | 1.49 | −19.38 | −29.50 |
| SO | 0.003 | 1.67 | 0.15 | −12.67 | −23.95 |
| Sr. No. | CS (mM) | Cf (mM) | Cp (μM) | R % | t (h) | Vp (mL) | J (L/hm2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDS | SO | SDS | SO | SDS | SO | SDS | SO | SDS | SO | |||
| 1 | 0.0 | 0.0 | 0.01 | 0.113 | 0.318 | 98.86 | 96.81 | 0.20 | 0.02 | 30 | 349 | 3262 |
| 2 | 8.3 | 2.2 | 0.01 | 0.099 | 0.102 | 99.00 | 98.97 | 0.22 | 0.15 | 30 | 324 | 458 |
| 3 | 8.6 | 2.5 | 0.01 | 0.098 | 0.092 | 99.01 | 99.07 | 0.23 | 0.19 | 30 | 306 | 377 |
| 4 | 9.0 | 3.0 | 0.01 | 0.097 | 0.088 | 99.02 | 99.11 | 0.24 | 0.20 | 30 | 298 | 348 |
| 5 | 10.0 | 4.0 | 0.01 | 0.096 | 0.087 | 99.03 | 99.12 | 0.25 | 0.22 | 30 | 278 | 324 |
| 6 | 11.0 | 5.0 | 0.01 | 0.094 | 0.084 | 99.05 | 99.15 | 0.26 | 0.23 | 30 | 267 | 303 |
| 7 | 12.0 | 6.0 | 0.01 | 0.090 | 0.080 | 99.09 | 99.19 | 0.28 | 0.25 | 30 | 247 | 261 |
| Sr. No. | [NaCl] (mM) | Cf (mM) | Cp (μM) | R % | t (h) | Vp (mL) | J (L/hm2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SDS | SO | SDS | SO | SDS | SO | SDS | SO | ||||
| 1 | 20 | 0.1 | 0.099 | 0.097 | 99.90 | 99.902 | 0.87 | 1.05 | 30 | 82.2 | 67.9 |
| 2 | 40 | 0.1 | 0.088 | 0.048 | 99.91 | 99.951 | 0.88 | 1.15 | 30 | 81.2 | 62.4 |
| 3 | 60 | 0.1 | 0.074 | 0.038 | 99.92 | 99.961 | 0.92 | 1.22 | 30 | 77.8 | 58.7 |
| 4 | 80 | 0.1 | 0.070 | 0.037 | 99.92 | 99.962 | 1.03 | 1.30 | 30 | 69.2 | 55.0 |
| 5 | 100 | 0.1 | 0.058 | 0.036 | 99.94 | 99.963 | 1.13 | 1.38 | 30 | 63.5 | 52.0 |
| 6 | 120 | 0.1 | 0.047 | 0.034 | 99.95 | 99.970 | 1.17 | 1.50 | 30 | 61.3 | 47.8 |
| Sr. No. | Pressure (Bar) | Cf (mM) | Cp (μM) | R % | t (h) | Vp (mL) | J (L/hm2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SDS | SO | SDS | SO | SDS | SO | SDS | SO | ||||
| 1 | 5 | 0.01 | 0.01 | 0.010 | 99.8 | 89.1 | 0.34 | 1.33 | 30 | 210 | 53 |
| 2 | 10 | 0.01 | 0.03 | 0.018 | 99.6 | 81.8 | 0.20 | 1.27 | 30 | 345 | 56 |
| 3 | 15 | 0.01 | 0.04 | 0.025 | 99.5 | 74.5 | 0.17 | 0.92 | 30 | 416 | 77 |
| 4 | 20 | 0.01 | 0.05 | 0.038 | 99.4 | 61.8 | 0.11 | 0.71 | 30 | 598 | 100 |
| 5 | 25 | 0.01 | 0.05 | 0.047 | 99.4 | 52.8 | 0.09 | 0.55 | 30 | 790 | 129 |
| 6 | 30 | 0.01 | 0.06 | 0.058 | 99.3 | 41.9 | 0.08 | 0.34 | 30 | 856 | 208 |
| Sr. No. | RPM | Cf (mM) | Cp (μM) | R % | T (h) | Vp (mL) | J (L/hm2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SDS | SO | SDS | SO | SDS | SO | SDS | SO | ||||
| 1 | 10 | 0.01 | 0.081 | 0.081 | 99.918 | 99.9991 | 0.154 | 0.307 | 30 | 464 | 233 |
| 2 | 20 | 0.01 | 0.083 | 0.087 | 99.916 | 99.99912 | 0.17 | 0.316 | 30 | 422 | 226 |
| 3 | 30 | 0.01 | 0.085 | 0.094 | 99.914 | 99.99905 | 0.174 | 0.353 | 30 | 412 | 202 |
| 4 | 40 | 0.01 | 0.091 | 0.097 | 99.908 | 99.99902 | 0.185 | 0.372 | 30 | 387 | 192 |
| 5 | 50 | 0.01 | 0.092 | 0.103 | 99.907 | 99.99896 | 0.200 | 0.388 | 30 | 358 | 184 |
| Sr. No. | pH | Cf (mM) | Cp (μM) | R % | t (h) | Vp (mL) | J (L/hm2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SDS | SO | SDS | SO | SDS | SO | SDS | SO | ||||
| 1 | 4 | 0.01 | 0.08 | 0.078 | 99.00 | 99.05 | 0.26 | 0.30 | 30 | 267 | 233 |
| 2 | 7 | 0.01 | 0.09 | 0.082 | 99.06 | 99.17 | 0.3 | 0.35 | 30 | 239 | 201 |
| 3 | 10 | 0.01 | 0.01 | 0.094 | 99.15 | 99.21 | 0.36 | 0.425 | 30 | 194 | 168 |
| Chemicals | CAS Reg. No. | Purity | Molecular Mass (gmol−1) | Supplier | Formulae | λmax | |
|---|---|---|---|---|---|---|---|
| This Work | Literature Reported | ||||||
| Cobalt (II) chloride | 7646-79-9 | 97% | 129.83 | Sigma Aldrich | CoCl2 | 512 | 512 |
| Sodium dodecyl sulfate (SDS) | 151-21-3 | 99% | 288.372 | Daejung Korea | NaC12H25SO4 | ||
| Sodium oleate (SO) | 148-19-1 | ≥99 | 304.4 | Daejung Korea | C18H33NaO2 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusaf, A.; Usman, M.; Ahmad, M.; Siddiq, M.; Mansha, A.; Al-Hussain, S.A.; Zaki, M.E.A.; Rehman, H.F. Highly Selective Methodology for Entrapment and Subsequent Removal of Cobalt (II) Ions under Optimized Conditions by Micellar-Enhanced Ultrafiltration. Molecules 2022, 27, 8332. https://doi.org/10.3390/molecules27238332
Yusaf A, Usman M, Ahmad M, Siddiq M, Mansha A, Al-Hussain SA, Zaki MEA, Rehman HF. Highly Selective Methodology for Entrapment and Subsequent Removal of Cobalt (II) Ions under Optimized Conditions by Micellar-Enhanced Ultrafiltration. Molecules. 2022; 27(23):8332. https://doi.org/10.3390/molecules27238332
Chicago/Turabian StyleYusaf, Amnah, Muhammad Usman, Matloob Ahmad, Muhammad Siddiq, Asim Mansha, Sami A. Al-Hussain, Magdi E. A. Zaki, and Hafiza Fatima Rehman. 2022. "Highly Selective Methodology for Entrapment and Subsequent Removal of Cobalt (II) Ions under Optimized Conditions by Micellar-Enhanced Ultrafiltration" Molecules 27, no. 23: 8332. https://doi.org/10.3390/molecules27238332
APA StyleYusaf, A., Usman, M., Ahmad, M., Siddiq, M., Mansha, A., Al-Hussain, S. A., Zaki, M. E. A., & Rehman, H. F. (2022). Highly Selective Methodology for Entrapment and Subsequent Removal of Cobalt (II) Ions under Optimized Conditions by Micellar-Enhanced Ultrafiltration. Molecules, 27(23), 8332. https://doi.org/10.3390/molecules27238332








