Role of Phenylpropanoids and Flavonoids in Plant Resistance to Pests and Diseases
Abstract
:1. Introduction
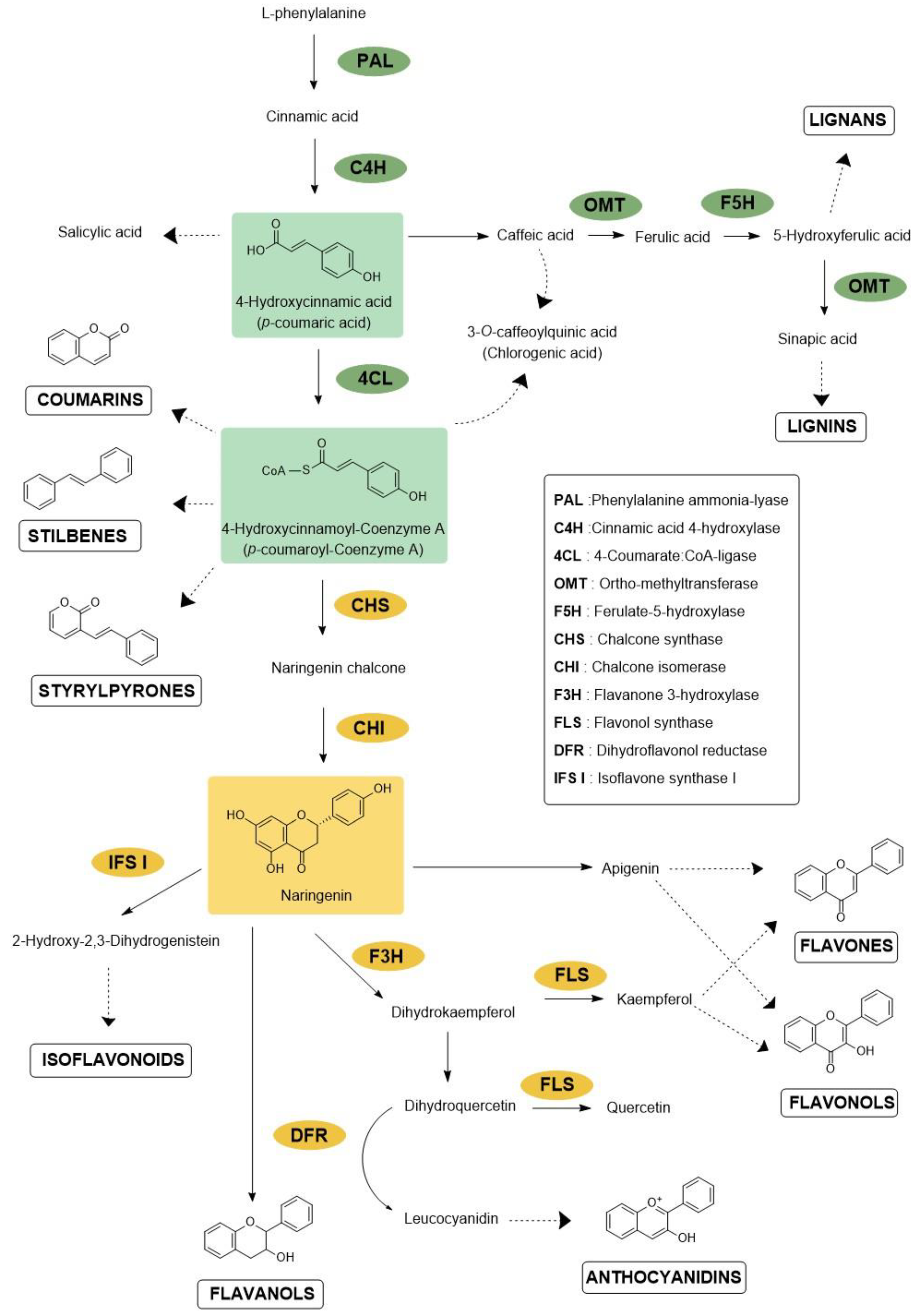
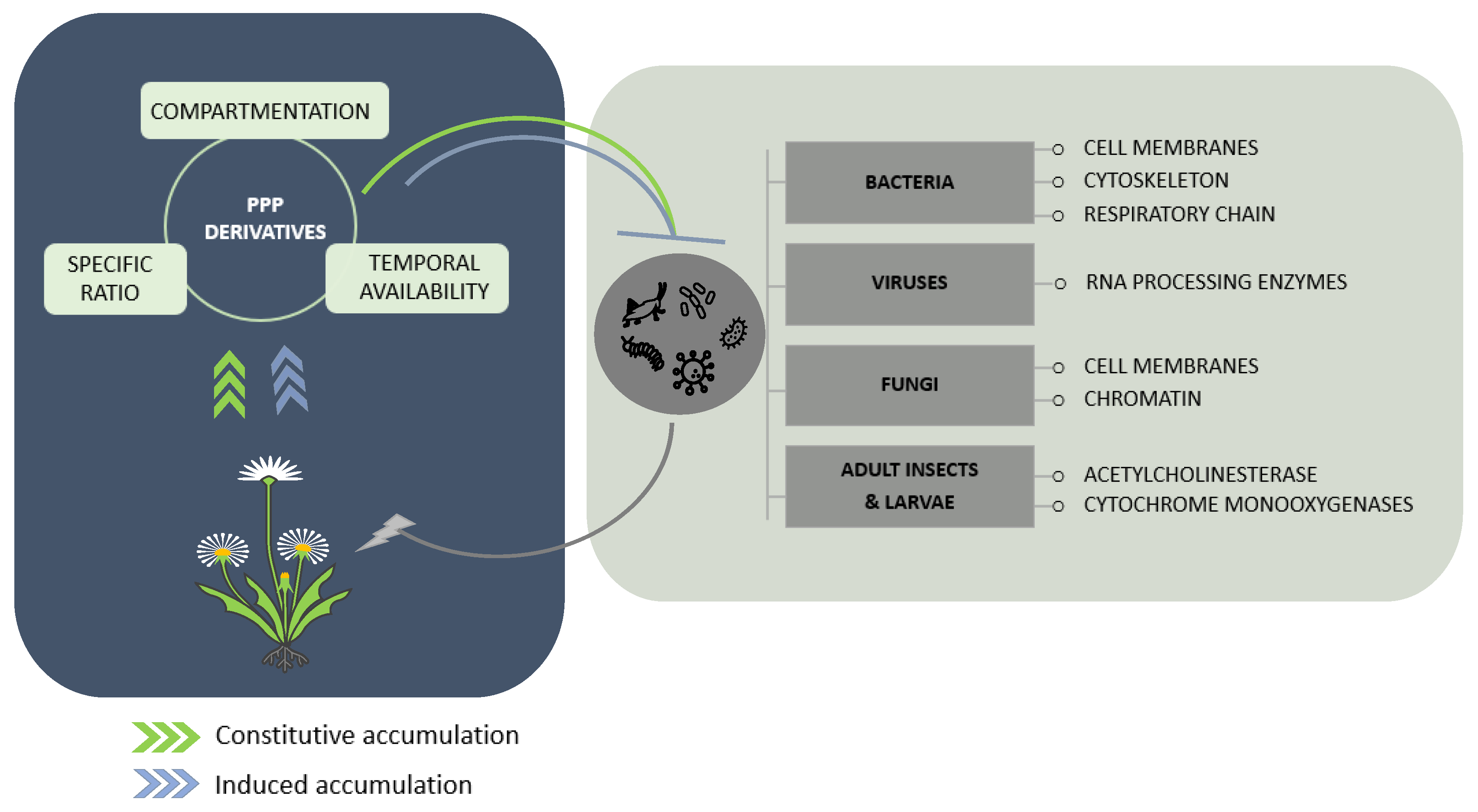
2. Protection against Microbes
2.1. Bacterial Targets
2.2. Fungal Targets
2.3. Viral Targets
3. Protection against Insects
3.1. Herbivore Targets
3.2. Vector Targets
4. Prospects
| Compound | Host | Targets | Concentration | Mechanisms | Ref. |
|---|---|---|---|---|---|
| Phenylpropanoids | |||||
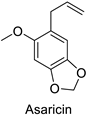 | Piper sarmentosum | Aedes aegypti | IC50 = 0.73 µg·mL−1 | Inhibition of acetylcholinesterase activity | [105] |
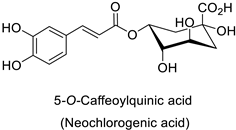 | Tobacco (Nicotiana tabacum) | Tobacco mosaic virus (TMV) | - | Accumulation at the infection site | [11] |
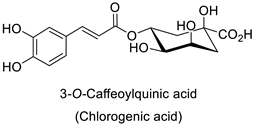 | Human | Escherichia coli | IC50 = 69.55 ± 3.6 µM | Inhibition of FtsZ polymerase (a cell division protein) | [55] |
| Shigella dysenteriae | MIC = 20 µg·mL−1 | Bacterial outer membrane disintegration | [52] | ||
| Candida albicans, Trichosporon beigelii and Malassezia furfur | MIC = 40–80 µg·mL−1 | Deterioration of membrane electrical potential | [76] | ||
| Groundnut (Arachis hypogaea) | Spodoptera litura (Fab.) | * MC50 = 0.67 µg·mL−1 | Larvicidal activity or limitation of larval development and adult malformation | [86] | |
| Chrysanthemum (Dendranthema grandiflora) | Frankliniella occidentalis | 5% in culture medium | Reduction of larval growth rate and adults survival rate | [85] | |
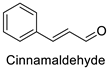 | Tomato (Solanum lycopersicum) | Alternaria alternata | MIC = 800 µg·mL−1 (Essential oil containing 30% of cinnamaldehyde and 44% of eugenol) | Cell wall invaginations and distortions | [75] |
 | Tomato (Solanum lycopersicum) | Alteranaria alternata | MIC = 800 µg·mL−1 (Essential oil containing 30% of cinnamaldehyde and 44% of eugenol) | Cell wall invaginations and distortions | [75] |
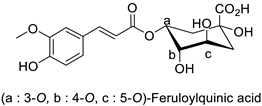 | Chrysanthemum (Dendranthema grandiflora) | Frankliniella occidentalis | 5% in culture medium | Reduction of larval growth rate and adults survival rate | [85] |
| Carrot (Daucus carota) | Alternaria dauci | - | - | [57] | |
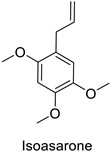 | Piper sarmentosum | Aedes aegypti | IC50 = 0.92 µg·mL−1 | Inhibition of acetylcholinesterase activity | [105] |
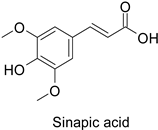 | Carrot (Daucus carota) | Psila rosae | 0.35–2.11 mg·g−1 | Insecticidal activity | [87] |
 | Piper sarmentosum | Aedes aegypti | IC50 = 15.75 µg·mL−1 | Inhibition of acetylcholinesterase activity | [105] |
| Flavonoids | |||||
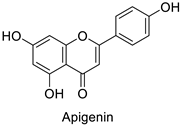 | Human | Candida albicans | 5µg·mL−1 | Cell shrinkage due to membrane disruption | [77] |
| Anopheles minimus | IC50 = 2.38 µM | Inhibition of cytochrome P450 monooxygenases | [104] | ||
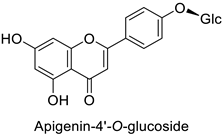 | Carrot (Daucus carota) | Alternaria dauci | - | - | [57] |
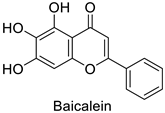 | Human | Murine leukemia viruses (MLVs) | 90% of inhibition at 1 µg·mL−1 | Inhibition of reverse transcriptase activity | [82] |
| Human immunodeficiency viruses (HIVs) | 90% of inhibition at 2 µg·mL−1 | ||||
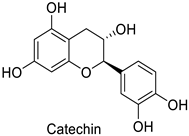 | Cotton (Gossypium hirsutum and Gossypium barbadense) | Verticillium dahliae | - | Confinement of the pathogen at the infection site | [61] |
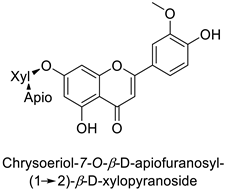 | Human | Staphylococcus aureus | MIC = 4–8 µg·mL−1 | Cytoplasmic membrane disruption | [48] |
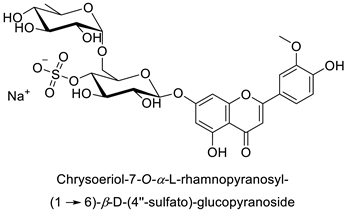 | |||||
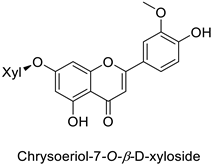 | |||||
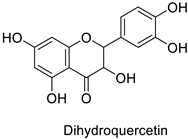 | - | Fusarium graminearum | IC50 = 124.27 mg·L−1 | Mycelial growth inhibition | [74] |
| - | Septoria zeicola | IC50 = 160.32 mg·L−1 | |||
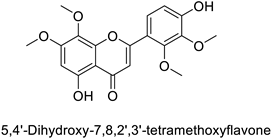 | Human | Anopheles minimus | IC50 = 5.91–16.6 µM | Inhibition of cytochrome P450 monooxygenases | [104] |
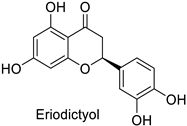 | - | Fusarium graminearum | IC50 = 162.71 mg·L−1 | Mycelial growth inhibition | [74] |
| - | Septoria zeicola | IC50 = 247.32 mg·L−1 | |||
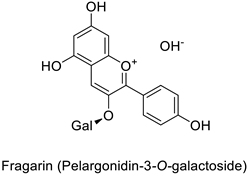 | Strawberry (Fragaria ananassa) | Clavibacter michiganensis | EC50 = 0.07 µM | Cell membrane permeabilization | [44,45] |
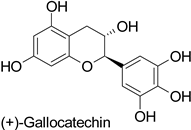 | Cotton (Gossypium hirsutum and Gossypium barbadense) | Verticillium dahliae | - | Confinement of the pathogen at the infection site | [61] |
 | Human | Candida glabrata | MIC = 4–16 µg·mL−1 | DNA fragmentation and chromatin condensation | [78] |
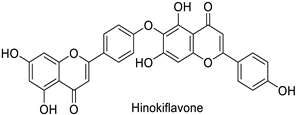 | Human | Human immunodeficiency virus | IC50 = 62 µM | Inhibition of reverse transcriptase activity | [83] |
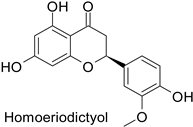 | - | Fusarium graminearum | IC50 = 274.78 mg·L−1 | Mycelial growth inhibition | [74] |
| - | Septoria zeicola | IC50 = 240.31 mg·L−1 | |||
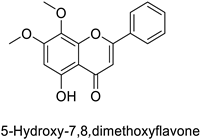 | Human | Anopheles minimus | IC50 = 7.24–8.90 µM | Inhibition of cytochrome P450 monooxygenases | [104] |
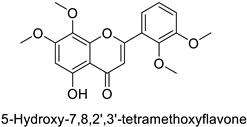 | IC50 = 6.45–8.35 µM | ||||
 | Human | Staphylococcus aureus | MIC = 4–8 µg·mL−1 | Cytoplasmic membrane disruption | [48] |
 | Tobacco (Nicotiana tabacum) | Tobacco mosaic virus (TMV) | - | Accumulation at SAR site | [11] |
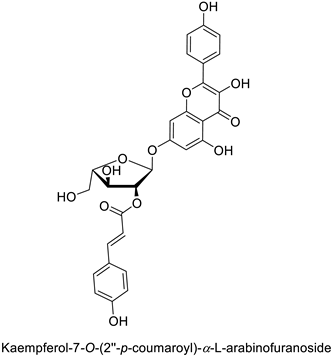 | Pine tree (Picea neoveitchii) | Rhizoctonia solani | 49.5% of growth inhibition at 1 mg·mL−1 | Mycelial growth inhibition | [58] |
| Fusarium oxysporum | 108.1% of growth inhibition at 1 mg·mL−1 | ||||
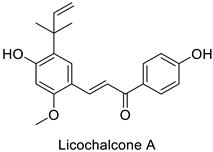 | Human | Micrococcus luteus | MIC = 1.56 µg mL−1 | Inhibition of NADH-cytochrome C reductase | [51] |
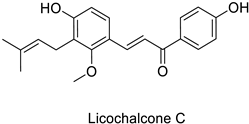 | MIC = 6.25 µg mL−1 | ||||
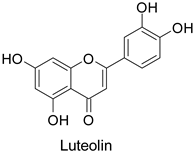 | Carrot (Daucus carota) | Psila rosae | 0.35–2.11 mg·g−1 | Insecticidal activity | [87] |
| - | Fusarium graminearum | IC50 = 56.38 mg·L−1 | Mycelial growth inhibition | [74] | |
| - | Septoria zeicola | IC50 = 81.48 mg·L−1 | |||
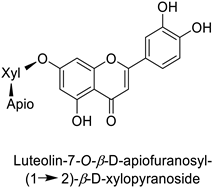 | Human | Staphylococcus aureus | MIC = 4–8 µg·mL−1 | Cytoplasmic membrane disruption | [48] |
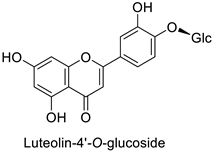 | Carrot (Daucus carota) | Alternaria dauci | - | - | [57] |
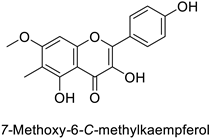 | Pine tree (Picea neoveitchii) | Rhizoctonia solani | 95.3% of growth inhibition at 1 mg·mL−1 | Mycelial growth inhibition | [58] |
 | Human | Staphylococcus aureus | MIC = 1.84 mM | Membrane fatty acid rearrangement leading to membrane integrity and fluidity alteration. | [49] |
| Escherichia coli | MIC = 3.64 mM | ||||
| Tomato (Solanum lycopersicum) | Bemisia tabaci | 10 µM | Oviposition deterrent | [95] | |
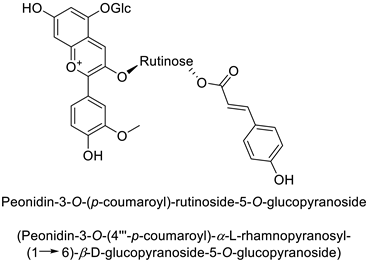 | Potato (Solanum tuberosum) | Erwinia carotovora | - | - | [42] |
 | |||||
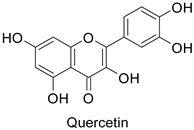 | Tobacco (Nicotiana tabacum) | Tobacco mosaic virus (TMV) | - | Accumulation at the infection site | [11] |
| Datura stramonium and Chenopodium amaranticolor | 250 µM | Putative host defense mediation through salicylic acid biosynthesis | [81] | ||
| Groundnut (Arachis hypogaea) | Spodoptera litura (Fab.) | * MC50 = 0.73 µg·mL−1 | Larvicidal activity or limitation of larval development and adult malformation | [86] | |
| Stored legumes | Callosobruchus chinensis L. | 3 mg·mL−1 (leaf extract mainly containing quercetin) | Insecticidal activity | [89] | |
| 6 mg·mL−1 (leaf extract mainly containing quercetin) | Oviposition deterrent Ovicidal activity | ||||
| Datura stramonium and Chenopodium amaranticolor | Tobacco mosaic virus (TMV) | 250 µM | Putative host defense mediation through kaempferol and salicylic acid biosynthesis | [81] | |
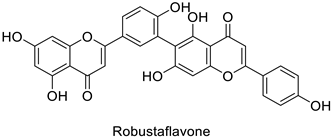 | Human | Human immunodeficiency virus | IC50 = 65 µM | Inhibition of reverse transcriptase activity | [83] |
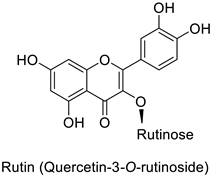 | Potato (Solanum tuberosum) | Pectobacterium atrosepticum | 88 µg·mL−1 | - | [41] |
| Groundnut (Arachis hypogaea) | Spodoptera litura (Fab.) | * MC50 = 0.60 µg·mL−1 | Larvicidal activity or limitation of larval development and adult malformation | [86] | |
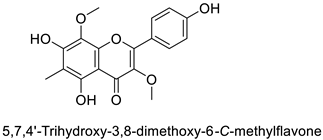 | Pine tree (Picea neoveitchii) | Rhizoctonia solani | 49.5% of growth inhibition at 1 mg·mL−1 | Mycelial growth inhibition | [58] |
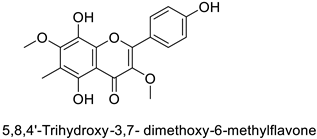 | 53.3% of growth inhibition at 1 mg·mL−1 | ||||
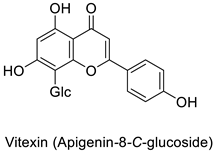 | Datura stramonium and Chenopodium amaranticolor | Tobacco mosaic virus (TMV) | 250 µM | Putative host defense mediation through kaempferol and salicylic acid biosynthesis | [81] |
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vining, L.C. Functions of Secondary Metabolites. Annu. Rev. Microbiol. 1990, 44, 395–427. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of Flavonoids in Plant Resistance: A Review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. CRC. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Bednarek, P. Chemical Warfare or Modulators of Defence Responses—The Function of Secondary Metabolites in Plant Immunity. Curr. Opin. Plant Biol. 2012, 15, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, A.; Jedrzejczak-Rey, N.; Bednarek, P. Secondary Metabolites in Plant Innate Immunity: Conserved Function of Divergent Chemicals. New Phytol. 2015, 206, 948–964. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P.C.; Nicolson, S.W.; Wright, G.A. Plant Secondary Metabolites in Nectar: Impacts on Pollinators and Ecological Functions. Funct. Ecol. 2017, 31, 65–75. [Google Scholar] [CrossRef]
- Pichersky, E.; Lewinsohn, E. Convergent Evolution in Plant Specialized Metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tissier, A.; Ziegler, J.; Vogt, T. Specialized Plant Metabolites: Diversity and Biosynthesis. In Ecological Biochemistry: Environmental and Interspecies Interactions; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 14–37. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived from the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar]
- Kim, J.; Buel, C.R. A Revolution in Plant Metabolism: Genome-Enabled Pathway Discovery. Plant Physiol. 2015, 169, 1532–1539. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.H.; Kim, H.K.; Linthorst, H.J.M.; Hollander, J.G.; Lefeber, A.W.M.; Erkelens, C.; Nuzillard, J.M.; Verpoorte, R. NMR Metabolomics to Revisit the Tobacco Mosaic Virus Infection in Nicotiana Tabacum Leaves. J. Nat. Prod. 2006, 69, 742–748. [Google Scholar] [CrossRef]
- Tugizimana, F.; Djami-Tchatchou, A.T.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Metabolomic Analysis of Defense-Related Reprogramming in Sorghum Bicolor in Response to Colletotrichum Sublineolum Infection Reveals a Functional Metabolic Web of Phenylpropanoid and Flavonoid Pathways. Front. Plant Sci. 2019, 9, 1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannoufa, A.; Varin, L.; Ibrahim, R.K. Spatial Distribution of Flavonoid Conjugates in Relation to Glucosyltransferase and Sulfotransferase Activities in Flaveria Bidentis. Plant Physiol. 1991, 97, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagrean-Tuza, C.; Mot, A.C.; Chmiel, T.; Bende, A.; Turcu, I. Sugar Matters: Sugar Moieties as Reactivity-Tuning Factors in Quercetin: O-Glycosides. Food Funct. 2020, 11, 5293–5307. [Google Scholar] [CrossRef] [PubMed]
- Bruneton, J. Pharmacognosie: Phytochimie et Plantes Médicinales; Tec & doc Cachan: Paris, France, 1999; ISBN 2-7430-0315-4. [Google Scholar]
- Samanta, A.; Das, G.; Das, S. Roles of Flavonoids in Plants. Carbon 2011, 100, 12–35. [Google Scholar]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [Green Version]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, H.; Yoshida, N.; Ishikawa, H.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Protection of Mitochondrial Functions against Oxidative Stresses by Isoflavans from Glycyrrhiza Glabra. J. Pharm. Pharmacol. 2010, 52, 219–223. [Google Scholar] [CrossRef]
- Rackova, L.; Firakova, S.; Kostalova, D.; Stefek, M.; Sturdik, E.; Majekova, M. Oxidation of Liposomal Membrane Suppressed by Flavonoids: Quantitative Structure-Activity Relationship. Bioorganic Med. Chem. 2005, 13, 6477–6484. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The Effect of Water Stress on Phytochemical Accumulation, Bioactive Compounds and Expression of Key Genes Involved in Flavonoid Biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The Biosynthesis of Flavonoids Is Enhanced Similarly by UV Radiation and Root Zone Salinity in L. vulgare Leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Flavonoids Are Determinants of Freezing Tolerance and Cold Acclimation in Arabidopsis Thaliana. Sci. Rep. 2016, 6, 34027. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Gravano, E.; Pinelli, P.; Mulinacci, N.; Romani, A. Flavonoids Accumulate in Leaves and Glandular Trichomes of Phillyrea Latifolia Exposed to Excess Solar Radiation. New Phytol. 2000, 148, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-Wide Analysis of Phenylpropanoid Defence Pathways. Mol. Plant Pathol. 2010, 11, 829–846. [Google Scholar] [CrossRef]
- Oros, G.; Kállai, Z. Phytoanticipins: The Constitutive Defense Compounds as Potential Botanical Fungicides. In Bioactive Molecules in Plant Defense: Signaling in Growth and Stress; Springer International Publishing: Cham, Switzerland, 2019; pp. 179–229. ISBN 9783030271657. [Google Scholar]
- Jeandet, P.; Hébrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the Role of Phytoalexins in Plant-Microorganism Interactions and Human Health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef] [Green Version]
- Hijaz, F.M.; Manthey, J.A.; Folimonova, S.Y.; Davis, C.L.; Jones, S.E.; Reyes-De-Corcuera, J.I. An HPLC-MS Characterization of the Changes in Sweet Orange Leaf Metabolite Profile Following Infection by the Bacterial Pathogen Candidatus Liberibacter Asiaticus. PLoS ONE 2013, 8, e79485. [Google Scholar] [CrossRef]
- Tan, B.A.; Daim, L.D.J.; Ithnin, N.; Ooi, T.E.K.; Md-Noh, N.; Mohamed, M.; Mohd-Yusof, H.; Appleton, D.R.; Kulaveerasingam, H. Expression of Phenylpropanoid and Flavonoid Pathway Genes in Oil Palm Roots during Infection by Ganoderma Boninense. Plant Gene 2016, 7, 11–20. [Google Scholar] [CrossRef]
- Zabala, G.; Zou, J.; Tuteja, J.; Gonzalez, D.O.; Clough, S.J.; Vodkin, L.O. Transcriptome Changes in the Phenylpropanoid Pathway of Glycine Max in Response to Pseudomonas Syringae Infection. BMC Plant Biol. 2006, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Szatmári, Á.; Zvara, Á.; Móricz, Á.M.; Besenyei, E.; Szabó, E.; Ott, P.G.; Puskás, L.G.; Bozsó, Z. Pattern Triggered Immunity (PTI) in Tobacco: Isolation of Activated Genes Suggests Role of the Phenylpropanoid Pathway in Inhibition of Bacterial Pathogens. PLoS ONE 2014, 9, e102869. [Google Scholar] [CrossRef]
- Matern, U.; Grimmig, B.; Kneusel, R.E. Plant Cell Wall Reinforcement in the Disease-Resistance Response: Molecular Composition and Regulation. Can. J. Bot. 1995, 73, 511–517. [Google Scholar] [CrossRef]
- Eynck, C.; Séguin-Swartz, G.; Clarke, W.E.; Parkin, I.A.P. Monolignol Biosynthesis Is Associated with Resistance to Sclerotinia Sclerotiorum in Camelina Sativa. Mol. Plant Pathol. 2012, 13, 887–899. [Google Scholar] [CrossRef] [PubMed]
- García-Lara, S.; Bergvinson, D.J.; Burt, A.J.; Ramputh, A.I.; Díaz-Pontones, D.M.; Arnason, J.T. The Role of Pericarp Cell Wall Components in Maize Weevil Resistance. Crop Sci. 2004, 44, 1546–1552. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Fauguel, C.M.; Vattuone, M.A.; Presello, D.A.; Catalán, C.A.N. Phenylpropanoids from Maize Pericarp: Resistance Factors to Kernel Infection and Fumonisin Accumulation by Fusarium Verticillioides. Eur. J. Plant Pathol. 2013, 135, 105–113. [Google Scholar] [CrossRef]
- Bily, A.C.; Reid, L.M.; Taylor, J.H.; Johnston, D.; Malouin, C.; Burt, A.J.; Bakan, B.; Regnault-Roger, C.; Pauls, K.P.; Arnason, J.T.; et al. Dehydrodimers of Ferulic Acid in Maize Grain Pericarp and Aleurone: Resistance Factors to Fusarium Graminearum. Phytopathology 2003, 93, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Rees, A.; Dodd, G.F.; Spencer, J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [Green Version]
- Crascì, L.; Basile, L.; Panico, A.; Puglia, C.; Bonina, F.P.; Basile, P.M.; Rizza, L.; Guccione, S. Correlating in Vitro Target-Oriented Screening and Docking: Inhibition of Matrix Metalloproteinases Activities by Flavonoids. Planta Med. 2017, 83, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Kröner, A.; Marnet, N.; Andrivon, D.; Val, F. Nicotiflorin, Rutin and Chlorogenic Acid: Phenylpropanoids Involved Differently in Quantitative Resistance of Potato Tubers to Biotrophic and Necrotrophic Pathogens. Plant Physiol. Biochem. 2012, 57, 23–31. [Google Scholar] [CrossRef]
- Lorenc-Kukuła, K.; Jafra, S.; Oszmiański, J.; Szopa, J. Ectopic Expression of Anthocyanin 5-O-Glucosyltransferase in Potato Tuber Causes Increased Resistance to Bacteria. J. Agric. Food Chem. 2005, 53, 272–281. [Google Scholar] [CrossRef]
- Yang, W.; Xu, X.; Li, Y.; Wang, Y.; Li, M.; Wang, Y.; Ding, X.; Chu, Z. Rutin-Mediated Priming of Plant Resistance to Three Bacterial Pathogens Initiating the Early SA Signal Pathway. PLoS ONE 2016, 11, e0146910. [Google Scholar] [CrossRef]
- Filippone, M.P.; Diaz-Ricci, J.C.; Castagnaro, A.P.; Farías, R.N. Effect of Fragarin on the Cytoplasmic Membrane of the Phytopathogen Clavibacter Michiganensis. Mol. Plant-Microbe Interact. 2001, 14, 925–928. [Google Scholar] [CrossRef]
- Filippone, M.P.; Diaz Ricci, J.; Mamaní De Marchese, A.; Farías, R.N.; Castagnaro, A. Isolation and Purification of a 316 Da Preformed Compound from Strawberry (Fragaria Ananassa) Leaves Active against Plant Pathogens. FEBS Lett. 1999, 459, 115–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Venegas, G.; Gómez-Mora, J.A.; Meraz-Rodríguez, M.A.; Flores-Sánchez, M.A.; Ortiz-Miranda, L.F. Effect of Flavonoids on Antimicrobial Activity of Microorganisms Present in Dental Plaque. Heliyon 2019, 5, e03013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagousop, C.N.; Tamokou, J.D.D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial Activities of Flavonoid Glycosides from Graptophyllum Grandulosum and Their Mechanism of Antibacterial Action. BMC Complement. Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef]
- Wang, L.H.; Zeng, X.A.; Wang, M.S.; Brennan, C.S.; Gong, D. Modification of Membrane Properties and Fatty Acids Biosynthesis-Related Genes in Escherichia Coli and Staphylococcus Aureus: Implications for the Antibacterial Mechanism of Naringenin. Biochim. Biophys. Acta Biomembr. 2018, 1860, 481–490. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial Activity of Flavonoids and Their Structure–Activity Relationship: An Update Review. Phyther. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of Antibacterial Action of Retrochalcones from Glycyrrhiza Inflata. Phytochemistry 1998, 48, 125–129. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial Activity and Mechanism of Plant Flavonoids to Gram-Positive Bacteria Predicted from Their Lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Soudaminikkutty, R.; Narasumani, M.L.; Doble, M. Phenylpropanoids Inhibit Protofilament Formation of Escherichia Coli Cell Division Protein FtsZ. J. Med. Microbiol. 2011, 60, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Bollina, V.; Kumaraswamy, G.K.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S.; Faubert, D.; Hamzehzarghani, H. Mass Spectrometry-Based Metabolomics Application to Identify Quantitative Resistance-Related Metabolites in Barley against Fusarium Head Blight. Mol. Plant Pathol. 2010, 11, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Koutouan, C.; Clerc, V.L.; Baltenweck, R.; Claudel, P.; Halter, D.; Hugueney, P.; Hamama, L.; Suel, A.; Huet, S.; Merlet, M.H.B.; et al. Link between Carrot Leaf Secondary Metabolites and Resistance to Alternaria Dauci. Sci. Rep. 2018, 8, 13746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.; Chen, W.; Du, X.; Zhang, H.; Lin, L.; Xu, H. Chemical Constituents of Picea Neoveitchii. Phytochemistry 2011, 72, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Wojakowska, A.; Muth, D.; Narożna, D.; Mądrzak, C.; Stobiecki, M.; Kachlicki, P. Changes of Phenolic Secondary Metabolite Profiles in the Reaction of Narrow Leaf Lupin (Lupinus angustifolius) Plants to Infections with Colletotrichum Lupini Fungus or Treatment with Its Toxin. Metabolomics 2013, 9, 575–589. [Google Scholar] [CrossRef] [Green Version]
- Yogendra, K.N.; Kushalappa, A.C.; Sarmiento, F.; Rodriguez, E.; Mosquera, T. Metabolomics Deciphers Quantitative Resistance Mechanisms in Diploid Potato Clones against Late Blight. Funct. Plant Biol. 2015, 42, 284–298. [Google Scholar] [CrossRef]
- Mace, M.E.; Bell, A.A.; Stipanovic, R.D. Histochemistry and Identification of Flavanols in Verticillium Wilt-Resistant and -Susceptible Cottons. Physiol. Plant Pathol. 1978, 13, 143–149. [Google Scholar] [CrossRef]
- Del Rio, J.A.; Gonzalez, A.; Fuster, M.D.; Botia, J.M.; Gomez, P.; Frias, V.; Ortuño, A. Tylose Formation and Changes in Phenolic Compounds of Grape. Phytopathol. Mediterr. 2001, 40, 394–399. [Google Scholar]
- Aist, J.R.; Gold, R.E.; Bayles, C.J.; Morrison, G.H.; Chandra, S.; Israel, H.W. Evidence That Molecular Components of Papillae May Be Involved in Ml-o Resistance to Barley Powdery Mildew. Physiol. Mol. Plant Pathol. 1988, 33, 17–32. [Google Scholar] [CrossRef]
- Ziouti, A.; EL Modafar, C.; Fleuriet, A.; EL Boustani, S.; Macheix, J.J. Phenolic Compounds in Date Palm Cultivars Sensitive and Resistant to Fusarium Oxysporum. Biol. Plant. 1996, 38, 451–457. [Google Scholar] [CrossRef]
- Maher, E.A.; Bate, N.J.; Ni, W.; Elkind, Y.; Dixon, R.A.; Lamb, C.J. Increased Disease Susceptibility of Transgenic Tobacco Plants with Suppressed Levels of Preformed Phenylpropanoid Products. Proc. Natl. Acad. Sci. USA 1994, 91, 7802–7806. [Google Scholar] [CrossRef] [PubMed]
- Ardila, H.D.; Martínez, S.T.; Higuera, B.L. Levels of Constitutive Flavonoid Biosynthetic Enzymes in Carnation (Dianthus Caryophyllus L.) Cultivars with Differential Response to Fusarium Oxysporum f. Sp. Dianthi. Acta Physiol. Plant. 2013, 35, 1233–1245. [Google Scholar] [CrossRef]
- Melake-Berhan, A.; Butler, L.G.; Ejeta, G.; Menkir, A. Grain Mold Resistance and Polyphenol Accumulation in Sorghum. J. Agric. Food Chem. 1996, 44, 2428–2434. [Google Scholar] [CrossRef]
- Wang, Y.C.; Qian, W.J.; Li, N.N.; Hao, X.Y.; Wang, L.; Xiao, B.; Wang, X.C.; Yang, Y.J. Metabolic Changes of Caffeine in Tea Plant (Camellia Sinensis (L.) O. Kuntze) as Defense Response to Colletotrichum Fructicola. J. Agric. Food Chem. 2016, 64, 6685–6693. [Google Scholar] [CrossRef]
- Shadle, G.L.; Wesley, S.V.; Korth, K.L.; Chen, F.; Lamb, C.; Dixon, R.A. Phenylpropanoid Compounds and Disease Resistance in Transgenic Tobacco with Altered Expression of L-Phenylalanine Ammonia-Lyase. Phytochemistry 2003, 64, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Lei, K.J.; Zhang, L.; Du, X.Y.; An, Y.; Chang, G.H.; An, G.Y. A Chalcone Synthase Controls the Verticillium Disease Resistance Response in Both Arabidopsis Thaliana and Cotton. Eur. J. Plant Pathol. 2018, 152, 769–781. [Google Scholar] [CrossRef]
- Lorenc-Kukuła, K.; Wróbel-Kwiatkowska, M.; Starzycki, M.; Szopa, J. Engineering Flax with Increased Flavonoid Content and Thus Fusarium Resistance. Physiol. Mol. Plant Pathol. 2007, 70, 38–48. [Google Scholar] [CrossRef]
- Wang, L.; Ran, L.; Hou, Y.; Tian, Q.; Li, C.; Liu, R.; Fan, D.; Luo, K. The Transcription Factor MYB115 Contributes to the Regulation of Proanthocyanidin Biosynthesis and Enhances Fungal Resistance in Poplar. New Phytol. 2017, 215, 351–367. [Google Scholar] [CrossRef] [Green Version]
- Fofana, B.; Benhamou, N.; Mcnally, D.J.; Labbé, C.; Séguin, A.; Bélanger, R.R. Suppression of Induced Resistance in Cucumber Through Disruption of the Flavonoid Pathway. Phytopathology 2004, 95, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wei, X.; Tian, Y.; Shen, L.; Xu, H.H. Antifungal Flavonoids from Ficus Sarmentosa Var. Henryi (King) Corner. Agric. Sci. China 2010, 9, 690–694. [Google Scholar] [CrossRef]
- Xu, S.; Yan, F.; Ni, Z.; Chen, Q.; Zhang, H.; Zheng, X. In Vitro and in Vivo Control of Alternaria Alternata in Cherry Tomato by Essential Oil from Laurus Nobilis of Chinese Origin. J. Sci. Food Agric. 2014, 94, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.S.; Lee, D.G. Antifungal Action of Chlorogenic Acid against Pathogenic Fungi, Mediated by Membrane Disruption. Pure Appl. Chem. 2010, 82, 219–226. [Google Scholar] [CrossRef]
- Lee, H.; Woo, E.R.; Lee, D.G. Apigenin Induces Cell Shrinkage in Candida Albicans by Membrane Perturbation. FEMS Yeast Res. 2018, 18, foy003. [Google Scholar] [CrossRef]
- Moazeni, M.; Hedayati, M.T.; Nabili, M.; Mousavi, S.J.; Abdollahi Gohar, A.; Gholami, S. Glabridin Triggers Over-Expression of MCA1 and NUC1 Genes in Candida Glabrata: Is It an Apoptosis Inducer? J. Mycol. Med. 2017, 27, 369–375. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, T.; Du, K.; Chen, H.; Cao, Y.; Xie, J.; Li, M.; Carr, J.P.; Wu, B.; Fan, Z.; et al. Maize Phenylalanine Ammonia-Lyases Contribute to Resistance to Sugarcane Mosaic Virus Infection, Most Likely through Positive Regulation of Salicylic Acid Accumulation. Mol. Plant Pathol. 2019, 20, 1365–1378. [Google Scholar] [CrossRef] [Green Version]
- Scholthof, K.-B. Tobacco Mosaic Virus. Plant Health Instr. 2000. [Google Scholar] [CrossRef]
- Krcatović, E.; Rusak, G.; Bezić, N.; Krajacić, M. Inhibition of Tobacco Mosaic Virus Infection by Quercetin and Vitexin. Acta Virol. 2008, 52, 119–124. [Google Scholar]
- Ono, K.; Nakane, H.; Fukushima, M.; Chermann, J.C.; Barré-Sinoussi, F. Inhibition of reverse transcriptase activity by a flavonoid compound, 5, 6, 7-trihydroxyflavone. Biochem. Biophys. Res. Commun. 1989, 160, 982–987. [Google Scholar] [CrossRef]
- Lin, Y.; Anderson, H.; Flavin, M.T.; Pai, Y.S.; Mata-greenwood, E.; Pengsuparp, T.; Pezzuto, J.M.; Schinazi, R.F.; Hughes, S.H.; Chen, F. In Vitro Anti-HIV Activity of Biflavonoids Isolated from Rhus Succedanea and Garcinia Multiflora. J. Nat. Prod. 1997, 3864, 884–888. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of Plant Defense against Insect Herbivores. Plant Signal. Behav. 2012, 7, 1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiss, K.A.; Maltese, F.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L. Identification of Chlorogenic Acid as a Resistance Factor for Thrips in Chrysanthemum. Plant Physiol. 2009, 150, 1567–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallikarjuna, N.; Kranthi, K.R.; Jadhav, D.R.; Kranthi, S.; Chandra, S. Influence of Foliar Chemical Compounds on the Development of Spodoptera Litura (Fab.) in Interspecific Derivatives of Groundnut. J. Appl. Entomol. 2004, 128, 321–328. [Google Scholar] [CrossRef]
- Leiss, K.A.; Cristofori, G.; van Steenis, R.; Verpoorte, R.; Klinkhamer, P.G.L. An Eco-Metabolomic Study of Host Plant Resistance to Western Flower Thrips in Cultivated, Biofortified and Wild Carrots. Phytochemistry 2013, 93, 63–70. [Google Scholar] [CrossRef]
- Simlat, M.; Stobiecki, M.; Szklarczyk, M. Accumulation of Selected Phenolics and Expression of PAL Genes in Carrots Differing in Their Susceptibility to Carrot Fly (Psila Rosae F.). Euphytica 2013, 190, 253–266. [Google Scholar] [CrossRef] [Green Version]
- Upasani, S.M.; Kotkar, H.M.; Mendki, P.S.; Maheshwari, V.L. Partial Characterization and Insecticidal Properties of Ricinus Communis L Foliage Flavonoids. Pest Manag. Sci. 2003, 59, 1349–1354. [Google Scholar] [CrossRef]
- Tak, J.H.; Isman, M.B. Penetration-Enhancement Underlies Synergy of Plant Essential Oil Terpenoids as Insecticides in the Cabbage Looper, Trichoplusia Ni. Sci. Rep. 2017, 7, 42432. [Google Scholar] [CrossRef] [Green Version]
- Tak, J.H.; Isman, M.B. Enhanced Cuticular Penetration as the Mechanism for Synergy of Insecticidal Constituents of Rosemary Essential Oil in Trichoplusia Ni. Sci. Rep. 2015, 5, 12690. [Google Scholar] [CrossRef] [Green Version]
- Feeny, P.; Sachdev, K.; Rosenberry, L.; Carter, M. Luteolin 7-O-(6″-O-Malonyl)-β-d-Glucopyranoside and Trans-Chlorogenic Acid: Oviposition Stimulants for the Black Swallowtail Butterfly. Phytochemistry 1988, 27, 3439–3448. [Google Scholar] [CrossRef]
- Almeida, R.P.P.; Nunney, L. How Do Plant Diseases Caused by Xylella Fastidiosa Emerge? Plant Dis. 2015, 99, 1457–1467. [Google Scholar] [CrossRef] [Green Version]
- Stoepler, T.M.; Wolf, T.K. North American Grapevine Yellows Disease: Current Knowledge and Management Recommendations for Wine Growers; Virginia Cooperative Extension: Blacksburg, VA, USA, 2013; Publication AREC-48P. [Google Scholar]
- Su, Q.; Chen, G.; Mescher, M.C.; Peng, Z.; Xie, W.; Wang, S.; Wu, Q.; Liu, J.; Li, C.; Wang, W.; et al. Whitefly Aggregation on Tomato Is Mediated by Feeding-Induced Changes in Plant Metabolites That Influence the Behaviour and Performance of Conspecifics. Funct. Ecol. 2018, 32, 1180–1193. [Google Scholar] [CrossRef]
- Yao, Q.; Peng, Z.; Tong, H.; Yang, F.; Xing, G.; Wang, L.; Zheng, J.; Zhang, Y.; Su, Q. Tomato Plant Flavonoids Increase Whitefly Resistance and Reduce Spread of Tomato Yellow Leaf Curl Virus. J. Econ. Entomol. 2019, 112, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Margaria, P.; Ferrandino, A.; Caciagli, P.; Kedrina, O.; Schubert, A.; Palmano, S. Metabolic and Transcript Analysis of the Flavonoid Pathway in Diseased and Recovered Nebbiolo and Barbera Grapevines (Vitis Vinifera L.) Following Infection by Flavescence Dorée Phytoplasma. Plant Cell Environ. 2014, 37, 2183–2200. [Google Scholar] [CrossRef] [PubMed]
- Gautam, K.; Kumar, P.; Poonia, S. Larvicidal Activity and GC-MS Analysis of Flavonoids of Vitex Negundo and Andrographis Paniculata against Two Vector Mosquitoes Anopheles Stephensi and Aedes Aegypti. J. Vector Borne Dis. 2013, 50, 171–178. [Google Scholar] [PubMed]
- El-Akhal, F.; Guemmouh, R.; Zerrouq, F.; Ez Zoubi, Y.; El Ouali Lalami, A. Struggle against Vector-Borne Diseases: Phytochemical Screening and Larvicidal Activity of Hydro-Ethanolic Extract of Ocimum Basilicum in North East of Morocco against the Larvae of Malaria Vector Mosquito Anopheles Labranchiae (Diptera: Culicidae). Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 284–288. [Google Scholar]
- Muema, J.M.; Njeru, S.N.; Colombier, C.; Marubu, R.M. Methanolic Extract of Agerantum Conyzoides Exhibited Toxicity and Growth Disruption Activities against Anopheles Gambiae Sensu Stricto and Anopheles Arabiensis Larvae. BMC Complement. Altern. Med. 2016, 16, 475. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, D.A.; Souza, M.S.R.; Teles, Y.C.F.; Oliveira, L.H.G.; Lima, J.B.; Conceição, A.S.; Nunes, F.C.; Silva, T.M.S.; De Fátima Vanderlei De Souza, M. New Sulphated Flavonoids and Larvicidal Activity of Helicteres Velutina K. Schum (Sterculiaceae). Molecules 2018, 23, 2784. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.D.L.; Fernandes, D.A.; Nunes, F.C.; Teles, Y.C.F.; Rolim, Y.M.; da Silva, C.M.; de Albuquerque, J.B.L.; Agra, M.D.F.; de Souza, M.D.F.V. Phytochemical Study of Waltheria Viscosissima and Evaluation of Its Larvicidal Activity against Aedes Aegypti. Rev. Bras. Farmacogn. 2019, 29, 582–590. [Google Scholar] [CrossRef]
- Da Silva, B.C.; Melo, D.R.; Franco, C.T.; Maturano, R.; Fabri, R.L.; Daemon, E. Evaluation of Eugenol and (E)-Cinnamaldehyde Insecticidal Activity against Larvae and Pupae of Musca Domestica (Diptera: Muscidae). J. Med. Entomol. 2020, 57, 181–186. [Google Scholar] [CrossRef]
- Kotewong, R.; Duangkaew, P.; Srisook, E.; Sarapusit, S.; Rongnoparut, P. Structure–Function Relationships of Inhibition of Mosquito Cytochrome P450 Enzymes by Flavonoids of Andrographis Paniculata. Parasitol. Res. 2014, 113, 3381–3392. [Google Scholar] [CrossRef]
- Hematpoor, A.; Liew, S.Y.; Chong, W.L.; Azirun, M.S.; Lee, V.S.; Awang, K. Inhibition and Larvicidal Activity of Phenylpropanoids from Piper Sarmentosum on Acetylcholinesterase against Mosquito Vectors and Their Binding Mode of Interaction. PLoS ONE 2016, 11, e155265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharibi, S.; Sayed Tabatabaei, B.E.; Saeidi, G.; Talebi, M.; Matkowski, A. The Effect of Drought Stress on Polyphenolic Compounds and Expression of Flavonoid Biosynthesis Related Genes in Achillea Pachycephala Rech.F. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Pandian, S.; Oh, Y.J.; Kang, H.J.; Cho, W.S.; Cho, Y.S. Metabolic Engineering of Isoflavones: An Updated Overview. Front. Plant Sci. 2021, 12, 897. [Google Scholar]
- Kubica, P.; Szopa, A.; Prokopiuk, B.; Komsta, Ł.; Pawłowska, B.; Ekiert, H. The Influence of Light Quality on the Production of Bioactive Metabolites—Verbascoside, Isoverbascoside and Phenolic Acids and the Content of Photosynthetic Pigments in Biomass of Verbena officinalis L. Cultured in Vitro. J. Photochem. Photobiol. B Biol. 2020, 203, 111768. [Google Scholar] [CrossRef]
- Luís, Â.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and Biofilm Inhibitory Activities of Gallic, Caffeic, and Chlorogenic Acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef]
- Vosman, B.; van’t Westende, W.P.C.; Henken, B.; van Eekelen, H.D.L.M.; de Vos, R.C.H.; Voorrips, R.E. Broad Spectrum Insect Resistance and Metabolites in Close Relatives of the Cultivated Tomato. Euphytica 2018, 214, 46. [Google Scholar] [CrossRef] [Green Version]
- Sanzani, S.M.; Schena, L.; De Girolamo, A.; Ippolito, A.; González-Candelas, L. Characterization of Genes Associated with Induced Resistance against Penicillium Expansum in Apple Fruit Treated with Quercetin. Postharvest Biol. Technol. 2010, 56, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; He, Y.; Kabahuma, M.; Chaya, T.; Kelly, A.; Borrego, E.; Bian, Y.; El Kasmi, F.; Yang, L.; Teixeira, P.; et al. A Gene Encoding Maize Caffeoyl-CoA O-Methyltransferase Confers Quantitative Resistance to Multiple Pathogens. Nat. Genet. 2017, 49, 1364–1372. [Google Scholar] [CrossRef]
- Muravnik, L.E.; Kostina, O.V.; Shavarda, A.L. Glandular Trichomes of Tussilago Farfara (Senecioneae, Asteraceae). Planta 2016, 244, 737–752. [Google Scholar] [CrossRef]
- Alvarez, D.V.; Hernández, M.S.; Hernández, V.A.G.; Engleman, E.M.; Damián Nava, A. Flavonoids in Psidium Guajava L. Leaves. Hortic. Int. J. 2021, 5, 38–41. [Google Scholar] [CrossRef]
- Schöpker, H.; Kneisel, M.; Beerhues, L.; Robenek, H.; Wiermann, R. Phenylalanine Ammonia-Lyase and Chalcone Synthase in Glands OfPrimula Kewensis (W. Wats): Immunofluorescence and Immunogold Localization. Planta 1995, 196, 712–719. [Google Scholar] [CrossRef]
- Tattini, M.; Guidi, L.; Morassi-Bonzi, L.; Pinelli, P.; Remorini, D.; Degl’Innocenti, E.; Giordano, C.; Massai, R.; Agati, G. On the Role of Flavonoids in the Integrated Mechanisms of Response of Ligustrum Vulgare and Phillyrea Latifolia to High Solar Radiation. New Phytol. 2005, 167, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R.; Veit, M.; Weissenböck, G.; Schnitzler, J.P. Tissue Localization of Phenolic Compounds in Plants by Confocal Laser Scanning Microscopy. J. Exp. Bot. 1998, 49, 953–965. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramaroson, M.-L.; Koutouan, C.; Helesbeux, J.-J.; Le Clerc, V.; Hamama, L.; Geoffriau, E.; Briard, M. Role of Phenylpropanoids and Flavonoids in Plant Resistance to Pests and Diseases. Molecules 2022, 27, 8371. https://doi.org/10.3390/molecules27238371
Ramaroson M-L, Koutouan C, Helesbeux J-J, Le Clerc V, Hamama L, Geoffriau E, Briard M. Role of Phenylpropanoids and Flavonoids in Plant Resistance to Pests and Diseases. Molecules. 2022; 27(23):8371. https://doi.org/10.3390/molecules27238371
Chicago/Turabian StyleRamaroson, Marie-Louisa, Claude Koutouan, Jean-Jacques Helesbeux, Valérie Le Clerc, Latifa Hamama, Emmanuel Geoffriau, and Mathilde Briard. 2022. "Role of Phenylpropanoids and Flavonoids in Plant Resistance to Pests and Diseases" Molecules 27, no. 23: 8371. https://doi.org/10.3390/molecules27238371
APA StyleRamaroson, M.-L., Koutouan, C., Helesbeux, J.-J., Le Clerc, V., Hamama, L., Geoffriau, E., & Briard, M. (2022). Role of Phenylpropanoids and Flavonoids in Plant Resistance to Pests and Diseases. Molecules, 27(23), 8371. https://doi.org/10.3390/molecules27238371









