Visible Light Promotes Cascade Trifluoromethylation/Cyclization, Leading to Trifluoromethylated Polycyclic Quinazolinones, Benzimidazoles and Indoles
Abstract
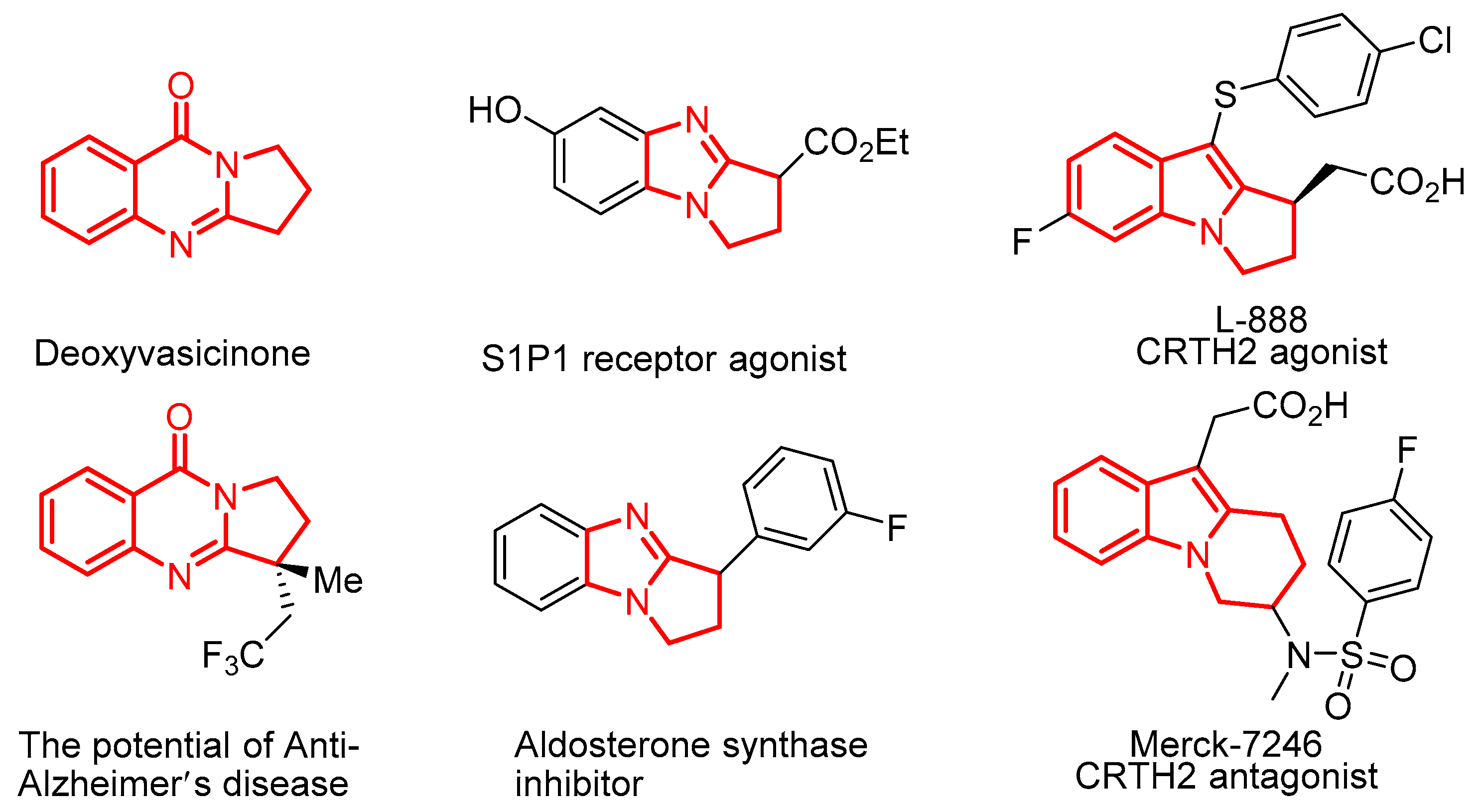
1. Introduction
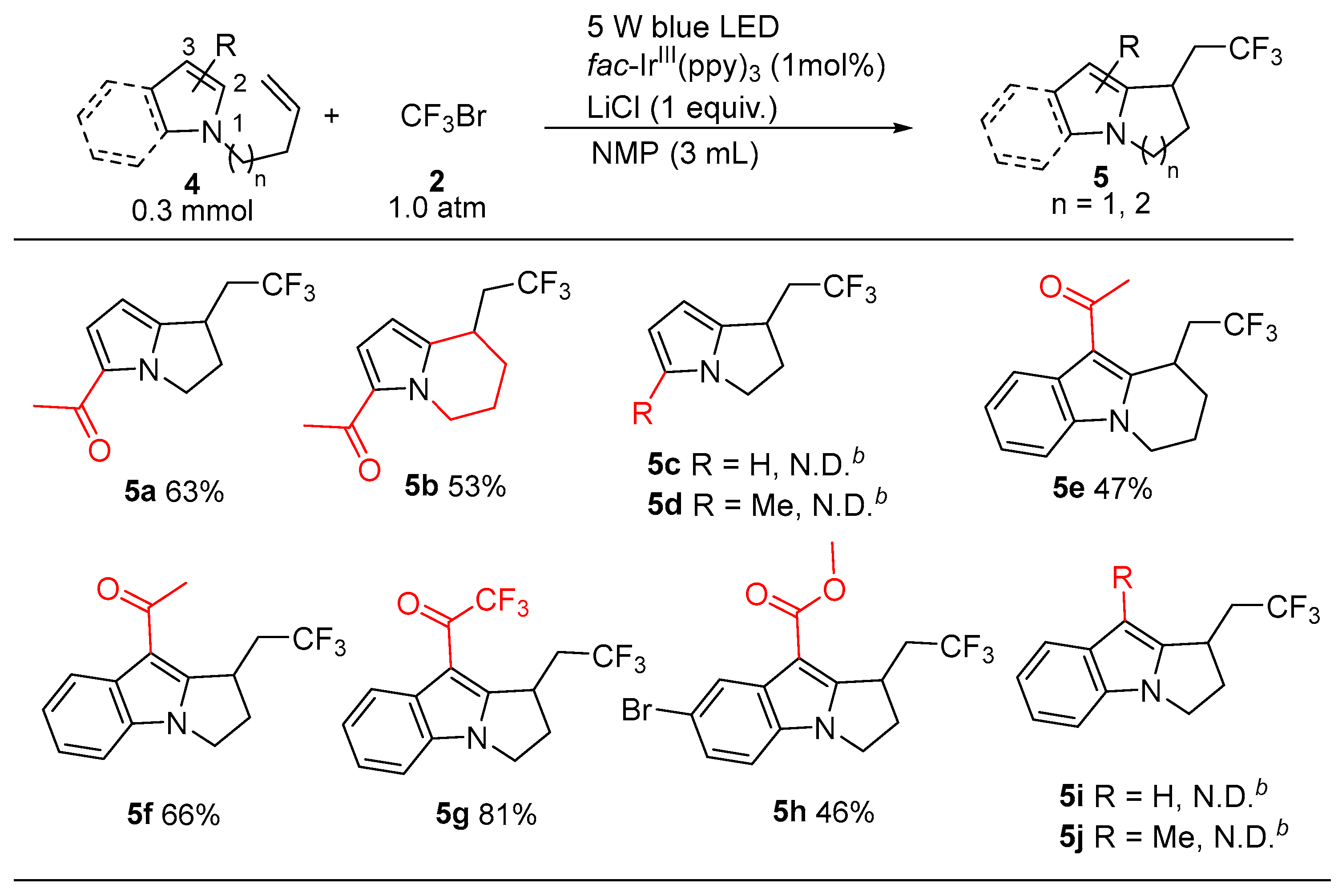
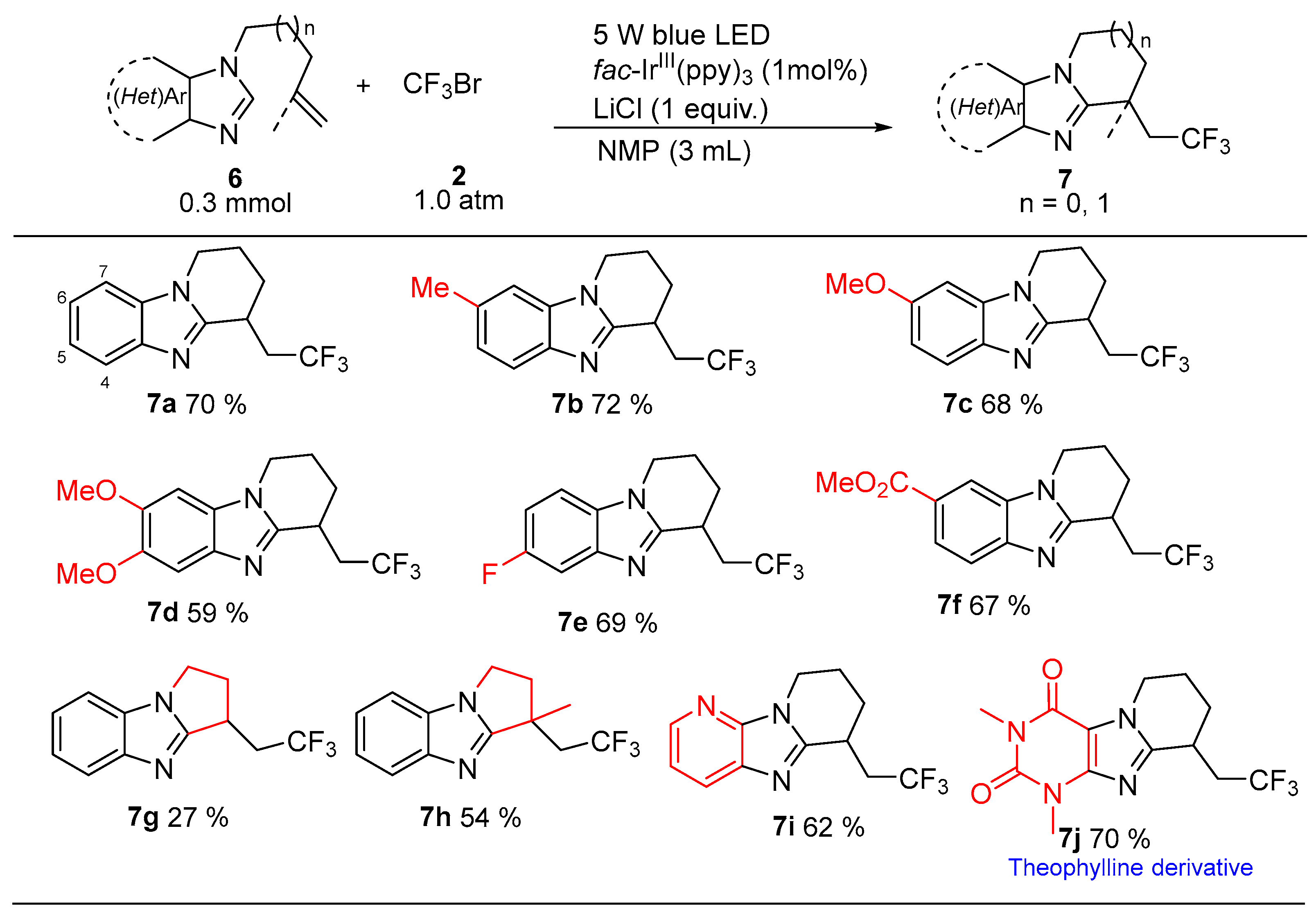
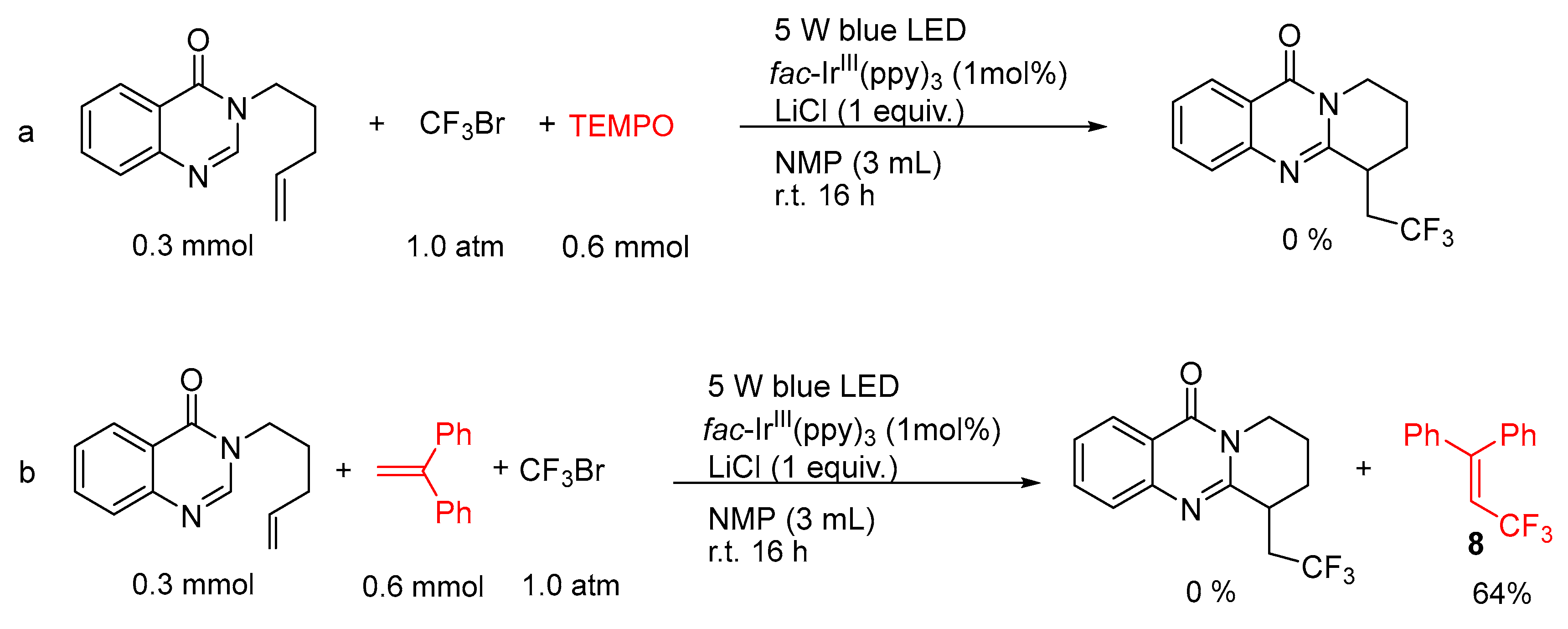
2. Results and Discussion
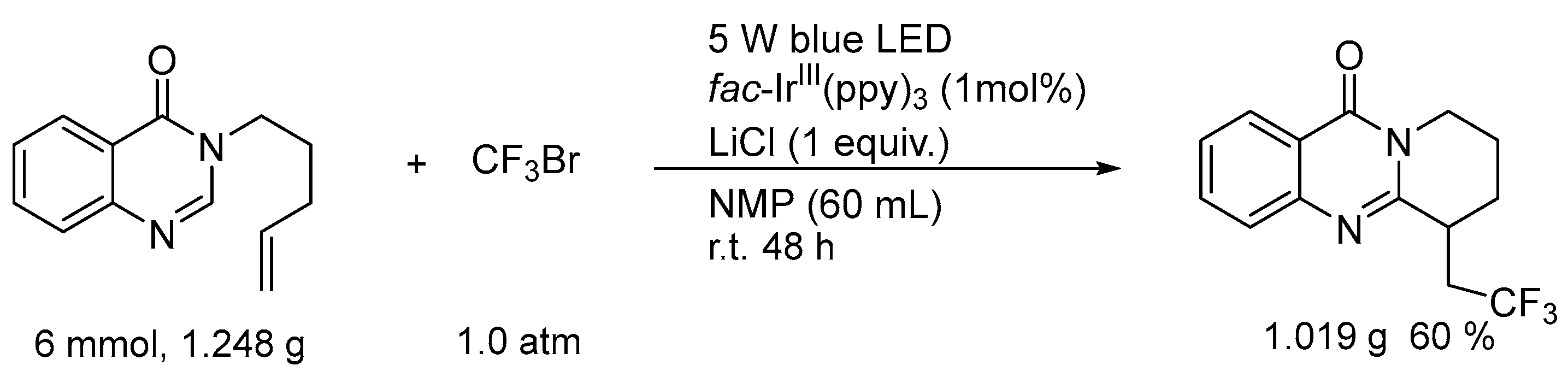
3. Gram-Scale Synthesis
4. Proposed Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef]
- Meanwell, N.A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef]
- Zafrani, Y.; Parvari, G.; Amir, D.; Ghindes-Azaria, L.; Elias, S.; Pevzner, A.; Fridkin, G.; Berliner, A.; Gershonov, E.; Eichen, Y.; et al. Modulation of the H-Bond Basicity of Functional Groups by α-Fluorine-Containing Functions and its Implications for Lipophilicity and Bioisosterism. J. Med. Chem. 2021, 64, 4516–4531. [Google Scholar] [CrossRef]
- Tiz, D.B.; Bagnoli, L.; Rosati, O.; Marini, F.; Sancineto, L.; Santi, C. New Halogen-Containing Drugs Approved by FDA in 2021: An Overview on Their Syntheses and Pharmaceutical Use. Molecules 2022, 27, 1643. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, K.; Fu, C.; Peng, H.; Hawker, C.J.; Whittaker, A.K. Biological Utility of Fluorinated Compounds: From Materials Design to Molecular Imaging, Therapeutics and Environmental Remediation. Chem. Rev. 2022, 122, 167–208. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 2020, 23, 101467. [Google Scholar] [CrossRef]
- Charpentier, J.; Früh, N.; Togni, A. Electrophilic Trifluoromethylation by Use of Hypervalent Iodine Reagents. Chem. Rev. 2015, 115, 650–682. [Google Scholar] [CrossRef]
- Wang, X.; Studer, A. Iodine(III) Reagents in Radical Chemistry. Acc. Chem. Res. 2017, 50, 1712–1724. [Google Scholar] [CrossRef]
- Zeng, H.; Luo, Z.; Han, X.; Li, C.-J. Metal-Free Construction of the C(sp3)–CF3 Bond: Trifluoromethylation of Hydrazones with Togni’s Reagent under Mild Conditions. Org. Let. 2019, 21, 5948–5951. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, T. Electrophilic Perfluoroalkylating Agents. Chem. Rev. 1996, 96, 1757–1778. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Yudin, A.K. Perfluoroalkylation with Organosilicon Reagents. Chem. Rev. 1997, 97, 757–786. [Google Scholar] [CrossRef]
- Liu, X.; Xu, C.; Wang, M.; Liu, Q. Trifluoromethyltrimethylsilane: Nucleophilic Trifluoromethylation and Beyond. Chem. Rev. 2015, 115, 683–730. [Google Scholar] [CrossRef]
- Shen, J.; Xu, J.; He, L.; Liang, C.; Li, W. Application of Langlois’ reagent (NaSO2CF3) in C–H functionalisation. Chin. Chem. Lett. 2022, 33, 1227–1235. [Google Scholar] [CrossRef]
- Zanardi, A.; Novikov, M.A.; Martin, E.; Benet-Buchholz, J.; Grushin, V.V. Direct Cupration of Fluoroform. J. Am. Chem. Soc. 2011, 133, 20901–20913. [Google Scholar] [CrossRef]
- Barthelemy, A.-L.; Anselmi, E.; Le, T.-N.; Vo-Thanh, G.; Guillot, R.; Miqueu, K.; Magnier, E. Divergent Synthesis of 1,2-Benzo[e]thiazine and Benzo[d]thiazole Analogues Containing a S-Trifluoromethyl Sulfoximine Group: Preparation and New Properties of the Adachi Reagent. J. Org. Chem. 2019, 84, 4086–4094. [Google Scholar] [CrossRef]
- Shi, G.; Shao, C.; Pan, S.; Yu, J.; Zhang, Y. Silver-Catalyzed C–H Trifluoromethylation of Arenes Using Trifluoroacetic Acid as the Trifluoromethylating Reagent. Org. Lett. 2015, 17, 38–41. [Google Scholar] [CrossRef]
- Lin, J.; Li, Z.; Kan, J.; Huang, S.; Su, W.; Li, Y. Photo-driven redox-neutral decarboxylative carbon-hydrogen trifluoromethylation of (hetero)arenes with trifluoroacetic acid. Nat. Commun. 2017, 8, 14353. [Google Scholar] [CrossRef]
- Yang, B.; Yu, D.; Xu, X.-H.; Qing, F.-L. Visible-Light Photoredox Decarboxylation of Perfluoroarene Iodine(III) Trifluoroacetates for C–H Trifluoromethylation of (Hetero)arenes. ACS Catal. 2018, 8, 2839–2843. [Google Scholar] [CrossRef]
- Beatty, J.W.; Douglas, J.J.; Cole, K.P.; Stephenson, C.R.J. A scalable and operationally simple radical trifluoromethylation. Nat. Commun. 2015, 6, 7919. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, J.; Huang, S.; Xu, B.; Lin, J.; Su, W. Photocatalytic fluoroalkylations of (hetero)arenes enabled by the acid-triggered reactivity umpolung of acetoxime esters. Chem Catal. 2022, 2, 1793–1806. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xu, X.-H.; Qing, F.-L. Trifluoromethanesulfonic Anhydride as a Low-Cost and Versatile Trifluoromethylation Reagent. Angew. Chem. Int. Ed. 2018, 57, 6926–6929. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.; Kim, N.; Kim, S.; Hong, S. Visible-Light-Enabled Trifluoromethylative Pyridylation of Alkenes from Pyridines and Triflic Anhydride. Angew. Chem. Int. Ed. 2020, 59, 13379–13384. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, J.-H.; Xiao, J.-C. A Readily Available Trifluoromethylation Reagent and Its Difunctionalization of Alkenes. Org. Lett. 2021, 23, 6079–6083. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Lin, J.-H.; Xiao, J.-C. Starting from Styrene: A Unified Protocol for Hydrotrifluoromethylation of Diversified Alkenes. Org. Lett. 2021, 23, 9277–9282. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Häring, A.P.; Berger, F.; Zhang, L.; Ritter, T. Trifluoromethyl Thianthrenium Triflate: A Readily Available Trifluoromethylating Reagent with Formal CF3+, CF3•, and CF3– Reactivity. J. Am. Chem. Soc. 2021, 143, 7623–7628. [Google Scholar] [CrossRef]
- Jia, H.; Ritter, T. α-Thianthrenium Carbonyl Species: The Equivalent of an α-Carbonyl Carbocation. Angew. Chem. Int. Ed. 2022, 61, e202208978. [Google Scholar] [CrossRef]
- Li, Y.; Liang, X.; Niu, K.; Gu, J.; Liu, F.; Xia, Q.; Wang, Q.; Zhang, W. Visible-Light-Induced Photocatalyst-Free Radical Trifluoromethylation. Org. Lett. 2022, 24, 5918–5923. [Google Scholar] [CrossRef]
- Sibille, S.; d’Incan, E.; Leport, L.; Perichon, J. Electrosynthesis of alcohols from organic halides and ketones or aldehydes. Tetrahedron Lett. 1986, 27, 3129–3132. [Google Scholar] [CrossRef]
- Surya Prakash, G.K.; Deffieux, D.; Yudin, A.K.; Olah, G.A. Convenient and Safe Electrochemical Synthesis of (Trifluoromethyl)trimethylsilane1a. Synlett 1994, 1994, 1057–1058. [Google Scholar] [CrossRef]
- Francèse, C.; Tordeux, M.; Wakselman, C. Synthesis of trifluoromethyl-substituted methanols: A Barbier procedure under pressure. J. Chem. Soc. Chem. Commun. 1987, 9, 642–643. [Google Scholar] [CrossRef]
- Grobe, J.; Hegge, J. Facile Aluminum Induced Synthesis of (Trifluoromethyl)trimethylsilane. Synlett 1995, 1995, 641–642. [Google Scholar] [CrossRef]
- Bürger, H.; Dittmar, T.; Pawelke, G. A simple one-pot synthesis of α-trifluoromethyl-substituted enamines by C-trifluoromethylation of dialkylamides with P(NEt2)3CF3Br. J. Fluorine Chem. 1995, 70, 89–93. [Google Scholar] [CrossRef]
- Qi, Q.; Shen, Q.; Lu, L. Polyfluoroalkylation of 2-aminothiazoles. J. Fluor. Chem. 2012, 133, 115–119. [Google Scholar] [CrossRef]
- Natte, K.; Jagadeesh, R.V.; He, L.; Rabeah, J.; Chen, J.; Taeschler, C.; Ellinger, S.; Zaragoza, F.; Neumann, H.; Brückner, A.; et al. Palladium-Catalyzed Trifluoromethylation of (Hetero)Arenes with CF3Br. Angew. Chem. Int. Ed. 2016, 55, 2782–2786. [Google Scholar] [CrossRef]
- Zhang, K.-F.; Bian, K.-J.; Li, C.; Sheng, J.; Li, Y.; Wang, X.-S. Nickel-Catalyzed Carbofluoroalkylation of 1,3-Enynes to Access Structurally Diverse Fluoroalkylated Allenes. Angew. Chem. Int. Ed. 2019, 58, 5069–5074. [Google Scholar] [CrossRef]
- Li, Q.; Fan, W.; Peng, D.; Meng, B.; Wang, S.; Huang, R.; Liu, S.; Li, S. Cobalt–Tertiary-Amine-Mediated Hydroxytrifluoromethylation of Alkenes with CF3Br and Atmospheric Oxygen. ACS Catal. 2020, 10, 4012–4018. [Google Scholar] [CrossRef]
- Peng, D.; Fan, W.; Zhao, X.; Chen, W.; Wen, Y.; Zhang, L.; Li, S. Zinc–Brønsted acid mediated practical hydrotrifluoromethylation of alkenes with CF3Br. Org. Chem. Front. 2021, 8, 6356–6363. [Google Scholar] [CrossRef]
- Ren, Y.-Y.; Zheng, X.; Zhang, X. Bromotrifluoromethane: A Useful Reagent for Hydrotrifluoromethylation of Alkenes and Alkynes. Synlett 2018, 29, 1028–1032. [Google Scholar]
- Ma, R.; Deng, Z.; Wang, K.-H.; Wang, J.; Huang, D.; Su, Y.; Hu, Y.; Lv, X. Photoinduced Trifluoromethylation with CF3Br as a Trifluoromethyl Source: Synthesis of α-CF3-Substituted Ketones. ACS Omega 2022, 7, 14357–14362. [Google Scholar] [CrossRef]
- Zheng, J.; Deng, Z.; Zhang, Y.; Cui, S. Copper-Catalyzed Divergent Trifluoromethylation/Cyclization of Unactivated Alkenes. Adv. Synth. Catal. 2016, 358, 746–751. [Google Scholar] [CrossRef]
- Hu, Q.; Yu, W.-L.; Luo, Y.-C.; Hu, X.-Q.; Xu, P.-F. A Photosensitizer–Free Radical Cascade for Synthesizing CF3-Containing Polycyclic Quinazolinones with Visible Light. J. Org. Chem. 2022, 87, 1493–1501. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, H.; Feng, X.; Guo, R.; Chen, X.; Pan, Z.; Zhang, L. Copper(I)-promoted trifluoromethylation of indole derivates with concomitant C–C bond formation to access fused tricyclic indoles. Tetrahedron Lett. 2015, 56, 1703–1705. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Wu, Z.; Shi, W.; Lei, Y.; Davies, P.W.; Shu, W. A Radical-Initiated Fragmentary Rearrangement Cascade of Ene-Ynamides to [1,2]-Annulated Indoles via Site-Selective Cyclization. Org. Lett. 2021, 23, 7209–7214. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Cui, J.; Chen, Y.; Li, Y. Copper-Catalyzed Direct Cycloaddition of Imidazoles and Alkenes to Trifluoromethylated Tricyclic Imidazoles. J. Org. Chem. 2021, 86, 15768–15776. [Google Scholar] [CrossRef]
- Sun, B.; Huang, P.; Yan, Z.; Shi, X.; Tang, X.; Yang, J.; Jin, C. Self-Catalyzed Phototandem Perfluoroalkylation/Cyclization of Unactivated Alkenes: Synthesis of Perfluoroalkyl-Substituted Quinazolinones. Org. Lett. 2021, 23, 1026–1031. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, W.; Xu, C.; He, J.; Xu, Z.; Yang, Z.; Ling, F.; Zhong, W. Electrosynthesis of CF3-Substituted Polycyclic Quinazolinones via Cascade Trifluoromethylation/Cyclization of Unactivated Alkene. Adv. Synth. Catal. 2022, 364, 1319–1325. [Google Scholar] [CrossRef]
- Yang, J.; Sun, B.; Ding, H.; Huang, P.-Y.; Tang, X.-L.; Shi, R.-C.; Yan, Z.-Y.; Yu, C.-M.; Jin, C. Photo-triggered self-catalyzed fluoroalkylation/cyclization of unactivated alkenes: Synthesis of quinazolinones containing the CF2R group. Green Chem. 2021, 23, 575–581. [Google Scholar] [CrossRef]
- Yang, R.; Yi, D.; Shen, K.; Fu, Q.; Wei, J.; Lu, J.; Yang, L.; Wang, L.; Wei, S.; Zhang, Z. Indole and Pyrrole Derivatives as Pre-photocatalysts and Substrates in the Sulfonyl Radical-Triggered Relay Cyclization Leading to Sulfonylated Heterocycles. Org. Lett. 2022, 24, 2014–2019. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Qi, S.-L.; Luan, Y.-X.; Han, X.-W.; Wang, S.; Chen, H.; Ye, M. Enantioselective Ni–Al Bimetallic Catalyzed exo-Selective C–H Cyclization of Imidazoles with Alkenes. J. Am. Chem. Soc. 2018, 140, 5360–5364. [Google Scholar] [CrossRef] [PubMed]



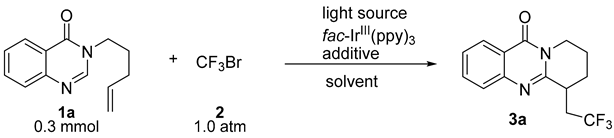 | |||||
|---|---|---|---|---|---|
| Entry | PC (x mol%) | Light Source | Additive | Solvent | Yield b (%) |
| 1 | 1.0 mol% | 5 W blue LED | - | CH3CN | 5% |
| 2 | 1.0 mol% | 5 W blue LED | - | DCM | 15% |
| 3 | 1.0 mol% | 5 W blue LED | - | Toluene | 3% |
| 4 | 1.0 mol% | 5 W blue LED | - | THF | 3% |
| 5 | 1.0 mol% | 5 W blue LED | - | DMSO | trace |
| 6 | 1.0 mol% | 5 W blue LED | - | DMF | 24% |
| 7 | 1.0 mol% | 5 W blue LED | - | NMP | 30% |
| 8 | 1.0 mol% | 10 W blue LED | - | NMP | 30% |
| 9 | 1.0 mol% | 15 W blue LED | - | NMP | 30% |
| 10 | 1.0 mol% | 5 W white LED | - | NMP | 21% |
| 11 | 1.0 mol% | 5 W blue LED | 20% Sc (OTf)3 | NMP | 45% |
| 12 | 1.0 mol% | 5 W blue LED | 20% In (OTf)3 | NMP | 38% |
| 13 | 1.0 mol% | 5 W blue LED | 100% LiF | NMP | 35% |
| 14 | 1.0 mol% | 5 W blue LED | 100% LiCl | NMP | 83% |
| 15 | 1.0 mol% | 5 W blue LED | 100% LiBr | NMP | 38% |
| 16 | 0.5 mol% | 5 W blue LED | 100% LiCl | NMP | 78% |
| 17 | 0.25 mol% | 5 W blue LED | 100% LiCl | NMP | 72% |
| 18 | - | 5 W blue LED | 100% LiCl | NMP | 0% |
| 19 | 1.0 mol% | - | 100% LiCl | NMP | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Ren, Y.; Deng, Z.; Wang, K.-H.; Wang, J.; Huang, D.; Hu, Y.; Lv, X. Visible Light Promotes Cascade Trifluoromethylation/Cyclization, Leading to Trifluoromethylated Polycyclic Quinazolinones, Benzimidazoles and Indoles. Molecules 2022, 27, 8389. https://doi.org/10.3390/molecules27238389
Ma R, Ren Y, Deng Z, Wang K-H, Wang J, Huang D, Hu Y, Lv X. Visible Light Promotes Cascade Trifluoromethylation/Cyclization, Leading to Trifluoromethylated Polycyclic Quinazolinones, Benzimidazoles and Indoles. Molecules. 2022; 27(23):8389. https://doi.org/10.3390/molecules27238389
Chicago/Turabian StyleMa, Ransong, Yuanyuan Ren, Zhoubin Deng, Ke-Hu Wang, Junjiao Wang, Danfeng Huang, Yulai Hu, and Xiaobo Lv. 2022. "Visible Light Promotes Cascade Trifluoromethylation/Cyclization, Leading to Trifluoromethylated Polycyclic Quinazolinones, Benzimidazoles and Indoles" Molecules 27, no. 23: 8389. https://doi.org/10.3390/molecules27238389
APA StyleMa, R., Ren, Y., Deng, Z., Wang, K.-H., Wang, J., Huang, D., Hu, Y., & Lv, X. (2022). Visible Light Promotes Cascade Trifluoromethylation/Cyclization, Leading to Trifluoromethylated Polycyclic Quinazolinones, Benzimidazoles and Indoles. Molecules, 27(23), 8389. https://doi.org/10.3390/molecules27238389







