New MgFeAl-LDH Catalysts for Claisen–Schmidt Condensation
Abstract
:1. Introduction
2. Results and Discussion
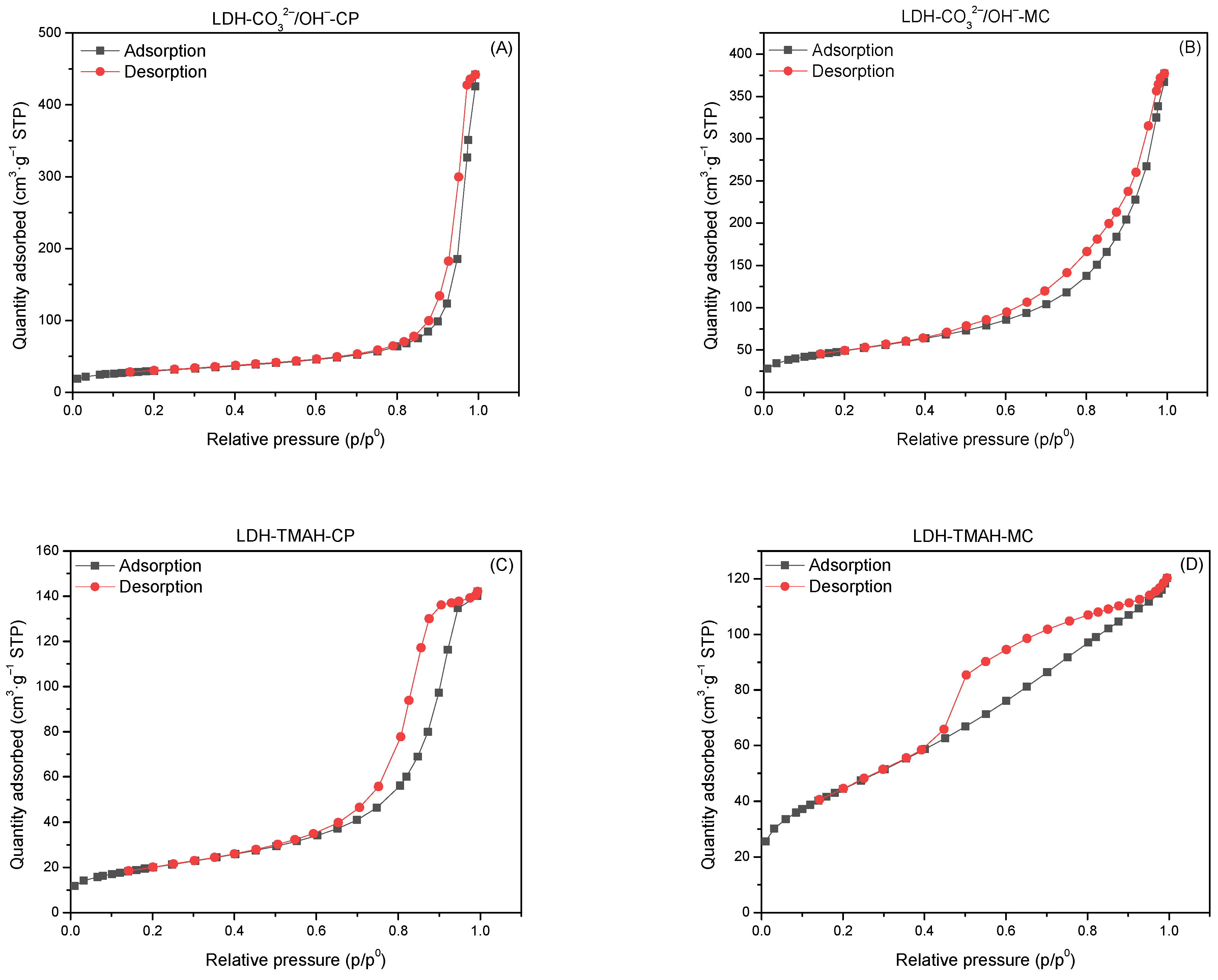
2.1. Characterization of Catalysts
2.2. Catalytic Activity
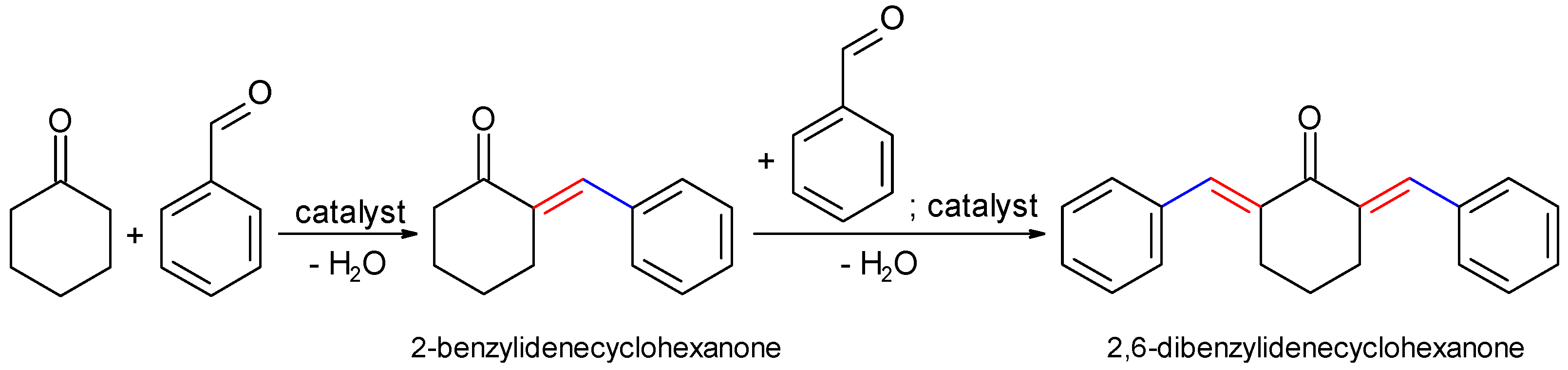
2.2.1. Claisen–Schmidt Condensation
2.2.2. Benzaldehyde Oxidation as Possible Side Reaction
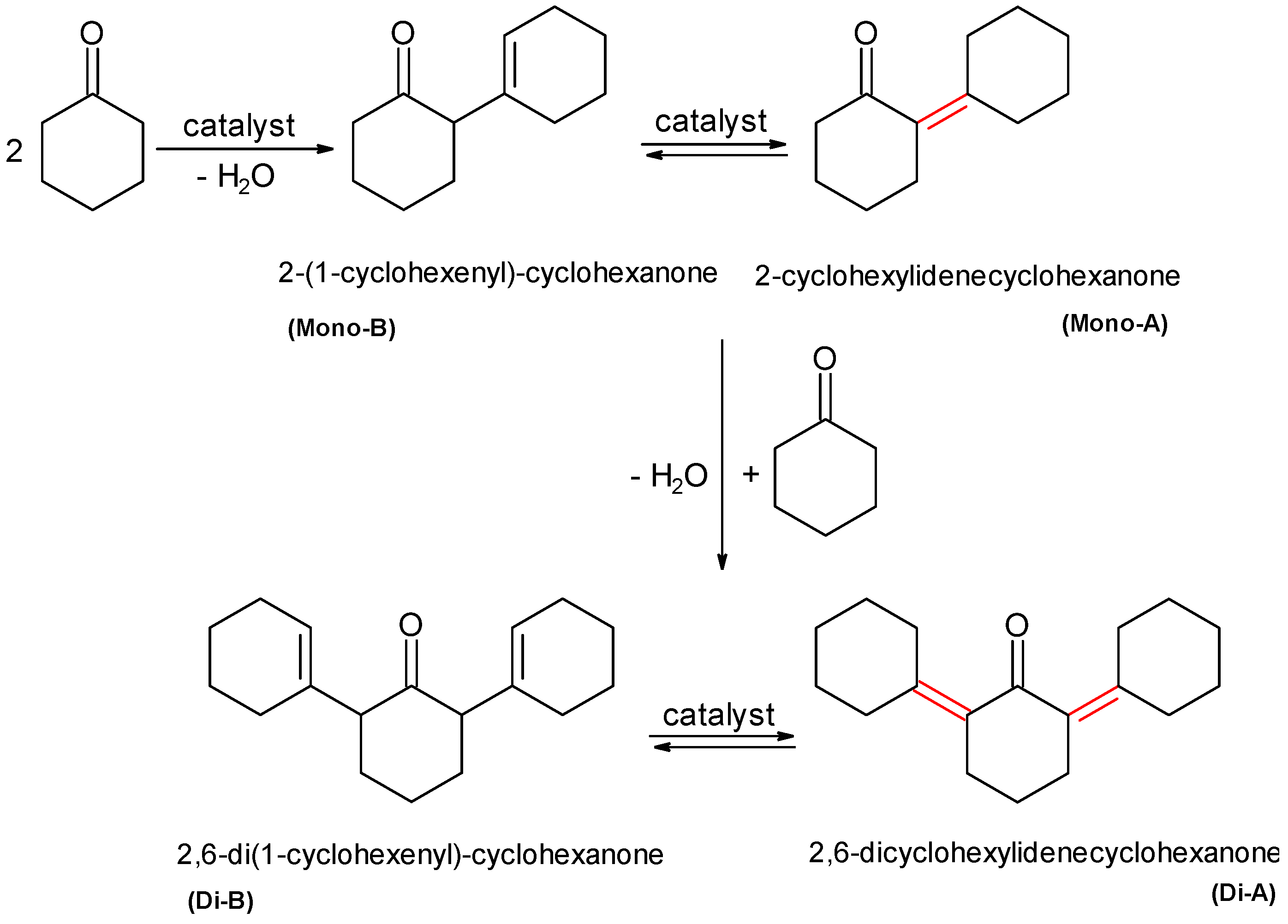
2.2.3. Self-Cyclohexanone Condensation as Possible Side Reaction
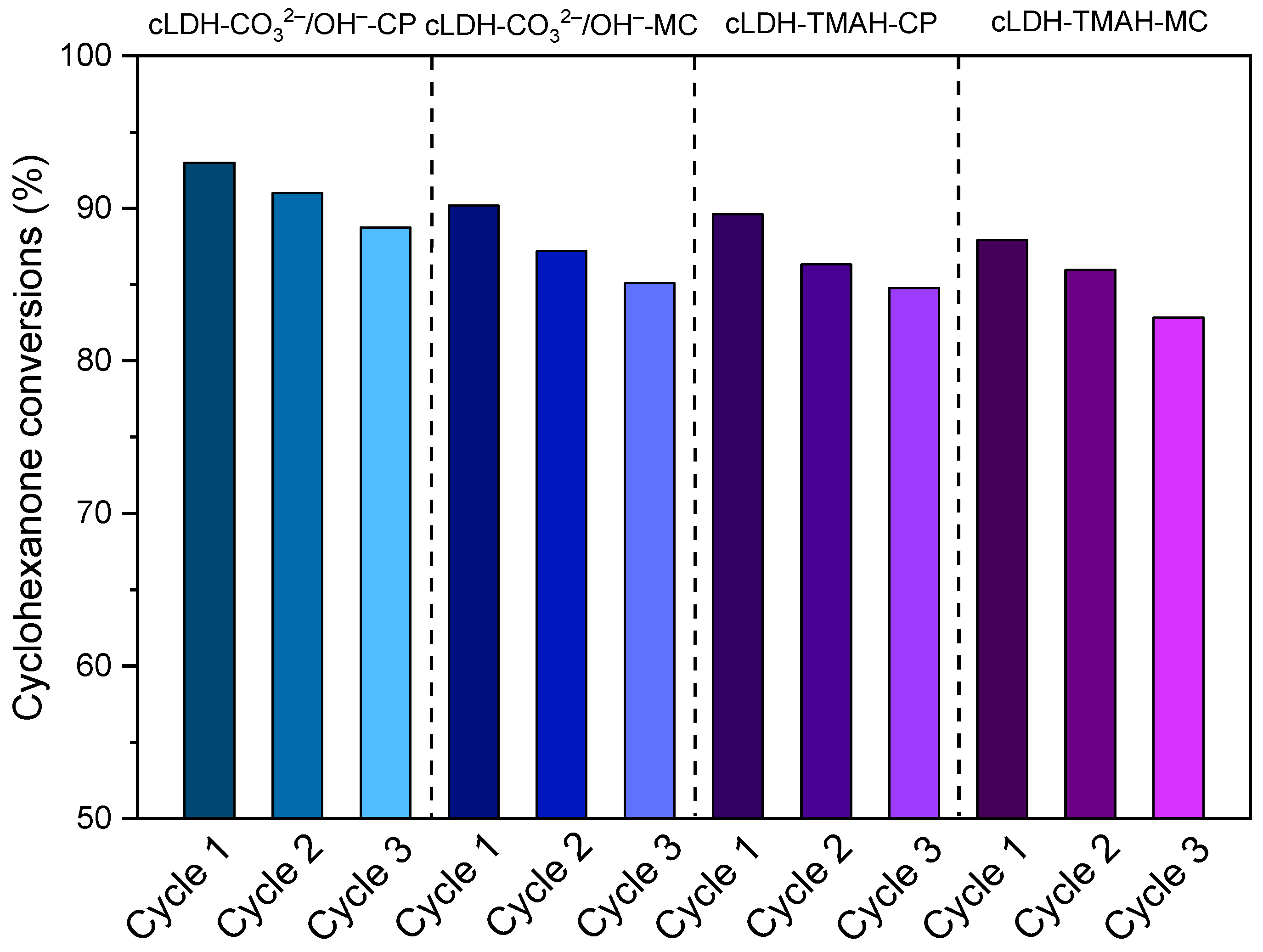
2.3. Catalyst Reusability
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
3.3.1. Claisen–Schmidt Condensation
3.3.2. The Aldol Cyclohexanone Self-Condensation
3.3.3. Benzaldehyde Oxidation
3.4. Catalyst Recycling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Bravo-Suárez, J.J.; Páez-Mozo, E.A.; Oyama, S.T. Review of the synthesis of layered double hydroxides: A thermodynamic approach. Quim. Nova 2004, 27, 601–614. [Google Scholar] [CrossRef]
- Angelescu, E.; Pavel, O.; Bîrjega, R.; Zăvoianu, R.; Costentin, G.; Che, M. Solid base catalysts obtained from hydrotalcite precursors, for Knoevenagel synthesis of cinamic acid and coumarin derivatives. Appl. Catal. A Gen. 2006, 308, 13–18. [Google Scholar] [CrossRef]
- Teodorescu, F.; Slabu, A.; Pavel, O.; Zăvoianu, R. A comparative study on the catalytic activity of ZnAl, NiAl, and CoAl mixed oxides derived from LDH obtained by mechanochemical method in the synthesis of 2-methylpyrazine. Catal. Commun. 2020, 133, 105829. [Google Scholar] [CrossRef]
- Bröcker, F.J.; Kainer, L. German Patent 2.024.282 (1970), to BASF AG, and UK Patent 1.342.020 (1971), to BASF AG.
- Jin, W.; Lee, D.; Jeon, Y.; Park, D.-H. Biocompatible Hydrotalcite Nanohybrids for Medical Functions. Minerals 2020, 10, 172. [Google Scholar] [CrossRef] [Green Version]
- Ulibarri, M.; Pavlovic, I.; Hermosín, M.; Cornejo, J. Hydrotalcite-like compounds as potential sorbents of phenols from water. Appl. Clay Sci. 1995, 10, 131–145. [Google Scholar] [CrossRef]
- Yamaoka, T.; Abe, M.; Tsuji, M. Synthesis of Cu–Al hydrotalcite like compound and its ion exchange property. Mater. Res. Bull. 1989, 24, 1183–1199. [Google Scholar] [CrossRef]
- Chaillot, D.; Bennici, S.; Brendlé, J. Layered double hydroxides and LDH-derived materials in chosen environmental applications: A review. Environ. Sci. Pollut. Res. 2021, 28, 24375–24405. [Google Scholar] [CrossRef]
- Vu, V.N.; Pham, T.H.T.; Chanthavong, M.; Do, T.H.; Nguyen, T.H.L.; Nguyen, Q.D.; Tran, T.K.N. Enhanced Photocatalytic Degradation of Rhodamine-B under Led Light Using CuZnAl Hydrotalcite Synthesized by Co-Precipitation Technique. Inorganics 2022, 10, 89. [Google Scholar] [CrossRef]
- van der Ven, L.; van Gemert, M.; Batenburg, L.; Keern, J.; Gielgens, L.; Koster, T.; Fischer, H. On the action of hydrotalcite-like clay materials as stabilizers in polyvinylchloride. Appl. Clay Sci. 2000, 17, 25–34. [Google Scholar] [CrossRef]
- Jing, C.; Dong, B.; Raza, A.; Zhang, T.; Zhang, Y. Corrosion inhibition of layered double hydroxides for metal-based systems. Nano Mater. Sci. 2020, 3, 47–67. [Google Scholar] [CrossRef]
- Tichit, D.; Coq, B. Catalysis by Hydrotalcites and Related Materials. CATTECH 2003, 7, 206–217. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, J.; Fan, Y.; Zhan, Y.; Chen, C.; Li, D.; Jiang, L. Mg–Al hydrotalcite-supported Pd catalyst for low-temperature CO oxidation: Effect of Pdn+ species and surface hydroxyl groups. Dalton Trans. 2018, 47, 14938–14944. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Huang, H.; Niederberger, M. Layered cobalt hydrotalcite as an advanced lithium-ion anode material with high capacity and rate capability. J. Mater. Chem. A 2019, 7, 21264–21269. [Google Scholar] [CrossRef] [Green Version]
- Trujillano, R.; González-García, I.; Morato, A.; Rives, V. Controlling the Synthesis Conditions for Tuning the Properties of Hydrotalcite-Like Materials at the Nano Scale. ChemEngineering 2018, 2, 31. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Fan, M.; DaCosta, H.F.M.; Russell, A.G.; Berchtold, K.A.; Dubey, M.K. Chapter 10–CO2 Sorption. In Coal Gasification and Its Applications; Bell, D.A., Towler, B.F., Fan, M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2011; pp. 293–339. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–766. [Google Scholar] [CrossRef]
- Ardhayanti, L.I.; Santosa, S.J. Synthesis of Magnetite-Mg/Al Hydrotalcite and Its Application as Adsorbent for Navy Blue and Yellow F3G Dyes. Procedia Eng. 2016, 148, 1380–1387. [Google Scholar] [CrossRef] [Green Version]
- Centi, G.; Perathoner, S. Behaviour of SOx-traps derived from ternary Cu/Mg/Al hydrotalcite materials. Catal. Today 2007, 127, 219–229. [Google Scholar] [CrossRef]
- Das, N.N.; Das, R. Synthesis, characterization and activation of quaternary layered double hydroxides for the one-pot synthesis of methyl isobutyl ketone. React. Kinet. Mech. Catal. 2010, 99, 397–408. [Google Scholar] [CrossRef]
- Barbosa, C.A.; Dias, P.M.; Ferreira, A.M.D.C.; Constantino, V.R. Mg–Al hydrotalcite-like compounds containing iron-phthalocyanine complex: Effect of aluminum substitution on the complex adsorption features and catalytic activity. Appl. Clay Sci. 2005, 28, 147–158. [Google Scholar] [CrossRef]
- Jana, S.K.; Kubota, Y.; Tatsumi, T. Cobalt-substituted polyoxometalate pillared hydrotalcite: Synthesis and catalysis in liquid-phase oxidation of cyclohexanol with molecular oxygen. J. Catal. 2008, 255, 40–47. [Google Scholar] [CrossRef]
- Li, Q.; Kirkpatrick, R.J. Organic anions in layered double hydroxides: An experimental investigation of citrate hydrotalcite. Am. Min. 2007, 92, 397–402. [Google Scholar] [CrossRef]
- Jana, A.; Roy, O.; Ravuru, S.S.; De, S. Tuning of graphene oxide intercalation in magnesium aluminium layered double hydroxide and their immobilization in polyacrylonitrile beads by single step mussel inspired phase inversion: A super adsorbent for lead. Chem. Eng. J. 2020, 391, 123587. [Google Scholar] [CrossRef]
- Duarte, M.F.; Rocha, I.M.; Figueiredo, J.L.; Freire, C.; Pereira, M.F.R. CoMn-LDH@carbon nanotube composites: Bifunctional electrocatalysts for oxygen reactions. Catal. Today 2018, 301, 17–24. [Google Scholar] [CrossRef]
- Ju, M.; Cai, R.; Ren, J.; Chen, J.; Qi, L.; Long, X.; Yang, S. Conductive Polymer Intercalation Tunes Charge Transfer and Sorption–Desorption Properties of LDH Enabling Efficient Alkaline Water Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 37063–37070. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, Q.; Li, X.; He, X.; Song, S. Mechanochemical approaches to synthesize layered double hydroxides: A review. Appl. Clay Sci. 2015, 119, 185–192. [Google Scholar] [CrossRef]
- Pavel, O.; Zăvoianu, R.; Bîrjega, R.; Angelescu, E.; Pârvulescu, V. Mechanochemical versus co-precipitated synthesized lanthanum-doped layered materials for olefin oxidation. Appl. Catal. A Gen. 2017, 542, 10–20. [Google Scholar] [CrossRef]
- Pavel, O.D.; Stamate, A.-E.; Zăvoianu, R.; Bucur, I.C.; Bîrjega, R.; Angelescu, E.; Pârvulescu, V.I. Mechano-chemical versus co-precipitation for the preparation of Y-modified LDHs for cyclohexene oxidation and Claisen-Schmidt condensations. Appl. Catal. A Gen. 2020, 605, 117797. [Google Scholar] [CrossRef]
- Pavel, O.D.; Stamate, A.-E.; Bacalum, E.; Cojocaru, B.; Zăvoianu, R.; Pârvulescu, V.I. Catalytic behavior of Li-Al-LDH prepared via mechanochemical and co-precipitation routes for cyanoethylation reaction. Catal. Today 2021, 366, 227–234. [Google Scholar] [CrossRef]
- Wang, C.; Li, F.; Sun, Z.; Song, Q. A highly active K/Cu-Mn-O catalyst for the removal of nitric oxide in indoor air. Indoor Built. Environ. 2019, 28, 7–16. [Google Scholar] [CrossRef]
- Ciotta, E.; Pizzoferrato, R.; Di Vona, M.L.; Ferrari, I.V.; Richetta, M.; Varone, A. Increasing the Electrical Conductivity of Layered Double Hydroxides by Intercalation of Ionic Liquids. Mater. Sci. Forum 2018, 941, 2209–2213. [Google Scholar] [CrossRef]
- Rong, F.; Zhao, J.; Yang, Q.; Li, C. Nanostructured hybrid NiFeOOH/CNT electrocatalysts for oxygen evolution reaction with low overpotential. RSC Adv. 2016, 6, 74536–74544. [Google Scholar] [CrossRef]
- Cojocaru, B.; Jurca, B.C.; Zăvoianu, R.; Bîrjega, R.; Pârvulescu, V.I.; Pavel, O.D. Tailored texture synthesized LDH catalysts in the presence of quaternary ammonium salts. Catal. Commun. 2022, 170, 106485. [Google Scholar] [CrossRef]
- Sels, B.F.; De Vos, D.E.; Jacobs, P.A. Hydrotalcite-like anionic clays in catalytic organic reactions. Catal. Rev. 2001, 43, 443–488. [Google Scholar] [CrossRef]
- Schmidt, J.G. Ueber die Einwirkung von Aldehyd auf Furfurol. Ber. Dtsch. Chem. Ges. 1880, 13, 2342–2345. [Google Scholar] [CrossRef] [Green Version]
- Claisen, L. Condensationen der Aldehyde mit Acetessig- und Malonsäureäther. Ber. Dtsch. Chem. Ges. 1881, 14, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Zăvoianu, R.; Mihăilă, S.-D.; Cojocaru, B.; Tudorache, M.; Pârvulescu, V.I.; Pavel, O.D.; Oikonomopoulos, S.; Jacobsen, E.E. An Advanced Approach for MgZnAl-LDH Catalysts Synthesis Used in Claisen-Schmidt Condensation. Catalysts 2022, 12, 759. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J.; Gu, X. Modified calcium oxide as stable solid base catalyst for Aldol condensation reaction. J. Chem. Sci. 2013, 125, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Jain, D.; Khatri, C.; Rani, A. Synthesis and characterization of novel solid base catalyst from fly ash. Fuel 2011, 90, 2083–2088. [Google Scholar] [CrossRef]
- Sluban, M.; Cojocaru, B.; Parvulescu, V.I.; Iskra, J.; Korošec, R.C.; Umek, P. Protonated titanate nanotubes as solid acid catalyst for aldol condensation. J. Catal. 2017, 346, 161–169. [Google Scholar] [CrossRef]
- Mortezaei, Z.; Zendehdel, M.; Bodaghifard, M.A. Synthesis and characterization of functionalized NaP Zeolite@CoFe2O4 hybrid materials: A micro–meso-structure catalyst for aldol condensation. Res. Chem. Intermed. 2020, 46, 2169–2193. [Google Scholar] [CrossRef]
- Rahman, A.F.M.M.; Ali, R.; Jahng, Y.; Kadi, A.A. A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones. Molecules 2012, 17, 571–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.G.; Liu, J.; Zeng, P.L.; Dong, Z.B. Synthesis of α,α′-bis(Substituted Benzylidene)Ketones Catalysed by a SOCl2/EtOH reagent. J. Chem. Res. 2004, 2004, 55–56. [Google Scholar] [CrossRef]
- Vashishtha, M.; Mishra, M.; Undre, S.; Singh, M.; Shah, D.O. Molecular mechanism of micellar catalysis of cross aldol reaction: Effect of surfactant chain length and surfactant concentration. J. Mol. Catal. A Chem. 2015, 396, 143–154. [Google Scholar] [CrossRef]
- Angelescu, E.; Birjega, R.; Pavel, O.D.; Che, M.; Costentin, G.; Popoiu, S. Hydrotalcites (HTs) and mesoporous mixed oxides obtained from HTs, basic solid catalysts for cyclohexanone condensation. Stud. Surf. Sci. Catal. 2005, 156, 257–264. [Google Scholar] [CrossRef]
- Peng, X.; Zeb, S.; Zhao, J.; Zhang, M.; Cui, Y.; Sun, G. Highly selective self-condensation of cyclohexanone: The distinct catalytic behaviour of HRF5015. R. Soc. Open Sci. 2020, 7, 200123. [Google Scholar] [CrossRef]
- Wu, X.; Xianmei, X.; Wu, Z.; Xie, X. Oxidation of Benzaldehyde to Benzoic Acid using Heterogenous Nial-Hydrotalcite-Like-Compounds as the Catalyst in Acetic Acid. Prog. React. Kinet. Mech. 2011, 36, 53–62. [Google Scholar] [CrossRef]
- Bîrjega, R.; Pavel, O.; Costentin, G.; Che, M.; Angelescu, E. Rare-earth elements modified hydrotalcites and corresponding mesoporous mixed oxides as basic solid catalysts. Appl. Catal. A: Gen. 2005, 288, 185–193. [Google Scholar] [CrossRef]
- Miyata, S. The Syntheses of Hydrotalcite-Like Compounds and Their Structures and Physico-Chemical Properties—I: The Systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Min. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Frost, R.L.; Cash, G.A.; Kloprogge, J. ‘Rocky Mountain leather’, sepiolite and attapulgite—an infrared emission spectroscopic study. Vib. Spectrosc. 1998, 16, 173–184. [Google Scholar] [CrossRef]
- Marchi, A.; Apesteguía, C. Impregnation-induced memory effect of thermally activated layered double hydroxides. Appl. Clay Sci. 1998, 13, 35–48. [Google Scholar] [CrossRef]
- Benziger, J.B.; Larson, L. An infrared spectroscopy study of the adsorption of CO on Fe/MgO. J. Catal. 1982, 77, 550–553. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Shi, R.; Waterhouse, G.I.N.; Wen, X.; Zhang, T. Fe-Based Catalysts for the Direct Photohydrogenation of CO 2 to Value-Added Hydrocarbons. Adv. Energy Mater. 2021, 11, 2002783. [Google Scholar] [CrossRef]
- Su, Y.; Wen, N.; Cheng, J.; Deng, W.; Zhou, H.; Zhao, B. Experimental Study on SCR-C3H6 Over Cu–Fe/Al-PILC Catalysts: Catalytic Performance, Characterization, and Mechanism. Ind. Eng. Chem. Res. 2020, 59, 14776–14788. [Google Scholar] [CrossRef]
- Chamritski, I.; Burns, G. Infrared- and Raman-Active Phonons of Magnetite, Maghemite, and Hematite: A Computer Simulation and Spectroscopic Study. J. Phys. Chem. B 2005, 109, 4965–4968. [Google Scholar] [CrossRef] [PubMed]
- Chourpa, I.; Douziech-Eyrolles, L.; Ngaboni-Okassa, L.; Fouquenet, J.-F.; Cohen-Jonathan, S.; Soucé, M.; Marchais, H.; Dubois, P. Molecular composition of iron oxide nanoparticles, precursors for magnetic drug targeting, as characterized by confocal Raman microspectroscopy. Analyst 2005, 130, 1395–1403. [Google Scholar] [CrossRef]
- Gunawardana, B.; Singhal, N.; Swedlund, P. Degradation of Chlorinated Phenols by Zero Valent Iron and Bimetals of Iron: A Review. Environ. Eng. Res. 2011, 16, 187–203. [Google Scholar] [CrossRef] [Green Version]
- Rafiee, E.; Rahimi, F. A green approach to the synthesis of chalcones via Claisen-Schmidt condensation reaction using cesium salts of 12-tungstophosphoric acid as a reusable nanocatalyst. Mon. Chem. 2013, 144, 361–367. [Google Scholar] [CrossRef]
- Li, J.-T.; Yang, W.-Z.; Chen, G.-F.; Li, T.-S. A Facile Synthesis of α,α′-bis(Substituted Benzylidene) Cycloalkanones Catalyzed by KF/Al2O3 Under Ultrasound Irradiation. Synth. Commun. 2003, 33, 2619–2625. [Google Scholar] [CrossRef]
- Debecker, D.P.; Gaigneaux, E.M.; Busca, G. Exploring, Tuning, and Exploiting the Basicity of Hydrotalcites for Applications in Heterogeneous Catalysis. Chem. Eur. J. 2009, 15, 3920–3935. [Google Scholar] [CrossRef]
- Parida, K.; Das, J. Mg/Al hydrotalcites: Preparation, characterisation and ketonisation of acetic acid. J. Mol. Catal. A Chem. 2000, 151, 185–192. [Google Scholar] [CrossRef]
- Pavel, O.; Zăvoianu, R.; Bîrjega, R.; Angelescu, E. The effect of ageing step elimination on the memory effect presented by Mg0.75Al0.25 hydrotalcites (HT) and their catalytic activity for cyanoethylation reaction. Catal. Commun. 2011, 12, 845–850. [Google Scholar] [CrossRef]
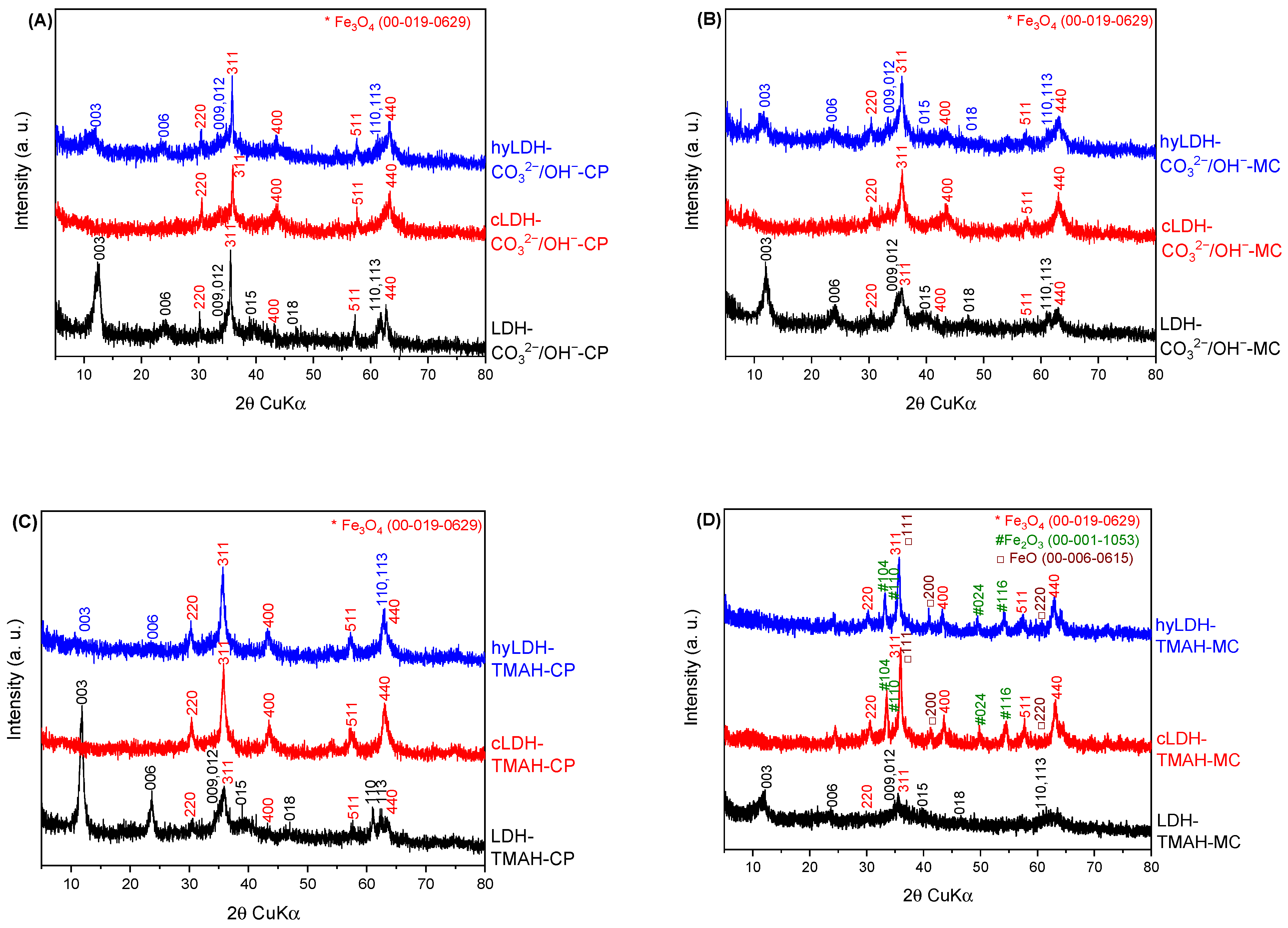

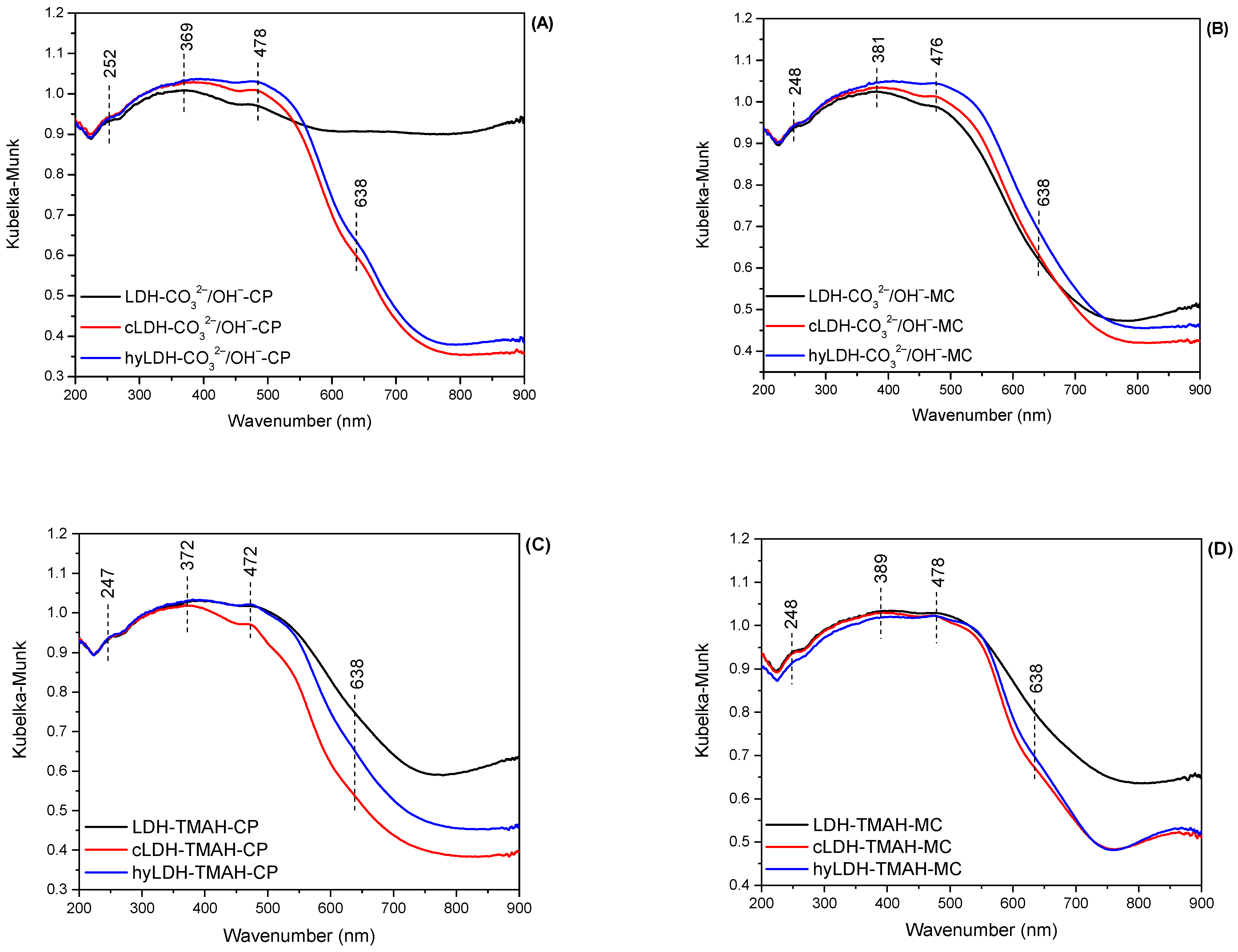







| Hydrotalcite Samples | Lattice Parameters | IFS 1 (Å) | 2θ003 (°) | I003/I006 | I003/I110 | FWHM003 | D 2 (Å) | |
|---|---|---|---|---|---|---|---|---|
| a (Å) | c (Å) | |||||||
| LDH-CO32−/OH−-CP | 3.004 | 22.039 | 2.55 | 12.6607 | 4.30 | 2.48 | 0.3120 | 256 |
| hyLDH-CO32−/OH−-CP | 3.036 | 22.412 | 2.67 | 11.8866 | 1.70 | 1.42 | 0.2667 | 300 |
| LDH-CO32−/OH−-MC | 3.040 | 22.492 | 2.70 | 11.9400 | 3.50 | 2.80 | 0.4400 | 182 |
| hyLDH-CO32−/OH−-MC | 3.041 | 23.029 | 2.88 | 11.4720 | 1.50 | 1.17 | 0.1040 | 667 |
| LDH-TMAH-CP | 3.034 | 22.575 | 2.72 | 11.7720 | 3.73 | 4.97 | 0.7200 | 111 |
| hyLDH-TMAH-CP | 3.033 | 23.035 | 2.88 | 11.5400 | 0.57 | 0.57 | 0.1200 | 665 |
| LDH-TMAH-MC | 3.038 | 22.920 | 2.84 | 11.2666 | 1.13 | 1.38 | 0.1333 | 600 |
| hyLDH-TMAH-MC | 3.033 | 22.283 | 2.63 | 11.9000 | 0.64 | 1.00 | 0.0534 | 1296 |
| Mixed oxide samples | a (Å) | 2θ311 (°) | I311 | FWHM311 | D 3 (Å) | |||
| cLDH-CO32−/OH−-CP | 4.995 | 35.9143 | 59 | 0.2087 | 400 | |||
| cLDH-CO32−/OH−-MC | 5.025 | 35.7083 | 60 | 0.7967 | 105 | |||
| cLDH-TMAH-CP | 5.021 | 35.7333 | 95 | 0.7333 | 114 | |||
| cLDH-TMAH-MC | 4.998 | 35.9074 | 123 | 0.4709 | 177 | |||
| Samples | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Width (Å) | Total Number of Base Sites (mmol·g−1) 1 |
|---|---|---|---|---|
| LDH-CO32−/OH−-CP | 103 | 0.505 | 191 | 7.21 |
| cLDH-CO32−/OH−-CP | 263 | 0.831 | 127 | 7.23 |
| hyLDH-CO32−/OH−-CP | 37 | 0.199 | 209 | 7.15 |
| LDH-CO32−/OH−-MC | 170 | 0.503 | 113 | 7.14 |
| cLDH-CO32−/OH−-MC | 274 | 0.875 | 103 | 7.25 |
| hyLDH-CO32−/OH−-MC | 42 | 0.213 | 222 | 6.96 |
| LDH-TMAH-CP | 69 | 0.217 | 120 | 7.16 |
| cLDH-TMAH-CP | 223 | 0.678 | 131 | 7.20 |
| hyLDH-TMAH-CP | 101 | 0.587 | 183 | 6.88 |
| LDH-TMAH-MC | 154 | 0.177 | 43 | 6.85 |
| cLDH-TMAH-MC | 231 | 0.764 | 139 | 7.15 |
| hyLDH-TMAH-MC | 139 | 0.438 | 111 | 6.73 |
| Catalysts | Benzaldehyde Conversion (%) |
|---|---|
| blank | 31.5 |
| LDH-CO32−/OH−-CP | 0.5 |
| cLDH-CO32−/OH−-CP | 0.6 |
| hyLDH-CO32−/OH−-CP | 0.2 |
| LDH-CO32−/OH−-MC | 0.4 |
| cLDH-CO32−/OH−-MC | 4.5 |
| hyLDH-CO32−/OH−-MC | 4.5 |
| LDH-TMAH-CP | 3.4 |
| cLDH-TMAH-CP | 4.8 |
| hyLDH-TMAH-CP | 3.4 |
| LDH-TMAH-MC | 2.4 |
| cLDH-TMAH-MC | 6.0 |
| hyLDH-TMAH-MC | 3.5 |
| Catalysts | Conv. C6H10O (%) | Sel. (%) Mono-A | Sel. (%) Mono-B | Sel. (%) Di-A | Sel. (%) Di-B |
|---|---|---|---|---|---|
| LDH-CO32−/OH−-CP | 0.8 | 60.1 | 37.8 | 1.1 | 1.0 |
| cLDH-CO32−/OH−-CP | 0.9 | 71.0 | 28.8 | 0.1 | 0.1 |
| hyLDH-CO32−/OH−-CP | 0.8 | 62.2 | 37.2 | 0.5 | 0.1 |
| LDH-CO32−/OH−-MC | 0.3 | 63.3 | 36.7 | 0.0 | 0.0 |
| cLDH-CO32−/OH−-MC | 0.7 | 78.5 | 21.5 | 0.0 | 0.0 |
| hyLDH-CO32−/OH−-MC | 0.5 | 70.8 | 29.2 | 0.0 | 0.0 |
| LDH-TMAH-CP | 1.2 | 75.6 | 13.0 | 5.5 | 5.9 |
| cLDH-TMAH-CP | 3.8 | 83.9 | 14.5 | 1.4 | 0.2 |
| hyLDH-TMAH-CP | 3.3 | 77.0 | 21.7 | 1.1 | 0.2 |
| LDH-TMAH-MC | 0.6 | 78.0 | 22.0 | 0.0 | 0.0 |
| cLDH-TMAH-MC | 1.0 | 81.1 | 18.9 | 0.0 | 0.0 |
| hyLDH-TMAH-MC | 0.9 | 80.0 | 20.0 | 0.0 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zăvoianu, R.; Tudorache, M.; Parvulescu, V.I.; Cojocaru, B.; Pavel, O.D. New MgFeAl-LDH Catalysts for Claisen–Schmidt Condensation. Molecules 2022, 27, 8391. https://doi.org/10.3390/molecules27238391
Zăvoianu R, Tudorache M, Parvulescu VI, Cojocaru B, Pavel OD. New MgFeAl-LDH Catalysts for Claisen–Schmidt Condensation. Molecules. 2022; 27(23):8391. https://doi.org/10.3390/molecules27238391
Chicago/Turabian StyleZăvoianu, Rodica, Mădălina Tudorache, Vasile I. Parvulescu, Bogdan Cojocaru, and Octavian D. Pavel. 2022. "New MgFeAl-LDH Catalysts for Claisen–Schmidt Condensation" Molecules 27, no. 23: 8391. https://doi.org/10.3390/molecules27238391