New TEMPO–Appended 2,2′-Bipyridine-Based Eu(III), Tb(III), Gd(III) and Sm(III) Complexes: Synthesis, Photophysical Studies and Testing Photoluminescence-Based Bioimaging Abilities
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Synthetic Design of the TEMPO-Lanthanide(III)-Based (bio)Thiol Probes
2.2. Synthesis of New TEMPO-Appended 2,2′-Bipyridine-Based Eu(III), Tb(III), Gd(III) and Sm(III) Complexes
2.3. Determination of the Structure of the Obtained Semi-Products and Lanthanide (III) Complexes
2.4. Photophysical Studies
2.5. Fluorescence and EPR Titration with Thio Compounds
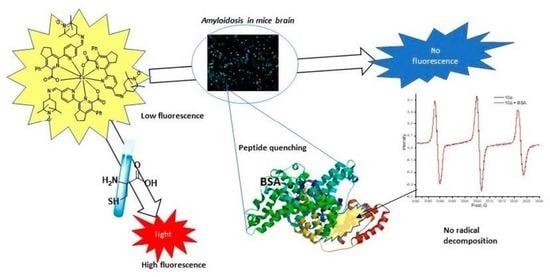
2.6. Tests on Tissue Sections of Mice with Amyloidosis
2.7. Vero Cell Culture Tests
2.8. Studies of Interaction between the Complex 10a and BSA by Means of EPR and Photophysical Methods
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Biological Studies
3.2.1. Ethics
3.2.2. Cell Cultivation
3.2.3. Cell and Tissue Staining
3.2.4. Microscopic Examination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Schwartz, L.N.; Shaffer, J.D.; Bukhman, G. The origins of the 4 × 4 framework for noncommunicable disease at the World Health Organization. SSM-Popul. Health 2021, 13, 100731. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H. Metabolism of sulfur-containing amino acids. Annu. Rev. Nutr. 1986, 6, 179–209. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.; Fritz, K.; McGinnis, C.; Marentette, J.; Roede, J. Alcohol metabolism induces a reduced hepatic cysteine proteome. Free Radic. Biol. Med. 2022, 192, 80. [Google Scholar] [CrossRef]
- Paul, B.D. Cysteine metabolism and hydrogen sulfide signaling in Huntington’s disease. Free Radic. Biol. Med. 2022, 186, 93–98. [Google Scholar] [CrossRef]
- Gunda, V.; Chhonker, Y.S.; Natesh, N.S.; Raut, P.; Muniyan, S.; Wyatt, T.A.; Murry, D.J.; Batra, S.K.; Rachagani, S. Nuclear factor kappa-B contributes to cigarette smoke tolerance in pancreatic ductal adenocarcinoma through cysteine metabolism. Biomed. Pharmacother. 2021, 144, 112312. [Google Scholar] [CrossRef]
- Da, D.; Pan, Z.; Zeng, L.; Dang, Y.; Dang, C.; Huang, Y.; Shi, D.; Li, H. Glutamate-cysteine ligase catalytic and its modifier function as novel immunotargets in gastric adenocarcinoma. Asian J. Surg. 2022, in press. [Google Scholar] [CrossRef]
- Zhang, H.F.; Hughes, C.S.; Li, W.; He, J.Z.; Surdez, D.; El-Naggar, A.M.; Cheng, H.; Prudova, A.; Delaidelli, A.; Negri, G.L.; et al. Proteomic screens for suppressors of anoikis identify IL1RAP as a promising surface target in ewing sarcoma. Cancer Discov. 2021, 11, 2884–2903. [Google Scholar] [CrossRef]
- Oates, P.J. Polyol pathway and diabetic peripheral neuropathy. Int. Rev. Neurobiol. 2002, 50, 325–392. [Google Scholar] [CrossRef]
- Araujo, T.F.; Cordeiro, A.V.; Vasconcelos, D.A.A.; Vitzel, K.F.; Silva, V.R.R. The role of cathepsin B in autophagy during obesity: A systematic review. Life Sci. 2018, 209, 274–281. [Google Scholar] [CrossRef]
- Barteková, M.; Adameová, A.; Görbe, A.; Ferenczyová, K.; Pecháňová, O.; Lazou, A.; Dhalla, N.S.; Ferdinandy, P.; Giricz, Z. Natural and synthetic antioxidants targeting cardiac oxidative stress and redox signaling in cardiometabolic diseases. Free Radic. Biol. Med. 2021, 169, 446–477. [Google Scholar] [CrossRef]
- Litke, R.; Garcharna, L.C.; Jiwani, S.; Neugroschl, J. Modifiable Risk Factors in Alzheimer Disease and Related Dementias: A Review. Clin. Ther. 2021, 43, 953–965. [Google Scholar] [CrossRef]
- Maina, M.B.; Al-Hilaly, Y.K.; Oakley, S.; Burra, G.; Khanom, T.; Biasetti, L.; Mengham, K.; Marshall, K.; Harrington, C.R.; Wischik, C.M.; et al. Dityrosine Cross-links are Present in Alzheimer’s Disease-derived Tau Oligomers and Paired Helical Filaments (PHF) which Promotes the Stability of the PHF-core Tau (297–391) In Vitro. J. Mol. Biol. 2022, 434, 167785. [Google Scholar] [CrossRef]
- Thal, D.R.; Tomé, S.O. The central role of tau in Alzheimer’s disease: From neurofibrillary tangle maturation to the induction of cell death. Brain Res. Bull. 2022, 190, 204–217. [Google Scholar] [CrossRef]
- Kageyama, Y.; Irie, Y.; Matsushima, Y.; Segawa, T.; Bellier, J.P.; Hidaka, K.; Sugiyama, H.; Kaneda, D.; Hashizume, Y.; Akatsu, H.; et al. Characterization of a Conformation-Restricted Amyloid β Peptide and Immunoreactivity of Its Antibody in Human AD brain. ACS Chem. Neurosci. 2021, 12, 3418–3432. [Google Scholar] [CrossRef]
- Schormann, N.; Murrell, J.R.; Benson, M.D. Tertiary structures of amyloidogenic and non-amyloidogenic transthyretin variants: New model for amyloid fibril formation. Amyloid 1998, 5, 175–187. [Google Scholar] [CrossRef]
- Amyloid-Beta Precursor Protein Isoform 1 Precursor [Mus Musculus]—Protein—NCBI. Available online: https://www.ncbi.nlm.nih.gov/protein/NP_001185752 (accessed on 24 October 2022).
- Kluska, K.; Adamczyk, J.; Krężel, A. Metal binding properties, stability and reactivity of zinc fingers. Coord. Chem. Rev. 2018, 367, 18–64. [Google Scholar] [CrossRef]
- Brambilla, D.; Le Droumaguet, B.; Nicolas, J.; Hashemi, S.H.; Wu, L.P.; Moghimi, S.M.; Couvreur, P.; Andrieux, K. Nanotechnologies for Alzheimer’s disease: Diagnosis, therapy, and safety issues. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 521–540. [Google Scholar] [CrossRef]
- Scarano, S.; Lisi, S.; Ravelet, C.; Peyrin, E.; Minunni, M. Detecting Alzheimer’s disease biomarkers: From antibodies to new bio-mimetic receptors and their application to established and emerging bioanalytical platforms—A critical review. Anal. Chim. Acta 2016, 940, 21–37. [Google Scholar] [CrossRef]
- Tooyama, I.; Yanagisawa, D.; Taguchi, H.; Kato, T.; Hirao, K.; Shirai, N.; Sogabe, T.; Ibrahim, N.F.; Inubushi, T.; Morikawa, S. Amyloid imaging using fluorine-19 magnetic resonance imaging (19F-MRI). Ageing Res. Rev. 2016, 30, 85–94. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, M. Radiolabeled bioactive benzoheterocycles for imaging β-amyloid plaques in Alzheimer’s disease. Eur. J. Med. Chem. 2014, 87, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, N.; Yang, X.; Ling, G.; Zhang, P. The roles of gold nanoparticles in the detection of amyloid-β peptide for Alzheimer’s disease. Colloid Interface Sci. Commun. 2022, 46, 100579. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, C.; Jiao, X.; Cai, S.; He, S.; Zhao, L.; Zeng, X.; Wang, T. Lysosome-targeted near-infrared fluorescent dye and its application in designing of probe for sensitive detection of cysteine in living cells. Dye. Pigment. 2021, 190, 109293. [Google Scholar] [CrossRef]
- Pan, Y.; Ban, L.; Li, J.; Liu, M.; Tang, L.; Yan, X. Cysteine recognition by a benzothiazole-derived fluorescent probe with “AIE+ESIPT” characteristics. Dye. Pigment. 2022, 203, 110305. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Xiang, H.; Ying, L.; Wu, C.; Zhou, H.; Liu, H. A new near-infrared ratiometric fluorescent probe based on quinoline-fused rhodamine dye for sensitive detection of cysteine and homocysteine in mitochondria. Dye. Pigment. 2020, 183, 108710. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Y.; Guan, X. Fluorescent Probes for Live Cell Thiol Detection. Molecules 2021, 26, 3575. [Google Scholar] [CrossRef]
- Aliaga, C.; Fuentealba, P.; Rezende, M.C.; Cárdenas, C. Mechanism of fluorophore quenching in a pre-fluorescent nitroxide probe: A theoretical illustration. Chem. Phys. Lett. 2014, 593, 89–92. [Google Scholar] [CrossRef]
- Aliaga, C.; Aspée, A.; Scaiano, J.C. A New Method to Study Antioxidant Capability: Hydrogen Transfer from Phenols to a Prefluorescent Nitroxide. Org. Lett. 2003, 5, 4145–4148. [Google Scholar] [CrossRef]
- Ivan, M.G.; Scaiano, J.C. A New Approach for the Detection of Carbon-centered Radicals in Enzymatic Processes Using Prefluorescent Probes. Photochem. Photobiol. 2003, 78, 416–419. [Google Scholar] [CrossRef]
- Jia, M.; Tang, Y.; Lam, Y.F.; Green, S.A.; Blough, N.V. Prefluorescent nitroxide probe for the highly sensitive determination of peroxyl and other radical oxidants. Anal. Chem. 2009, 81, 8033–8040. [Google Scholar] [CrossRef]
- Dong, H.; Ma, S.; Zhong, Q.; Zhu, M. Crystal structure, magnetic properties and luminescent behavior of four mononuclear lanthanide-radical complexes. J. Mol. Struct. 2022, 1252, 132195. [Google Scholar] [CrossRef]
- Ma, S.; Deng, X.; Zhong, M.; Zhu, M.; Zhang, L. Three lanthanide–nitronyl nitroxide complexes: Syntheses, crystal structures, magnetic properties and fluorescence of selective sensing of Fe (III) ions. Polyhedron 2020, 179, 114370. [Google Scholar] [CrossRef]
- Train, C.; Norel, L.; Baumgarten, M. Organic radicals, a promising route towards original molecule-based magnetic materials. Coord. Chem. Rev. 2009, 253, 2342–2351. [Google Scholar] [CrossRef]
- Karbowiak, M.; Rudowicz, C.; Nakamura, T.; Murakami, R.; Ishida, T. Spectroscopic and magnetic studies of erbium(III)-TEMPO complex as a potential single-molecule magnet: Interplay of the crystal-field and exchange coupling effects. Chem. Phys. Lett. 2016, 662, 163–168. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Jeoung, S.; Moon, H.R.; Kim, M. Dual-fixations of europium cations and TEMPO species on metal-organic frameworks for the aerobic oxidation of alcohols. Dalt. Trans 2020, 49, 8060. [Google Scholar] [CrossRef]
- Kitajima, S.; Kurioka, M.; Yoshimoto, T.; Shindo, M.; Kanaori, K.; Tajima, K.; Oda, K. A cysteine residue near the propionate side chain of heme is the radical site in ascorbate peroxidase. FEBS J. 2008, 275, 470–480. [Google Scholar] [CrossRef]
- Krinochkin, A.P.; Kopchuk, D.S.; Kozhevnikov, D.N. Luminescent neutral lanthanide complexes of 5-aryl-2,2′-bipyridine-6-carboxylic acids, synthesis and properties. Polyhedron 2015, 102, 556–561. [Google Scholar] [CrossRef]
- Foster, R.A.A.; Willis, M.C. Tandem inverse-electron-demand hetero-/retro-Diels–Alder reactions for aromatic nitrogen heterocycle synthesis. Chem. Soc. Rev. 2012, 42, 63–76. [Google Scholar] [CrossRef]
- Prokhorov, A.M.; Kozhevnikov, D.N. Reactions of triazines and tetrazines with dienophiles (review). Chem. Heterocycl. Compd. 2012, 48, 1153–1176. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Charushin, V.N. Recent advances in the field of nucleophilic aromatic substitution of hydrogen. Tetrahedron Lett. 2016, 57, 2665–2672. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Charushin, V.N. Nucleophilic C-H functionalization of arenes: A new logic of organic synthesis Expanding the scope of nucleophilic substitution of hydrogen in aromatics. Pure Appl. Chem. 2017, 89, 1195–1208. [Google Scholar] [CrossRef]
- Krinochkin, A.P.; Kopchuk, D.S.; Chepchugov, N.V.; Kovalev, I.S.; Zyryanov, G.V.; Rusinov, V.L.; Chupakhin, O.N. Effect of substituent in pyridine-2-carbaldehydes on their heterocyclization to 1,2,4-triazines and 1,2,4-triazine 4-oxides. Russ. J. Org. Chem. 2017, 53, 963–970. [Google Scholar] [CrossRef]
- Kozhevnikov, V.N.; Kozhevnikov, D.N.; Nikitina, T.V.; Rusinov, V.L.; Chupakhin, O.N.; Zabel, M.; König, B. A versatile strategy for the synthesis of functionalized 2,2′-bi- and 2,2′:6′,2′-terpyridines via their 1,2,4-triazine analogues. J. Org. Chem. 2003, 68, 2882–2888. [Google Scholar] [CrossRef] [PubMed]
- Kopchuk, D.S.; Pavlyuk, D.E.; Kovalev, I.S.; Zyryanov, G.V.; Rusinov, V.L.; Chupakhin, O.N. Synthesis of a new DTTA-and 5-phenyl-2,2′-bipyridine-based ditopic ligand and its Eu3+ complex. Can. J. Chem. 2016, 94, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Kopchuk, D.S.; Kim, G.A.; Kovalev, I.S.; Santra, S.; Zyryanov, G.V.; Majee, A.; Rusinov, V.L.; Chupakhin, O.N. Tripod-type 2,2′-bipyridine ligand for lanthanide cations: Synthesis and photophysical studies on coordination to transition metal cations. Can. J. Chem. 2018, 96, 419–424. [Google Scholar] [CrossRef]
- Gillies, D.G.; Sutcliffe, L.H.; Wu, X.; Belton, P.S. Molecular motion of a water-soluble nitroxyl radical in gelatin gels. Food Chem. 1996, 55, 349–352. [Google Scholar] [CrossRef]
- Shin, B.K.; Saxena, S. Insight into potential cu(II)-binding motifs in the four pseudorepeats of tau protein. J. Phys. Chem. B 2011, 115, 15067–15078. [Google Scholar] [CrossRef]
- Nandi, N.B.; Purkayastha, A.; Roy, S.; Kłak, J.; Ganguly, R.; Alkorta, I.; Misra, T.K. Tetranuclear copper(II) cubane complexes derived from self-assembled 1,3-dimethyl-5-(o-phenolate-azo)-6-aminouracil: Structures, non-covalent interactions and magnetic property. New J. Chem. 2021, 45, 2742–2753. [Google Scholar] [CrossRef]
- Ghosh, S.; Garcia, V.; Singewald, K.; Damo, S.M.; Saxena, S. Cu(II) EPR Reveals Two Distinct Binding Sites and Oligomerization of Innate Immune Protein Calgranulin C. Appl. Magn. Reson. 2018, 49, 1299–1311. [Google Scholar] [CrossRef]
- Hong, J.; Zhuang, Y.; Ji, X.; Guo, X. A long-lived luminescence and EPR bimodal lanthanide-based probe for free radicals. Analyst 2011, 136, 2464–2470. [Google Scholar] [CrossRef]
- Leguerrier, D.M.D.; Barré, R.; Molloy, J.; Thomas, F. Lanthanide complexes as redox and ROS/RNS probes: A new paradigm that makes use of redox-reactive and redox non-innocent ligands. Coord. Chem. Rev. 2021, 446, 214133. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Gumy, F.; Scopelliti, R.; Bünzli, J.C.G. Highly luminescent homoleptic europium chelates. Inorg. Chem. 2009, 48, 5611–5613. [Google Scholar] [CrossRef]
- Armelao, L.; Quici, S.; Barigelletti, F.; Accorsi, G.; Bottaro, G.; Cavazzini, M.; Tondello, E. Design of luminescent lanthanide complexes: From molecules to highly efficient photo-emitting materials. Coord. Chem. Rev. 2010, 254, 487–505. [Google Scholar] [CrossRef]
- Kopchuk, D.S.; Krinochkin, A.P.; Kim, G.A.; Kozhevnikov, D.N. Europium complex of 5-(4-dodecyloxyphenyl)2,2′-bipyridine-6′-carboxylic acid. Mendeleev Commun. 2017, 27, 394–396. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ohmagari, H.; Tanaka, H.; Machida, K. Luminescence of lanthanide complexes: From fundamental to prospective approaches related to water- and molecular-stimuli. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100484. [Google Scholar] [CrossRef]
- Aebischer, A.; Gumy, F.; Bünzli, J.C.G. Intrinsic quantum yields and radiative lifetimes of lanthanide tris(dipicolinates). Phys. Chem. Chem. Phys. 2009, 11, 1346–1353. [Google Scholar] [CrossRef]
- Barré, R.; Leguerrier, D.M.D.; Fedele, L.; Imbert, D.; Molloy, J.K.; Thomas, F. Luminescent pro-nitroxide lanthanide complexes for the detection of reactive oxygen species. Chem. Commun. 2020, 56, 435–438. [Google Scholar] [CrossRef]
- Molloy, J.K.; Jarjayes, O.; Philouze, C.; Fedele, L.; Imbert, D.; Thomas, F. A redox active switch for lanthanide luminescence in phenolate complexes. Chem. Commun. 2017, 53, 605–608. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Kundu, S.; Das, R. Distance-dependent formation of electronic charge-transfer states in the ground states of anthracene and pyrene covalently linked to a TEMPO free radical. Phys. Chem. Chem. Phys. 2018, 21, 77–88. [Google Scholar] [CrossRef]
- Joksimović, N.; Baskić, D.; Popović, S.; Zarić, M.; Kosanić, M.; Ranković, B.; Stanojković, T.; Novaković, S.B.; Davidović, G.; Bugarčić, Z.; et al. Synthesis, characterization, biological activity, DNA and BSA binding study: Novel copper(II) complexes with 2-hydroxy-4-aryl-4-oxo-2-butenoate. Dalt. Trans. 2016, 45, 15067–15077. [Google Scholar] [CrossRef]
- Nosrati, H.; Abhari, F.; Charmi, J.; Davaran, S.; Danafar, H. Multifunctional nanoparticles from albumin for stimuli-responsive efficient dual drug delivery. Bioorg. Chem. 2019, 88, 102959. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.S.; Na, K. Caffeic acid-coated multifunctional magnetic nanoparticles for the treatment and bimodal imaging of tumours. J. Photochem. Photobiol. B Biol. 2016, 160, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Rathi, S.; Arora, D. Fluorescence spectral studies on interaction of fluorescent probes with Bovine Serum Albumin (BSA). J. Lumin. 2016, 175, 135–140. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 0387312781. [Google Scholar]
- Topală, T.; Bodoki, A.; Oprean, L.; Oprean, R. Bovine Serum Albumin Interactions with Metal Complexes. Clujul Med. 2014, 87, 215. [Google Scholar] [CrossRef] [Green Version]
- Varshney, A.; Sen, P.; Ahmad, E.; Rehan, M.; Subbarao, N.; Khan, R.H. Ligand binding strategies of human serum albumin: How can the cargo be utilized? Chirality 2010, 22, 77–87. [Google Scholar] [CrossRef]
- Rudra, S.; Dasmandal, S.; Patra, C.; Kundu, A.; Mahapatra, A. Binding affinities of Schiff base Fe(II) complex with BSA and calf-thymus DNA: Spectroscopic investigations and molecular docking analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 166, 84–94. [Google Scholar] [CrossRef]
- Akhuli, A.; Chakraborty, D.; Agrawal, A.K.; Sarkar, M. Probing the Interaction of Bovine Serum Albumin with Copper Nanoclusters: Realization of Binding Pathway Different from Protein Corona. Langmuir 2021, 37, 1823–1837. [Google Scholar] [CrossRef]
- Ustyugov, A.A.; Sipyagina, N.A.; Malkova, A.N.; Straumal, E.A.; Yurkova, L.L.; Globa, A.A.; Lapshina, M.A.; Chicheva, M.M.; Chaprov, K.D.; Maksimkin, A.V.; et al. 3D Neuronal Cell Culture Modeling Based on Highly Porous Ultra-High Molecular Weight Polyethylene. Molecules 2022, 27, 2087. [Google Scholar] [CrossRef]
- Dey, B.B. C—Hydrazoximes of methyl- and phenyl-glyoxals. J. Chem. Soc. Trans. 1914, 105, 1039–1046. [Google Scholar] [CrossRef]

















| Complex | Absorption Wavelength, λMax, nm (ε, 10−3 M−1cm−1) a | Φb, % | τ c, ms |
|---|---|---|---|
| 10a | 227, 311 (42.7) | 2.4 | 1.61 |
| 10b | 227, 310 (44.5) | - | - |
| 10c | 227, 310 (55.9) | - | - |
| 10d | 227, 310 (47.0) | - | - |
| 11a [38] | 309 | 6.4 | - |
| 11b [38] | 309 | 11.0 | - |
| 11c [38] | 232, 295 | 5.7 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slovesnova, N.V.; Minin, A.S.; Belousova, A.V.; Ustyugov, A.A.; Chaprov, K.D.; Krinochkin, A.P.; Valieva, M.I.; Shtaitz, Y.K.; Starnovskaya, E.S.; Nikonov, I.L.; et al. New TEMPO–Appended 2,2′-Bipyridine-Based Eu(III), Tb(III), Gd(III) and Sm(III) Complexes: Synthesis, Photophysical Studies and Testing Photoluminescence-Based Bioimaging Abilities. Molecules 2022, 27, 8414. https://doi.org/10.3390/molecules27238414
Slovesnova NV, Minin AS, Belousova AV, Ustyugov AA, Chaprov KD, Krinochkin AP, Valieva MI, Shtaitz YK, Starnovskaya ES, Nikonov IL, et al. New TEMPO–Appended 2,2′-Bipyridine-Based Eu(III), Tb(III), Gd(III) and Sm(III) Complexes: Synthesis, Photophysical Studies and Testing Photoluminescence-Based Bioimaging Abilities. Molecules. 2022; 27(23):8414. https://doi.org/10.3390/molecules27238414
Chicago/Turabian StyleSlovesnova, Nataliya V., Artem S. Minin, Anna V. Belousova, Aleksey A. Ustyugov, Kirill D. Chaprov, Alexey P. Krinochkin, Maria I. Valieva, Yaroslav K. Shtaitz, Ekaterina S. Starnovskaya, Igor L. Nikonov, and et al. 2022. "New TEMPO–Appended 2,2′-Bipyridine-Based Eu(III), Tb(III), Gd(III) and Sm(III) Complexes: Synthesis, Photophysical Studies and Testing Photoluminescence-Based Bioimaging Abilities" Molecules 27, no. 23: 8414. https://doi.org/10.3390/molecules27238414