Activity of Mono-, Bi-, and Trimetallic Catalysts Pt-Ni-Cr/C in the Bicyclohexyl Dehydrogenation Reaction
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Characterization of Catalysts
3.3. Dehydrogenation of Bicyclohexyl
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bourane, A.; Elanany, M.; Pham, T.V.; Katikaneni, S.P. An overview of organic liquid phase hydrogen carriers. Int. J. Hydrogen Energy 2016, 41, 23075. [Google Scholar] [CrossRef]
- Biniwale, R.B.; Rayalu, S.; Devotta, S.; Ichikawa, M. Chemical hydrides: A solution to high capacity hydrogen storage and supply. Int. J. Hydrogen Energy 2008, 33, 360. [Google Scholar] [CrossRef]
- Geburtig, D.; Preuster, P.; Bosmann, A.; Muller, K.; Wasserscheid, P. Chemical utilization of hydrogen from fluctuating energy sources-Catalytic transfer hydrogenation from charged Liquid Organic Hydrogen Carrier systems. Int. J. Hydrogen Energy 2016, 41, 1010. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Current research trends ana perspectives on materials-based hydrogen storage solution: A critical review. Int. J. Hydrogen Energy 2017, 42, 289. [Google Scholar] [CrossRef]
- Hodoshima, S.; Hiroshi, A.; Shigeki, T.; Yasukazu, S. Catalytic decalin dehydrogenation/naphthalene hydrogenation pair as a hydrogen source for fuel-cell vehicle. Int. J. Hydrogen Energy 2003, 28, 1255. [Google Scholar] [CrossRef]
- Kustov, L.M.; Kalenchuk, A.N.; Bogdan, V.I. Systems for accumulation, storage and release of hydrogen. Rus. Chem. Rev. 2020, 89, 897. [Google Scholar] [CrossRef]
- Kalenchuk, A.N.; Bogdan, V.I.; Dunaev, S.F.; Kustov, L.M. Influence of steric factors on reversible reactions of hydrogenation-dehydrogenation of polycyclic aromatic hydrocarbons on a Pt/C catalyst in hydrogen storage systems. Fuel 2020, 280, 118625. [Google Scholar] [CrossRef]
- Kalenchuk, A.N.; Bogdan, V.I.; Dunaev, S.F.; Kustov, L.M. Dehydrogenation of Polycyclic Naphthenes on a Pt/C Catalyst for Hydrogen Storage in Liquid Organic Hydrogen Carriers. Fuel Proc. Tech. 2018, 169, 94. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Kustov, L.M. Effects of the support on the morphology and electronic properties ofsupported metal clusters: Modern concepts and progress in 1990s. Appl. Catal. A Gen. 1999, 188, 3. [Google Scholar] [CrossRef]
- Biniwale, R.B.; Kariya, N.; Ichikawa, M. Dehydrogenation of cyclohexane over Ni based catalysts supported on activated carbon using spray-pulsed reactor and enhancement in activity by addition of a small amount of Pt. Catal. Lett 2005, 105, 83. [Google Scholar] [CrossRef]
- Sachtler, W.M.H.; Stakheev, A.Y. Electron-deficient palladium clusters and bifunctional sites in zeolites. Catal. Today 1992, 12, 283. [Google Scholar] [CrossRef]
- Surovikin, V.F.; Surovikin, U.V.; Cehanovich, M.C. New directions in the technology of carbon-carbon materials. Application of carbon-carbon materials. Mend. Commun. 2007, I, 111. [Google Scholar]
- Rodriguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Suslova, E.V.; Ivanov, A.S.; Egorov, A.V.; Maslakov, K.I.; Savilov, S.V.; Lunin, V.V. Co catalysts supported on oxidized CNTs: Evolution of structure during prepa-ration, reduction and catalytic test in Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2016, 523, 221. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Ivanov, A.S.; Strokova, N.E.; Maslakov, K.I.; Savilov, S.V.; Lunin, V.V. Mechanism of thermal defunctionalization of oxidized carbon nanotubes. J. Phys. Chem. C 2016, 120, 17465. [Google Scholar] [CrossRef]
- Kalenchuk, A.N.; Bogdan, V.I.; Dunaev, S.F.; Kustov, L.M. Effect of surface hydrophilization on Pt/Sibunit catalytic activity in bicyclohexyl dehydrogenation in hydrogen storage application. Int. J. Hydrogen Energy 2018, 43, 6191. [Google Scholar] [CrossRef]
- Kalenchuk, A.N.; Davchan, N.A.; Bogdan, V.I.; Dunaev, S.F.; Kustov, L.M. Reversible hydrogenation- dehydrogenation reactions of meta-terphenyl on catalyst with various supports. Rus. Chem. Bull. 2018, 67, 28. [Google Scholar] [CrossRef]
- Kustov, L.M.; Tarasov, A.L.; Sung, J.; Godovsky, D.Y. Hydrogen storage materials. Mend. Commun. 2014, 24, 1. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; García-Moreno, L.; Vizcaíno, A.J. Production of renewable hydrogen from glycerol steam reforming over bimetallic Ni-(Cu,Co,Cr) catalysts supported on SBA-15 silica. Catalysts 2017, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Rouibah, K.; Barama, A.; Benrabaa, R.; Guerrero-Caballero, J.; Kane, T.; Vannier, R.N.; Rubbens, A.; Lofberg, A. Dry reforming of methane on nickel-chrome, nickel-cobalt and nickel-manganese catalysts. Int. J. Hydrogen Energy 2017, 30, 1. [Google Scholar] [CrossRef]
- Mukerjee, S.; Srinivasan, S.; Soriaga, M.P.; McBreen, J. Effect of preparation conditions of Pt alloys on their electronic, structural, and electrocatalytic activities for oxygen reduction-XRD, XAS, and Electrochemical Studies. J. Phys. Chem 1995, 99, 4577. [Google Scholar] [CrossRef]
- Kaewsai, D.; Hunsom, M. Comparative study of the ORR activity and stability of Pt and PtM (M=Ni, Co, Cr, Pd) supported on polyaniline/carbon nanotubes in a PEM fuel cell. Nanomaterials 2018, 8, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, M.; Cho, J.; Cho, K.; Kim, H. Particle size and alloying effects of Pt-based alloy catalysts for fuel cell applications. Electrochim. Acta 2000, 45, 4211. [Google Scholar] [CrossRef]
- Tarasov, A.L.; Tkachenko, O.P.; Kustov, L.M. Mono and Bimetallic Pt–(M)/Al2O3 Catalysts for Dehydrogenation of Perhydro-N-ethylcarbazole as the Second Stage of Hydrogen Storage. Catal. Lett 2018, 148, 472. [Google Scholar] [CrossRef]
- Baglio, V.; Stassi, A.; Blasi, A.D.; D’Urso, C.; Antonucci, V.; Arico, A.S. Investigation of bimetallic Pt–M/C as DMFC cathode catalysts. Electrochim. Acta 2007, 53, 1360. [Google Scholar] [CrossRef]
- Scott, Y.K.; Cheng, H. Fabrication and evaluation of Pt–Fe alloys as methanol tolerant cathode materials for direct methanol fuel cells. J. Power Sources 2006, 163, 323. [Google Scholar]
- Xiong, L.; Manthiram, A. Effect of atomic ordering on the catalytic activity of carbon supported PtM (M=Fe, Co, Ni, and Cu) alloys for oxygen reduction in PEMFCs. J. Electrochem. Soc 2005, 152, 697. [Google Scholar] [CrossRef]
- Colon-Mercado, H.R.; Popov, B.N. The stability of Pt-M (M = first row transition metal) alloy catalysts and its effect on the activity in low temperature fuel cells. J. Power Sources 2006, 155, 253. [Google Scholar]
- Jeon, M.K.; McGinn, P.J. Composition dependence of ternary PtNiCr/C catalyst activity for methanol electro-oxidation reaction. J. Power Sources 2009, 194, 737. [Google Scholar] [CrossRef]
- Jeon, M.K.; Zhang, Y.; McGinn, P.J. Effect of reduction conditions on electrocatalytic activity of a ternary PtNiCr/C catalyst for methanol electro-oxidation. Electrochim. Acta 2009, 54, 2837. [Google Scholar] [CrossRef]
- Mom, R.; Frevel, L.; Velasco-Velez, J.J.; Pladinec, M.; Knop-Gericke, A.; Schlogl, R. The oxidation of platinum under wet conditions observed by electrochemical X-ray photoelectron spectroscopy. J. Am. Chem. Soc 2019, 141, 6537. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, M.Y.; Vovk, E.I.; Kalinkin, A.V.; Simonov, P.A.; Gerasimov, E.Y.; Bukhtiyarov, V.I. Formation of surface platinum oxides in the Interaction of the Pt/Sibunit catalysts with NO2: Estimates of the width of oxide shell from XPS data. Kinet. Catal. 2018, 59, 663. [Google Scholar] [CrossRef]
- Kalenchuk, A.N.; Leonov, A.V.; Bogdan, V.I.; Kustov, L.M. Dehydrogenation of Bicyclohexyl over Ni/Oxidized Sibunit Catalyst. Rus. J. Phys. Chem. A 2019, 93, 652. [Google Scholar]
- Bogdan, V.I.; Kalenchuk, A.N.; Chernavsky, P.A.; Bogdan, T.V.; Mishanin, I.I.; Kustov, L.M. Synergistic effect of metal components of the low-loaded Pt-Ni-Cr/C catalyst in the bicyclohexyl dehydrogenation reaction. Int. J. Hydrogen Energy 2021, 46, 1. [Google Scholar] [CrossRef]
- Kalenchuk, A.N.; Maslakov, K.I.; Bogdan, T.V.; Chernavskiy, P.A.; Bogdan, V.I. Formation of the surface of Pt—Ni—Cr/C catalysts under reducing conditions. Rus. Chem. Bull 2021, 70, 323. [Google Scholar] [CrossRef]
- Bogdan, T.V.; Kalenchuk, A.N.; Maksimov, S.V.; Bogdan, V.I. Surface structure of Pt-Ni-Cr/C catalysts. Rus. J. Phys. Chem. A 2021, 95, 523. [Google Scholar] [CrossRef]
- Holzl, J.; Schulte, F.K. Work Functions of Metals. Solid Surface Physics; Springer: Berlin, Germany, 1979; pp. 1–150. [Google Scholar] [CrossRef]
- Vitos, L.; Ruban, A.V.; Skriver, H.L.; Kollár, J. The surface energy of metals. Surf. Sci 1998, 411, 186. [Google Scholar] [CrossRef]
- Ellner, M.; Kolatschek, K.; Predel, B. On the Partial Atomic Volume and the Partial Molar Enthalpy of Aluminium in Some Phases with Cu and Cu3Au Structures. J. Less Common Met. 1991, 170, 171. [Google Scholar] [CrossRef]
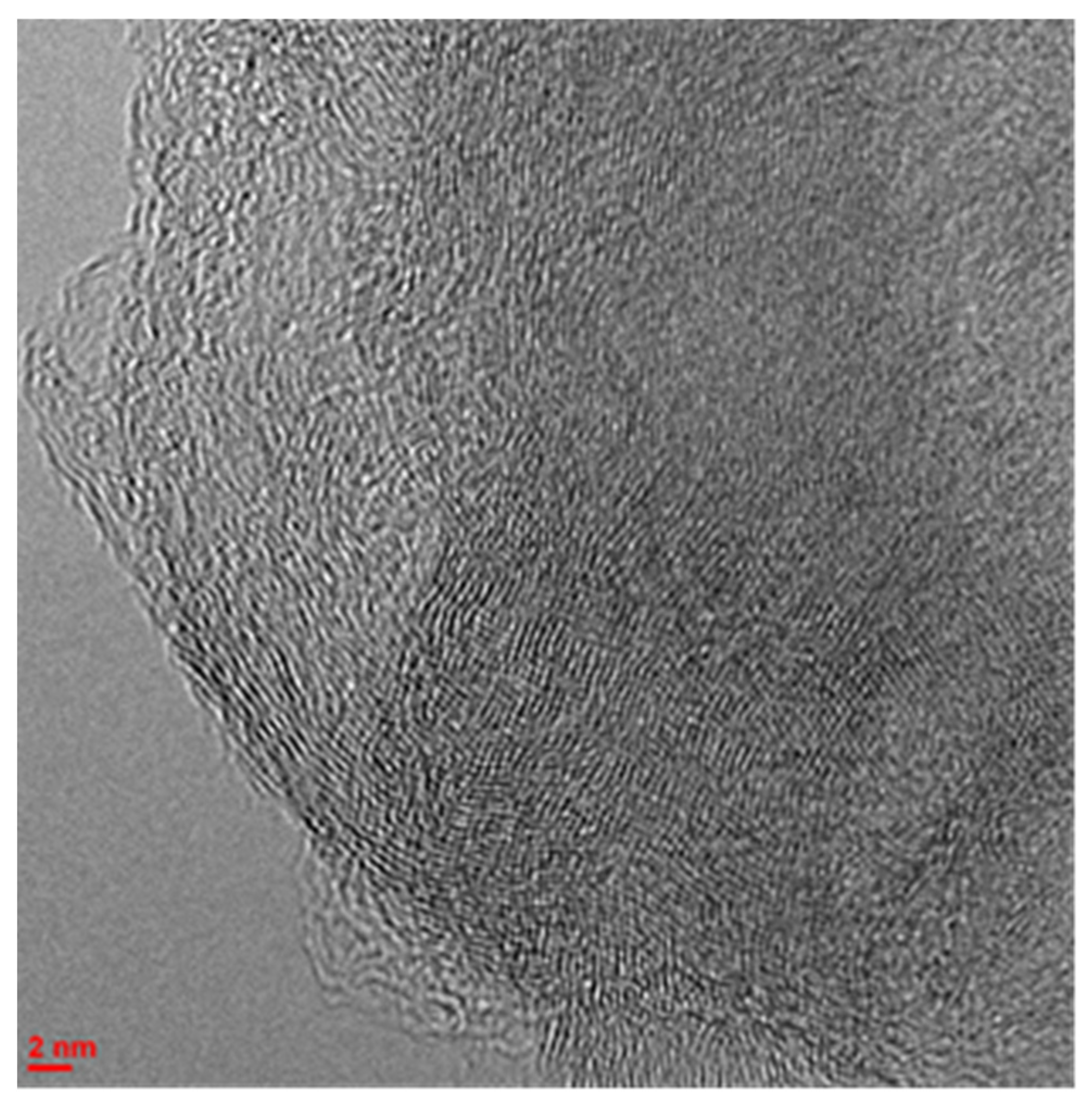

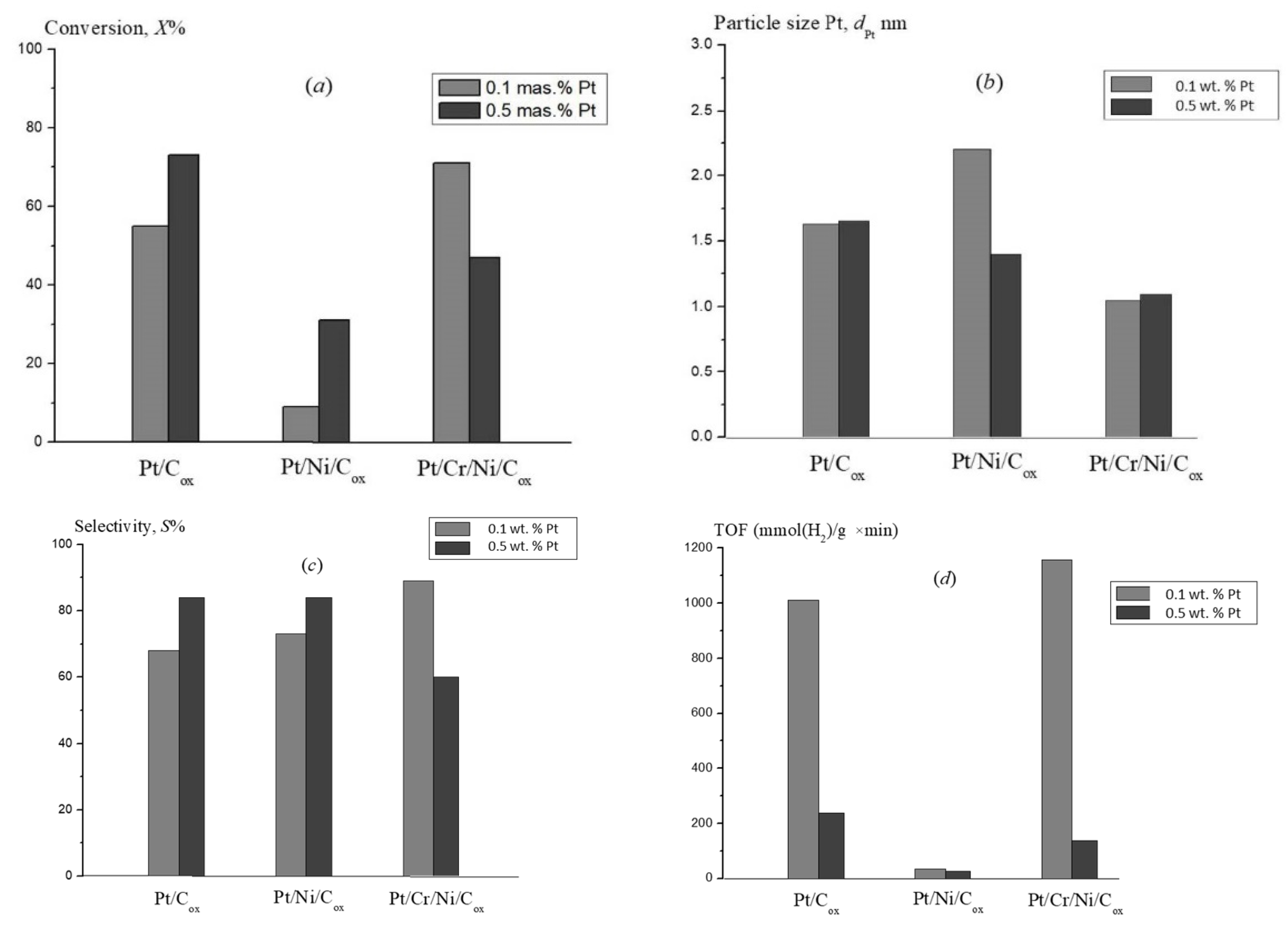
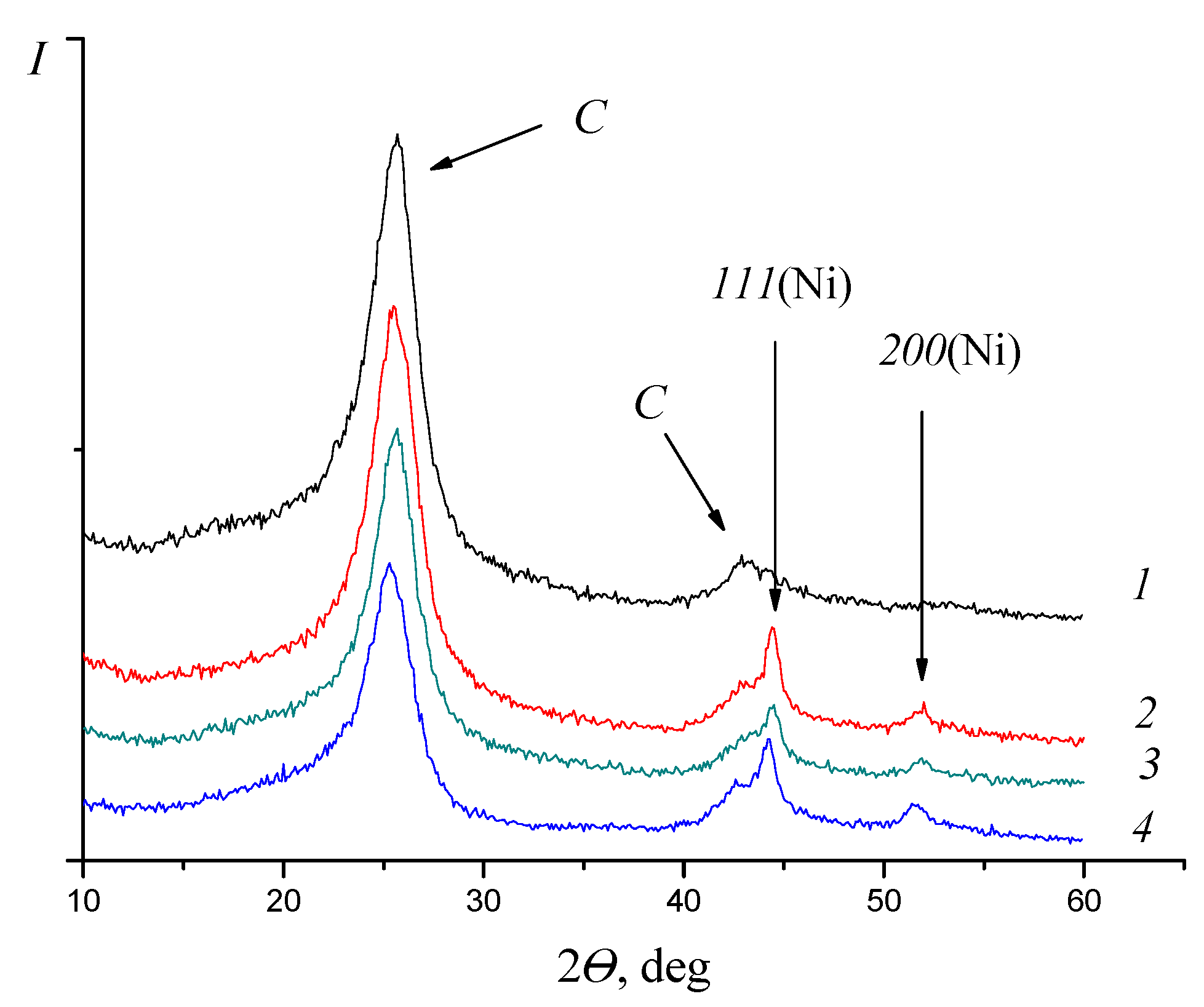
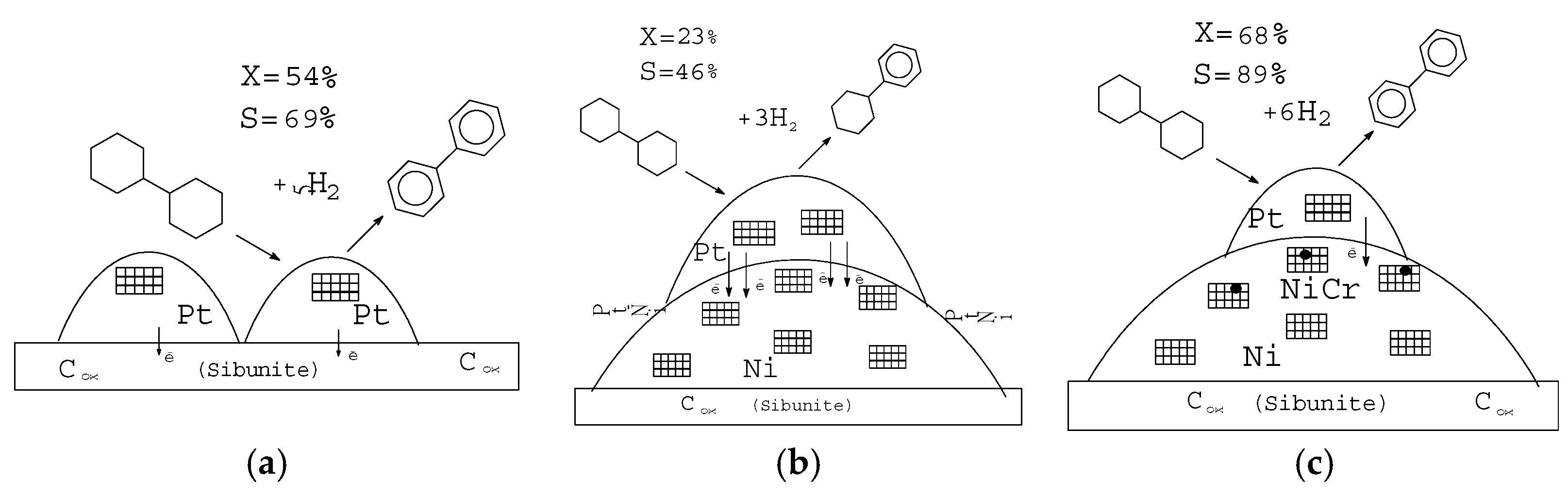
| Substrate | Catalyst | TOF, (mmol(H2)/gPt × min) | Ref. |
|---|---|---|---|
| Cyclohexane | 3.82 wt% Pt/AC (activated carbon) | 1800 | [2] |
| Methylcyclohexane | 3.82 wt% Pt/AC | 1700 | [2] |
| Decalin | 5 wt% Pt/C | 444.4 | [2] |
| Decalin | (Pt-Ir)/C (5 wt% metal) | 25.2 | [2] |
| Perhydro-meta-terphenyl | 5Pt/Al2O3 | 7.1 | [17] |
| Perhydro-meta-terphenyl | 4.8Pd/C | 15.1 | [17] |
| Perhydro-meta-terphenyl | 3Pt/C(Sibunit) | 56.2 | [17] |
| SBET, m2/g | Vpore, cm3/g | rpore, nm | C-C (sp2) | C-C (sp3) | C–O | C=O | O=C-O | |
|---|---|---|---|---|---|---|---|---|
| C | 360 | 0.55 | 3.8 | ~98% | - | - | - | 1–2% |
| Cox | 240 | 0.45 | 4.2 | ~88% | 5–7% | 3–4% | 0–1% | 3–4% |
| Parameter | Catalyst | |||||
|---|---|---|---|---|---|---|
| 0.5Pt/C | 0.5Pt/Cox | 0.3Pt/C | 0.3Pt/Cox | 0.1Pt/C | 0.1Pt/Cox | |
| XC12H22, % (±5%) | 63 | 73 | 57 | 64 | 26 | 55 |
| SC12H10, % | 61 | 84 | 75 | 79 | 58 | 68 |
| TOF, (mmol(H2)/gPt × min) | 184 | 238 | 287 | 359 | 855 | 1012 |
| Metal | Work Function, eV [37] | Surface Energy, J/m2 [38] |
|---|---|---|
| Pt | 5.93 | 2.067 |
| Ni | 5.04 | 2.450 |
| Cr | 4.50 | 2.300 |
| Catalyst | The Sequence of Preparation Stages |
|---|---|
| 3Ni/C; 3Ni/1.5Cr/C | Impregnation (C; 1.5Cr/C) with an aqueous solution of Ni(NO3)2 6H2O (Sigma Aldrich), drying (120 °C), calcination in N2 (500 °C), reduction of Ni in H2 (500 °C) |
| 1.5Cr/C 1.5Cr/3Ni/C | Impregnation (C; 3Ni/C) with an aqueous solution of Cr(NO3)3 9H2O (Sigma Aldrich, Saint-Louis, MO, USA), drying (120 °C), calcination in N2 (500 °C), reduction of Cr in H2 (500 °C) |
| (1.5Cr-3Ni)/C | Co-impregnation (C) with aqueous solutions of Ni(NO3)2 6H2O and Cr(NO3)3 9H2O, drying (120 °C), calcination in N2 (500 °C), reduction of Ni and Cr in H2 (500 °C) |
| 0.1Pt/C; 0.1Pt/3Ni/C; 0.1Pt/1.5Cr/C; 0.1Pt//3Ni/1.5Cr/C; 0.1Pt/1.5Cr/3Ni/C; 0.1Pt/(1.5Cr-3Ni)/C | Impregnation (C; 3Ni/C; 1.5Cr/C; 3Ni/1.5Cr/C; 1.5Cr/3Ni/C; (1.5Cr-3Ni)/C) with an aqueous solution of H2PtCl6 6H2O (Alfa-Aesar, Stoughton, MA, USA), drying (120 °C), calcination in N2 (350 °C), reduction of Pt in H2 (320 °C) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalenchuk, A.N.; Kustov, L.M. Activity of Mono-, Bi-, and Trimetallic Catalysts Pt-Ni-Cr/C in the Bicyclohexyl Dehydrogenation Reaction. Molecules 2022, 27, 8416. https://doi.org/10.3390/molecules27238416
Kalenchuk AN, Kustov LM. Activity of Mono-, Bi-, and Trimetallic Catalysts Pt-Ni-Cr/C in the Bicyclohexyl Dehydrogenation Reaction. Molecules. 2022; 27(23):8416. https://doi.org/10.3390/molecules27238416
Chicago/Turabian StyleKalenchuk, Alexander N., and Leonid M. Kustov. 2022. "Activity of Mono-, Bi-, and Trimetallic Catalysts Pt-Ni-Cr/C in the Bicyclohexyl Dehydrogenation Reaction" Molecules 27, no. 23: 8416. https://doi.org/10.3390/molecules27238416





