Advances in Molecular Pathology of Obstructive Sleep Apnea
Abstract
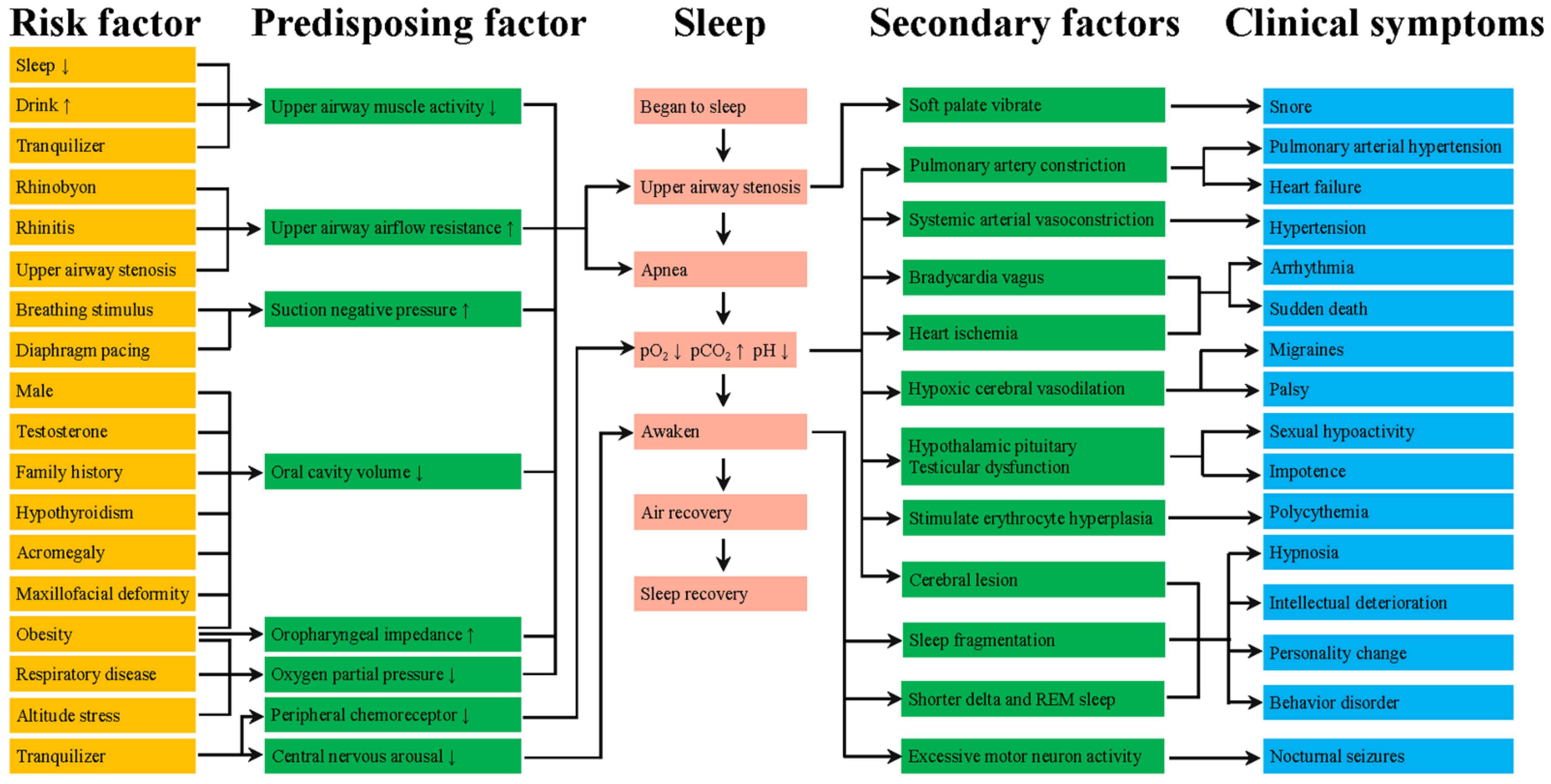
1. Introduction
2. The Pathogenesis of OSA and Pharyngeal Muscle Fatigue
3. OSA Correlated Signaling Pathway
4. MicroRNA (miRNA) in OSA
5. Long Noncoding RNAs (lncRNAs) in OSA
6. DNA Methylation in OSA
7. Chemical Compounds for OSA Treatment
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryan, S.; Cummins, E.P.; Farre, R.; Gileles-Hillel, A.; Jun, J.; Oster, H.; Pepin, J.-L.; Ray, D.W.; Reutrakul, S.; Sanchez-De-La-Torre, M.; et al. Understanding the pathophysiological mechanisms of cardiometabolic complications in obstructive sleep apnoea: Towards personalised treatment approaches. Eur. Respir. J. 2020, 56, 1902295. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lin, B.M.; Markt, S.C.; Stampfer, M.J.; Laden, F.; Hu, F.B.; Tworoger, S.S.; Redline, S. Sex differences in the associations of obstructive sleep apnoea with epidemiological factors. Eur. Respir. J. 2018, 51, 1702421. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.R.; Gold, A.R.; Schubert, N.; Stryzak, A.; Wise, R.A.; Permutt, S.; Smith, P.L. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am. Rev. Respir. Dis. 1991, 144 Pt 1, 494–498. [Google Scholar] [CrossRef]
- Ng, S.S.S.; Tam, W.W.S.; Lee, R.W.W.; Chan, T.O.; Yiu, K.; Yuen, B.T.Y.; Wong, K.T.; Woo, J.; Ma, R.C.W.; Chan, K.K.P.; et al. Effect of Weight Loss and Continuous Positive Airway Pressure on Obstructive Sleep Apnea and Metabolic Profile Stratified by Craniofacial Phenotype: A Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2022, 205, 711–720. [Google Scholar] [CrossRef]
- Strohl, K.P. Con: Sleep apnea is not an anatomic disorder. Am. J. Respir. Crit. Care Med. 2003, 168, 271–272;discussion 272–273. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef]
- White, D.P. Pathogenesis of obstructive and central sleep apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 1363–1370. [Google Scholar] [CrossRef]
- Xu, L.; Keenan, B.T.; Wiemken, A.S.; Chi, L.; Staley, B.; Wang, Z.; Wang, J.; Benedikstdottir, B.; Juliusson, S.; Pack, A.I.; et al. Differences in three-dimensional upper airway anatomy between Asian and European patients with obstructive sleep apnea. Sleep 2020, 43, zsz273. [Google Scholar] [CrossRef]
- Dahlin, L.B.; Lundborg, G. Vibration-induced hand problems: Role of the peripheral nerves in the pathophysiology. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2001, 35, 225–232. [Google Scholar] [CrossRef]
- Zhu, L.; Chamberlin, N.L.; Arrigoni, E. Muscarinic Inhibition of Hypoglossal Motoneurons: Possible Implications for Upper Airway Muscle Hypotonia during REM Sleep. J. Neurosci. 2019, 39, 7910–7919. [Google Scholar] [CrossRef]
- Cori, J.M.; Nicholas, C.L.; Avraam, J.; Lee, V.V.; Schembri, R.; Jackson, M.L.; Jordan, A.S. The Effects of Experimental Sleep Fragmentation and Sleep Deprivation on the Response of the Genioglossus Muscle to Inspiratory Resistive Loads. J. Clin. Sleep Med. 2018, 14, 715–724. [Google Scholar] [CrossRef]
- Salman, L.A.; Shulman, R.; Cohen, J.B. Obstructive Sleep Apnea, Hypertension, and Cardiovascular Risk: Epidemiology, Pathophysiology, and Management. Curr. Cardiol. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Liu, H.M.; Chiang, I.J.; Kuo, K.N.; Liou, C.M.; Chen, C. The effect of acetazolamide on sleep apnea at high altitude: A systematic review and meta-analysis. Ther. Adv. Respir. Dis. 2017, 11, 20–29. [Google Scholar] [CrossRef]
- Hao, T.; Liu, Y.H.; Li, Y.Y.; Lu, Y.; Xu, H.Y. Transcriptomic Analysis of Physiological Significance of Hypoxia-inducible Factor-1α in Myogenesis and Carbohydrate Metabolism of Genioglossus in Mice. Chin. Med. J. 2017, 130, 1570–1577. [Google Scholar] [CrossRef]
- Schulz, R.; Hummel, C.; Heinemann, S.; Seeger, W.; Grimminger, F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am. J. Respir. Crit. Care Med. 2002, 165, 67–70. [Google Scholar] [CrossRef]
- Ryan, S.; McNicholas, W.T. Intermittent hypoxia and activation of inflammatory molecular pathways in OSAS. Arch. Physiol. Biochem. 2008, 114, 261–266. [Google Scholar] [CrossRef]
- El-Solh, A.A.; Mador, M.J.; Sikka, P.; Dhillon, R.S.; Amsterdam, D.; Grant, B.J. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest 2002, 121, 1541–1547. [Google Scholar] [CrossRef]
- Aydin, Ş.; Özdemir, C.; Küçükali, C.I.; Sökücü, S.N.; Giriş, M.; Akcan, U.; Tüzün, E. Reduced Peripheral Blood Mononuclear Cell ROCK1 and ROCK2 Levels in Obstructive Sleep Apnea Syndrome. In Vivo 2018, 32, 319–325. [Google Scholar]
- Ugur, K.S.; Acar, M.; Ozol, D.; Dagli, E.; Oznur, M.; Kosus, A.; Gunduz, M. Gene Expression Profiles of Tumor Necrosis Factor-α and Endothelin-1 in Obstructive Sleep Apnea. ORL-J. Oto-Rhino-Laryngol. Head Neck Surg. 2019, 81, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Serrano, F.; Fenik, P.; Hsu, R.; Kong, L.; Pratico, D.; Klann, E.; Veasey, S.C. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L. Obstructive sleep apnoea syndrome—An oxidative stress disorder. Sleep Med. Rev. 2003, 7, 35–51. [Google Scholar] [CrossRef]
- Ip, M.S.; Lam, B.; Chan, L.Y.; Zheng, L.; Tsang, K.W.; Fung, P.C.; Lam, W.K. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am. J. Respir. Crit. Care Med. 2000, 162, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Priou, P.; Gagnadoux, F.; Tesse, A.; Mastronardi, M.L.; Agouni, A.; Meslier, N.; Racineux, J.L.; Martinez, M.C.; Trzepizur, W.; Andriantsitohaina, R. Endothelial dysfunction and circulating microparticles from patients with obstructive sleep apnea. Am. J. Pathol. 2010, 177, 974–983. [Google Scholar] [CrossRef]
- Jelic, S.; Padeletti, M.; Kawut, S.M.; Higgins, C.; Canfield, S.M.; Onat, D.; Colombo, P.C.; Basner, R.C.; Factor, P.; LeJemtel, T.H. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 2008, 117, 2270–2278. [Google Scholar] [CrossRef]
- Wu, X.; Chang, S.C.; Jin, J.; Gu, W.; Li, S. NLRP3 inflammasome mediates chronic intermittent hypoxia-induced renal injury implication of the microRNA-155/FOXO3a signaling pathway. J. Cell. Physiol. 2018, 233, 9404–9415. [Google Scholar] [CrossRef]
- Xu, L.H.; Xie, H.; Shi, Z.H.; Du, L.D.; Wing, Y.K.; Li, A.M.; Ke, Y.; Yung, W.H. Critical Role of Endoplasmic Reticulum Stress in Chronic Intermittent Hypoxia-Induced Deficits in Synaptic Plasticity and Long-Term Memory. Antioxid. Redox Signal. 2015, 23, 695–710. [Google Scholar] [CrossRef]
- Dyugovskaya, L.; Polyakov, A.; Cohen-Kaplan, V.; Lavie, P.; Lavie, L. Bax/Mcl-1 balance affects neutrophil survival in intermittent hypoxia and obstructive sleep apnea: Effects of p38MAPK and ERK1/2 signaling. J. Transl. Med. 2012, 10, 211. [Google Scholar] [CrossRef]
- Shan, X.; Chi, L.; Ke, Y.; Luo, C.; Qian, S.; Gozal, D.; Liu, R. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol. Dis. 2007, 28, 206–215. [Google Scholar] [CrossRef]
- Seo, Y.J.; Ju, H.M.; Lee, S.H.; Kwak, S.H.; Kang, M.J.; Yoon, J.H.; Kim, C.H.; Cho, H.J. Damage of Inner Ear Sensory Hair Cells via Mitochondrial Loss in a Murine Model of Sleep Apnea With Chronic Intermittent Hypoxia. Sleep 2017, 40, zsx106. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, X.; Dong, Y.; Zhang, X.; Ding, N.; Liu, J.; Hutchinson, S.Z.; Lu, G.; Zhang, X. Adiponectin alleviates genioglossal mitochondrial dysfunction in rats exposed to intermittent hypoxia. PLoS ONE 2014, 9, e109284. [Google Scholar] [CrossRef]
- Stål, P.S.; Johansson, B. Abnormal mitochondria organization and oxidative activity in the palate muscles of long-term snorers with obstructive sleep apnea. Respiration 2012, 83, 407–417. [Google Scholar] [CrossRef]
- Zhu, Y.; Fenik, P.; Zhan, G.; Sanfillipo-Cohn, B.; Naidoo, N.; Veasey, S.C. Eif-2a protects brainstem motoneurons in a murine model of sleep apnea. J. Neurosci. 2008, 28, 2168–2178. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, X.; Zheng, Y.; Miao, X.; Feng, W.; Cai, J.; Cai, L. Metallothionein prevents intermittent hypoxia-induced cardiac endoplasmic reticulum stress and cell death likely via activation of Akt signaling pathway in mice. Toxicol. Lett. 2014, 227, 113–123. [Google Scholar] [CrossRef]
- Jelic, S.; Lederer, D.J.; Adams, T.; Padeletti, M.; Colombo, P.C.; Factor, P.H.; Le Jemtel, T.H. Vascular inflammation in obesity and sleep apnea. Circulation 2010, 121, 1014–1021. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 824–830. [Google Scholar] [CrossRef]
- Li, K.; Wei, P.; Qin, Y.; Wei, Y. Is C-reactive protein a marker of obstructive sleep apnea?: A meta-analysis. Medicine 2017, 96, e6850. [Google Scholar] [CrossRef]
- Nadeem, R.; Molnar, J.; Madbouly, E.M.; Nida, M.; Aggarwal, S.; Sajid, H.; Naseem, J.; Loomba, R. Serum inflammatory markers in obstructive sleep apnea: A meta-analysis. J. Clin. Sleep Med. 2013, 9, 1003–1012. [Google Scholar] [CrossRef]
- Toujani, S.; Kaabachi, W.; Mjid, M.; Hamzaoui, K.; Cherif, J.; Beji, M. Vitamin D deficiency and interleukin-17 relationship in severe obstructive sleep apnea-hypopnea syndrome. Ann. Thorac. Med. 2017, 12, 107–113. [Google Scholar] [CrossRef]
- Yokoe, T.; Minoguchi, K.; Matsuo, H.; Oda, N.; Minoguchi, H.; Yoshino, G.; Hirano, T.; Adachi, M. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 2003, 107, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Kales, A.; Tyson, K.; Chrousos, G.P. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J. Clin. Endocrinol. Metab. 1997, 82, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Fleming, W.E.; Holty, J.C.; Bogan, R.K.; Hwang, D.; Ferouz-Colborn, A.S.; Budhiraja, R.; Redline, S.; Mensah-Osman, E.; Osman, N.I.; Li, Q.; et al. Use of blood biomarkers to screen for obstructive sleep apnea. Nat. Sci. Sleep 2018, 10, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, N.; Heizhati, M.; Yao, X.; Abdireim, A.; Wang, Y.; Abulikemu, Z.; Zhang, D.; Chang, G.; Kong, J.; et al. What do changes in concentrations of serum surfactant proteins A and D in OSA mean? Sleep Breath. 2015, 19, 955–962. [Google Scholar] [CrossRef]
- Akinnusi, M.; Jaoude, P.; Kufel, T.; El-Solh, A.A. Toll-like receptor activity in patients with obstructive sleep apnea. Sleep Breath. 2013, 17, 1009–1016. [Google Scholar] [CrossRef]
- Cherneva, R.V.; Cherneva, Z.V.; Georgiev, O.B.; Petrova, D.S.; Petrova, J.I. 8-isoprostanes and resistin as markers of vascular damage in non-hypersomnolent obstructive sleep apnoea patients. Clin. Physiol. Funct. Imaging 2017, 37, 695–702. [Google Scholar] [CrossRef]
- Gautier-Veyret, E.; Bäck, M.; Arnaud, C.; Belaïdi, E.; Tamisier, R.; Lévy, P.; Arnol, N.; Perrin, M.; Pépin, J.L.; Stanke-Labesque, F. Cysteinyl-leukotriene pathway as a new therapeutic target for the treatment of atherosclerosis related to obstructive sleep apnea syndrome. Pharmacol. Res. 2018, 134, 311–319. [Google Scholar] [CrossRef]
- Kim, J.; Bhattacharjee, R.; Snow, A.B.; Capdevila, O.S.; Kheirandish-Gozal, L.; Gozal, D. Myeloid-related protein 8/14 levels in children with obstructive sleep apnoea. Eur. Respir. J. 2010, 35, 843–850. [Google Scholar] [CrossRef]
- Minoguchi, K.; Yokoe, T.; Tazaki, T.; Minoguchi, H.; Oda, N.; Tanaka, A.; Yamamoto, M.; Ohta, S.; O’Donnell, C.P.; Adachi, M. Silent brain infarction and platelet activation in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2007, 175, 612–617. [Google Scholar] [CrossRef]
- Cortese, R.; Gileles-Hillel, A.; Khalyfa, A.; Almendros, I.; Akbarpour, M.; Khalyfa, A.A.; Qiao, Z.; Garcia, T.; Andrade, J.; Gozal, D. Aorta macrophage inflammatory and epigenetic changes in a murine model of obstructive sleep apnea: Potential role of CD36. Sci. Rep. 2017, 7, 43648. [Google Scholar] [CrossRef]
- Shamsuzzaman, A.; Amin, R.S.; Calvin, A.D.; Davison, D.; Somers, V.K. Severity of obstructive sleep apnea is associated with elevated plasma fibrinogen in otherwise healthy patients. Sleep Breath. 2014, 18, 761–766. [Google Scholar] [CrossRef]
- Hayashi, M.; Fujimoto, K.; Urushibata, K.; Takamizawa, A.; Kinoshita, O.; Kubo, K. Hypoxia-sensitive molecules may modulate the development of atherosclerosis in sleep apnoea syndrome. Respirology 2006, 11, 24–31. [Google Scholar] [CrossRef]
- Steffanina, A.; Proietti, L.; Antonaglia, C.; Palange, P.; Angelici, E.; Canipari, R. The Plasminogen System and Transforming Growth Factor-β in Subjects With Obstructive Sleep Apnea Syndrome: Effects of CPAP Treatment. Respir. Care 2015, 60, 1643–1651. [Google Scholar] [CrossRef]
- Ding, X.; Yu, C.; Liu, Y.; Yan, S.; Li, W.; Wang, D.; Sun, L.; Han, Y.; Li, M.; Zhang, S.; et al. Chronic obstructive sleep apnea accelerates pulmonary remodeling via TGF-β/miR-185/CoLA1 signaling in a canine model. Oncotarget 2016, 7, 57545–57555. [Google Scholar] [CrossRef]
- Lederer, D.J.; Jelic, S.; Basner, R.C.; Ishizaka, A.; Bhattacharya, J. Circulating KL-6, a biomarker of lung injury, in obstructive sleep apnoea. Eur. Respir. J. 2009, 33, 793–796. [Google Scholar] [CrossRef]
- Aihara, K.; Oga, T.; Harada, Y.; Chihara, Y.; Handa, T.; Tanizawa, K.; Watanabe, K.; Tsuboi, T.; Hitomi, T.; Mishima, M.; et al. Comparison of biomarkers of subclinical lung injury in obstructive sleep apnea. Respir. Med. 2011, 105, 939–945. [Google Scholar] [CrossRef]
- Li, J.; Thorne, L.N.; Punjabi, N.M.; Sun, C.K.; Schwartz, A.R.; Smith, P.L.; Marino, R.L.; Rodriguez, A.; Hubbard, W.C.; O’Donnell, C.P.; et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ. Res. 2005, 97, 698–706. [Google Scholar] [CrossRef]
- Li, J.; Grigoryev, D.N.; Ye, S.Q.; Thorne, L.; Schwartz, A.R.; Smith, P.L.; O’Donnell, C.P.; Polotsky, V.Y. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J. Appl. Physiol. 2005, 99, 1643–1648. [Google Scholar] [CrossRef]
- Savransky, V.; Jun, J.; Li, J.; Nanayakkara, A.; Fonti, S.; Moser, A.B.; Steele, K.E.; Schweitzer, M.A.; Patil, S.P.; Bhanot, S.; et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ. Res. 2008, 103, 1173–1180. [Google Scholar] [CrossRef]
- Zirlik, S.; Hildner, K.M.; Targosz, A.; Neurath, M.F.; Fuchs, F.S.; Brzozowski, T.; Konturek, P.C. Melatonin and omentin: Influence factors in the obstructive sleep apnoea syndrome? J. Physiol. Pharmacol. 2013, 64, 353–360. [Google Scholar]
- Kurt, O.K.; Tosun, M.; Alcelik, A.; Yilmaz, B.; Talay, F. Serum omentin levels in patients with obstructive sleep apnea. Sleep Breath. 2014, 18, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.L.; Meng, B.; Ding, J.H. The expression of serum hepatocyte growth factor in OSAHS. J. Clin. Otorhinolaryngol. Head Neck Surg. 2017, 31, 690–693. [Google Scholar]
- Jeon, B.; Luyster, F.S.; Sereika, S.M.; DiNardo, M.M.; Callan, J.A.; Chasens, E.R. Comorbid obstructive sleep apnea and insomnia and its associations with mood and diabetes-related distress in type 2 diabetes mellitus. J. Clin. Sleep Med. 2022, 18, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Lesser, D.J.; Bhatia, R.; Tran, W.H.; Oliveira, F.; Ortega, R.; Keens, T.G.; Mittelman, S.D.; Khoo, M.C.; Davidson Ward, S.L. Sleep fragmentation and intermittent hypoxemia are associated with decreased insulin sensitivity in obese adolescent Latino males. Pediatric Res. 2012, 72, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.X.; Cai, W.; Sun, J.F.; Liao, W.J.; Liu, Y.; Xiao, J.R.; Zhu, L.Y.; Liu, J.Y.; Zhang, W. Serum advanced glycation end products are associated with insulin resistance in male nondiabetic patients with obstructive sleep apnea. Sleep Breath. 2015, 19, 827–833. [Google Scholar] [CrossRef]
- Wu, W.; Li, Z.; Tang, T.; Wu, J.; Liu, F.; Gu, L. 5-HTR2A and IL-6 polymorphisms and obstructive sleep apnea-hypopnea syndrome. Biomed. Rep. 2016, 4, 203–208. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, L.; Cao, Y.; Chen, P.; Chen, Y.; Zong, D.; Ouyang, R. Relation between serum leptin levels, lipid profiles and neurocognitive deficits in Chinese OSAHS patients. Int. J. Neurosci. 2017, 127, 981–987. [Google Scholar] [CrossRef]
- Imayama, I.; Prasad, B. Role of Leptin in Obstructive Sleep Apnea. Ann. Am. Thorac. Soc. 2017, 14, 1607–1621. [Google Scholar] [CrossRef]
- Schiza, S.E.; Mermigkis, C.; Bouloukaki, I. Leptin and leptin receptor gene polymorphisms and obstructive sleep apnea syndrome: Is there an association? Sleep Breath. 2015, 19, 1079–1080. [Google Scholar] [CrossRef][Green Version]
- Gharib, S.A.; Hayes, A.L.; Rosen, M.J.; Patel, S.R. A pathway-based analysis on the effects of obstructive sleep apnea in modulating visceral fat transcriptome. Sleep 2013, 36, 23–30. [Google Scholar] [CrossRef]
- Badran, M.; Gozal, D. PAI-1: A Major Player in the Vascular Dysfunction in Obstructive Sleep Apnea? Int. J. Mol. Sci. 2022, 23, 5516. [Google Scholar] [CrossRef]
- Chen, H.H.; Lu, J.; Guan, Y.F.; Li, S.J.; Hu, T.T.; Xie, Z.S.; Wang, F.; Peng, X.H.; Liu, X.; Xu, X.; et al. Estrogen/ERR-α signaling axis is associated with fiber-type conversion of upper airway muscles in patients with obstructive sleep apnea hypopnea syndrome. Sci. Rep. 2016, 6, 27088. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y. Effects of genistein and estrogen on the genioglossus in rats exposed to chronic intermittent hypoxia may be HIF-1α dependent. Oral Dis. 2013, 19, 702–711. [Google Scholar] [CrossRef]
- Ma, X.R.; Wang, Y.; Sun, Y.C. Imbalance of osteoprotegerin/receptor activator of nuclear factor-κB ligand and oxidative stress in patients with obstructive sleep apnea-hypopnea syndrome. Chin. Med. J. 2019, 132, 25–29. [Google Scholar] [CrossRef]
- Ragia, G.; Archontogeorgis, K.; Simmaco, M.; Gentile, G.; Borro, M.; Zissimopoulos, A.; Froudarakis, M.; Manolopoulos, V.G.; Steiropoulos, P. Genetics of Obstructive Sleep Apnea: Vitamin D Receptor Gene Variation Affects Both Vitamin D Serum Concentration and Disease Susceptibility. Omics-A J. Integr. Biol. 2019, 23, 45–53. [Google Scholar] [CrossRef]
- Qin, B.; Sun, Z.; Liang, Y.; Yang, Z.; Zhong, R. The association of 5-HT2A, 5-HTT, and LEPR polymorphisms with obstructive sleep apnea syndrome: A systematic review and meta-analysis. PLoS ONE 2014, 9, e95856. [Google Scholar] [CrossRef]
- Zhang, X.B.; Zeng, Y.M.; Chen, X.Y.; Zhang, Y.X.; Ding, J.Z.; Xue, C. Decreased expression of hepatic cytochrome P450 1A2 (CYP1A2) in a chronic intermittent hypoxia mouse model. J. Thorac. Dis. 2018, 10, 825–834. [Google Scholar] [CrossRef]
- Liu, Z.L.; Wu, X.; Luo, Y.J.; Wang, L.; Qu, W.M.; Li, S.Q.; Huang, Z.L. Signaling mechanism underlying the histamine-modulated action of hypoglossal motoneurons. J. Neurochem. 2016, 137, 277–286. [Google Scholar] [CrossRef]
- Grace, K.P.; Hughes, S.W.; Shahabi, S.; Horner, R.L. K+ channel modulation causes genioglossus inhibition in REM sleep and is a strategy for reactivation. Respir. Physiol. Neurobiol. 2013, 188, 277–288. [Google Scholar] [CrossRef]
- Chamberlin, N.L.; Bocchiaro, C.M.; Greene, R.W.; Feldman, J.L. Nicotinic excitation of rat hypoglossal motoneurons. Neuroscience 2002, 115, 861–870. [Google Scholar] [CrossRef]
- Kubin, L. Sleep-wake control of the upper airway by noradrenergic neurons, with and without intermittent hypoxia. Prog. Brain Res. 2014, 209, 255–274. [Google Scholar] [PubMed]
- Ling, J.; Yu, Q.; Li, Y.; Yuan, X.; Wang, X.; Liu, W.; Guo, T.; Duan, Y.; Li, L. Edaravone Improves Intermittent Hypoxia-Induced Cognitive Impairment and Hippocampal Damage in Rats. Biol. Pharm. Bulletin 2020, 43, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Liaw, S.F.; Chiu, C.H.; Lin, M.W. Effects of continuous positive airway pressure on exhaled transforming growth factor-β and vascular endothelial growth factor in patients with obstructive sleep apnea. J. Thorac. Disease 2020, 12, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; Tsai, C.N.; Lee, Y.S.; Chu, S.F.; Chen, N.H. Gene expression profiles in peripheral blood mononuclear cells of Asian obstructive sleep apnea patients. Biomed. J. 2014, 37, 60–70. [Google Scholar]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.L. Obstructive sleep apnoea syndrome. Nature reviews. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef]
- Xu, W.; Chi, L.; Row, B.W.; Xu, R.; Ke, Y.; Xu, B.; Luo, C.; Kheirandish, L.; Gozal, D.; Liu, R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 2004, 126, 313–323. [Google Scholar] [CrossRef]
- Chang, H.R.; Lien, C.F.; Jeng, J.R.; Hsieh, J.C.; Chang, C.W.; Lin, J.H.; Yang, K.T. Intermittent Hypoxia Inhibits Na+-H+ Exchange-Mediated Acid Extrusion Via Intracellular Na+ Accumulation in Cardiomyocytes. Cell. Physiol. Biochem. 2018, 46, 1252–1262. [Google Scholar] [CrossRef]
- Veasey, S.C.; Davis, C.W.; Fenik, P.; Zhan, G.; Hsu, Y.J.; Pratico, D.; Gow, A. Long-term intermittent hypoxia in mice: Protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004, 27, 194–201. [Google Scholar] [CrossRef]
- Ryan, S.; Arnaud, C.; Fitzpatrick, S.F.; Gaucher, J.; Tamisier, R.; Pépin, J.L. Adipose tissue as a key player in obstructive sleep apnoea. Eur. Respir. Rev. 2019, 28, 190006. [Google Scholar] [CrossRef]
- Lam, S.Y.; Liu, Y.; Ng, K.M.; Lau, C.F.; Liong, E.C.; Tipoe, G.L.; Fung, M.L. Chronic intermittent hypoxia induces local inflammation of the rat carotid body via functional upregulation of proinflammatory cytokine pathways. Histochem. Cell Biol. 2012, 137, 303–317. [Google Scholar] [CrossRef]
- Grieve, D.J.; Shah, A.M. Oxidative stress in heart failure. More than just damage. Eur. Heart J. 2003, 24, 2161–2163. [Google Scholar] [CrossRef]
- Lavie, L.; Lavie, P. Molecular mechanisms of cardiovascular disease in OSAHS: The oxidative stress link. Eur. Respir. J. 2009, 33, 1467–1484. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef]
- Pilkauskaite, G.; Miliauskas, S.; Sakalauskas, R. Reactive oxygen species production in peripheral blood neutrophils of obstructive sleep apnea patients. Sci. World J. 2013, 2013, 421763. [Google Scholar] [CrossRef]
- Lavie, L.; Lavie, P. CrossTalk opposing view: Most cardiovascular diseases in sleep apnoea are not caused by sympathetic activation. J. Physiol.-Lond. 2012, 590, 2817–2819;discussion 2821. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, G.; Huang, J.; Chen, L.; Liu, Y.; Huang, J.; Zhang, S.; Lin, Q. Inhibition of miR-193a-3p protects human umbilical vein endothelial cells against intermittent hypoxia-induced endothelial injury by targeting FAIM2. Aging 2020, 12, 1899–1909. [Google Scholar] [CrossRef]
- Periasamy, S.; Hsu, D.Z.; Fu, Y.H.; Liu, M.Y. Sleep deprivation, oxidative stress and inflammation. Adv. Protein Chem. Struct. Biol. 2020, 119, 309–336. [Google Scholar]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kwak, J.W.; Lee, K.E.; Cho, H.S.; Lim, S.J.; Kim, K.S.; Yang, H.S.; Kim, H.J. Can mitochondrial dysfunction be a predictive factor for oxidative stress in patients with obstructive sleep apnea? Antioxid. Redox Signaling 2014, 21, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Cheresh, P.; Jablonski, R.P.; Williams, D.B.; Kamp, D.W. The Role of Mitochondrial DNA in Mediating Alveolar Epithelial Cell Apoptosis and Pulmonary Fibrosis. Int. J. Mol. Sci. 2015, 16, 21486–24519. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, J.H.; Stewart, J.B. Mitochondrial DNA: Radically free of free-radical driven mutations. Biochim. Et Biophys. Acta 2015, 1847, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Groenendyk, J.; Agellon, L.B.; Michalak, M. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu. Rev. Physiol. 2013, 75, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Tessema, B.; Sack, U.; König, B.; Serebrovska, Z.; Egorov, E. Effects of Intermittent Hypoxia in Training Regimes and in Obstructive Sleep Apnea on Aging Biomarkers and Age-Related Diseases: A Systematic Review. Front. Aging Neurosci. 2022, 14, 878278. [Google Scholar] [CrossRef]
- Kong, W.; Zheng, Y.; Xu, W.; Gu, H.; Wu, J. Biomarkers of Alzheimer’s disease in severe obstructive sleep apnea-hypopnea syndrome in the Chinese population. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 865–872. [Google Scholar] [CrossRef]
- Xu, H.; Liu, F.; Li, Z.; Li, X.; Liu, Y.; Li, N.; Zhang, X.; Gao, Z.; Zhang, X.; Liu, Y.; et al. Genome-Wide Association Study of Obstructive Sleep Apnea and Objective Sleep-Related Traits Identifies Novel Risk Loci in Han Chinese Individuals. Am. J. Respir. Crit. Care Med. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Strausz, S.; Ruotsalainen, S.; Ollila, H.M.; Karjalainen, J.; Kiiskinen, T.; Reeve, M.; Kurki, M.; Mars, N.; Havulinna, A.S.; Luonsi, E.; et al. Genetic analysis of obstructive sleep apnoea discovers a strong association with cardiometabolic health. Eur. Respir. J. 2021, 57, 2003091. [Google Scholar] [CrossRef]
- Li, J.; Lv, Q.; Sun, H.; Yang, Y.; Jiao, X.; Yang, S.; Yu, H.; Qin, Y. Combined Association Between ADIPOQ, PPARG, and TNF Genes Variants and Obstructive Sleep Apnea in Chinese Han Population. Nat. Sci. Sleep 2022, 14, 363–372. [Google Scholar] [CrossRef]
- Tanizawa, K.; Chin, K. Genetic factors in sleep-disordered breathing. Respir. Investig. 2018, 56, 111–119. [Google Scholar] [CrossRef]
- Wang, H.; Cade, B.E.; Sofer, T.; Sands, S.A.; Chen, H.; Browning, S.R.; Stilp, A.M.; Louie, T.L.; Thornton, T.A.; Johnson, W.C.; et al. Admixture mapping identifies novel loci for obstructive sleep apnea in Hispanic/Latino Americans. Hum. Mol. Genet. 2019, 28, 675–687. [Google Scholar] [CrossRef]
- Kalra, M.; Pal, P.; Kaushal, R.; Amin, R.S.; Dolan, L.M.; Fitz, K.; Kumar, S.; Sheng, X.; Guha, S.; Mallik, J.; et al. Association of ApoE genetic variants with obstructive sleep apnea in children. Sleep Med. 2008, 9, 260–265. [Google Scholar] [CrossRef]
- Gozal, D.; Khalyfa, A.; Capdevila, O.S.; Kheirandish-Gozal, L.; Khalyfa, A.A.; Kim, J. Cognitive function in prepubertal children with obstructive sleep apnea: A modifying role for NADPH oxidase p22 subunit gene polymorphisms? Antioxid. Redox Signal. 2012, 16, 171–177. [Google Scholar] [CrossRef]
- Cade, B.E.; Chen, H.; Stilp, A.M.; Gleason, K.J.; Sofer, T.; Ancoli-Israel, S.; Arens, R.; Bell, G.I.; Below, J.E.; Bjonnes, A.C.; et al. Genetic Associations with Obstructive Sleep Apnea Traits in Hispanic/Latino Americans. Am. J. Respir. Crit. Care Med. 2016, 194, 886–897. [Google Scholar] [CrossRef]
- Sánchez-de-la-Torre, M.; Khalyfa, A.; Sánchez-de-la-Torre, A.; Martinez-Alonso, M.; Martinez-García, M.Á.; Barceló, A.; Lloberes, P.; Campos-Rodriguez, F.; Capote, F.; Diaz-de-Atauri, M.J.; et al. Precision Medicine in Patients With Resistant Hypertension and Obstructive Sleep Apnea: Blood Pressure Response to Continuous Positive Airway Pressure Treatment. J. Am. Coll. Cardiol. 2015, 66, 1023–1032. [Google Scholar] [CrossRef]
- Goodchild, T.T.; Lefer, D.J. Obstructive Sleep Apnea: The Not-So-Silent Killer. Circ. Res. 2020, 126, 229–231. [Google Scholar] [CrossRef]
- Pinilla, L.; Barbé, F.; de Gonzalo-Calvo, D. MicroRNAs to guide medical decision-making in obstructive sleep apnea: A review. Sleep Med. Rev. 2021, 59, 101458. [Google Scholar] [CrossRef]
- Li, K.; Wei, P.; Qin, Y.; Wei, Y. MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in obstructive sleep apnea patients. Medicine 2017, 96, e7917. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, S.; Yang, G.; Zou, L.; Huang, X.; Liu, S. The Role of miRNAs during Endoplasmic Reticulum Stress Induced Apoptosis in Digestive Cancer. J. Cancer 2021, 12, 6787–6795. [Google Scholar] [CrossRef]
- Liu, K.X.; Chen, Q.; Chen, G.P.; Huang, J.C.; Huang, J.F.; He, X.R.; Lin, T.; Lin, Q.C. Inhibition of microRNA-218 reduces HIF-1α by targeting on Robo1 in mice aortic endothelial cells under intermittent hypoxia. Oncotarget 2017, 8, 104359–104366. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Han, Z.; Huang, S.; Bai, R.; Ge, X.; Chen, F.; Lei, P. Intermittent hypoxia caused cognitive dysfunction relate to miRNAs dysregulation in hippocampus. Behav. Brain Res. 2017, 335, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, W.; Li, G.C.; Jin, M.; You, Z.X.; Liu, H.G.; Hu, Y. Atorvastatin Attenuates Myocardial Hypertrophy Induced by Chronic Intermittent Hypoxia In Vitro Partly through miR-31/PKCε Pathway. Curr. Med. Sci. 2018, 38, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, K.; Li, X.; Ma, Z.; Zhang, Y.; Yuan, M.; Suo, Y.; Liang, X.; Tse, G.; Goudis, C.A.; et al. Doxycycline attenuates chronic intermittent hypoxia-induced atrial fibrosis in rats. Cardiovasc. Ther. 2018, 36, e12321. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, Z.; Qin, Y.; Wei, Y. MiR-664a-3p expression in patients with obstructive sleep apnea: A potential marker of atherosclerosis. Medicine 2018, 97, e9813. [Google Scholar] [CrossRef]
- An, Z.; Wang, D.; Yang, G.; Zhang, W.Q.; Ren, J.; Fu, J.L. Role of microRNA-130a in the pathogeneses of obstructive sleep apnea hypopnea syndrome-associated pulmonary hypertension by targeting the GAX gene. Medicine 2017, 96, e6746. [Google Scholar] [CrossRef]
- Hao, S.; Jiang, L.; Fu, C.; Wu, X.; Liu, Z.; Song, J.; Lu, H.; Wu, X.; Li, S. 2-Methoxyestradiol attenuates chronic-intermittent-hypoxia-induced pulmonary hypertension through regulating microRNA-223. J. Cell. Physiol. 2019, 234, 6324–6335. [Google Scholar] [CrossRef]
- Yu, C.; Liu, Y.; Sun, L.; Wang, D.; Wang, Y.; Zhao, S.; Dai, H.; Zhao, J.; Zhang, S.; Li, M.; et al. Chronic obstructive sleep apnea promotes aortic remodeling in canines through miR-145/Smad3 signaling pathway. Oncotarget 2017, 8, 37705–37716. [Google Scholar] [CrossRef][Green Version]
- Khalyfa, A.; Kheirandish-Gozal, L.; Khalyfa, A.A.; Philby, M.F.; Alonso-Álvarez, M.L.; Mohammadi, M.; Bhattacharjee, R.; Terán-Santos, J.; Huang, L.; Andrade, J.; et al. Circulating Plasma Extracellular Microvesicle MicroRNA Cargo and Endothelial Dysfunction in Children with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2016, 194, 1116–1126. [Google Scholar] [CrossRef]
- Liu, K.X.; Chen, G.P.; Lin, P.L.; Huang, J.C.; Lin, X.; Qi, J.C.; Lin, Q.C. Detection and analysis of apoptosis- and autophagy-related miRNAs of mouse vascular endothelial cells in chronic intermittent hypoxia model. Life Sci. 2018, 193, 194–199. [Google Scholar] [CrossRef]
- Bi, R.; Dai, Y.; Ma, Z.; Zhang, S.; Wang, L.; Lin, Q. Endothelial cell autophagy in chronic intermittent hypoxia is impaired by miRNA-30a-mediated translational control of Beclin-1. J. Cell. Biochem. 2019, 120, 4214–4224. [Google Scholar] [CrossRef]
- Lv, X.; Wang, K.; Tang, W.; Yu, L.; Cao, H.; Chi, W.; Wang, B. miR-34a-5p was involved in chronic intermittent hypoxia-induced autophagy of human coronary artery endothelial cells via Bcl-2/beclin 1 signal transduction pathway. J. Cell. Biochem. 2019, 120, 18871–18882. [Google Scholar] [CrossRef]
- Lin, G.; Huang, J.; Chen, Q.; Chen, L.; Feng, D.; Zhang, S.; Huang, X.; Huang, Y.; Lin, Q. miR-146a-5p Mediates Intermittent Hypoxia-Induced Injury in H9c2 Cells by Targeting XIAP. Oxidative Med. Cell. Longev. 2019, 2019, 6581217. [Google Scholar] [CrossRef]
- Yang, X.; Niu, X.; Xiao, Y.; Lin, K.; Chen, X. MiRNA expression profiles in healthy OSAHS and OSAHS with arterial hypertension: Potential diagnostic and early warning markers. Respir. Res. 2018, 19, 194. [Google Scholar] [CrossRef]
- Gu, W.; Gong, L.; Wu, X.; Yao, X. Hypoxic TAM-derived exosomal miR-155-5p promotes RCC progression through HuR-dependent IGF1R/AKT/PI3K pathway. Cell Death Discov. 2021, 7, 147. [Google Scholar] [CrossRef]
- Yuan, K.; Lan, J.; Xu, L.; Feng, X.; Liao, H.; Xie, K.; Wu, H.; Zeng, Y. Long noncoding RNA TLNC1 promotes the growth and metastasis of liver cancer via inhibition of p53 signaling. Mol. Cancer 2022, 21, 105. [Google Scholar] [CrossRef]
- Nojima, T.; Proudfoot, N.J. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat. Rev. Mol. Cell Biol. 2022, 23, 389–406. [Google Scholar] [CrossRef]
- Johnsson, P.; Ziegenhain, C.; Hartmanis, L.; Hendriks, G.J.; Hagemann-Jensen, M.; Reinius, B.; Sandberg, R. Transcriptional kinetics and molecular functions of long noncoding RNAs. Nat. Genet. 2022, 54, 306–317. [Google Scholar] [CrossRef]
- DiStefano, J.K.; Gerhard, G.S. Long Noncoding RNAs and Human Liver Disease. Annu. Rev. Pathol. -Mech. Disease 2022, 17, 1–21. [Google Scholar] [CrossRef]
- Mentis, A.A.; Dardiotis, E.; Katsouni, E.; Chrousos, G.P. From warrior genes to translational solutions: Novel insights into monoamine oxidases (MAOs) and aggression. Transl. Psychiatry 2021, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Z.; Wang, Y.; Wei, L.; Chen, H. Overexpressed long noncoding RNA CPS1-IT alleviates pulmonary arterial hypertension in obstructive sleep apnea by reducing interleukin-1β expression via HIF1 transcriptional activity. J. Cell. Physiol. 2019, 234, 19715–19727. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Liu, J.; Liu, F.; Sun, Y.; Yang, R. Long non-coding RNA ROR mitigates cobalt chloride-induced hypoxia injury through regulation of miR-145. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lin, G.; Huang, J.; Chen, G.; Huang, X.; Lin, Q. Expression profile of long non-coding RNAs in rat models of OSA-induced cardiovascular disease: New insight into pathogenesis. Sleep Breath. 2019, 23, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.T.; Chen, Y.C.; Tseng, C.C.; Chang, H.C.; Su, M.C.; Wang, T.Y.; Lin, Y.Y.; Zheng, Y.X.; Chang, J.C.; Chin, C.H.; et al. Aberrant DNA methylation of the toll-like receptors 2 and 6 genes in patients with obstructive sleep apnea. PLoS ONE 2020, 15, e0228958. [Google Scholar] [CrossRef] [PubMed]
- Perikleous, E.; Steiropoulos, P.; Tzouvelekis, A.; Nena, E.; Koffa, M.; Paraskakis, E. DNA Methylation in Pediatric Obstructive Sleep Apnea: An Overview of Preliminary Findings. Front. Pediatrics 2018, 6, 154. [Google Scholar] [CrossRef]
- Chen, Y.C.; Huang, K.T.; Su, M.C.; Hsu, P.Y.; Chin, C.H.; Lin, I.C.; Liou, C.W.; Wang, T.Y.; Lin, Y.Y.; Hsiao, C.C.; et al. Aberrant DNA methylation levels of the formyl peptide receptor 1/2/3 genes are associated with obstructive sleep apnea and its clinical phenotypes. Am. J. Transl. Res. 2020, 12, 2521–2537. [Google Scholar]
- Chen, W.; Ye, J.; Han, D.; Yin, G.; Wang, B.; Zhang, Y. Association of prepro-orexin polymorphism with obstructive sleep apnea/hypopnea syndrome. Am. J. Otolaryngol. 2012, 33, 31–36. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, T.W.; Su, M.C.; Chen, C.J.; Chen, K.D.; Liou, C.W.; Tang, P.; Wang, T.Y.; Chang, J.C.; Wang, C.C.; et al. Whole Genome DNA Methylation Analysis of Obstructive Sleep Apnea: IL1R2, NPR2, AR, SP140 Methylation and Clinical Phenotype. Sleep 2016, 39, 743–755. [Google Scholar] [CrossRef]
- Kim, J.; Bhattacharjee, R.; Khalyfa, A.; Kheirandish-Gozal, L.; Capdevila, O.S.; Wang, Y.; Gozal, D. DNA methylation in inflammatory genes among children with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2012, 185, 330–338. [Google Scholar] [CrossRef]
- L Kheirandish-Gozal, L.; Khalyfa, A.; Gozal, D.; Bhattacharjee, R.; Wang, Y. Endothelial dysfunction in children with obstructive sleep apnea is associated with epigenetic changes in the eNOS gene. Chest 2013, 143, 971–977. [Google Scholar] [CrossRef]
- Nanduri, J.; Peng, Y.J.; Wang, N.; Khan, S.A.; Semenza, G.L.; Kumar, G.K.; Prabhakar, N.R. Epigenetic regulation of redox state mediates persistent cardiorespiratory abnormalities after long-term intermittent hypoxia. J. Physiol.-Lond. 2017, 595, 63–77. [Google Scholar] [CrossRef]
- Cortese, R.; Almendros, I.; Wang, Y.; Gozal, D. Tumor circulating DNA profiling in xenografted mice exposed to intermittent hypoxia. Oncotarget 2015, 6, 556–569. [Google Scholar] [CrossRef]
- Chu, A.; Gozal, D.; Cortese, R.; Wang, Y. Cardiovascular dysfunction in adult mice following postnatal intermittent hypoxia. Pediatric Res. 2015, 77, 425–433. [Google Scholar] [CrossRef][Green Version]
- Sanz-Rubio, D.; Sanz, A.; Varona, L.; Bolea, R.; Forner, M.; Gil, A.V.; Cubero, P.; Marin-Oto, M.; Martin-Burriel, I.; Marin, J.M.; et al. Forkhead Box P3 Methylation and Expression in Men with Obstructive Sleep Apnea. Int. J. Mol. Sci. 2020, 21, 2233. [Google Scholar] [CrossRef]
- Lambert, A.A.; Parker, A.M.; Moon, K.K. High-dose N-acetylcysteine in chronic obstructive pulmonary disease, prone positioning in acute respiratory distress syndrome, and continuous positive airway pressure and exhaled nitric oxide in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2014, 189, 223–224. [Google Scholar] [CrossRef]
- Lu, W.; Kang, J.; Hu, K.; Tang, S.; Zhou, X.; Xu, L.; Li, Y.; Yu, S. The role of the Nox4-derived ROS-mediated RhoA/Rho kinase pathway in rat hypertension induced by chronic intermittent hypoxia. Sleep Breath. 2017, 21, 667–677. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Z.; Zhang, J.; Wang, S.; Ge, W.; Li, X.; Mou, W.; Wang, X.; Chai, W.; Zhao, J.; et al. Tim-3 is a potential regulator that inhibits monocyte inflammation in response to intermittent hypoxia in children with obstructive sleep apnea syndrome. Clin. Immunol. 2021, 222, 108641. [Google Scholar] [CrossRef]
- Harki, O.; Tamisier, R.; Pépin, J.L.; Bailly, S.; Mahmani, A.; Gonthier, B.; Salomon, A.; Vilgrain, I.; Faury, G.; Briançon-Marjollet, A. VE-cadherin cleavage in sleep apnoea: New insights into intermittent hypoxia-related endothelial permeability. Eur. Respir. J. 2021, 58, 2004518. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, C.L.; Yu, C.C. Trazodone improves obstructive sleep apnea after ischemic stroke: A randomized, double-blind, placebo-controlled, crossover pilot study. J. Neurol. 2021, 268, 2951–2960. [Google Scholar] [CrossRef]
- Moderie, C.; Carrier, J.; Dang-Vu, T.T. Sleep disorders in patients with a neurocognitive disorder. Enceph. -Rev. De Psychiatr. Clin. Biol. Et Ther. 2022, 48, 325–334. [Google Scholar]
- Jaffuel, D.; Mallet, J.P.; Dauvilliers, Y.; Bourdin, A. Is the Muscle the Only Potential Target of Desipramine in Obstructive Sleep Apnea Syndrome? Am. J. Respir. Crit. Care Med. 2017, 195, 1677–1678. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.S.; Santos-Carvalho, A.; Santos, B.; Carvalhas-Almeida, C.; Barros-Viegas, A.T.; Oliveiros, B.; Donato, H.; Santos, C.; Moita, J.; Cavadas, C.; et al. Peripheral biomarkers to diagnose obstructive sleep apnea in adults: A systematic review and meta-analysis. Sleep Med. Rev. 2022, 64, 101659. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; Malhotra, A.; Wellman, A.; White, D.P. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep 2014, 37, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Veasey, S.C.; Fenik, P.; Panckeri, K.; Pack, A.I.; Hendricks, J.C. The effects of trazodone with L-tryptophan on sleep-disordered breathing in the English bulldog. Am. J. Respir. Crit. Care Med. 1999, 160 Pt 1, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, J.H.; Wang, M.; Huang, M.; Wang, C.Y.; Xia, H.; Xu, J.G.; Li, M.X.; Wang, S. Injection of L-glutamate into the insular cortex produces sleep apnea and serotonin reduction in rats. Sleep Breath. 2012, 16, 845–853. [Google Scholar] [CrossRef]
- Carley, D.W.; Prasad, B.; Reid, K.J.; Malkani, R.; Attarian, H.; Abbott, S.M.; Vern, B.; Xie, H.; Yuan, C.; Zee, P.C. Pharmacotherapy of Apnea by Cannabimimetic Enhancement, the PACE Clinical Trial: Effects of Dronabinol in Obstructive Sleep Apnea. Sleep 2018, 41, zsx184. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Lu, Y.; Zhao, B. Inhibitory effects of 17β-estradiol or a resveratrol dimer on hypoxia-inducible factor-1α in genioglossus myoblasts: Involvement of ERα and its downstream p38 MAPK pathways. Int. J. Mol. Med. 2017, 40, 1347–1356. [Google Scholar] [CrossRef]
- Dasu, M.R.; Riosvelasco, A.C.; Jialal, I. Candesartan inhibits Toll-like receptor expression and activity both in vitro and in vivo. Atherosclerosis 2009, 202, 76–83. [Google Scholar] [CrossRef]
- Nakatsuka, R.; Nozaki, T.; Uemura, Y.; Matsuoka, Y.; Sasaki, Y.; Shinohara, M.; Ohura, K.; Sonoda, Y. 5-Aza-2’-deoxycytidine treatment induces skeletal myogenic differentiation of mouse dental pulp stem cells. Arch. Oral Biol. 2010, 55, 350–357. [Google Scholar] [CrossRef]
- Ohike, Y.; Kozaki, K.; Iijima, K.; Eto, M.; Kojima, T.; Ohga, E.; Santa, T.; Imai, K.; Hashimoto, M.; Yoshizumi, M.; et al. Amelioration of vascular endothelial dysfunction in obstructive sleep apnea syndrome by nasal continuous positive airway pressure--possible involvement of nitric oxide and asymmetric NG, NG-dimethylarginine. Circ. J. 2005, 69, 221–226. [Google Scholar] [CrossRef]
- Roizenblatt, S.; Guilleminault, C.; Poyares, D.; Cintra, F.; Kauati, A.; Tufik, S. A double-blind, placebo-controlled, crossover study of sildenafil in obstructive sleep apnea. Arch. Intern. Med. 2006, 166, 1763–1767. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Messineo, L.; Sands, S.A.; Azarbarzin, A.; Marques, M.; Edwards, B.A.; Eckert, D.J.; White, D.P.; Wellman, A. The Combination of Atomoxetine and Oxybutynin Greatly Reduces Obstructive Sleep Apnea Severity. A Randomized, Placebo-controlled, Double-Blind Crossover Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 1267–1276. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Edwards, B.A.; Sands, S.A.; Marques, M.; Eckert, D.J.; White, D.P.; Wellman, A. Desipramine Increases Genioglossus Activity and Reduces Upper Airway Collapsibility during Non-REM Sleep in Healthy Subjects. Am. J. Respir. Crit. Care Med. 2016, 194, 878–885. [Google Scholar] [CrossRef]
- Zhao, F.; Meng, Y.; Wang, Y.; Fan, S.; Liu, Y.; Zhang, X.; Ran, C.; Wang, H.; Lu, M. Protective effect of Astragaloside IV on chronic intermittent hypoxia-induced vascular endothelial dysfunction through the calpain-1/SIRT1/AMPK signaling pathway. Front. Pharmacol. 2022, 13, 920977. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, H.; Cui, Z.; Tai, X.; Chu, Y.; Guo, X. Tauroursodeoxycholic acid attenuates endoplasmic reticulum stress and protects the liver from chronic intermittent hypoxia induced injury. Exp. Ther. Med. 2017, 14, 2461–2468. [Google Scholar] [CrossRef]
- Peng, L.; Li, Y.; Li, X.; Du, Y.; Li, L.; Hu, C.; Zhang, J.; Qin, Y.; Wei, Y.; Zhang, H. Extracellular Vesicles Derived from Intermittent Hypoxia-Treated Red Blood Cells Impair Endothelial Function Through Regulating eNOS Phosphorylation and ET-1 Expression. Cardiovasc. Drugs Therapy. 2021, 35, 901–913. [Google Scholar] [CrossRef]
- Sukys-Claudino, L.; Moraes, W.; Guilleminault, C.; Tufik, S.; Poyares, D. Beneficial effect of donepezil on obstructive sleep apnea: A double-blind, placebo-controlled clinical trial. Sleep Med. 2012, 13, 290–296. [Google Scholar] [CrossRef]
- Hedner, J.; Kraiczi, H.; Peker, Y.; Murphy, P. Reduction of sleep-disordered breathing after physostigmine. Am. J. Respir. Crit. Care Med. 2003, 168, 1246–1251. [Google Scholar] [CrossRef]
- Chan, E.; Steenland, H.W.; Liu, H.; Horner, R.L. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am. J. Respir. Crit. Care Med. 2006, 174, 1264–1273. [Google Scholar] [CrossRef]
- Aoki, C.R.; Liu, H.; Downey, G.P.; Mitchell, J.; Horner, R.L. Cyclic nucleotides modulate genioglossus and hypoglossal responses to excitatory inputs in rats. Am. J. Respir. Crit. Care Med. 2006, 173, 555–565. [Google Scholar] [CrossRef]
- Berry, R.B.; Yamaura, E.M.; Gill, K.; Reist, C. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep 1999, 22, 1087–1092. [Google Scholar] [CrossRef] [PubMed][Green Version]
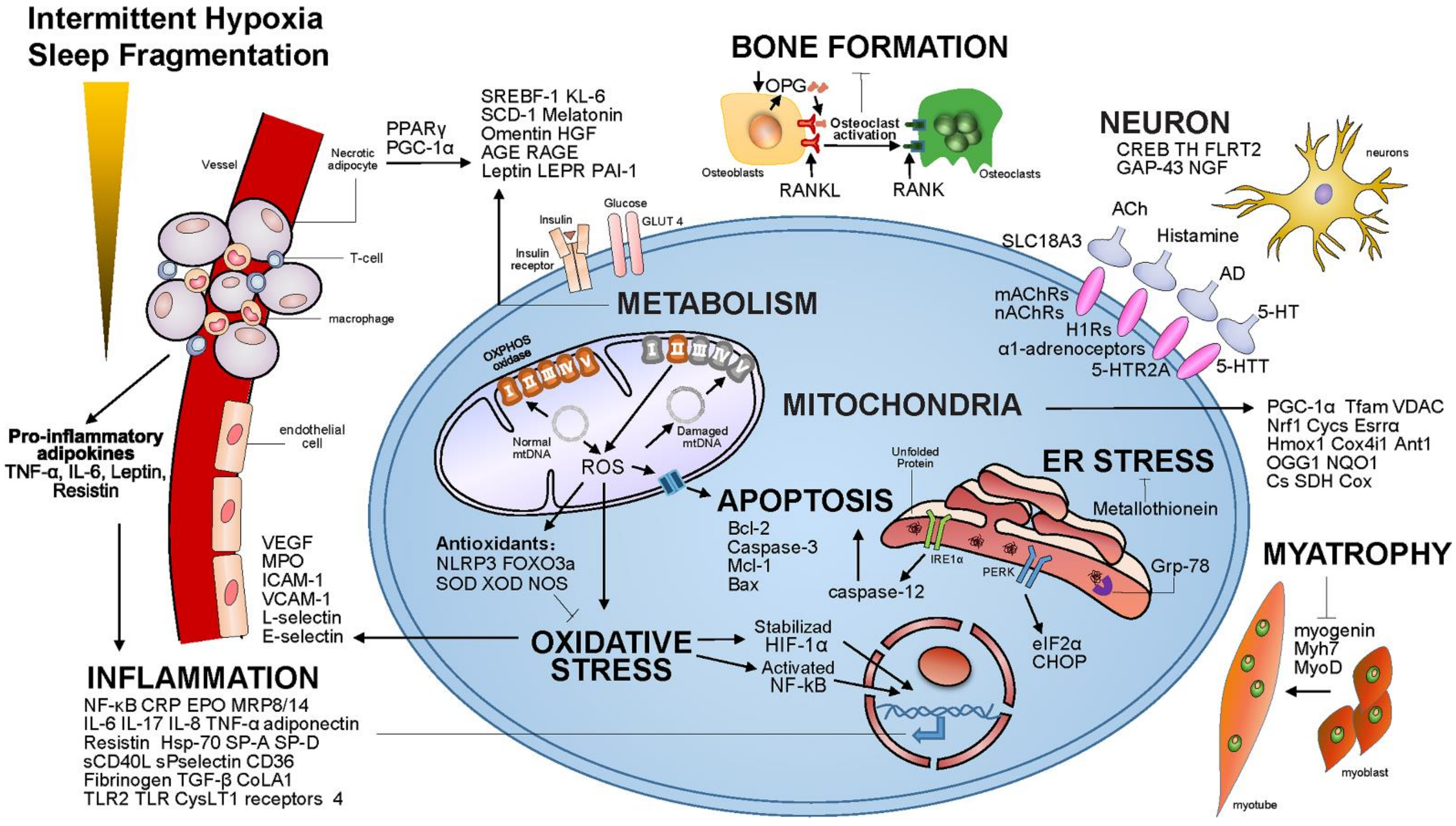
| Signaling Pathway | Gene | Main Function in OSA |
|---|---|---|
| Oxidative stress | HIF-1α | HIF-1α was upregulated in patients with OSA. HIF-1α can enhance types I, Ⅱa, and Ⅱx fiber generation during the process of myogenic differentiation and suppress Ⅱb fiber generation [15]. |
| VEGF | VEGF was reported to be increased in the serum and plasma of OSA patients. Serum levels of VEGF are elevated in severely hypoxic patients with OSA and are related to the degree of nocturnal oxygen desaturation. This might constitute an adaptive mechanism to counterbalance the emergence of OSA-related cardiovascular disease [16]. | |
| MPO, ICAM-1, VCAM-1, L-selectin, E-selectin | The increase in ICAM-1, VCAM-1, L-selectin, E-selectin, and MPO in peripheral blood is one of the mechanisms of cardiovascular damage in elderly patients with OSA [17,18]. | |
| ROCK1, ROCK2 | OSA patients showed significantly lower PBMC ROCK1 and ROCK2 levels than healthy controls in the morning but not in the evening [19]. | |
| TNFα,EN-1 | EN-1 and TNF-α gene expression levels were significantly higher in the OSA group than in the control group [20]. | |
| NADPH oxidase | Long-term IH increased NADPH oxidase gene and protein responses in wake-active brain regions [21]. | |
| NOS | Circulating nitric oxide is suppressed in OSA. Baseline endothelial expression of eNOS and phosphorylated eNOS were reduced in patients with OSA compared with control subjects [22,23,24,25]. | |
| NLRP3, FOXO3a, SOD, XOD | miR-155 might be a positive regulator of the NLRP3 pathway by inhibiting the targeted FOXO3a gene. Chronic OSA also strikingly increased NLRP3, SOD, and XOD [26]. | |
| Apoptosis | Bcl-2, Caspase-3 | Bcl-2 and cleaved caspase-3 play a critical role in underlying memory deficits in obstructive sleep apnea (OSA)-associated IH, and were upregulated after chronic IH treatment [27]. |
| Mcl-1, Bax | Hypoxia upregulated the anti-apoptotic Mcl-1 and downregulated the pro-apoptotic Bax. IH induced ERK1/2 and p38 MAPKs phosphorylation, whereas SH induced only p38 MAPK phosphorylation [28]. | |
| Mitochondria | MnSOD | The data from the in vitro and in vivo experiments indicate that CIH-mediated mitochondrial oxidative stress may play a major role in neuronal cell loss and neurocognitive dysfunction in OSA. Overexpression of MnSOD decreased CIH-mediated cortical neuronal apoptosis [29]. |
| PGC-1α, Tfam, VDAC | The expressions of PGC-1α, Tfam, and VDAC were higher in inner ear sensory hair cells in the CIH group, and there is an association between OSA and mitochondria [30]. | |
| Nrf1, Cycs, Esrrα | Levels of mRNAs were implicated in mitochondrial biogenesis based on quantitative real-time RT-PCR performed on RNA isolated from genioglossus muscle from three groups [31]. | |
| Hmox1, Cox4i1, Ant1, OGG1, NQO1, Cs | The mRNA levels of genes related to mitochondrial function, such as Hmox1, Cs, Cox4i1, Ant1, 8-OGG1, and NQO1, were all markedly lower in the genioglossus of the CIH group [31]. | |
| SDH, Cox | Stained genioglossi sections demonstrated a lower number of Cox- and SDH-positive muscle fibers and reduced intensity of SDH and Cox staining in the CIH group [32]. | |
| ER stress | Grp-78, caspase-12, CHOP | Upregulation of Grp-78, caspase-12, and CHOP occurred after IH treatment, which was prevented by the injection of TUDCA [27]. |
| CHOP, eIF-2a | Protection of eIF-2 phosphorylation with systemically administered salubrinal throughout hypoxia/reoxygenation exposure prevented CHOP/GADD153 activation in susceptible motoneurons. The augmentation of eIF-2a phosphorylation minimizes motoneuronal injury in hypoxia [33]. | |
| MT | M MT protection from ER-stress-induced apoptosis was mediated by upregulating Akt phosphorylation since the inhibition of Akt phosphorylation abolished MT’s protection from ER stress and apoptosis [34]. | |
| Inflammation | p50, p52, p65, c-REL, REL B, NF-kB | Chronic IH ability to induce cardiac ER stress, cell death, and inflammation can be prevented by MT, probably via upregulation of the Akt function [35,36]. |
| CRP | The increase in serum hs-crp content is closely related to the inflammation degree of OSA patients, which can promote the synthesis and release of chemokines and induce the expression of adhesion molecules in vascular endothelial cells to some extent, thus causing damage to the cardiovascular system. The SNP of CRP is correlated with hypertension in OSA patients [37]. | |
| IL-6 | Levels of IL6 were increased in the serum of OSA patients. The serum IL-6 level can be decreased in OSA patients using an effective treatment [38]. | |
| IL-17 | Vitamin D deficiency in patients with severe OSA is common with a negative association between IL-17 and vitamin D serum levels [39]. | |
| IL-8 | IL-8 precedes the development of systemic inflammatory markers in young children with sleep-related CIH [40]. | |
| TNFα | TNFa was elevated in OSA patients [41]. | |
| EPO | EPO is activated solely in response to hypoxia and, therefore, represents a better marker for HIF-1 activation [42]. | |
| SP-A, SP-D | OSA pathogenesis was associated with changes in SP-A and SP-D decreased expression levels [43]. | |
| TLR2, TLR4 | OSA is associated with enhanced expression and signaling events downstream of TLR2 and TLR4 in circulating monocytes [44]. | |
| Resistin | Resistin production can be enhanced by hypoxic stress during sleep, possibly mediating systemic inflammatory processes [45]. | |
| CysLT1 receptors | CysLT1 receptors play a regulatory role in the pathogenesis of OSA in children [46]. | |
| MRP8/14 | Plasma MRP8/14 levels are associated with pediatric OSA and may reflect an increased risk for cardiovascular morbidity [47]. | |
| sCD40L, sPselectin | Serum levels of sCD40L and sP-selectin are elevated in patients with moderate-to-severe OSA [48]. | |
| CD36 | In CIH-exposed mice that closely mimic the chronicity of human OSA, the increased accumulation and proliferation of pro-inflammatory metabolic M1-like macrophages highly expressing CD36 emerged in the aorta [49]. | |
| Fibrinogen | Fibrinogen levels were significantly elevated in patients with severe OSA. Fibrinogen levels were directly related to AHI and the arousal index and inversely related to the mean and lowest oxygen saturation during sleep [50]. | |
| Hsp-70 | Hsp-70 was upregulated by repetitive hypoxemia in OSA and may be involved in the development of the atherogenic process in OSAHS [51]. | |
| TGF-β, CoLA1 | Serum TGF-β level was lower in OSA patients [52]. OSA can accelerate the progression of pulmonary remodeling through TGF-β/miR-185/CoLA1 signaling [53]. | |
| Metabolism | KL-6 | Circulating KL-6 is a biomarker of lung injury in OSA [54,55]. |
| SREBF-1, SCD-1 | CIH induces fasting dyslipidemia in both lean and obese mice due to the activation of SREBF-1 and SCD-1 [56,57,58]. In human subjects, hepatic SCD mRNA levels correlate with the degree of nocturnal hypoxemia [58]. | |
| Melatonin | Circulating melatonin levels are elevated in OSA patients [59]. | |
| Omentin | Circulating omentin levels are elevated in OSA patients and seem to be involved in the pathogenesis of OSAS [59,60]. | |
| HGF | Combined detection of serum HGF concentrations in patients with OSA has a clinical value in judging the condition and curative effect and evaluating the cardiovascular damage [61]. | |
| AGE, RAGE | AGEs may play an important role in insulin resistance in OSA and serve as a biomarker for patients with OSA with a high risk of type 2 diabetes mellitus [62,63,64,65]. | |
| Leptin, LEPR | OSA patients have significantly higher levels of leptin. Leptin affects the sleep architecture, ventilation, and the defense of upper airway patency. The association between leptin and leptin receptor gene polymorphisms and susceptibility to OSA remains poorly defined due to conflicting data [66,67,68,69]. | |
| PPARγ | PPARγ was downregulated in subjects with OSA [70]. | |
| PAI-1 | PAI-1 was significantly higher in subjects with OSA. Gene set enrichment analysis (GSEA) identified several gene sets that are upregulated in the adipose tissue of OSA patients, including the pro-inflammatory NF-κB pathway and the proteolytic ubiquitin/proteasome module [71]. | |
| Myatrophy | Myh7 | Myh7 were both downregulated in palatopharyngeal tissues from OSA patients [72]. |
| MyoD, myogenin | The MyoD and myogenin mRNA in the CIH group was significantly lower compared with the control. When the oxygen level was normal, the myosin heavy chain (MHC), myogenin, and MyoD expression increased [73]. | |
| Bone formation | OPG/RANKL | The serum level of OPG and the OPG/RANKL ratio were lower in the OSA group [74]. |
| VDR | A low vitamin D serum concentration was reportedly linked to OSA susceptibility [75]. | |
| Neuron | 5-HTR2A, 5-HTT | 5-HT activity is required to maintain upper airway stability in OSA models. 5-HTR2A and 5-HTT genes may be susceptible markers to develop for OSA [76,77]. |
| H1Rs | Histamine excited HMN with an inward current under a voltage clamp and a depolarization membrane potential under a current clamp via H1Rs. This contributes an excitatory drive to the GG muscle involved in the pathogenesis of OSA [78]. | |
| mAChRs | The mAChRs mechanism linked to GIRK channels would suppress HM activity, largely in REM sleep [79]. | |
| nAChRs | The nAChRs activation on HMNs may contribute to the central maintenance of upper airway patency and prevent airway obstruction [80]. | |
| α1-adrenoceptors | Chronic IH increases the noradrenergic drive to XII motoneurons including the sprouting of noradrenergic terminals in the XII nucleus and increased expression of α1-adrenoceptors [81]. | |
| CREB | IH induced significant decreases in Ser-133-phosphorylated CREB without changes in the total CREB [82]. | |
| GAP-43, TH, NGF | GAP-43, TH, and NGF were highly expressed in OSA groups. OSA can accelerate the progression of pulmonary remodeling through TGF-β/miR-185/CoLA1 signaling [83]. | |
| SLC18A3, FLRT2 | SLC18A3 gene expression was significantly upregulated in peripheral blood from patients with OSA, while FLRT2 was significantly depressed in patients with severe OSA [84]. |
| Genes | miRNA | Function in OSA |
|---|---|---|
| Unknown | miR-664a-3p | miR-664a-3p levels are positively associated with AHI, LOS, and CIMT, and thus, it has a possible role in the pathogenesis of atherosclerosis in OSA patients and as a noninvasive marker of these related conditions [125]. |
| GAX | miR-130a | miR-130a may be involved in the progression of OSA-associated PHT by downregulating the GAX gene [126]. |
| Unknown | miR-223 | CIH decreased the expression of miR-223, whereas 2-methoxyestradiol reversed the downregulation of miR-223, both in vivo and in vitro [127]. |
| CoLA1 | miR-185 | OSA could activate the expression of TGF-β, which subsequently suppressed miR-185 and promoted CoLA1 expression [83,128]. |
| Smad3 | miR-145 | miR-145/Smad3 signaling pathway might promote aortic remodeling during OSA [128]. |
| Nrf2, AMP kinase, and tight junction pathways | miR-630 | The expression of exosomal miRNA-630 was reduced in children with endothelial dysfunction and was normalized after therapy, along with restoration of endothelial function [129]. |
| Autophagy and apoptosis | miR-16, miR-718, miR-1249, miR-193, miR-218, miR-30B | Four (miR-1249, miR-193, miR-218, and miR-30B) were upregulated and two (miR-16 and miR-718) were downregulated markedly in CIH [130]. |
| Beclin-1 | miR-30a | Suppression of miR-30a via the expression of the antisense of miR-30a significantly increased Beclin-1 levels to enhance endothelial cell autophagy in vitro and in vivo, which improved endothelial cell survival against CIH [131]. |
| Unknown | miR-26b, miR-207 | miR-26b and miR-207 could be involved in OSA-induced cognitive impairments [122]. |
| PANK CAD | miR-107, miR-485-5p, miR-574-5p, miR-199-3p | These different microRNAs also play a significant role in metabolism, hypoxia, and oxidative stress, and might participate in OSA [119]. |
| Bcl-2 | miR-34a-5p | The overexpression of miR-34a-5p activated Beclin 1 through Bcl-2 inhibition in CIH and participated in CIH-induced autophagy [132]. |
| XIAP | miR-146a-5p | miR-146a-5p could attenuate viability and promote the apoptosis of H9c2 by targeting XIAP, thus aggravating the H9c2 cell injury induced by IH [133]. |
| Unknown | miR-126-3p, let-7d-5p, miR-7641, miR-1233-5p, miR-320b, miR-145-5p, miR-107, miR-26a-5p | miR-145-5p and let-7d-5p in combination can identify healthy OSA, and the presence of miR-126-3p, miR-26a-5p, and miR-107 was strongly indicative of OSA with arterial hypertension [134]. |
| FOXO3a | miR-155 | miR-155 might be a positive regulator of the NLRP3 pathway by inhibiting the targeted FOXO3a gene [135]. |
| lncRNAs | Function in OSA |
|---|---|
| lncRNA-CPS1-IT | CPS1-IT was downregulated in an OSA rat model. Overexpressed CPS1-IT increased the activity of NO, NOS, and SOD, as well as α-SMA expression, whereas decreases in LPO activity, PCNA expression and IL-1β expression occurred through NF-κB signaling pathway via inhibiting the HIF1 transcriptional activity [141]. |
| lncRNA-ROR | lncRNA-ROR revealed properties that are useful for regulating the hypoxia response. CoCl2 increased the expression of ROR. ROR overexpression upregulated the anti-apoptotic protein Bcl-2; decreased p53, Bax, cleaved caspase-3, miR-145, and the phosphorylation of MAPK; and increased the expression of HIF-α and the phosphorylation of ERK [142]. |
| XR_596701, XR_344474,XR_600374, ENSRNOT00000065561, XR_590196, XR_597099 | Three lncRNAs (XR_596701, XR_344474, and ENSRNOT00000065561) increased and three lncRNAs (XR_600374, XR_590196, and XR_597099) decreased in the heart samples of rats exposed to eight weeks of CIH [143]. |
| Target Genes | Function in OSA |
|---|---|
| AR, NPR2, L1R2, SP140 | OSA-related hypoxia leads to the altering in the promoter methylation of AR, NPR2, L1R2 and SP140 [147,148]. |
| FOXP3 | The FOXP3 gene, which regulates expression of T regulatory lymphocytes, ismore likely todisplay increasedmethylation among children with OSA who exhibit increased systemic inflammatory responses [149]. |
| eNOS | A CpG site showed significantly higher methylation levels. eNOS mRNA expression levels were significantly reduced [150]. |
| AOEs | Long term IH (IH) increased DNA methylation of genes encoding AOEs. Treatment with decitabine, a DNA methylation inhibitor, prevented DNA methylation, normalized the expression of AOE genes and ROS levels [151]. |
| Rab3a | Mice engrafted with TC1 epithelial lung cancer cells and controls were exposed to IH. Increased Rab3a showed significant plasma cirDNA modification, increasing tumor invasion [152]. |
| Ace1, Atg | IH-exposed mice showed higher lever of DNA methylation patterns of the Ace1 and the Agt genes CD31+ endothelial cells [153]. |
| Targets | Chemical Compounds | Main Functions in OSA |
|---|---|---|
| Nox1 and Nox4 | GKT137831 | Nox1 and Nox4 inhibitor [153]. |
| ROS scavenger, antioxidant, anti-inflammatory, and mucolytic effects | NAC | Limiting ROS production by NAC could suppress ER stress activation [155]. |
| RhoA inhibitor | Y27632 | Treatment with Y27632 reduced both Systolic blood pressure and renal sympathetic nerve activity in rats exposed to chronic IH [156]. |
| Lipid-lowering medicine | Statin | Inhibition of the inflammatory response by statins may be due to the down-regulation of TLR4 and TLR2 expression, there by reducing the release of downstream effectors [167]. |
| TLR2 and TLR4 | Candesartan | TLR2 and TLR4 expression at mRNA and protein levels are inhibited by candesartan both in vitro and in vivo [168]. |
| CysLT1 receptors | LTD4 | LTD4 can promote T cell proliferation in adenoid tissues via activation of CysLT1 receptors in children with OSA [47]. |
| Antioxidant and anti-inflammatory | ALA | ALA attenuates endothelial dysfunction by preventing oxidative stress and inflammation and restoring nitric oxide bioavailability in mice exposed to CIH [169]. |
| NOS inhibitor | ADMA | Nasal CPAP improves endothelial function, in part by the decreasing ADMA concentration, thereby potentiating NO production [170]. |
| Inhibits cyclic guanosine monophosphate-specific phosphodiesterase 5. | Sildenafil | In patients with severe obstructive sleep apnea, a single 50-mg dose of sildenafil at bedtime worsens respiratory and desaturation events [171]. |
| Norepinephrine reuptake inhibitor antimuscarinic | Atomoxetine, oxybutynin | A combination of noradrenergic and antimuscarinic agents administered orally before bedtime on one night greatly reduced OSA severity [172]. |
| An inhibitor of NET and SERT, and prevents the reduction in genioglossus activity | Desipramine | Desipramine reduces the state-related drop in tonic genioglossus muscleactivity that occurs from wakefulness to non-REM sleep and reduces airway collapsibility [173]. |
| AD, as an adipocyte-specific protein, regulates metabolism | AD | Impaired mitochondrial structure and function was significantly improved and a percentage of type I fiber was elevated. Moreover, a significant decrease in phosphorylation of LKB1, AMPK, and PGC1-α, whereas there was significant rescue of such reduction in phosphorylation [174]. |
| TUDCA and 4-PBA, which are two chemical chaperones that reduce ER stress by facilitating proper protein folding | TUDCA, 4-PBA | Attenuators of ER stress may serve as novel adjunct therapeutic agents for ameliorating OSA-induced neurocognitive impairment [175]. |
| A specific inhibitor of MEK1/2 and blocks ERK1/2 activation of a competitive p38MAPK inhibitor | U0126, SB202190 | Both ERK and p38MAPK inhibitors attenuated the IH-induced Mcl-1 increase. In SH, only p38MAPK inhibition decreased Mcl-1 expression [176]. |
| E2 and RD inhibited the overexpression of HIF-1α | E2, RD | ERα may be responsible for downregulation of HIF-1α by E2 or RD via activation of downstream p38 MAPK pathways [167]. |
| miR-223 | 2-methoxyestradio | CIH decreased the expression of miR-223, whereas 2-methoxyestradiol reversed the downregulation of miR-223 both in vivo and in vitro [127]. |
| AChEI | Donezepil, Physostigmine | A cholinesterase inhibitor, promotes cholinergic transmission [177,178]. |
| Nicotinic agonist | 1,1-dimethyl-4-phenylpiperazinium iodide | Excited hypoglossal motoneurons via a Ca2+-sensitive and TTX-insensitive inward current [80]. |
| The alpha1 receptor antagonist | terazosin | Provides noradrenergic activation and significantly decreases GG activity in wakefulness and non-REM sleep [179]. |
| Adenylyl cyclase activator | Forskolin | Increases cAMP at the HMN, as well as respiratory-related and tonic genioglossus activities, during wakefulness and non-REM sleep but not REM sleep [180]. |
| A weak SSRI | Trazodone | Simultaneously inhibits SERT, 5-HT2A, and 5-HT2C receptors, reduces levels of serotonin thus improve apnea and hypopnea episodes in OSA patients [163]. |
| SSRI | Paroxetine | Block 5-HT re-uptake, can increase the peak sleep inspiratory velocity and the activity of genioglossal muscle in OSA patients [181]. |
| Serotonin antagonists | Methicillin, Ritanserin | Reductions in plasma 5-HT levels, and induced apnea [164,165]. |
| Non-selective CB1/CB2 receptor agonist | Dronabinol | Reduced the frequency of spontaneous central apneas in a rodent model of sleep-related breathing disorder [166]. |
| The carbonic anhydrase inhibitor | Acetazolamide | Acetazolamide improves sleep apnea at high altitude by decreasing AHI and percentage of periodic breathing time and increasing nocturnal oxygenation [15]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Lu, Y.; Sheng, L.; Han, X.; Yu, L.; Zhang, W.; Liu, S.; Liu, Y. Advances in Molecular Pathology of Obstructive Sleep Apnea. Molecules 2022, 27, 8422. https://doi.org/10.3390/molecules27238422
Zhang M, Lu Y, Sheng L, Han X, Yu L, Zhang W, Liu S, Liu Y. Advances in Molecular Pathology of Obstructive Sleep Apnea. Molecules. 2022; 27(23):8422. https://doi.org/10.3390/molecules27238422
Chicago/Turabian StyleZhang, Menghan, Yun Lu, Lu Sheng, Xinxin Han, Liming Yu, Weihua Zhang, Shangfeng Liu, and Yuehua Liu. 2022. "Advances in Molecular Pathology of Obstructive Sleep Apnea" Molecules 27, no. 23: 8422. https://doi.org/10.3390/molecules27238422
APA StyleZhang, M., Lu, Y., Sheng, L., Han, X., Yu, L., Zhang, W., Liu, S., & Liu, Y. (2022). Advances in Molecular Pathology of Obstructive Sleep Apnea. Molecules, 27(23), 8422. https://doi.org/10.3390/molecules27238422





