Deep Eutectic Solvents as à-la-Carte Medium for Transition-Metal-Catalyzed Organic Processes
Abstract
1. Introduction
- Type I: formed by a quaternary ammonium salt and a metal chloride.
- Type II: differs from type I in having hydrated metal halides instead of non-hydrated ones.
- Type III: formed from quaternary ammonium salt and hydrogen bond donors (HBDs), e.g., alcohols, amino acids or amides.
- Type IV: formed from transition-metal salts and HBDs.
- Type V: formed exclusively by non-ionic components.
2. RedOx Processes
| Entry | Reaction | DES | Conditions | Product | Ref. |
|---|---|---|---|---|---|
| 1 | Hydrolysis and oxidation | FeCl3·6H2O:(CH2OH)2 (1:2) | 120 °C | Gluconic acid | [4] |
| 2 | Alcohol Oxidation | d-fructose:urea (3:2) | 25–40 °C | Ketones/Aldehydes | [7] |
| 3 | Hydroformylation | DMU: RAME-ß-CD | CO/H2 (50 bar) | Aldehydes | [12] |
| 4 | Hydrogenations | ChCl:glycerol (1:2) | Pd/C, H2 | Diarylpyrazines | [13] |
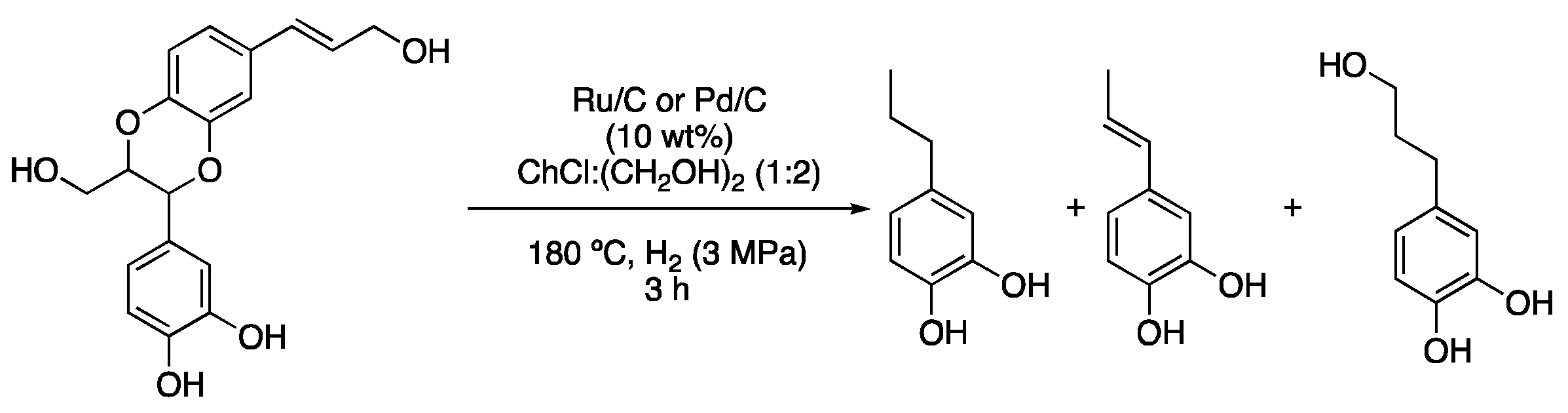
| 5 | Hydrogenolysis | ChCl:(CH2OH)2 (1:2) | Ru/C or Pd/C, H2 | Resorcinol derivatives | [15] |
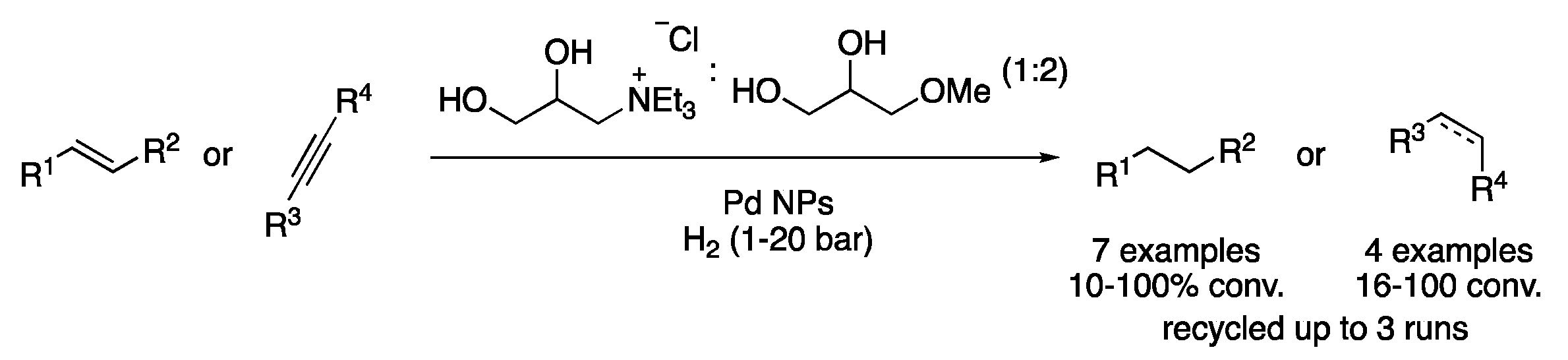
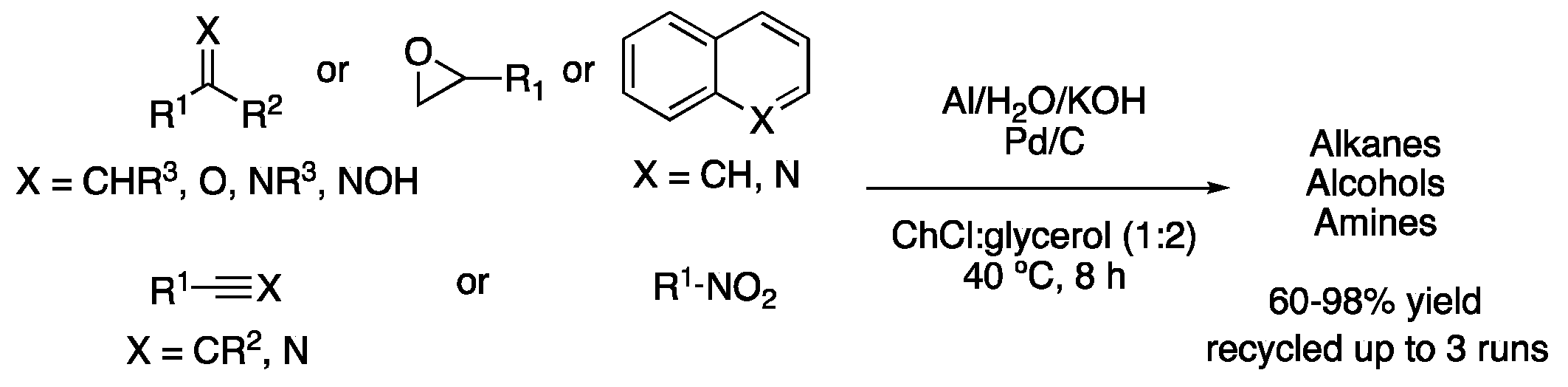
| 6 | Hydrogenation | ChCl:glycerol (1:2) | Al, KOH, H2O | Alkanes/alcohols/amines | [21] |
| 7 | Transfer hydrogenation | TBAB:HCOOH (1:1) | [Ru], NEt3, CPME | Secondary alcohols | [22] |
3. Cyclization Reactions
3.1. Cycloadditions
3.2. Cyclizations through Condensation
| Entry | Reaction | DES | Conditions | Product | Ref. |
|---|---|---|---|---|---|
| 1 | Cycloisomerization of alkynoic acids | ChCl:urea (1:2) | [Au] | Enol-lactones | [23,26] |
| 2 | Cycloisomerization of Z-enynols | ChCl:glycerol (1:2) | [Au] | Furans | [24] |
| 3 | 3+2 Cycloaddition | ChCl:ZnCl2 (1:2) | 100–140 °C | 1H-Tetrazoles | [27,28] |
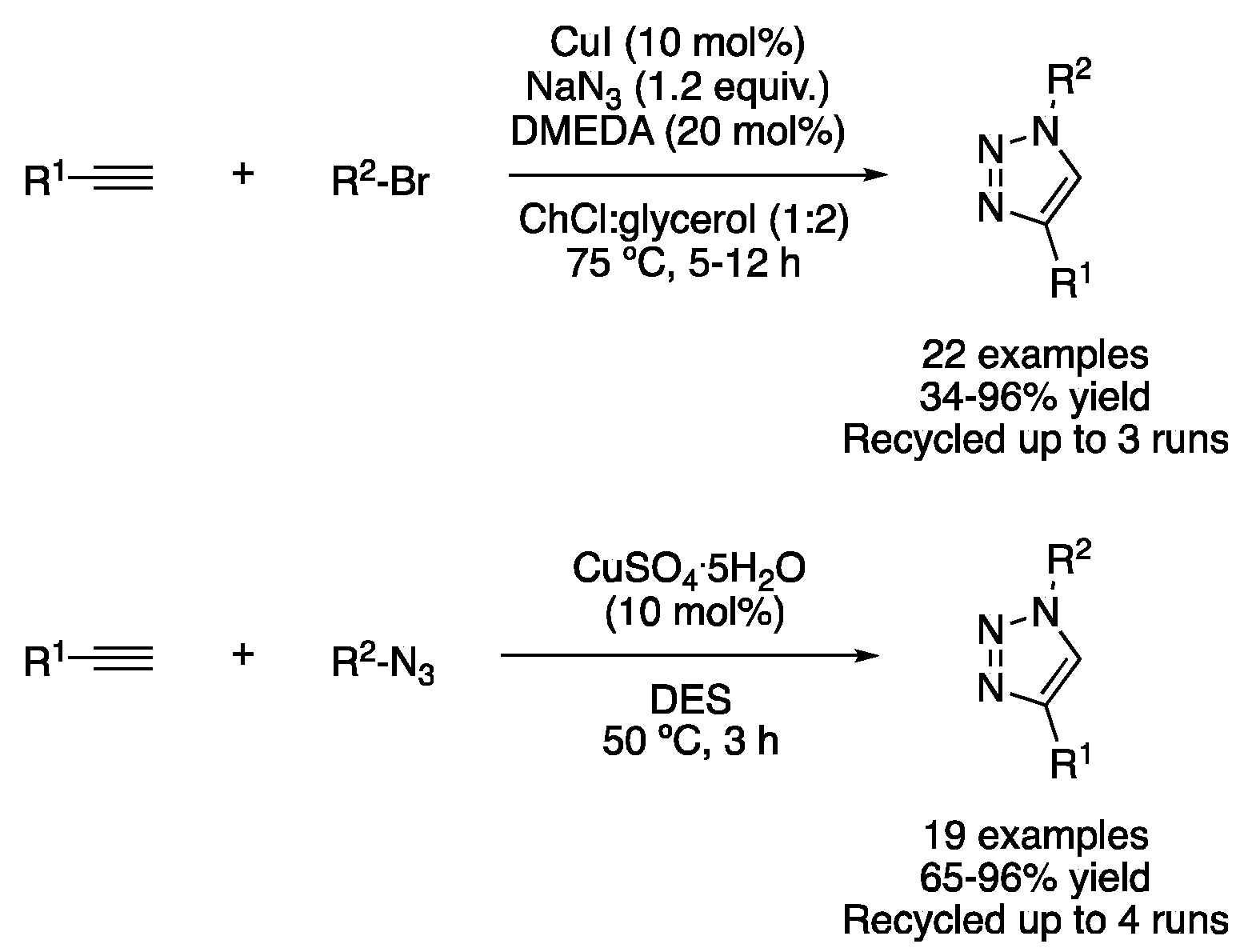
| 4 | 3+2 Cycloaddition | ChCl:glycerol (1:2) | [Cu], 50–75 °C | Triazoles | [29,30] |
| 5 | A3 coupling | Citric acid:DMU (7:2) | CuFeO2 | imidazo[1,2a]pyridines | [32,33] |
| 6 | Condensation | ChCl:urea (1:2) | [Fe/Mo], MW | spirooxyndoles | [34] |
| 7 | S-heterocyclization | ChCl:glycerol (1:2) | PdI2/KI | Thiophene derivatives | [38] |
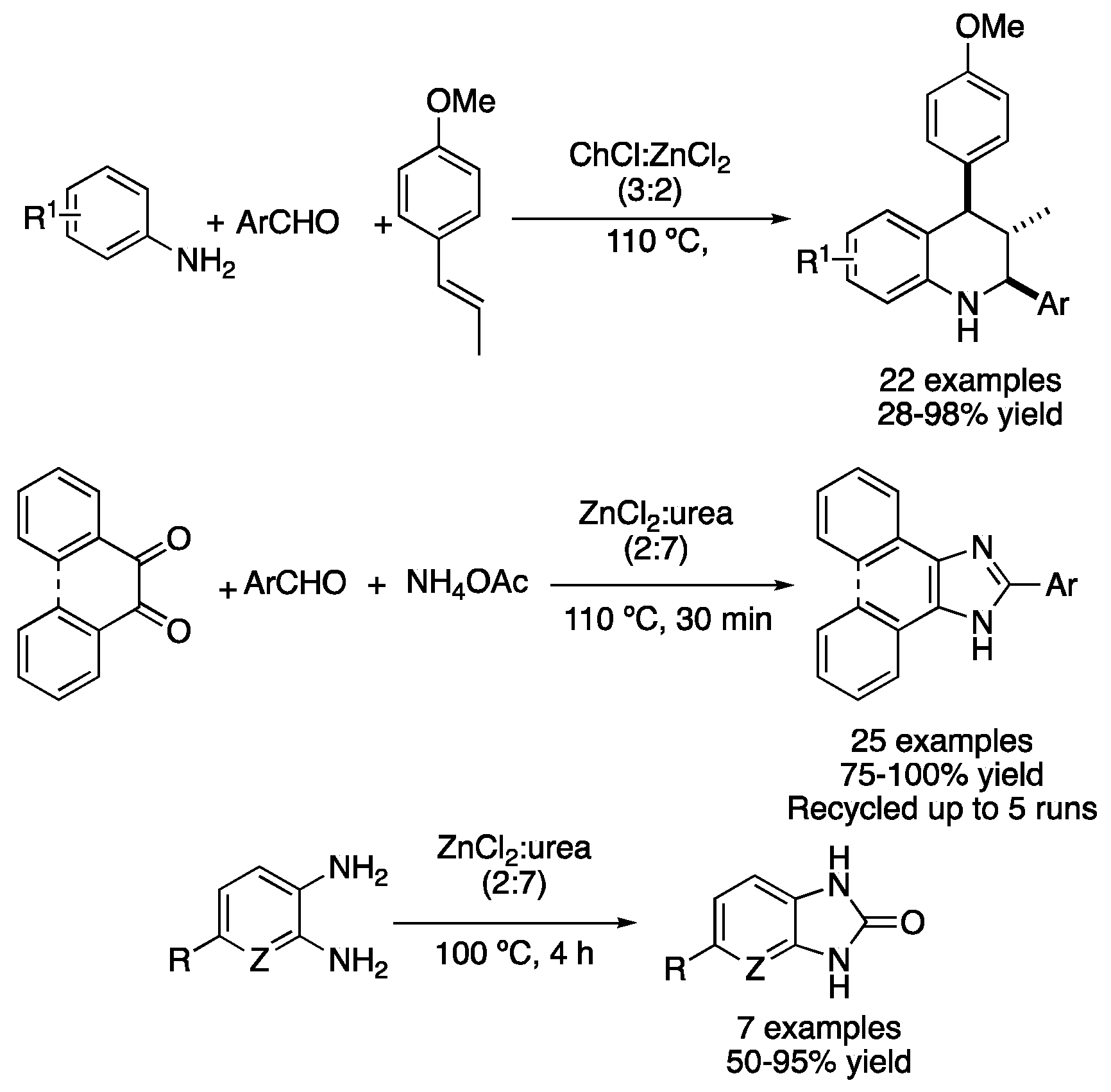
| 8 | Condensation + D.A. | ChCl:ZnCl2 (3:2) | 110 °C | Tetrahydroisoquinolines | [39] |
| 9 | Condensation | ZnCl2:urea (2:7) | 110 °C | phenantro[9,10a]imidazoles | [41] |
| 10 | Condensation | ZnCl2:urea (2:7) | 100 °C | 2-benzimidazolones | [42] |
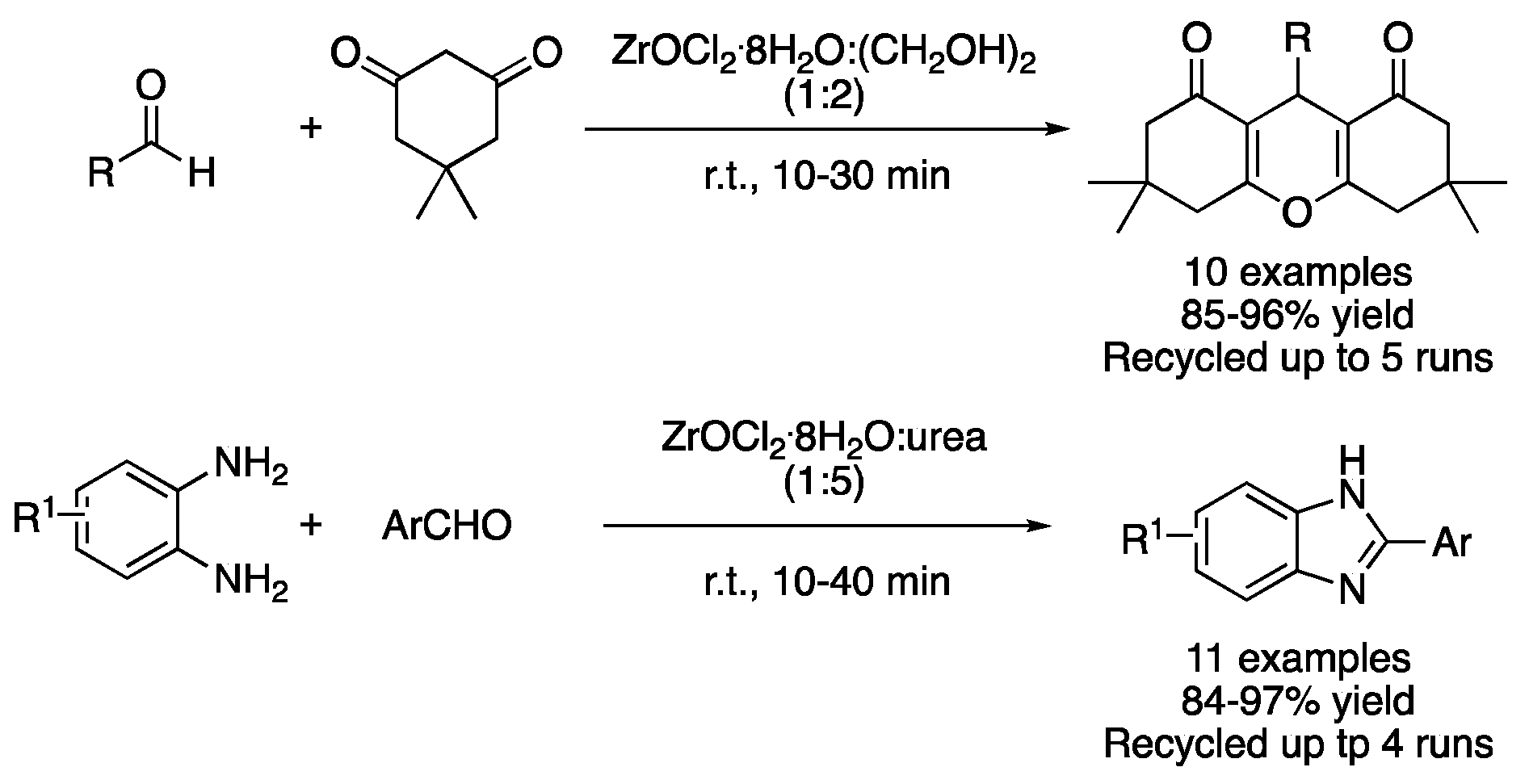
| 11 | Condensation | ZrOCl2:(CH2OH)2 (1:2) | r.t. | dioxooctahydroxanthenes | [43] |
| 12 | Condensation | ZrOCl2:urea (1:5) | r.t. | benzimidazoles | [44] |
4. Cross-Coupling Reactions
5. C-H Functionalization
| Entry | Reaction | DES | Conditions | Product | Ref. |
|---|---|---|---|---|---|
| 1 | CDC | ChCl:(CH2OH)2 (1:2) | [Cu], 50 °C | 1-ArTetrahydroisoquinolines | [85] |
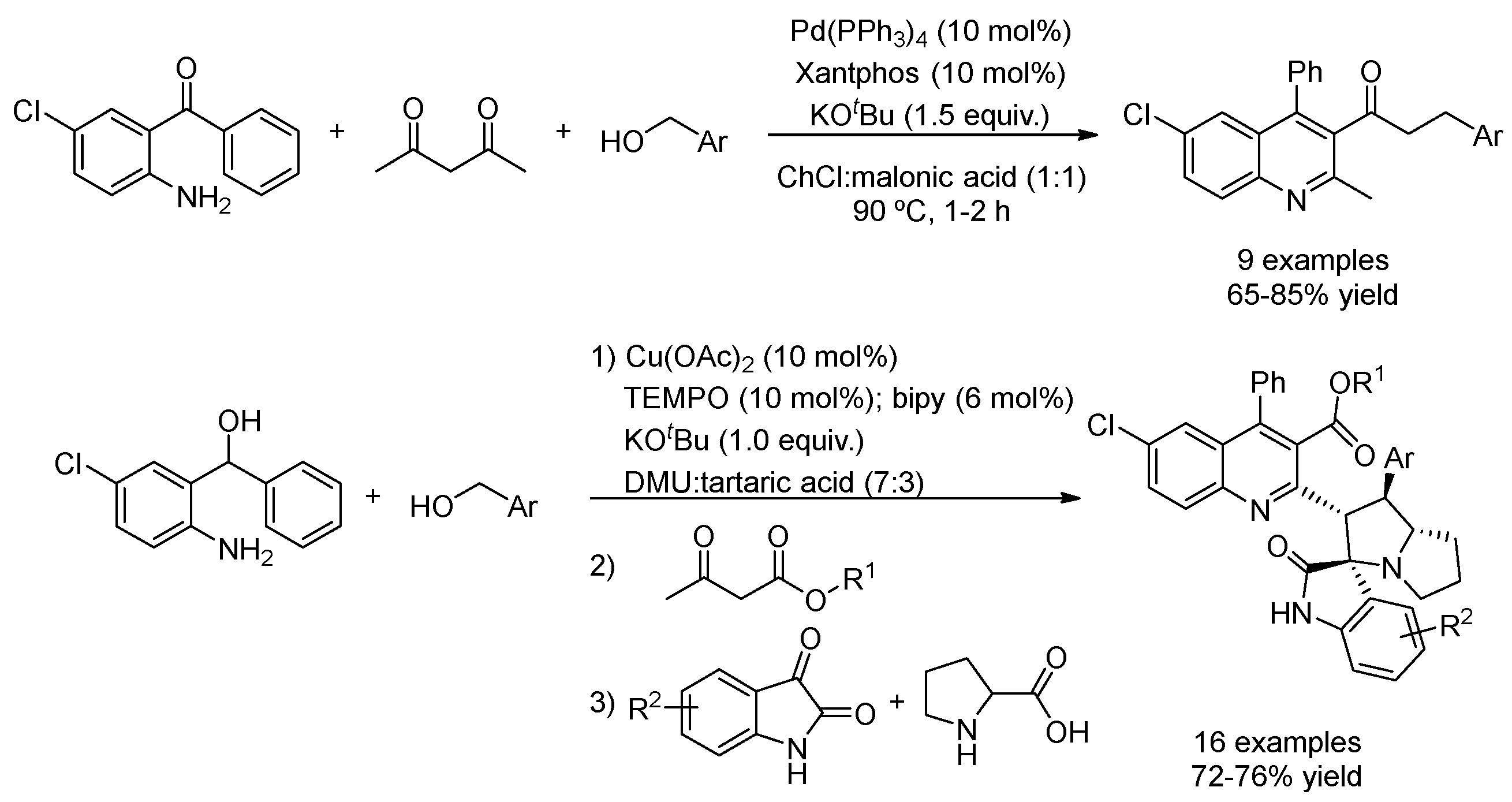
| 2 | Hydrogen auto-transfer | ChCl:malonic acid (1:1) | [Pd], 90 °C | Quinolines | [86] |
| 3 | Hydrogen auto-transfer | DMU:tartaric acid (7:3) | [Cu] + TEMPO | Spiroindoles | [88] |
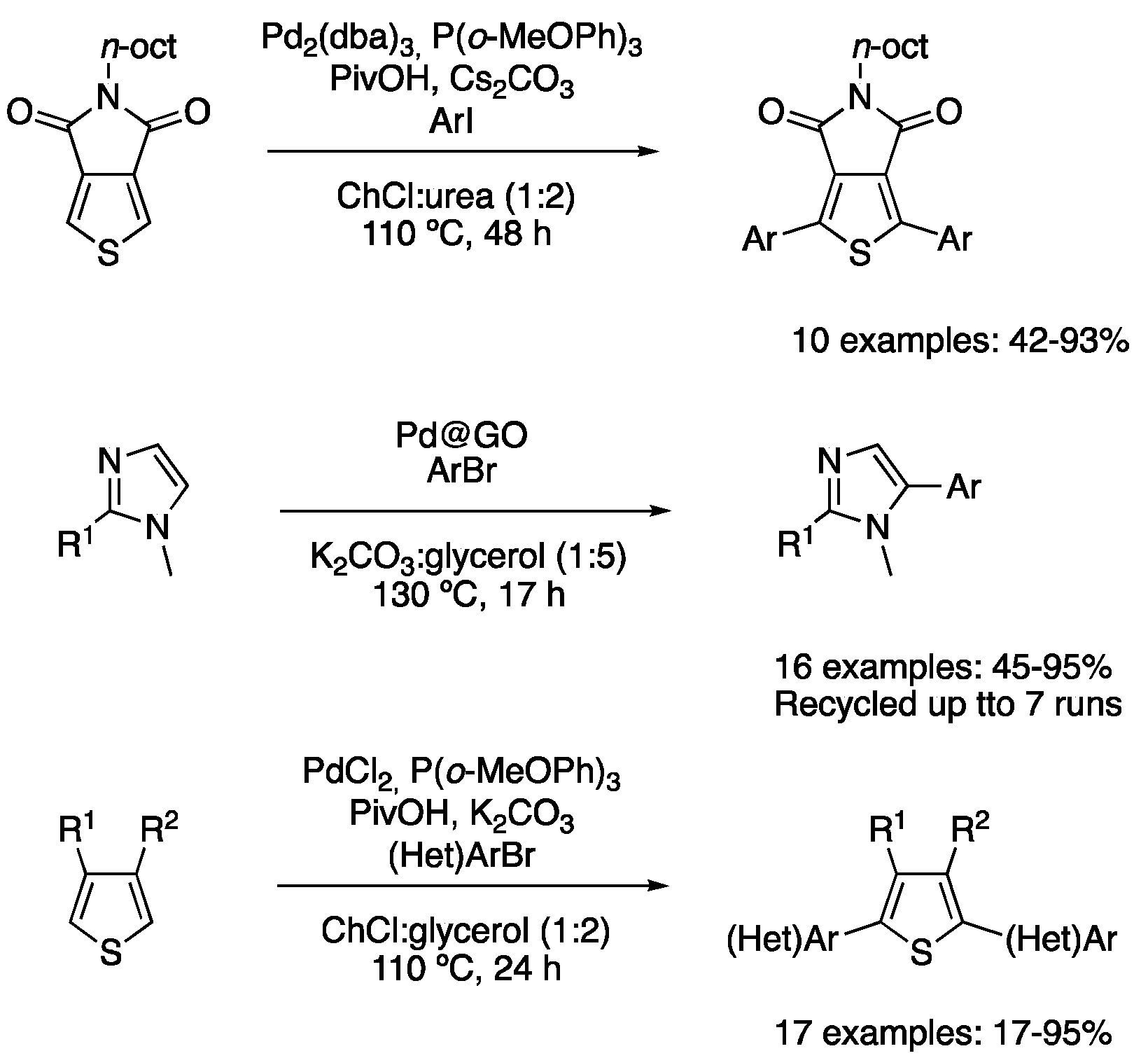
| 4 | Direct arylation | ChCl:urea (1:2) | [Pd], 110 °C | Thienyl derivatives | [89] |
| 5 | Direct arylation | ChCl:glycerol (1:2) | [Pd], 110 °C | Thienyl derivatives | [91] |
| 6 | Direct arylation | K2CO3:glycerol (1:5) | [Pd], 130 °C | Imidazole derivatives | [90] |
| 7 | Csp2-H activation | ChCl:(CH2OH)2 (1:2)Betaine:HFIP (1:2) | [Ru], 70–120 °C | (Het)Ar derivatives | [92] |
| 8 | Csp3-H activation | ChCl:acetamide (1:2)Betaine:HFIP (1:2) | [Pd], 110 °C | arylated amides derivatives | [93] |
6. Multicomponent Reactions
| Entry | Reaction | DES | Conditions | Product | Ref. |
|---|---|---|---|---|---|
| 1 | Kabachnik–Fields | ChCl:ZnCl2 (1:2)ZrOCl2:urea (1:5) | r.t. | Aminophosphonates | [94] |
| 2 | Mannich | ChCl:ZnCl2 (1:2) | r.t. | Aminocarbonyls | [96] |
| 3 | Condensation | ChCl:ZnCl2 (1:3) | r.t. | 3-indole derivatives | [97] |
| 4 | A3 coupling | ZnCl2:DMU (2:7) | r.t. | Propargylamines | [98] |
| 5 | A3 coupling | ChCl:urea (1:2) ChCl:(CH2OH)2 (1:2) | [Cu], 60–80 °C | Propargylamines | [99,100] |
| 6 | A3 coupling | PAA:AO12 (1:1) | [Ag], 60 °C | Propargylamines | [101] |
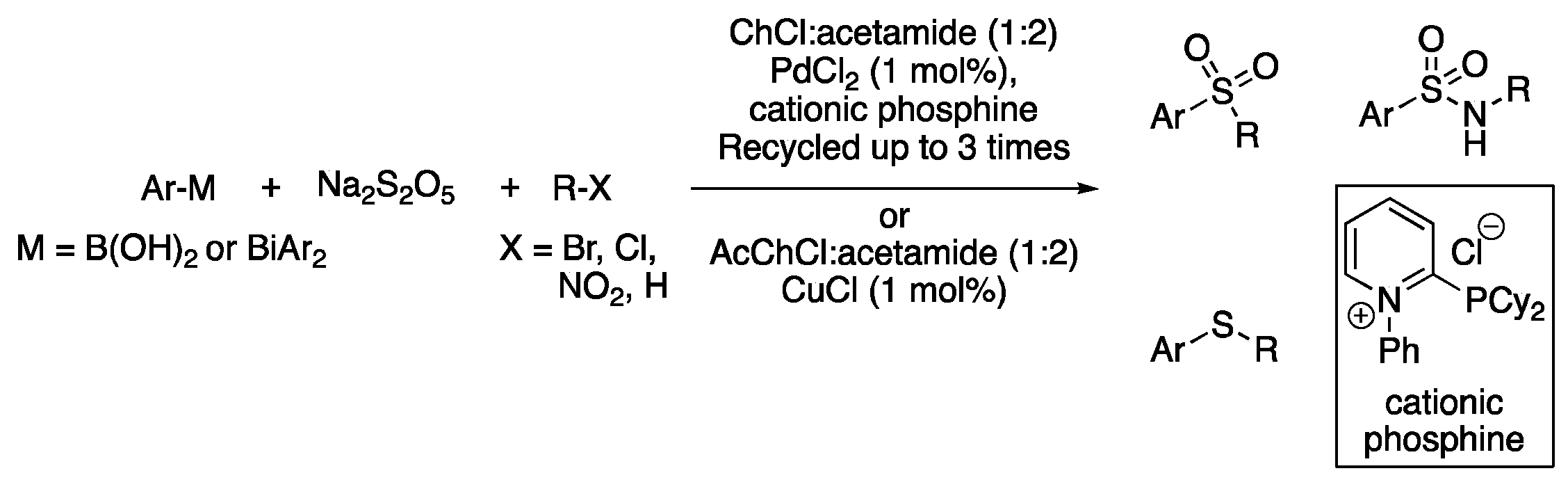
| 7 | Sulfonylation | (Ac)ChCl:acetamide (1:2) | [Pd] or [Cu], 80 °C | Sulfones/sulfonamides | [103,104] |
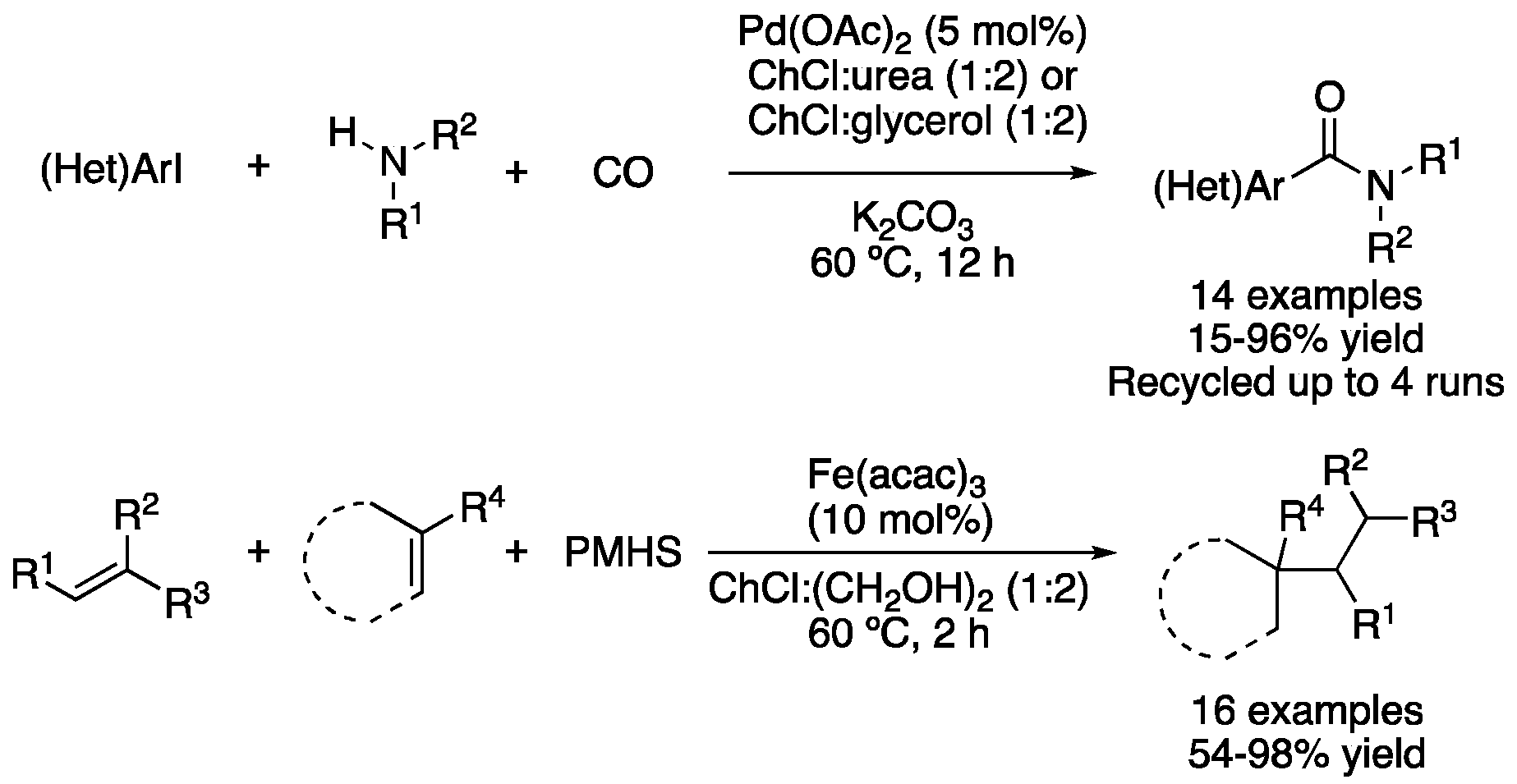
| 8 | Aminocarbonylation | ChCl:urea (1:2)ChCl:glycerol (1:2) | [Pd], 60 °C | (Het)Arylamides | [105] |
| 9 | Conjugate addition | ChCl:(CH2OH)2 (1:2) | [Fe]. 60 °C | Addition products | [106] |
7. Miscellaneous
| Entry | Reaction | DES | Conditions | Product | Ref. |
|---|---|---|---|---|---|
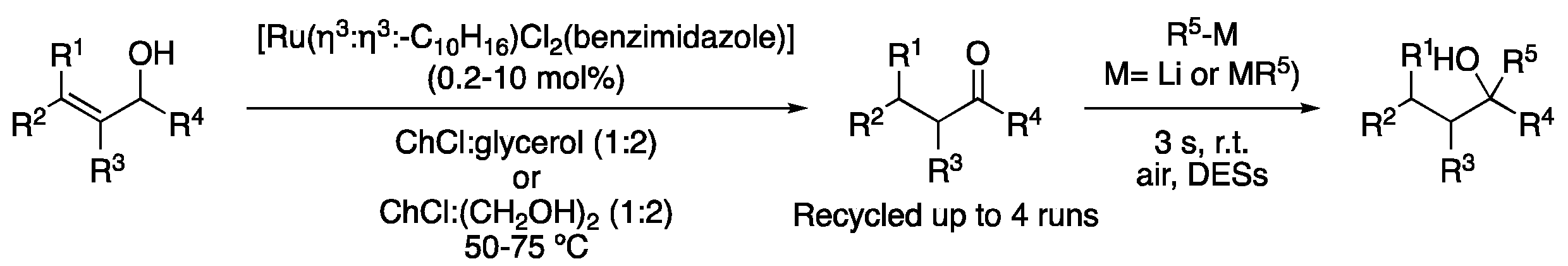
| 1 | Allylic alcohol isomerization | ChCl:glycerol (1:2) | [Ru], 50–75 °C | ketones | [107,108] |
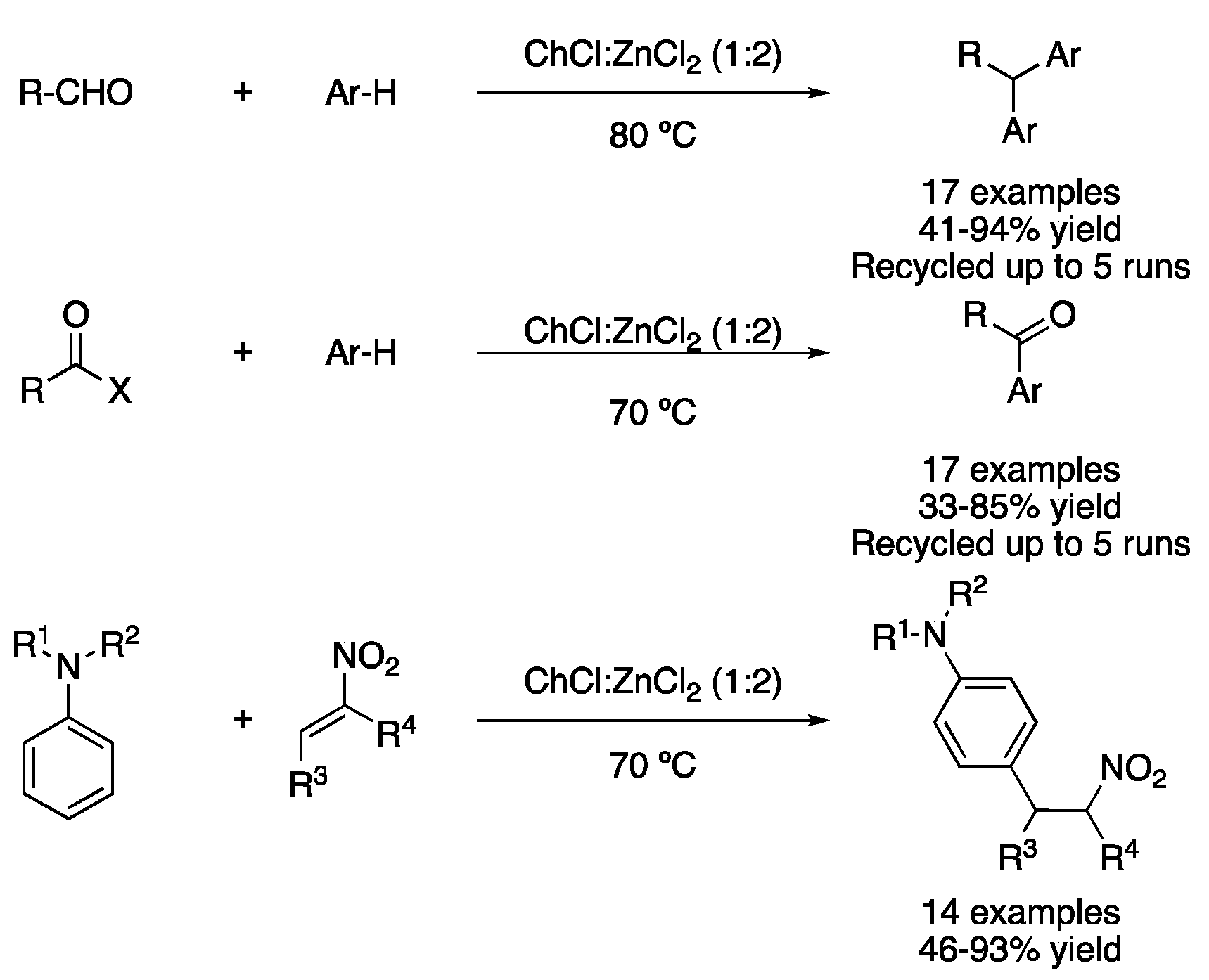
| 2 | Friedel–Crafts alkylation | ChCl:ZnCl2 (1:2) | 80 °C | Ar-R | [112] |
| 3 | Friedel–Crafts acylation | ChCl:ZnCl2 (1:2) | 70 °C | ArCOR | [113] |
| 4 | Friedel–Crafts alkylation | ChCl:ZnCl2 (1:2) | 70 °C | Alkylated anilines | [114] |
| 5 | Meyer–Schuster | FeCl3.6H2O:glycerol (3:1) | r.t. | Ketones/aldehydes | [115] |
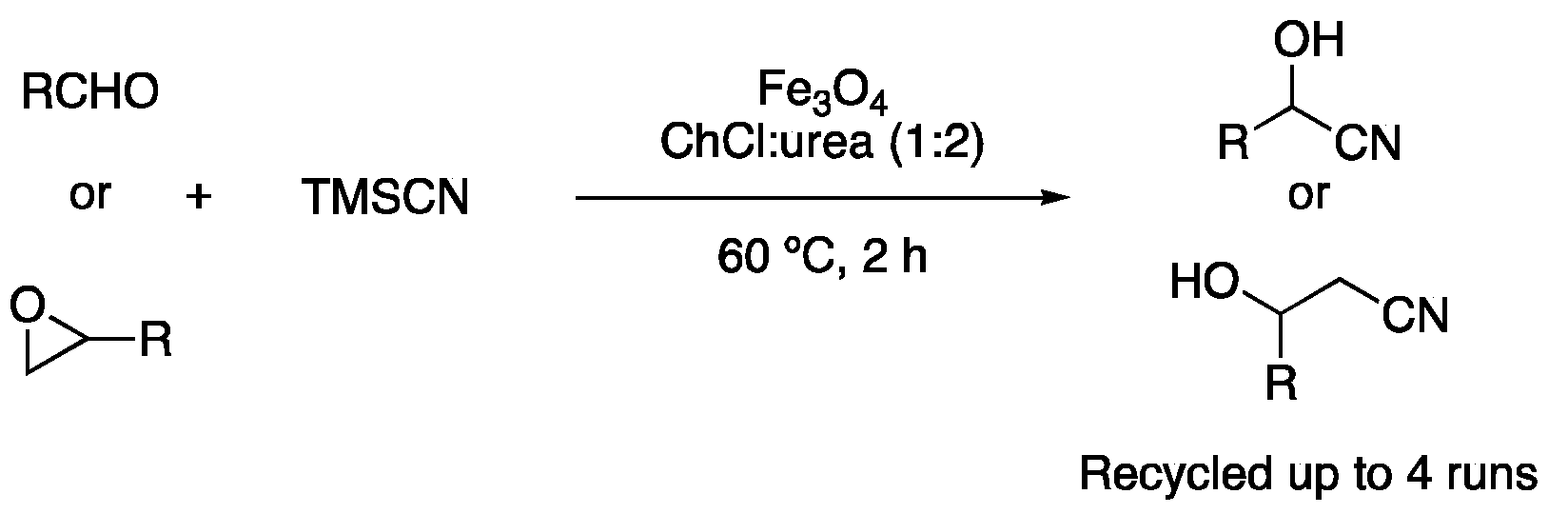
| 6 | Cyanosilylation | ChCl:urea (1:2) | [Fe], r.t. | cyanohydrins | [119] |
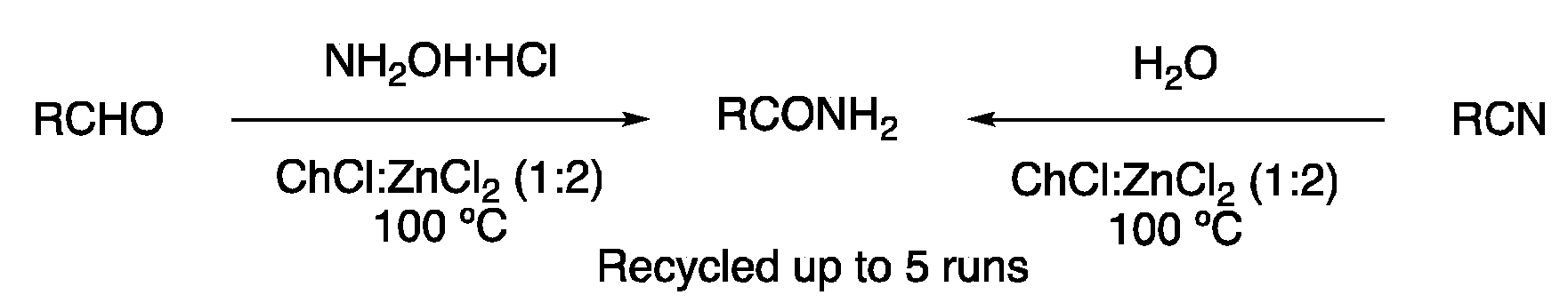
| 7 | Amide synthesis | ChCl:ZnCl2 (1:2) | 100 °C | Primary amides | [121] |
| 8 | Substitution | ChCl:ZnCl2 (1:2) | [Fe], 60–130 °C | Carbamates/ureas | [122,123,124] |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| acac | acetylacetonate |
| AcChCl | Acetylcholine chloride |
| API | Active Pharmaceutical Ingredient |
| β-CD | β-cyclodextrin |
| ChCl | Choline chloride |
| CPME | Cyclopentyl ethyl ether |
| CuAAC | Cu(I)-catalyzed azide–alkyne cycloaddition |
| DABCO | 1,4-Diazabicyclo[2.2.2]octane |
| dba | Dibenzylideneacetone |
| DCC | Dicyclohexylcarbodiimide |
| DES | Deep Eutectic Solvent |
| DFT | Density Functional Theory |
| DMF | Dimethyl formamide |
| DMU | 1,3-Dimethylurea |
| dppf | 1,1′-Ferrocenediyl-bis(diphenylphosphine) |
| GO | Graphene Oxide |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| HFIP | 1,1,1,3,3,3-Hexafluoro-2-propanol |
| HMF | 5-Hydroxymethyl furfural |
| MCR | Multicomponent Reaction |
| MW | microwave |
| NADES | Natural Deep Eutectic Solvent |
| NP | Nanoparticle |
| PEG | Polyethylene glycol |
| PivOH | Pivalic acid |
| TBAB | Tetrabuthyl ammonium bromide |
| TEM | Transition Electron Microscopy |
| TEMPO | 2,2,6,6-Tetramethylpiperidine 1-oxyl |
| TIPS | triisopropylsilane |
| TPPTS | Triphenylphosphine-3,3′,3′′-trisulfonic acid trisodium salt |
| VOC | Volatile Organic Compound |
| XPS | X-ray photoelectron spectroscopy |
References
- Constable, D.J.C.; Jiménez-González, C.; Henderson, R.K. Perspective on Solvent Use in the Pharmaceutical Industry. Org. Proc. Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Abranches, D.O.; Coutinho, J.A.P. Type V deep eutectic solvents: Design and applications. Curr. Opin. Green Sustain. Chem. 2022, 35, 100612. [Google Scholar] [CrossRef]
- Liu, F.; Xue, Z.; Zhao, X.; Mou, H.; He, J.; Mu, T. Catalytic deep eutectic solvents for highly efficient conversion of cellulose to gluconic acid with gluconic acid self-precipitation separation. Chem. Commun. 2018, 54, 6140–6143. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, H.; Si, Y.; Lyu, X.; Cheng, Y.; Zheng, L.; Wang, L.; Li, X. Conversion of Glucose to 5-Hydroxymethylfurfural in Deep Eutectic Solvent of Choline Chloride–Chromium Chloride. Ind. Eng. Chem. Res. 2022, 61, 7216–7224. [Google Scholar] [CrossRef]
- Dutta, A.; Garg, A.; Borah, J.; Borah, R.P.; Sarma, D. Deep eutectic solvent mediated controlled and selective oxidation of organic sulfides and hydroxylation of arylboronic acids. Curr. Res. Green Sustain. Chem. 2021, 4, 100107. [Google Scholar] [CrossRef]
- Cicco, L.; Roggio, M.; López-Aguilar, M.; Ramos-Martín, M.; Perna, F.M.; García-Álvarez, J.; Vitale, P.; Capriati, V. Selective Aerobic Oxidation of Alcohols in Low Melting Mixtures and Water and Use for Telescoped One-Pot Hybrid Reactions. ChemistryOpen 2022, 11, e202200160. [Google Scholar] [CrossRef]
- Feng, Y.; Yan, G.; Wang, T.; Jia, W.; Zeng, X.; Sperry, J.; Sun, Y.; Tang, X.; Lei, T.; Lin, L. Synthesis of MCM-41-Supported Metal Catalysts in Deep Eutectic Solvent for the Conversion of Carbohydrates into 5-Hydroxymethylfurfural. ChemSusChem 2019, 12, 978–982. [Google Scholar] [CrossRef]
- Zhao, M.-Y.; Zhu, J.-N.; Li, P.; Li, W.; Cai, T.; Cheng, F.-F.; Xiong, W.-W. Structural variation of transition metal–organic frameworks using deep eutectic solvents with different hydrogen bond donors. Dalton Trans. 2019, 48, 10199–10209. [Google Scholar] [CrossRef]
- Jérôme, F.; Ferreira, M.; Bricout, H.; Menuel, S.; Monflier, E.; Tilloy, S. Low melting mixtures based on β-cyclodextrin derivatives and N,N′-dimethylurea as solvents for sustainable catalytic processes. Green Chem. 2014, 16, 3876–3880. [Google Scholar] [CrossRef]
- Ferreira, M.; Jérôme, F.; Bricout, H.; Menuel, S.; Landy, D.; Fourmentin, S.; Tilloy, S.; Monflier, E. Rhodium catalyzed hydroformylation of 1-decene in low melting mixtures based on various cyclodextrins and N,N′-dimethylurea. Catal. Commun. 2015, 63, 62–65. [Google Scholar] [CrossRef]
- Esteban, J.; Warmeling, H.; Vorholt, A.J. Utilization of deep eutectic solvents based on choline chloride in the biphasic hydroformylation of 1-decene with rhodium complexes. Catal. Commun. 2019, 129, 105721. [Google Scholar] [CrossRef]
- Vitale, P.; Cicco, L.; Messa, F.; Perna, F.M.; Salomone, A.; Capriati, V. Streamlined Routes to Phenacyl Azides and 2,5-Diarylpyrazines Enabled by Deep Eutectic Solvents. Eur. J. Org. Chem. 2019, 2019, 5557–5562. [Google Scholar] [CrossRef]
- Garg, G.; Masdeu-Bultó, A.M.; Farfán, N.; Ordóñez-Hernández, J.; Gómez, M.; Medina-González, Y. Palladium Nanoparticles in Glycerol/Ionic Liquid/Carbon Dioxide Medium as Hydrogenation Catalysts. ACS Appl. Nano Mater. 2020, 3, 12240–12249. [Google Scholar] [CrossRef]
- Liu, C.; Wang, S.; Wang, B.; Song, G. Catalytic hydrogenolysis of castor seeds C-lignin in deep eutectic solvents. Ind. Crops Prod. 2021, 169, 113666. [Google Scholar] [CrossRef]
- Leal-Duaso, A.; Favier, I.; Pla, D.; Pires, E.; Gómez, M. Design of Glycerol-Based Solvents for the Immobilization of Palladium Nanocatalysts: A Hydrogenation Study. ACS Sustain. Chem. Eng. 2021, 9, 6875–6885. [Google Scholar] [CrossRef]
- Iwanow, M.; Finkelmeyer, J.; Söldner, A.; Kaiser, M.; Gärtner, T.; Sieber, V.; König, B. Preparation of Supported Palladium Catalysts using Deep Eutectic Solvents. Chem. A Eur. J. 2017, 23, 12467–12470. [Google Scholar] [CrossRef]
- Batarseh, C.; Levi-Zada, A.; Abu-Reziq, R. Preparation of catalytic deep eutectic solvent-based silica microreactors using a non-aqueous sol–gel route. J. Mater. Chem. A 2019, 7, 2242–2252. [Google Scholar] [CrossRef]
- Sadjadi, S.; Akbari, M.; Kahangi, F.G.; Heravi, M.M. Pd immobilized on the composite of halloysite and low-cost bio-based eutectic solvent-derived carbon: An efficient catalyst for hydrogenation in the presence of cyclodextrin. Appl. Clay Sci. 2020, 192, 105640. [Google Scholar] [CrossRef]
- O’Neill, M.; Raghuwanshi, V.S.; Wendt, R.; Wollgarten, M.; Hoell, A.; Rademann, K. Gold Nanoparticles in Novel Green Deep Eutectic Solvents: Self-Limited Growth, Self-Assembly & Catalytic Implications. Z. Für Phys. Chem. 2015, 229, 221–234. [Google Scholar]
- Messa, F.; Dilauro, G.; Paparella, A.N.; Silvestri, L.; Furlotti, G.; Iacoangeli, T.; Perrone, S.; Salomone, A. Deep eutectic solvents meet safe, scalable and sustainable hydrogenations enabled by aluminum powder and Pd/C. Green Chem. 2022, 24, 4388–4394. [Google Scholar] [CrossRef]
- Cavallo, M.; Arnodo, D.; Mannu, A.; Blangetti, M.; Prandi, C.; Baratta, W.; Baldino, S. Deep eutectic solvents as H2-sources for Ru(II)-catalyzed transfer hydrogenation of carbonyl compounds under mild conditions. Tetrahedron 2021, 83, 131997. [Google Scholar] [CrossRef]
- Rodríguez-Álvarez, M.J.; Vidal, C.; Díez, J.; García-Álvarez, J. Introducing deep eutectic solvents as biorenewable media for Au(i)-catalysed cycloisomerisation of γ-alkynoic acids: An unprecedented catalytic system. Chem. Commun. 2014, 50, 12927–12929. [Google Scholar] [CrossRef]
- Vidal, C.; Merz, L.; García-Álvarez, J. Deep eutectic solvents: Biorenewable reaction media for Au(i)-catalysed cycloisomerisations and one-pot tandem cycloisomerisation/Diels–Alder reactions. Green Chem. 2015, 17, 3870–3878. [Google Scholar] [CrossRef]
- Rodríguez-Álvarez, M.J.; Vidal, C.; Schumacher, S.; Borge, J.; García-Álvarez, J. AuI Iminophosphorane Complexes as Efficient Catalysts for the Cycloisomerization of Alkynyl Amides under Air, at Room Temperature, and in Aqueous or Eutectic-Mixture Solutions. Chem. A Eur. J. 2017, 23, 3425–3431. [Google Scholar] [CrossRef]
- Saavedra, B.; Pérez, J.M.; Rodríguez-Álvarez, M.J.; García-Álvarez, J.; Ramón, D.J. Impregnated palladium on magnetite as a water compatible catalyst for the cycloisomerization of alkynoic acid derivatives. Green Chem. 2018, 20, 2151–2157. [Google Scholar] [CrossRef]
- Padvi, S.A.; Dalal, D.S. Choline chloride–ZnCl2: Recyclable and efficient deep eutectic solvent for the [2+3] cycloaddition reaction of organic nitriles with sodium azide. Synth. Commun. 2017, 47, 779–787. [Google Scholar] [CrossRef]
- Xiong, X.; Yi, C.; Liao, X.; Lai, S. A practical multigram-scale method for the green synthesis of 5-substituted-1H-tetrazoles in deep eutectic solvent. Tetrahedron Lett. 2019, 60, 402–406. [Google Scholar] [CrossRef]
- Kafle, A.; Handy, S.T. A one-pot, copper-catalyzed azidation/click reaction of aryl and heteroaryl bromides in an environmentally friendly deep eutectic solvent. Tetrahedron 2017, 73, 7024–7029. [Google Scholar] [CrossRef]
- Giofrè, S.V.; Tiecco, M.; Ferlazzo, A.; Romeo, R.; Ciancaleoni, G.; Germani, R.; Iannazzo, D. Base-Free Copper-Catalyzed Azide-Alkyne Click Cycloadditions (CuAAc) in Natural Deep Eutectic Solvents as Green and Catalytic Reaction Media**. Eur. J. Org. Chem. 2021, 2021, 4777–4789. [Google Scholar] [CrossRef]
- Mirshafiee, S.; Salamatmanesh, A.; Heydari, A. A sustainable approach for efficient one-pot synthesis of 1-aryl 1,2,3-triazoles using copper iodide supported on 3-thionicotinyl-urea-modified magnetic nanoparticles in DES. Appl. Organomet. Chem. 2021, 35, e6255. [Google Scholar] [CrossRef]
- Lu, J.; Li, X.-T.; Ma, E.-Q.; Mo, L.-P.; Zhang, Z.-H. Superparamagnetic CuFeO2 Nanoparticles in Deep Eutectic Solvent: An Efficient and Recyclable Catalytic System for the Synthesis of Imidazo[1,2-a]pyridines. ChemCatChem 2014, 6, 2854–2859. [Google Scholar] [CrossRef]
- Salamatmanesh, A.; Heydari, A. Magnetic nanostructure-anchored mixed-donor ligand system based on carboxamide and N-heterocyclic thiones: An efficient support of CuI catalyst for synthesis of imidazo[1,2-a]pyridines in eutectic medium. Appl. Catal. A: Gen. 2021, 624, 118306. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.-H.; Shang, Z.-R.; Hu, H.-C.; Zhang, Z.-H. Supported molybdenum on graphene oxide/Fe3O4: An efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal. Commun. 2017, 88, 39–44. [Google Scholar] [CrossRef]
- Maleki, A.; Kari, T.; Aghaei, M. Fe3O4@SiO2@TiO2-OSO3H: An efficient hierarchical nanocatalyst for the organic quinazolines syntheses. J. Porous Mater. 2017, 24, 1481–1496. [Google Scholar] [CrossRef]
- Maleki, A.; Aghaei, M.; Kari, T. Facile Synthesis of 7-Aryl-benzo[h]tetrazolo[5,1-b]quinazoline-5,6-dione Fused Polycyclic Compounds by Using a Novel Magnetic Polyurethane Catalyst. Polycycl. Aromat. Compd. 2019, 39, 266–278. [Google Scholar] [CrossRef]
- Akbarian, M.; Sanchooli, E.; Oveisi, A.R.; Daliran, S. Choline chloride-coated UiO-66-Urea MOF: A novel multifunctional heterogeneous catalyst for efficient one-pot three-component synthesis of 2-amino-4H-chromenes. J. Mol. Liq. 2021, 325, 115228. [Google Scholar] [CrossRef]
- Mancuso, R.; Maner, A.; Cicco, L.; Perna, F.M.; Capriati, V.; Gabriele, B. Synthesis of thiophenes in a deep eutectic solvent: Heterocyclodehydration and iodocyclization of 1-mercapto-3-yn-2-ols in a choline chloride/glycerol medium. Tetrahedron 2016, 72, 4239–4244. [Google Scholar] [CrossRef]
- Peñaranda Gómez, A.; Rodríguez Bejarano, O.; Kouznetsov, V.V.; Ochoa-Puentes, C. One-Pot Diastereoselective Synthesis of Tetrahydroquinolines from Star Anise Oil in a Choline Chloride/Zinc Chloride Eutectic Mixture. ACS Sustain. Chem. Eng. 2019, 7, 18630–18639. [Google Scholar] [CrossRef]
- Damghani, K.F.; Kiyani, H.; Pourmousavi, A.S. Green Three-component Synthesis of Merocyanin Dyes Based on 4-Arylideneisoxazol-5(4H)-ones. Curr. Green Chem. 2020, 7, 217–225. [Google Scholar] [CrossRef]
- Higuera, N.L.; Peña-Solórzano, D.; Ochoa-Puentes, C. Urea–Zinc Chloride Eutectic Mixture-Mediated One-Pot Synthesis of Imidazoles: Efficient and Ecofriendly Access to Trifenagrel. Synlett 2019, 30, 225–229. [Google Scholar]
- Aghapoor, K.; Mohsenzadeh, F.; Darabi, H.R.; Sayahi, H.; Jalali, M.R. ZnCl2/Urea Eutectic Solvent as Stable Carbonylation Source for Benign Synthesis of 2–Benzimidazolones and 2–Imidazolones: An Effective Strategy for Preventing NH3 Gas Evolution. ChemistrySelect 2019, 4, 11093–11097. [Google Scholar] [CrossRef]
- Shaibuna, M.; Abbas, A.; Kariyottu Kuniyil, M.J.; Sreekumar, K. Sustainable synthesis of 1,8-dioxooctahydroxanthenes in deep eutectic solvents (DESs). New J. Chem. 2021, 45, 8335–8344. [Google Scholar] [CrossRef]
- Shaibuna, M.; Hiba, K.; Shebitha, A.M.; Kariyottu Kuniyil, M.J.; Sherly mole, P.B.; Sreekumar, K. Sustainable and selective synthesis of benzimidazole scaffolds using deep eutectic solvents. Curr. Res. Green Sustain. Chem. 2022, 5, 100285. [Google Scholar] [CrossRef]
- Imperato, G.; Vasold, R.; König, B. Stille Reactions with Tetraalkylstannanes and Phenyltrialkylstannanes in Low Melting Sugar-Urea-Salt Mixtures. Adv. Synth. Catal. 2006, 348, 2243–2247. [Google Scholar] [CrossRef]
- Imperato, G.; Höger, S.; Lenoir, D.; König, B. Low melting sugar–urea–salt mixtures as solvents for organic reactions—Estimation of polarity and use in catalysis. Green Chem. 2006, 8, 1051–1055. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Lu, M. β-cyclodextrin-capped palladium nanoparticle-catalyzed ligand-free Suzuki and Heck couplings in low-melting β-cyclodextrin/NMU mixtures. Appl. Organomet. Chem. 2014, 28, 635–640. [Google Scholar] [CrossRef]
- Marset, X.; Khoshnood, A.; Sotorrios, L.; Gomez-Bengoa, E.; Alonso, D.A.; Ramón, D.J. Deep Eutectic Solvent Compatible Metallic Catalysts: Cationic Pyridiniophosphine Ligands in Palladium Catalyzed Cross-Coupling Reactions. ChemCatChem 2017, 9, 1269–1275. [Google Scholar] [CrossRef]
- Paris, J.; Ríos-Lombardía, N.; Morís, F.; Gröger, H.; González-Sabín, J. Novel Insights into the Combination of Metal- and Biocatalysis: Cascade One-Pot Synthesis of Enantiomerically Pure Biaryl Alcohols in Deep Eutectic Solvents. ChemCatChem 2018, 10, 4417–4423. [Google Scholar] [CrossRef]
- Paris, J.; Telzerow, A.; Ríos-Lombardía, N.; Steiner, K.; Schwab, H.; Morís, F.; Gröger, H.; González-Sabín, J. Enantioselective One-Pot Synthesis of Biaryl-Substituted Amines by Combining Palladium and Enzyme Catalysis in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 5486–5493. [Google Scholar] [CrossRef]
- Dilauro, G.; García, S.M.; Tagarelli, D.; Vitale, P.; Perna, F.M.; Capriati, V. Ligand-Free Bioinspired Suzuki–Miyaura Coupling Reactions using Aryltrifluoroborates as Effective Partners in Deep Eutectic Solvents. ChemSusChem 2018, 11, 3495–3501. [Google Scholar] [CrossRef]
- Delaye, P.-O.; Pénichon, M.; Boudesocque-Delaye, L.; Enguehard-Gueiffier, C.; Gueiffier, A. Natural Deep Eutectic Solvents as Sustainable Solvents for Suzuki –Miyaura Cross-Coupling Reactions Applied to Imidazo-Fused Heterocycles. SynOpen 2018, 02, 0306–0311. [Google Scholar] [CrossRef]
- Saavedra, B.; González-Gallardo, N.; Meli, A.; Ramón, D.J. A Bipyridine-Palladium Derivative as General Pre-Catalyst for Cross-Coupling Reactions in Deep Eutectic Solvents. Adv. Synth. Catal. 2019, 361, 3868–3879. [Google Scholar] [CrossRef]
- Marset, X.; Saavedra, B.; González-Gallardo, N.; Beaton, A.; León, M.M.; Luna, R.; Ramón, D.J.; Guillena, G. Palladium Mesoionic Carbene Pre-catalyst for General Cross-Coupling Transformations in Deep Eutectic Solvents. Front. Chem. 2019, 7, 700. [Google Scholar] [CrossRef]
- Ghinato, S.; Dilauro, G.; Perna, F.M.; Capriati, V.; Blangetti, M.; Prandi, C. Directed ortho-metalation–nucleophilic acyl substitution strategies in deep eutectic solvents: The organolithium base dictates the chemoselectivity. Chem. Commun. 2019, 55, 7741–7744. [Google Scholar] [CrossRef]
- Pelliccioli, V.; Dilauro, G.; Grecchi, S.; Arnaboldi, S.; Graiff, C.; Perna, F.M.; Vitale, P.; Licandro, E.; Aliprandi, A.; Cauteruccio, S.; et al. Ligand-Free Suzuki–Miyaura Cross-Coupling Reactions in Deep Eutectic Solvents: Synthesis of Benzodithiophene Derivatives and Study of their Optical and Electrochemical Performance. Eur. J. Org. Chem. 2020, 2020, 6981–6988. [Google Scholar] [CrossRef]
- Krishna, T.; Reddy, T.; Eppakayala, L.; Kalita, D. Nickel-catalyzed suzuki-miyaura cross- coupling reactions: One-pot synthesis of 2-arylthiophenes. Rasayan J. Chem. 2020, 13, 2438–2444. [Google Scholar] [CrossRef]
- Ilgen, F.; König, B. Organic reactions in low melting mixtures based on carbohydrates and l-carnitine—A comparison. Green Chem. 2009, 11, 848–854. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Zhu, T. Various morphologies of hydrogen-substituted graphynes: The importance of reaction solvents. J. Mol. Liq. 2019, 296, 111958. [Google Scholar] [CrossRef]
- Thiyagamurthy, P.; Khan, F.R.N. A Base-Free Pd-Precatalyst Mediated Suzuki-Miyaura and Sonogashira Cross-Coupling in Deep Eutectic Solvents. ChemistrySelect 2020, 5, 2610–2617. [Google Scholar] [CrossRef]
- Mancuso, R.; Lettieri, M.; Strangis, R.; Russo, P.; Piccionello, A.P.; De Angelis, S.; Gabriele, B. Iodocyclization of 2-Methylthiophenylacetylenes to 3-Iodobenzothiophenes and their coupling Reactions under More Sustainable Conditions. Asian J. Org. Chem. 2022, 11, e202200353. [Google Scholar] [CrossRef]
- Ríos-Lombardía, N.; Rodríguez-Álvarez, M.J.; Morís, F.; Kourist, R.; Comino, N.; López-Gallego, F.; González-Sabín, J.; García-Álvarez, J. DESign of Sustainable One-Pot Chemoenzymatic Organic Transformations in Deep Eutectic Solvents for the Synthesis of 1,2-Disubstituted Aromatic Olefins. Front. Chem. 2020, 8, 139. [Google Scholar] [CrossRef]
- Heidari, B.; Heravi, M.M.; Nabid, M.R.; Sedghi, R.; Hooshmand, S.E. Novel palladium nanoparticles supported on β-cyclodextrin@graphene oxide as magnetically recyclable catalyst for Suzuki–Miyaura cross-coupling reaction with two different approaches in bio-based solvents. Appl. Organomet. Chem. 2019, 33, e4632. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mruthunjayappa, M.H.; Aruchamy, K.; Singh, N.; Prasad, K.; Kalpana, D.; Ghosh, D.; Sanna Kotrappanavar, N.; Mondal, D. Facile Process for Metallizing DNA in a Multitasking Deep Eutectic Solvent for Ecofriendly C–C Coupling Reaction and Nitrobenzene Reduction. ACS Sustain. Chem. Eng. 2019, 7, 14225–14235. [Google Scholar] [CrossRef]
- Salamatmanesh, A.; Heydari, A.; Nahzomi, H.T. Stabilizing Pd on magnetic phosphine-functionalized cellulose: DFT study and catalytic performance under deep eutectic solvent assisted conditions. Carbohydr. Polym. 2020, 235, 115947. [Google Scholar] [CrossRef]
- Niakan, M.; Masteri-Farahani, M.; Karimi, S.; Shekaari, H. Hydrophilic role of deep eutectic solvents for clean synthesis of biphenyls over a magnetically separable Pd-catalyzed Suzuki-Miyaura coupling reaction. J. Mol. Liq. 2021, 324, 115078. [Google Scholar] [CrossRef]
- Niakan, M.; Masteri-Farahani, M. An efficient clean and sustainable methodology for catalytic C-C coupling process over a Pd-free magnetically recoverable cobalt catalyst. J. Mol. Liq. 2022, 355, 118932. [Google Scholar] [CrossRef]
- Messa, F.; Dilauro, G.; Perna, F.M.; Vitale, P.; Capriati, V.; Salomone, A. Sustainable Ligand-Free Heterogeneous Palladium-Catalyzed Sonogashira Cross-Coupling Reaction in Deep Eutectic Solvents. ChemCatChem 2020, 12, 1979–1984. [Google Scholar] [CrossRef]
- Niakan, M.; Masteri-Farahani, M.; Shekaari, H.; Karimi, S. Pd supported on clicked cellulose-modified magnetite-graphene oxide nanocomposite for C-C coupling reactions in deep eutectic solvent. Carbohydr. Polym. 2021, 251, 117109. [Google Scholar] [CrossRef]
- Leal-Duaso, A.; Mayoral, J.A.; Pires, E. Steps Forward toward the Substitution of Conventional Solvents in the Heck–Mizoroki Coupling Reaction: Glycerol-Derived Ethers and Deep Eutectic Solvents as Reaction Media. ACS Sustain. Chem. Eng. 2020, 8, 13076–13084. [Google Scholar] [CrossRef]
- Dang, M.-H.D.; Nguyen, T.T.T.; Le, B.Q.G.; Nguyen, L.H.T.; Mai, N.X.D.; Nguyen, M.V.; Tran, P.H.; Doan, T.L.H. An effective combination of reusable Pd@MOF catalyst and deep eutectic solvents for high-performance C–C coupling reaction. J. Ind. Eng. Chem. 2022, 111, 111–120. [Google Scholar] [CrossRef]
- Afshari, R.; Emad Hooshmand, S.; Atharnezhad, M.; Shaabani, A. An insight into the novel covalent functionalization of multi-wall carbon nanotubes with pseudopeptide backbones for palladium nanoparticles immobilization: A versatile catalyst towards diverse cross-coupling reactions in bio-based solvents. Polyhedron 2020, 175, 114238. [Google Scholar] [CrossRef]
- Paparella, A.N.; Messa, F.; Dilauro, G.; Troisi, L.; Perrone, S.; Salomone, A. A Glycerol-Based Deep Eutectic Solvent as Natural Medium and Organic Reductant for Homocoupling of (Hetero)Aryl Chlorides: A Green Route to 2,2′-Bipyridine and Biaryl Scaffolds. ChemistrySelect 2022, 7, e202203438. [Google Scholar] [CrossRef]
- Marset, X.; De Gea, S.; Guillena, G.; Ramón, D.J. NCN–Pincer–Pd Complex as Catalyst for the Hiyama Reaction in Biomass-Derived Solvents. ACS Sustain. Chem. Eng. 2018, 6, 5743–5748. [Google Scholar] [CrossRef]
- Dilauro, G.; Azzollini, C.S.; Vitale, P.; Salomone, A.; Perna, F.M.; Capriati, V. Scalable Negishi Coupling between Organozinc Compounds and (Hetero)Aryl Bromides under Aerobic Conditions when using Bulk Water or Deep Eutectic Solvents with no Additional Ligands. Angew. Chem. Int. Ed. 2021, 60, 10632–10636. [Google Scholar] [CrossRef]
- Quivelli, A.F.; Vitale, P.; Perna, F.M.; Capriati, V. Reshaping Ullmann Amine Synthesis in Deep Eutectic Solvents: A Mild Approach for Cu-Catalyzed C–N Coupling Reactions With No Additional Ligands. Front. Chem. 2019, 7, 723. [Google Scholar] [CrossRef]
- Cicco, L.; Hernández-Fernández, J.A.; Salomone, A.; Vitale, P.; Ramos-Martín, M.; González-Sabín, J.; Presa Soto, A.; Perna, F.M.; Capriati, V.; García-Álvarez, J. Copper-catalyzed Goldberg-type C–N coupling in deep eutectic solvents (DESs) and water under aerobic conditions. Org. Biomol. Chem. 2021, 19, 1773–1779. [Google Scholar] [CrossRef]
- Quivelli, A.F.; Rossi, F.V.; Vitale, P.; García-Álvarez, J.; Perna, F.M.; Capriati, V. Sustainable and Scalable Two-Step Synthesis of Thenfadil and Some Analogs in Deep Eutectic Solvents: From Laboratory to Industry. ACS Sustain. Chem. Eng. 2022, 10, 4065–4072. [Google Scholar] [CrossRef]
- Shaabani, A.; Afshari, R. Magnetic Ugi-functionalized graphene oxide complexed with copper nanoparticles: Efficient catalyst toward Ullman coupling reaction in deep eutectic solvents. J. Colloid Interface Sci. 2018, 510, 384–394. [Google Scholar] [CrossRef]
- Bagheri, S.; Pazoki, F.; Radfar, I.; Heydari, A. Copper(I)–creatine complex on magnetic nanoparticles as a green catalyst for N- and O-arylation in deep eutectic solvent. Appl. Organomet. Chem. 2020, 34, e5447. [Google Scholar] [CrossRef]
- Pazoki, F.; Heydari, A. Magnetic acyclovir-copper nanoparticle: DFT study and application as an efficient, magnetically separable and recyclable catalyst for N-arylation of amines under green condition. Inorg. Chem. Commun. 2020, 122, 108240. [Google Scholar] [CrossRef]
- Bagheri, S.; Pazoki, F.; Esfandiary, N.; Fadaei, M.M.; Heydari, A. Synthesis and characterization of Pd(II)–vitamin B6 complex supported on magnetic nanoparticle as an efficient and recyclable catalyst system for C–N cross coupling of amides in deep eutectic solvents. Appl. Organomet. Chem. 2020, 34, e5723. [Google Scholar] [CrossRef]
- Quivelli, A.F.; Marinò, M.; Vitale, P.; García-Álvarez, J.; Perna, F.M.; Capriati, V. Ligand-Free Copper-Catalyzed Ullmann-Type C−O Bond Formation in Non-Innocent Deep Eutectic Solvents under Aerobic Conditions. ChemSusChem 2022, 15, e202102211. [Google Scholar] [CrossRef]
- Niakan, M.; Karimi, S.; Masteri-Farahani, M.; Shekaari, H. An efficient, cost-effective, and magnetically recoverable copper catalyst for O-arylation of phenols with aryl halides in choline chloride-based deep eutectic solvents. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 620, 126603. [Google Scholar] [CrossRef]
- Marset, X.; Pérez, J.M.; Ramón, D.J. Cross-dehydrogenative coupling reaction using copper oxide impregnated on magnetite in deep eutectic solvents. Green Chem. 2016, 18, 826–833. [Google Scholar] [CrossRef]
- Teja, C.; Nawaz Khan, F.R. Choline Chloride-Based Deep Eutectic Systems in Sequential Friedländer Reaction and Palladium-Catalyzed sp3 CH Functionalization of Methyl Ketones. ACS Omega 2019, 4, 8046–8055. [Google Scholar] [CrossRef]
- Prameela, S.; Nawaz Khan, F.-R. Ir(I)-Catalyzed Synthesis of (E)-4-Benzylidenylacridines and (E)-2-Styrylquinoline-3-carboxamide through Sequential Suzuki–Miyaura Coupling, Dehydrogenative Friedländer Reaction, and sp3-C–H Activation. Eur. J. Org. Chem. 2020, 2020, 5394–5410. [Google Scholar] [CrossRef]
- Pavithra, D.; Ethiraj, K.R.; Nawaz Khan, F.-R. Cu-TEMPO Catalyzed Dehydrogenative Friedlander Annulation/sp3 C–H Functionalization/Spiroannulation towards Spiro[indoline-3,3′-pyrrolizin]-2′-yl)-4-phenylquinoline-3-Carboxylates. Eur. J. Org. Chem. 2020, 2020, 7035–7050. [Google Scholar] [CrossRef]
- Punzi, A.; Coppi, D.I.; Matera, S.; Capozzi, M.A.M.; Operamolla, A.; Ragni, R.; Babudri, F.; Farinola, G.M. Pd-Catalyzed Thiophene–Aryl Coupling Reaction via C–H Bond Activation in Deep Eutectic Solvents. Org. Lett. 2017, 19, 4754–4757. [Google Scholar] [CrossRef]
- Shariatipour, M.; Salamatmanesh, A.; Jadidi Nejad, M.; Heydari, A. Imidazole-aryl coupling reaction via CH bond activation catalyzed by palladium supported on modified magnetic reduced graphene oxide in alkaline deep eutectic solvent. Catal. Commun. 2020, 135, 105890. [Google Scholar] [CrossRef]
- D’Amico, F.; Papucci, C.; Franchi, D.; Reginato, G.; Calamante, M.; Zani, L.; Dessì, A.; Mordini, A. Sustainable Pd-Catalyzed Direct Arylation of Thienyl Derivatives with (Hetero)aromatic Bromides under Air in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2022, 10, 3037–3047. [Google Scholar] [CrossRef]
- González-Gallardo, N.; Saavedra, B.; Guillena, G.; Ramón, D.J. A jackpot C–H activation protocol using simple ruthenium catalyst in deep eutectic solvents. Green Chem. 2022, 24, 4941–4951. [Google Scholar] [CrossRef]
- Marset, X. Palladium-catalysed Csp3–H functionalisation of unactivated 8-aminoquinoline amides in deep eutectic solvents. Org. Biomol. Chem. 2022, 20, 7071–7075. [Google Scholar] [CrossRef]
- Disale, S.T.; Kale, S.R.; Kahandal, S.S.; Srinivasan, T.G.; Jayaram, R.V. Choline chloride·2ZnCl2 ionic liquid: An efficient and reusable catalyst for the solvent free Kabachnik–Fields reaction. Tetrahedron Lett. 2012, 53, 2277–2279. [Google Scholar] [CrossRef]
- Keshavarzipour, F.; Tavakol, H. Deep Eutectic Solvent as a Recyclable Catalyst for Three-Component Synthesis of β-Amino Carbonyls. Catal. Lett. 2015, 145, 1062–1066. [Google Scholar] [CrossRef]
- Azizi, N.; Edrisi, M. Deep eutectic solvent immobilized on SBA-15 as a novel separable catalyst for one-pot three-component Mannich reaction. Microporous Mesoporous Mater. 2017, 240, 130–136. [Google Scholar] [CrossRef]
- Tran, M.-N.T.; Nguyen, X.-T.T.; Nguyen, H.T.; Chau, D.-K.N.; Tran, P.H. Deep eutectic solvent: An efficient and green catalyst for the three-component condensation of indoles, aromatic aldehydes, and activated methylene compounds. Tetrahedron Lett. 2020, 61, 151481. [Google Scholar] [CrossRef]
- Obst, M.; Srivastava, A.; Baskaran, S.; König, B. Preparation of Propargyl Amines in a ZnCl2–Dimethylurea Deep-Eutectic Solvent. Synlett 2018, 29, 185–188. [Google Scholar] [CrossRef]
- Abtahi, B.; Tavakol, H. Choline chloride-urea deep eutectic solvent as an efficient media for the synthesis of propargylamines via organocuprate intermediate. Appl. Organomet. Chem. 2020, 34, e5895. [Google Scholar] [CrossRef]
- Abtahi, B.; Tavakol, H. CuI-catalyzed, one-pot synthesis of 3-aminobenzofurans in deep eutectic solvents. Appl. Organomet. Chem. 2021, 35, e6433. [Google Scholar] [CrossRef]
- Brambilla, E.; Bortolla, A.; Pirovano, V.; Caselli, A.; Tiecco, M.; Abbiati, G. Silver-catalysed A3-coupling reactions in phenylacetic acid/alkylamine N-oxide eutectic mixture under dielectric heating: An alternative approach to propargylamines. Appl. Organomet. Chem. 2022, 36, e6669. [Google Scholar] [CrossRef]
- Shaibuna, M.; Sreekumar, K. Experimental Investigation on the Correlation between the Physicochemical Properties and Catalytic Activity of Six DESs in the Kabachnik-Fields Reaction. ChemistrySelect 2020, 5, 13454–13460. [Google Scholar] [CrossRef]
- Marset, X.; Guillena, G.; Ramón, D.J. Deep Eutectic Solvents as Reaction Media for the Palladium-Catalysed C−S Bond Formation: Scope and Mechanistic Studies. Chem. A Eur. J. 2017, 23, 10522–10526. [Google Scholar] [CrossRef]
- Marset, X.; Torregrosa-Crespo, J.; Martínez-Espinosa, R.M.; Guillena, G.; Ramón, D.J. Multicomponent synthesis of sulfonamides from triarylbismuthines, nitro compounds and sodium metabisulfite in deep eutectic solvents. Green Chem. 2019, 21, 4127–4132. [Google Scholar] [CrossRef]
- Messa, F.; Perrone, S.; Capua, M.; Tolomeo, F.; Troisi, L.; Capriati, V.; Salomone, A. Towards a sustainable synthesis of amides: Chemoselective palladium-catalysed aminocarbonylation of aryl iodides in deep eutectic solvents. Chem. Commun. 2018, 54, 8100–8103. [Google Scholar] [CrossRef]
- Saavedra, B.; Ramón, D.J. Deep Eutectic Solvent as a Sustainable Medium for C–C Bond Formation Via Multicomponent Radical Conjugate Additions. ACS Sustain. Chem. Eng. 2021, 9, 7941–7947. [Google Scholar] [CrossRef]
- Vidal, C.; Suárez, F.J.; García-Álvarez, J. Deep eutectic solvents (DES) as green reaction media for the redox isomerization of allylic alcohols into carbonyl compounds catalyzed by the ruthenium complex [Ru(η3:η3-C10H16)Cl2(benzimidazole)]. Catal. Commun. 2014, 44, 76–79. [Google Scholar] [CrossRef]
- Cicco, L.; Rodríguez-Álvarez, M.J.; Perna, F.M.; García-Álvarez, J.; Capriati, V. One-pot sustainable synthesis of tertiary alcohols by combining ruthenium-catalysed isomerisation of allylic alcohols and chemoselective addition of polar organometallic reagents in deep eutectic solvents. Green Chem. 2017, 19, 3069–3077. [Google Scholar] [CrossRef]
- Dilauro, G.; Francesca Quivelli, A.; Vitale, P.; Capriati, V.; Perna, F.M. Water and Sodium Chloride: Essential Ingredients for Robust and Fast Pd-Catalysed Cross-Coupling Reactions between Organolithium Reagents and (Hetero)aryl Halides. Angew. Chem. Int. Ed. 2019, 58, 1799–1802. [Google Scholar] [CrossRef]
- Cicco, L.; Ríos-Lombardía, N.; Rodríguez-Álvarez, M.J.; Moris, F.; Perna, F.M.; Capriati, V.; García-Álvarez, J.; González-Sabín, J. Programming cascade reactions interfacing biocatalysis with transition-metal catalysis in Deep Eutectic Solvents as biorenewable reaction media. Green Chem. 2018, 20, 3468–3475. [Google Scholar] [CrossRef]
- Wang, J.; Han, J.; Khan, M.Y.; He, D.; Peng, H.; Chen, D.; Xie, X.; Xue, Z. Deep eutectic solvents for green and efficient iron-mediated ligand-free atom transfer radical polymerization. Polym. Chem. 2017, 8, 1616–1627. [Google Scholar] [CrossRef]
- Wang, A.; Xing, P.; Zheng, X.; Cao, H.; Yang, G.; Zheng, X. Deep eutectic solvent catalyzed Friedel–Crafts alkylation of electron-rich arenes with aldehydes. RSC Adv. 2015, 5, 59022–59026. [Google Scholar] [CrossRef]
- Jin, X.; Wang, A.; Cao, H.; Zhang, S.; Wang, L.; Zheng, X.; Zheng, X. A new efficient method for the preparation of intermediate aromatic ketones by Friedel–Crafts acylation. Res. Chem. Intermed. 2018, 44, 5521–5530. [Google Scholar] [CrossRef]
- Ranjbari, M.A.; Tavakol, H.; Manoukian, M. Regioselective and solvent-free arylation of β-nitrostyrenes with mono- and dialkyl anilines. Res. Chem. Intermed. 2021, 47, 709–721. [Google Scholar] [CrossRef]
- Ríos-Lombardía, N.; Cicco, L.; Yamamoto, K.; Hernández-Fernández, J.A.; Morís, F.; Capriati, V.; García-Álvarez, J.; González-Sabín, J. Deep eutectic solvent-catalyzed Meyer–Schuster rearrangement of propargylic alcohols under mild and bench reaction conditions. Chem. Commun. 2020, 56, 15165–15168. [Google Scholar] [CrossRef]
- Rokade, S.M.; Bhate, P.M. One-pot synthesis of per-O-acetylated hemiacetals from free sugars in a deep eutectic solvent. Carbohydr. Res. 2015, 416, 21–23. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Yang, Q.; Chen, L.; Deng, J.; Chen, W.; Lian, H.; Mota-Morales, J.D.; Liimatainen, H. Choline chloride-zinc chloride deep eutectic solvent mediated preparation of partial O-acetylation of chitin nanocrystal in one step reaction. Carbohydr. Polym. 2019, 220, 211–218. [Google Scholar] [CrossRef]
- Cao, J.; Qi, B.; Liu, J.; Shang, Y.; Liu, H.; Wang, W.; Lv, J.; Chen, Z.; Zhang, H.; Zhou, X. Deep eutectic solvent choline chloride·2CrCl3·6H2O: An efficient catalyst for esterification of formic and acetic acid at room temperature. RSC Adv. 2016, 6, 21612–21616. [Google Scholar] [CrossRef]
- Azizi, N.; Rahimi, Z.; Alipour, M. A magnetic nanoparticle catalyzed eco-friendly synthesis of cyanohydrins in a deep eutectic solvent. RSC Adv. 2015, 5, 61191–61198. [Google Scholar] [CrossRef]
- Patil, U.B.; Singh, A.S.; Nagarkar, J.M. Choline chloride based eutectic solvent: An efficient and reusable solvent system for the synthesis of primary amides from aldehydes and from nitriles. RSC Adv. 2014, 4, 1102–1106. [Google Scholar] [CrossRef]
- Azizi, N.; Edrisi, M.; Abbasi, F. Mesoporous silica SBA-15 functionalized with acidic deep eutectic solvent: A highly active heterogeneous N-formylation catalyst under solvent-free conditions. Appl. Organomet. Chem. 2018, 32, e3901. [Google Scholar] [CrossRef]
- Dindarloo Inaloo, I.; Majnooni, S. Deep Eutectic Solvents (DES) as Green and Efficient Solvent/Catalyst Systems for the Synthesis of Carbamates and Ureas from Carbonates. ChemistrySelect 2019, 4, 7811–7817. [Google Scholar] [CrossRef]
- Inaloo, I.D.; Majnooni, S.; Esmaeilpour, M. Superparamagnetic Fe3O4 Nanoparticles in a Deep Eutectic Solvent: An Efficient and Recyclable Catalytic System for the Synthesis of Primary Carbamates and Monosubstituted Ureas. Eur. J. Org. Chem. 2018, 2018, 3481–3488. [Google Scholar] [CrossRef]
- Abbasi, S.; Miraki, M.K.; Radfar, I.; Karimi, M.; Heydari, A. Efficient Synthesis of N-Acylureas Using Copper Oxide Supported on Magnetic Nanoparticles in Deep Eutectic Solvent. ChemistrySelect 2018, 3, 77–80. [Google Scholar] [CrossRef]
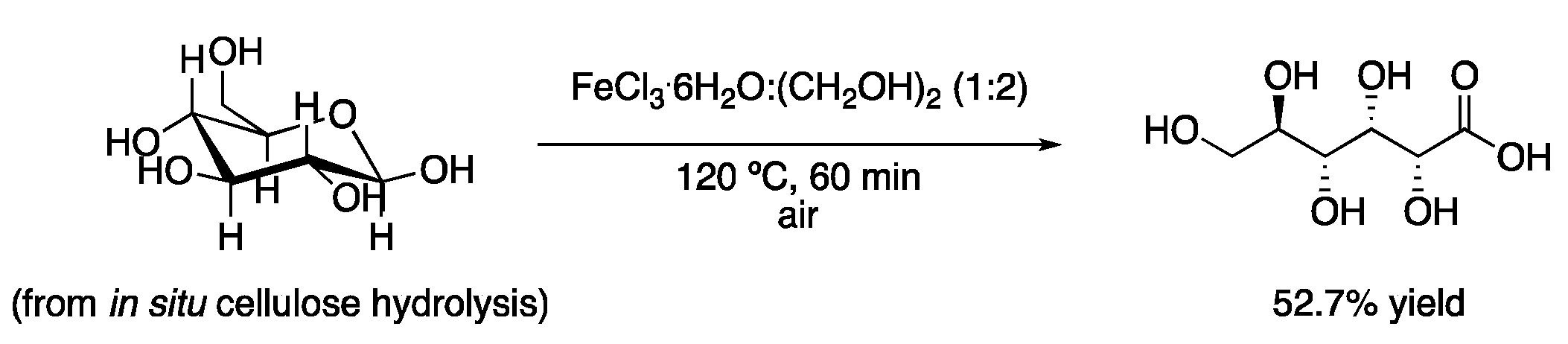

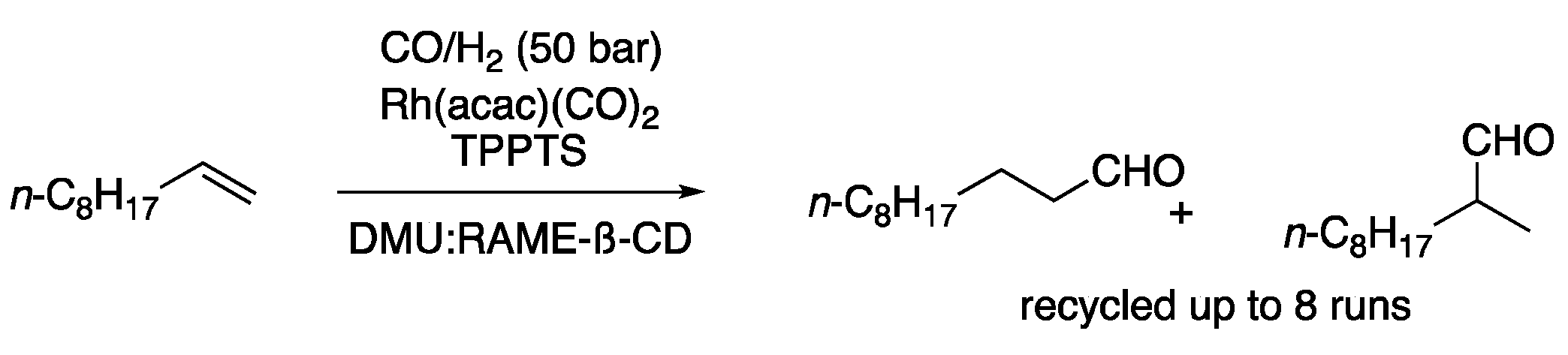
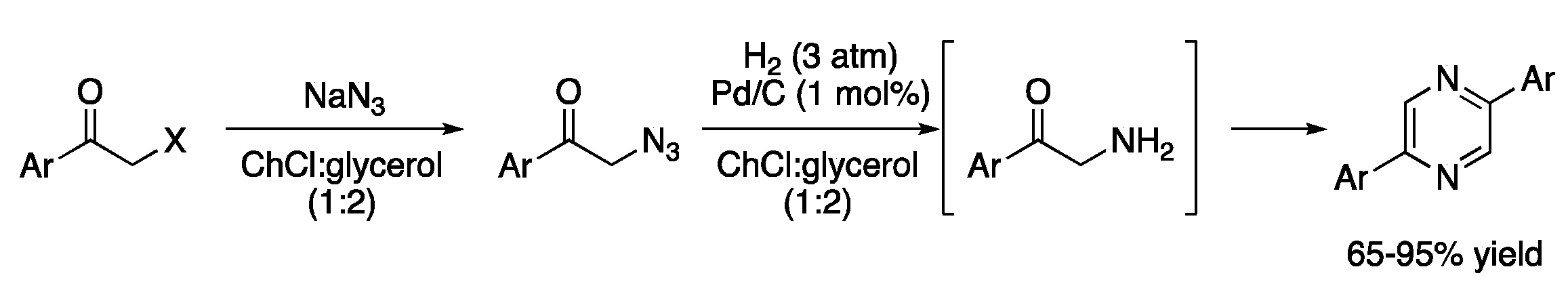


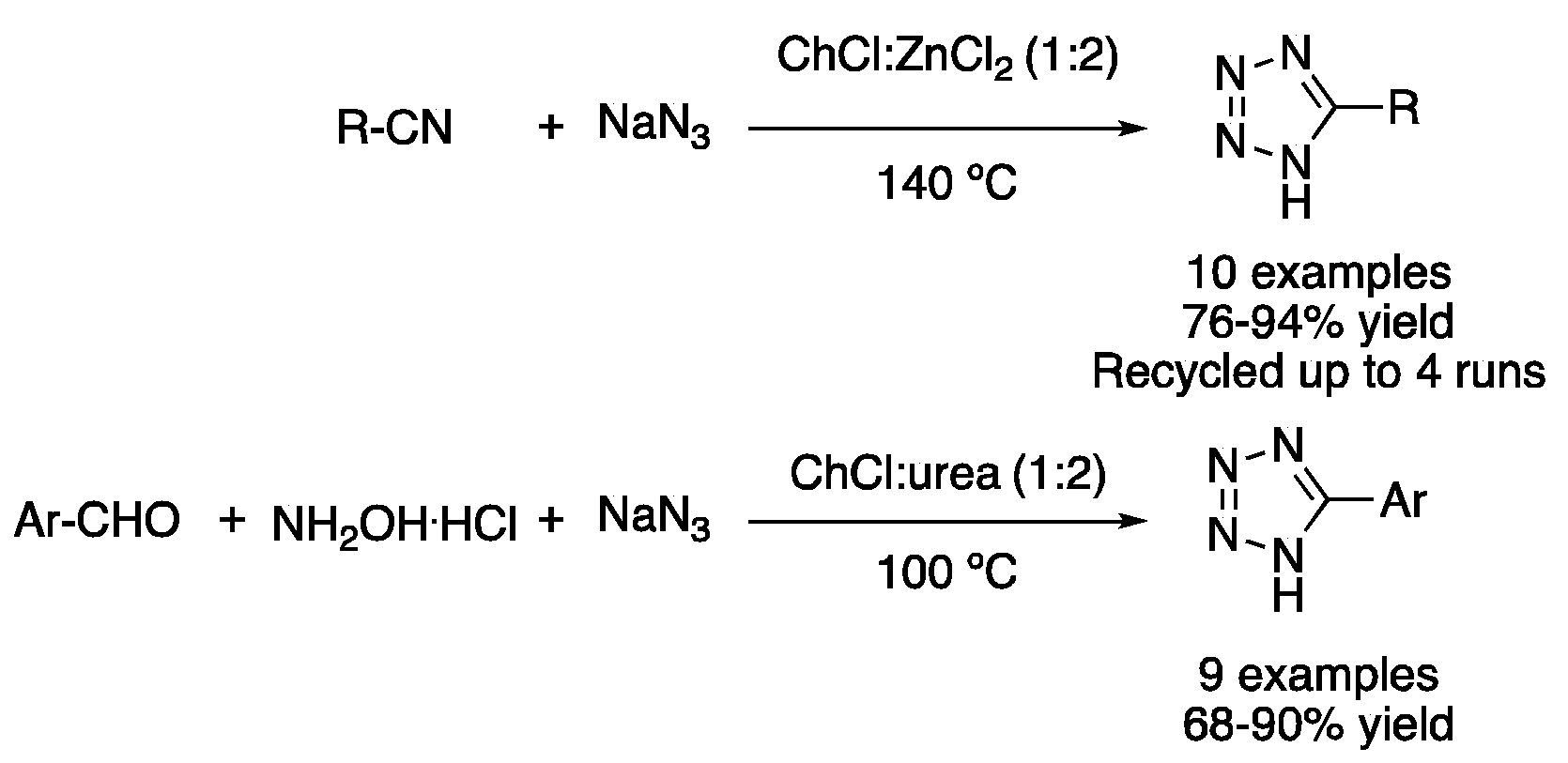


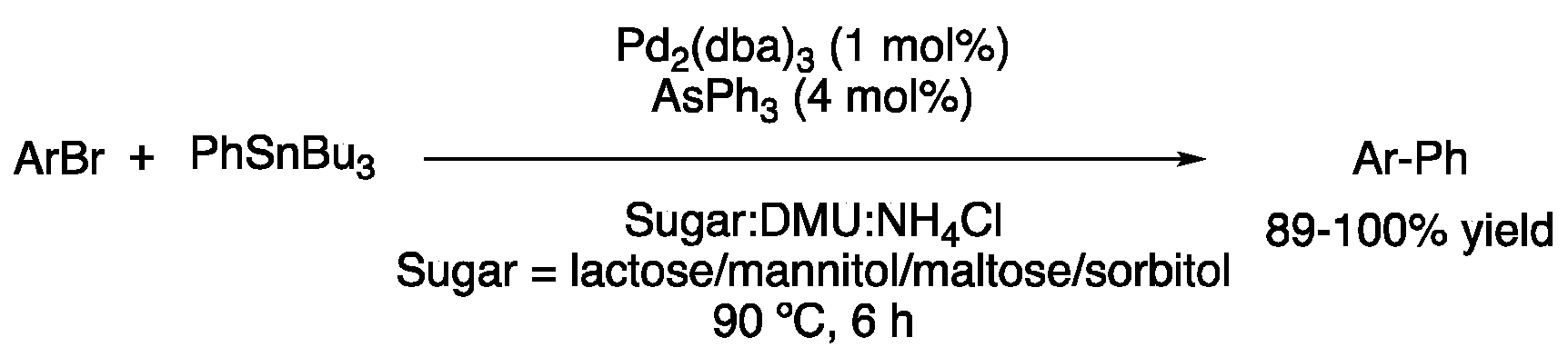
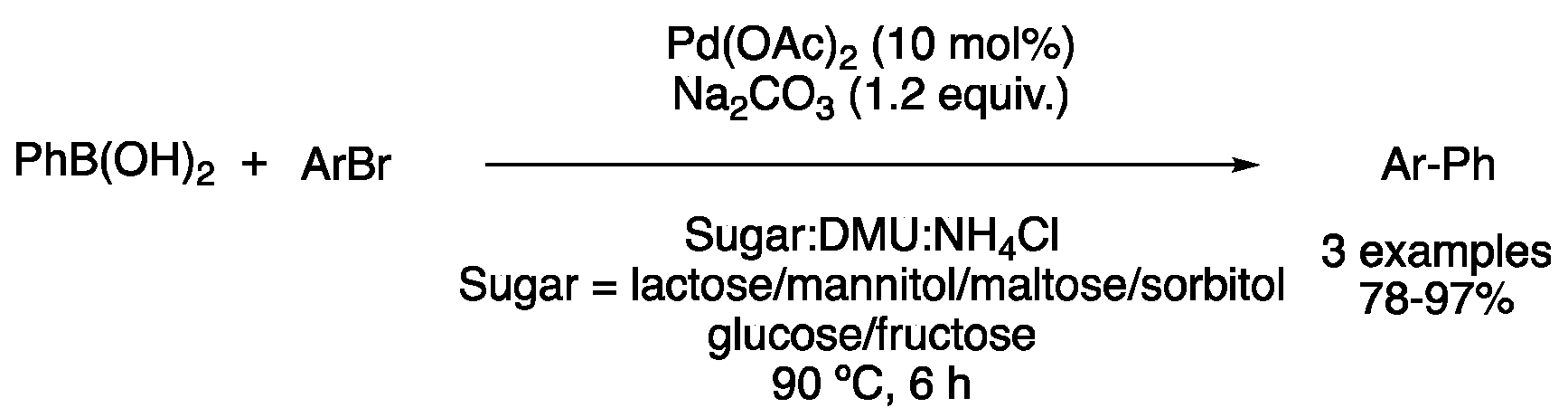

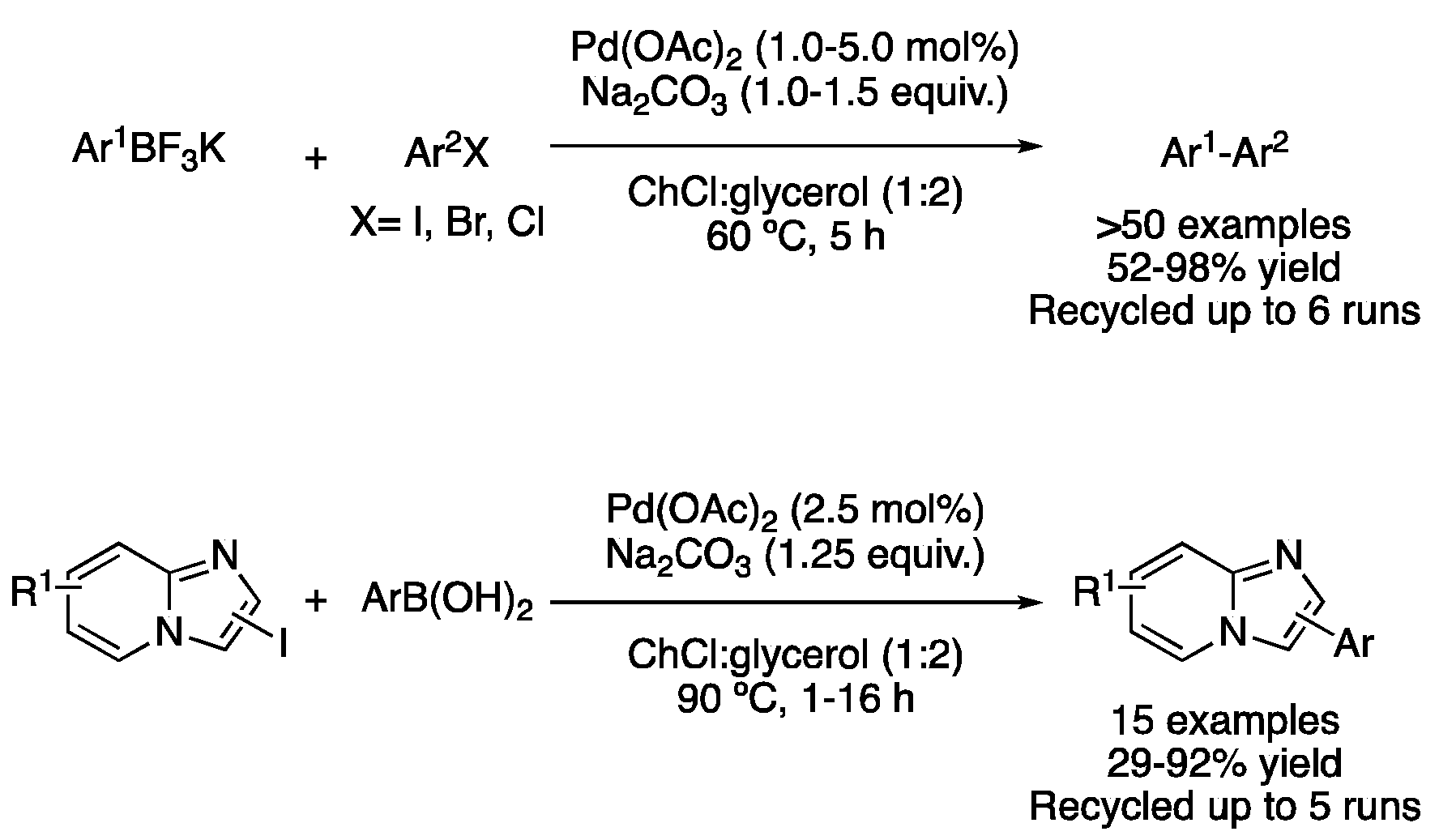

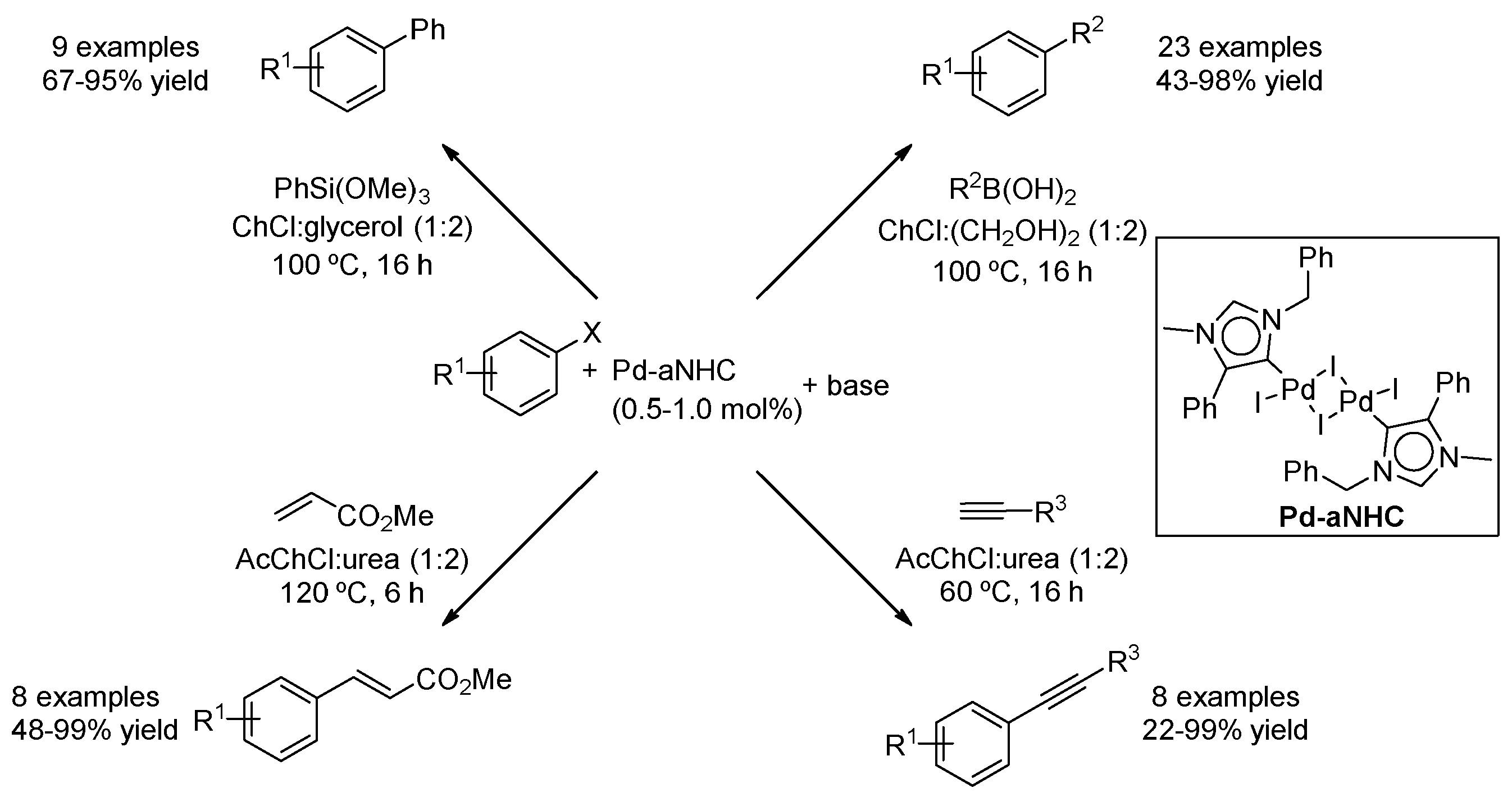


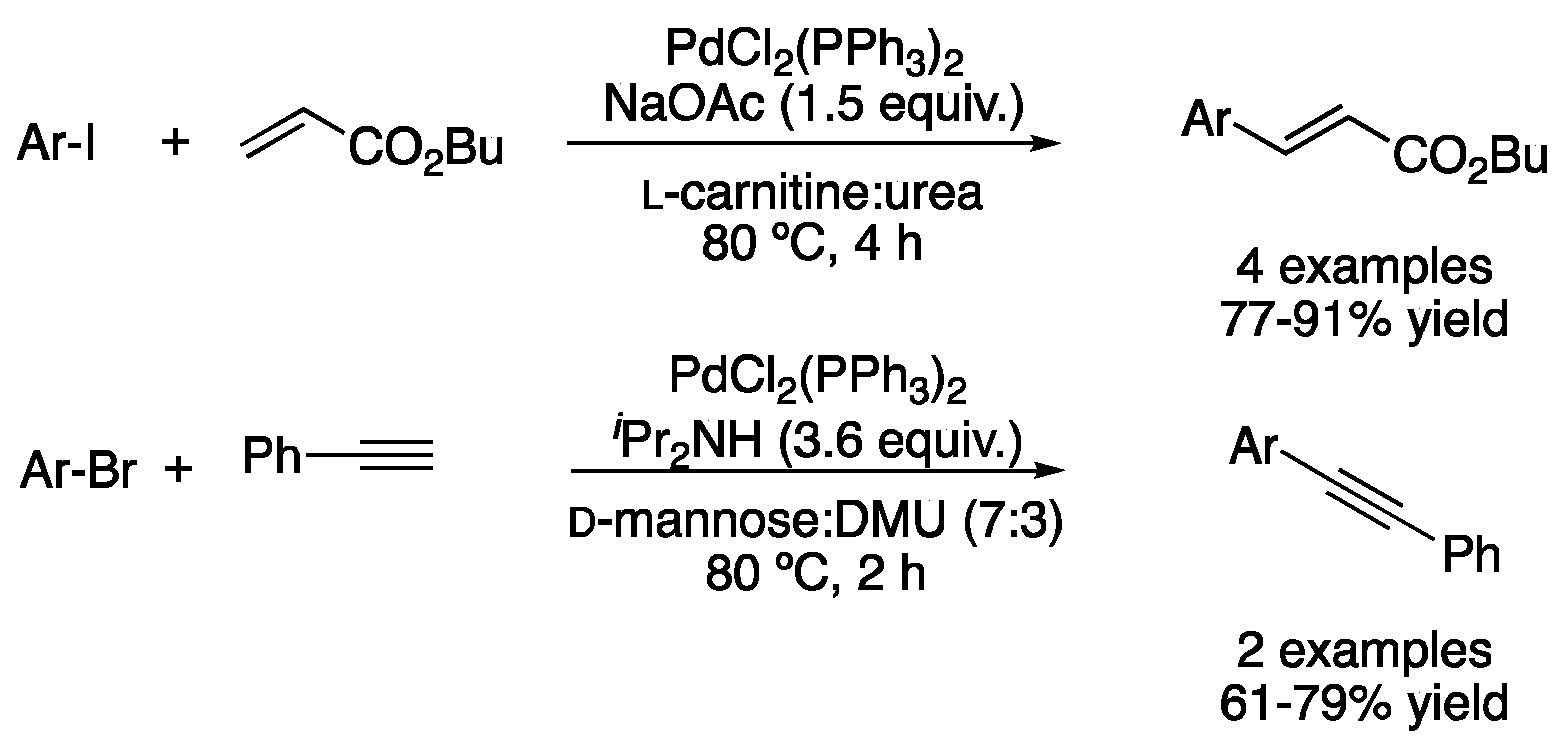
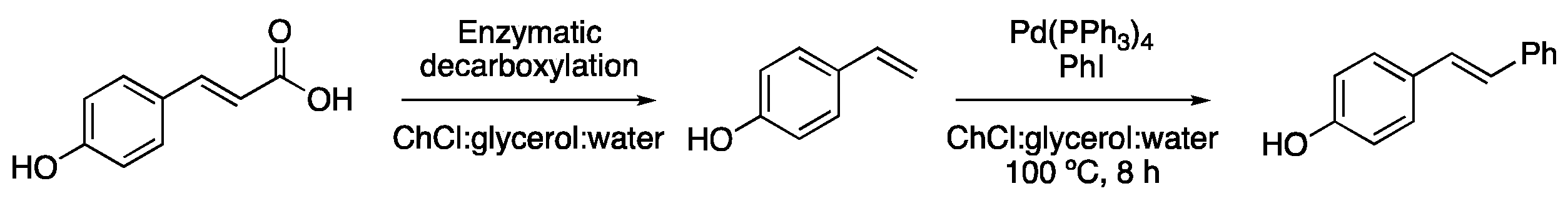



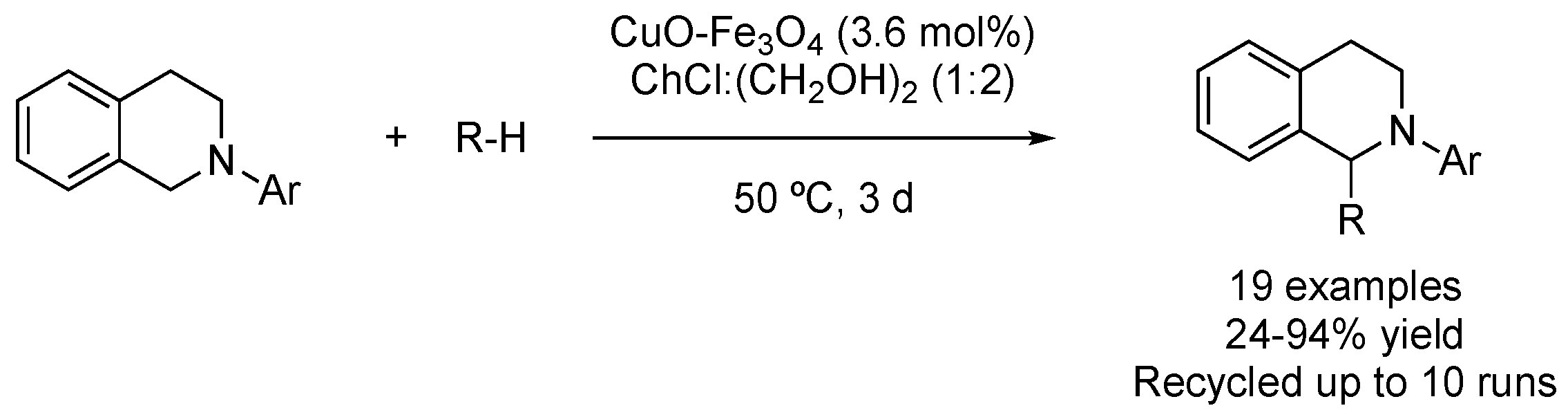




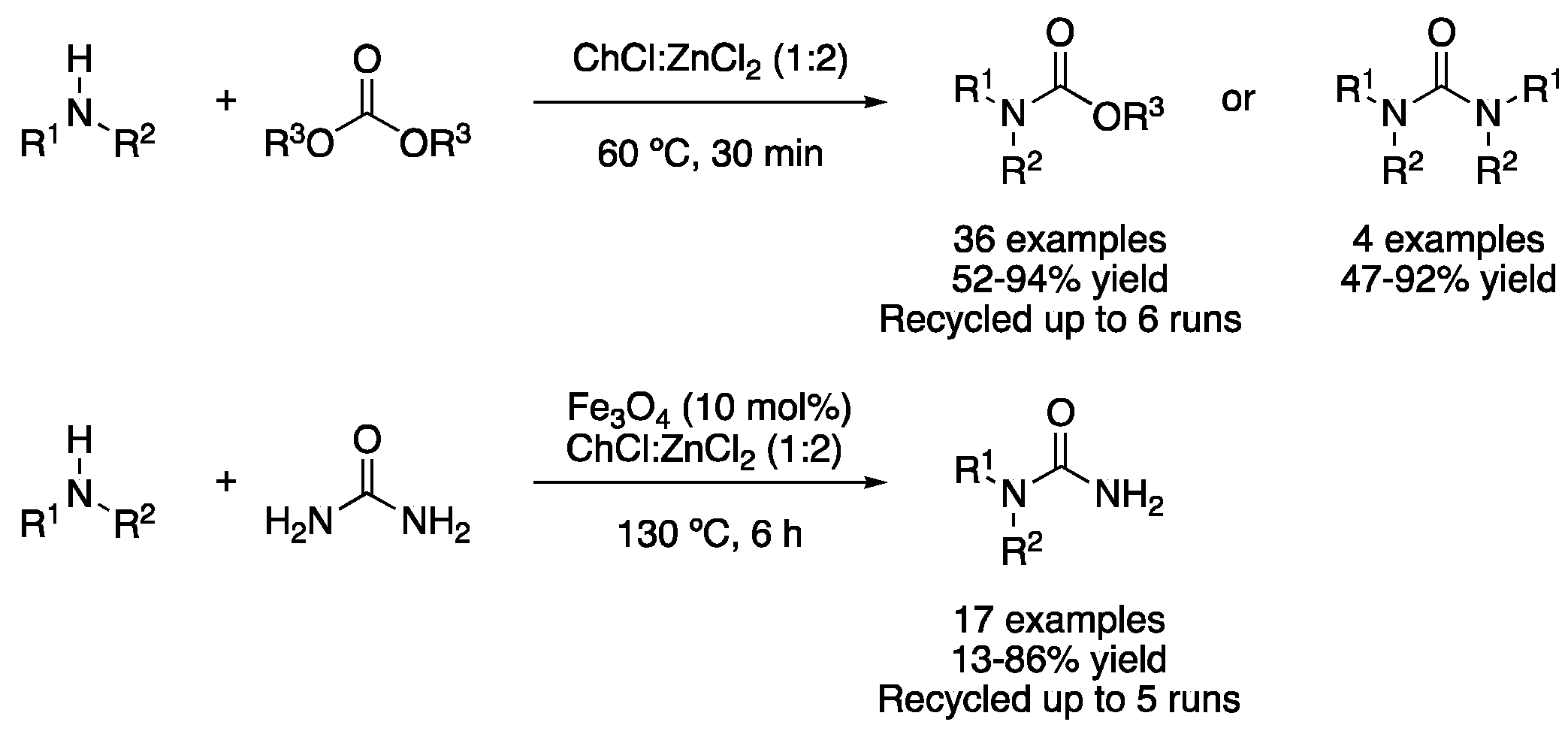
| Entry | Reaction | DES | Conditions | Product | Ref. |
|---|---|---|---|---|---|
| 1 | Stille | Sugar:DMU:NH4Cl | [Pd], 90 °C | Biaryl | [45] |
| 2 | Suzuki | Sugar:DMU:NH4Cl | [Pd], 90 °C | Biaryl | [46] |
| 3 | Suzuki | ChCl:glycerol (1:2) | [Pd], 60–100 °C | (Het)biaryl | [48,49,50,56,61] |
| 4 | Suzuki | ChCl:(CH2OH)2 (1:2) | [Pd], 100 °C | Biaryl | [53,54] |
| 5 | Suzuki | ChCl:glycerol (1:2) | [Pd], 90 °C | Imidazole-fused deriv. | [52] |
| 6 | Suzuki | ChCl:urea (1:2) | [Ni], 60 °C | (Het)biaryl | [57] |
| 7 | Heck | l-carnitine:urea | [Pd], 80 °C | Ar-CH=CHR | [58] |
| 8 | Heck | ChCl:glycerol (1:2) | [Pd], 80–120 °C | Ar-CH=CHR | [48,62] |
| 9 | Heck | ChCl:(CH2OH)2 (1:2) | [Pd], 120 °C | Ar-CH=CHR | [53] |
| 10 | Heck | AcChCl:urea (1:2) | [Pd],120 °C | Ar-CH=CHR | [54] |
| 11 | Sonogashira | d-mannose:DMU (7:3) | [Pd], 80 °C | Ar-C≡C-Ar’ | [58] |
| 12 | Sonogashira | Ph3PMeBr:glycerol (1:2) | [Pd], 60 °C | Ar-C≡C-Ar’ | [48,53] |
| 13 | Sonogashira | AcChCl:urea (1:2) | [Pd], 60 °C | Ar-C≡C-Ar’ | [54] |
| 14 | Hiyama | ChCl:glycerol (1:2) | [Pd], 100 °C | Ar-R | [53,54,74] |
| 15 | Tsuji–Trost | RAME-ß-CD:urea (3:7) | [Pd], 90 °C | Allylamine | [10] |
| 16 | Negishi | ChCl:urea (1:2) | [Pd], 60 °C | Ar-R | [75] |
| 17 | Ullmann C-N | ChCl:glycerol (1:2) | [Cu], 60–100 °C | ArNR1R | [76,78] |
| 18 | Goldberg | ChCl:H2O (1:2) | [Cu], 80 °C | Amides | [77] |
| 19 | Ullmann C-O | ChCl:ROH | [Cu], 80 °C | Ar-OR | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marset, X.; Guillena, G. Deep Eutectic Solvents as à-la-Carte Medium for Transition-Metal-Catalyzed Organic Processes. Molecules 2022, 27, 8445. https://doi.org/10.3390/molecules27238445
Marset X, Guillena G. Deep Eutectic Solvents as à-la-Carte Medium for Transition-Metal-Catalyzed Organic Processes. Molecules. 2022; 27(23):8445. https://doi.org/10.3390/molecules27238445
Chicago/Turabian StyleMarset, Xavier, and Gabriela Guillena. 2022. "Deep Eutectic Solvents as à-la-Carte Medium for Transition-Metal-Catalyzed Organic Processes" Molecules 27, no. 23: 8445. https://doi.org/10.3390/molecules27238445
APA StyleMarset, X., & Guillena, G. (2022). Deep Eutectic Solvents as à-la-Carte Medium for Transition-Metal-Catalyzed Organic Processes. Molecules, 27(23), 8445. https://doi.org/10.3390/molecules27238445







