Geniposidic Acid from Eucommia ulmoides Oliver Staminate Flower Tea Mitigates Cellular Oxidative Stress via Activating AKT/NRF2 Signaling
Abstract
1. Introduction
2. Results
2.1. Total Phenolic and Total Flavonoid Content
2.2. Comparison of the In Vitro Antioxidant Activities of Different Fractions of ESF Tea
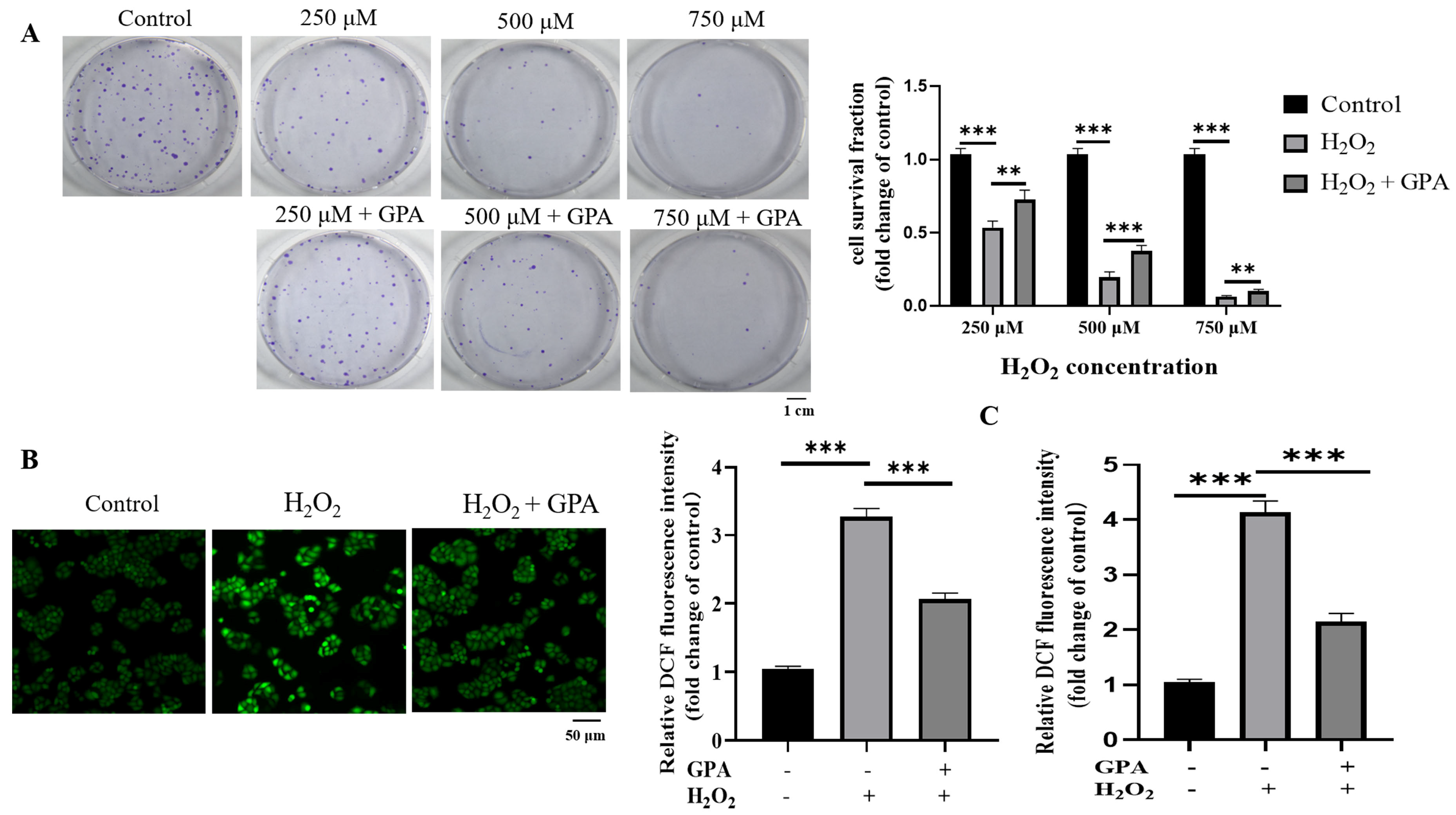
2.3. Cytoprotective Activity against H2O2-Induced Oxidative Injury of ESF Tea
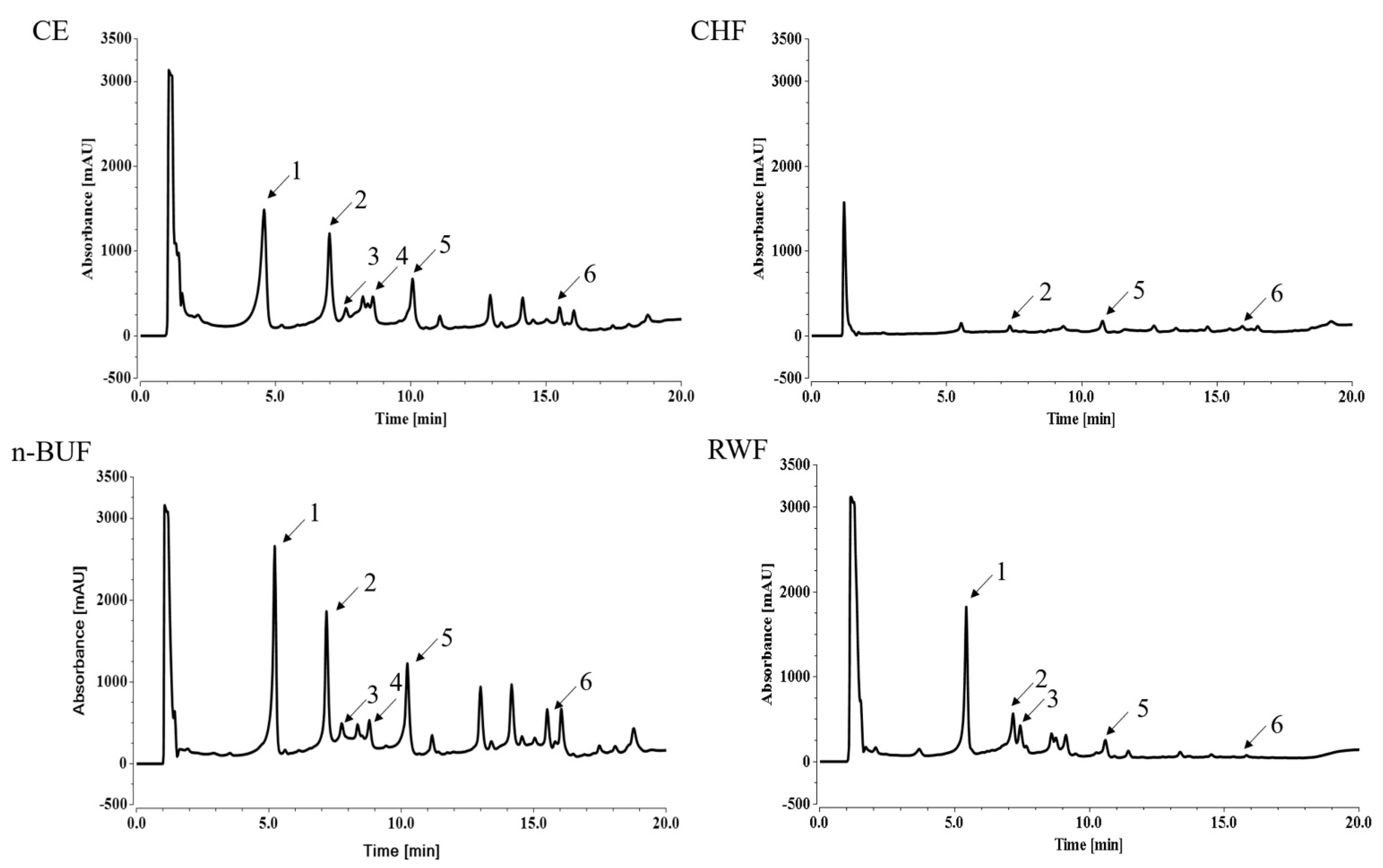
2.4. Qualitative and Quantitative Analyzes of Different Fraction Compounds
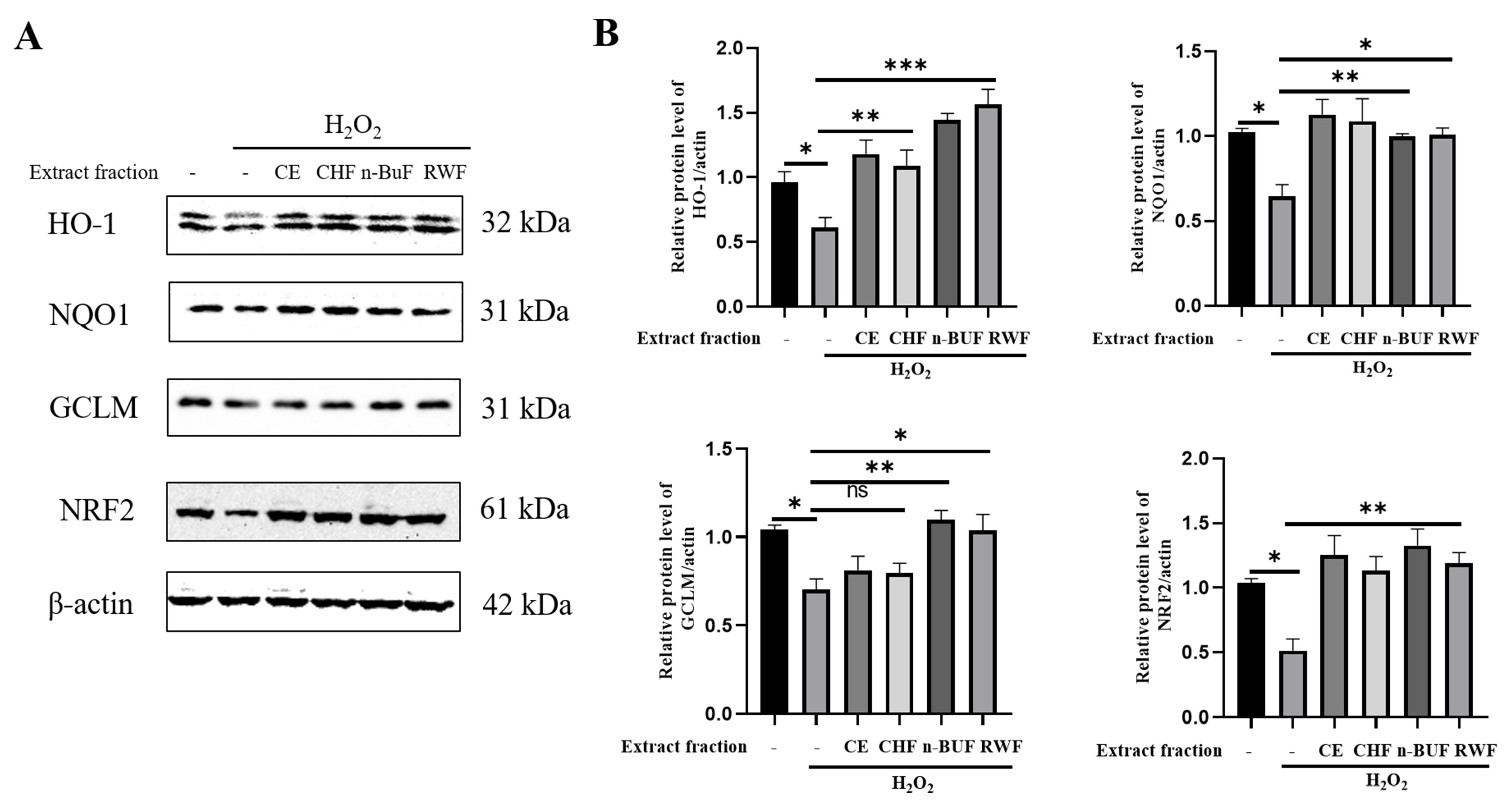
2.5. GPA Alleviated H2O2-Induced Oxidative Stress via AKT/NRF2/OGG1 Signaling Pathway
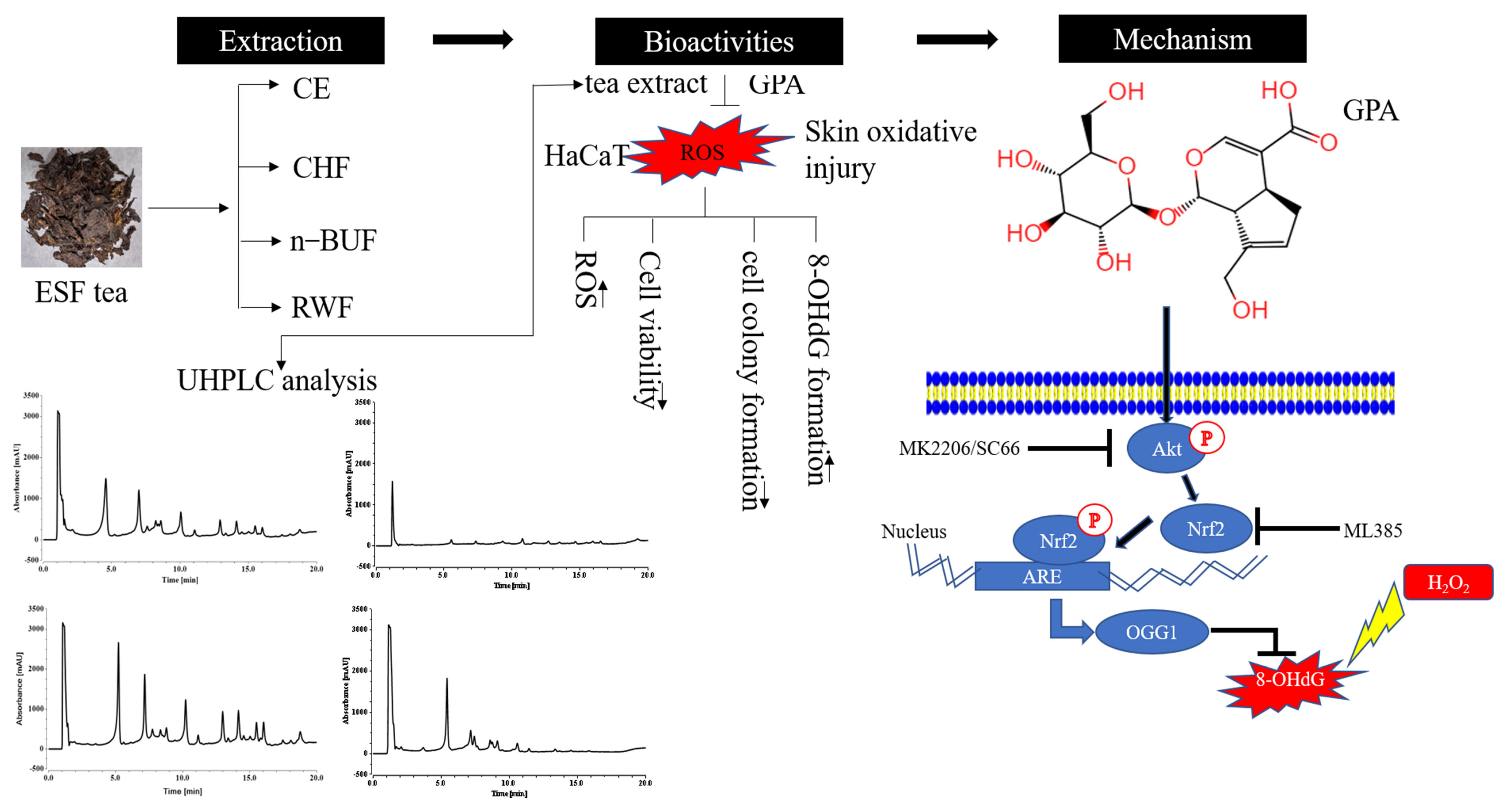
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sequential Extraction Process
4.3. Total Phenolics and Total Flavonoids Content
4.4. Antioxidant Activity
4.5. Cell Culture and Viability Tests
4.6. Analysis of Intracellular ROS
4.7. Western Blotting Analysis
4.8. UHPLC-DAD Analysis
4.9. Clonogenic Surviving Assay
4.10. Immunofluorescence Staining
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, X.; Wang, J.; Li, M.; Hao, D.; Yang, Y.; Zhang, C.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Wang, Y.; Yang, Y.Y. Research Progress in Study of Sheng Ji Zong Lu in Last Ten Years. Chin. J. Basic Med. Tradit. Chin. Med. 2022, 28, 486–490. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Wang, J.; Xiao, W.; Wang, Z.; Zhang, J.; Yang, Y.; Zhang, S.; Ai, C. Investigation into the mechanism of Eucommia ulmoides Oliv. based on a systems pharmacology approach. J. Ethnopharmacol. 2014, 151, 452–460. [Google Scholar] [CrossRef]
- Li, Y.; Kamo, S.; Metori, K.; Koike, K.; Che, Q.-M.; Takahashi, S. The Promoting Effect of Eucommiol from Eucommiae Cortex on Collagen Synthesis. Biol. Pharm. Bull. 2000, 23, 54–59. [Google Scholar] [CrossRef][Green Version]
- Ding, Y.-X.; Li, Q.; Zhang, H.; Kim, Y.-H.; Dou, D.-Q. Iridoid constituents from the male flower ofEucommia ulmoidesand their promotion proliferation on ESF-1. J. Asian Nat. Prod. Res. 2015, 17, 867–875. [Google Scholar] [CrossRef]
- Ho, J.N.; Cho, H.Y.; Lim, E.J.; Kim, H.K. Effects of Aucubin Isolated from Eucommia ulmoides on UVB-induced Oxidative Stress in Human Keratinocytes HaCaT. Food Sci. Biotechnol. 2009, 18, 475–480. [Google Scholar]
- Jimbo, N.; Kawada, C.; Nomura, Y. Herb extracts and collagen hydrolysate improve skin damage resulting from ultraviolet-induced aging in hairless mice. Biosci. Biotechnol. Biochem. 2015, 79, 1624–1628. [Google Scholar] [CrossRef]
- Zhu, M.-Q.; Sun, R.-C. Eucommia ulmoides Oliver: A Potential Feedstock for Bioactive Products. J. Agric. Food Chem. 2018, 66, 5433–5438. [Google Scholar] [CrossRef]
- Call, V.B.; Dilcher, D.L. The fossil record of Eucommia (Eucommiaceae) in North America. Am. J. Bot. 1997, 84, 798–814. [Google Scholar] [CrossRef]
- Dong, J.; Ma, X.; Fu, Z.; Guo, Y. Effects of microwave drying on the contents of functional constituents of Eucommia ulmoides flower tea. Ind. Crop. Prod. 2011, 34, 1102–1110. [Google Scholar] [CrossRef]
- Li, Q.; Du, H.E.; Lou, L.J.; Fu, J.M.; Du, L.Y.; Cheng, B.Q. Effect of Eucommia ulmoides Oliver male flower tea on SOD, GSH-PX activity and MDA level in D-galactose-induced aging mice. Chin. Tradit. Pat. Med. 2011, 33, 331–333. [Google Scholar]
- Li, Q.; Jin, X.L.; Du, H.Y. Study on anti-fatigue effects of Eucommia ulmoides Oliv. male flower tea on mice. Food Sci. 2008, 7, 432–434. [Google Scholar]
- Liu, C.; Guo, H.; Dain, J.A.; Wan, Y.; Gao, X.-H.; Chen, H.-D.; Seeram, N.P.; Ma, H. Cytoprotective effects of a proprietary red maple leaf extract and its major polyphenol, ginnalin A, against hydrogen peroxide and methylglyoxal induced oxidative stress in human keratinocytes. Food Funct. 2020, 11, 5105–5114. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Lee, K.T.; Choi, Y.H.; Kim, G.-Y. Anthocyanins from Hibiscus syriacus L. Inhibit Oxidative Stress-Mediated Apoptosis by Activating the Nrf2/HO-1 Signaling Pathway. Antioxidants 2020, 9, 42. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Guo, N.; Zhao, L.; Zhao, Y.; Li, Q.; Xue, X.; Wu, L.; Escalada, M.G.; Wang, K.; Peng, W. Comparison of the Chemical Composition and Biological Activity of Mature and Immature Honey: An HPLC/QTOF/MS-Based Metabolomic Approach. J. Agric. Food Chem. 2020, 68, 4062–4071. [Google Scholar] [CrossRef]
- Giudice, A.; Montella, M. Activation of the Nrf2–ARE signaling pathway: A promising strategy in cancer prevention. BioEssays 2006, 28, 169–181. [Google Scholar] [CrossRef]
- Boiteux, S.; Coste, F.; Castaing, B. Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases. Free Radic. Biol. Med. 2017, 107, 179–201. [Google Scholar] [CrossRef]
- Klungland, A.; Bjelland, S. Oxidative damage to purines in DNA: Role of mammalian Ogg1. DNA Repair 2007, 6, 481–488. [Google Scholar] [CrossRef]
- Li, M.; Wu, C.; Muhammad, J.S.; Yan, D.; Tsuneyama, K.; Hatta, H.; Cui, Z.-G.; Inadera, H. Melatonin sensitises shikonin-induced cancer cell death mediated by oxidative stress via inhibition of the SIRT3/SOD2-AKT pathway. Redox Biol. 2020, 36, 101632. [Google Scholar] [CrossRef]
- Ma, Q. Transcriptional responses to oxidative stress: Pathological and toxicological implications. Pharmacol. Ther. 2010, 125, 376–393. [Google Scholar] [CrossRef]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef]
- Guo, T.; Wei, L.; Sun, J.; Hou, C.-L.; Fan, L. Antioxidant activities of extract and fractions from Tuber indicum Cooke & Massee. Food Chem. 2011, 127, 1634–1640. [Google Scholar] [CrossRef]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Gruber, J.V.; Holtz, R. Examining the Genomic Influence of Skin AntioxidantsIn Vitro. Mediat. Inflamm. 2010, 2010, 1–10. [Google Scholar] [CrossRef]
- Lee, K.-H.; Do, H.-K.; Kim, D.-Y.; Kim, W. Impact of chlorogenic acid on modulation of significant genes in dermal fibroblasts and epidermal keratinocytes. Biochem. Biophys. Res. Commun. 2021, 583, 22–28. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, R.; Wang, M.; Zhai, L.; Liu, J.; Xu, X.; Sun, L.; Zhao, D. Ginsenosides repair UVB-induced skin barrier damage in BALB/c hairless mice and HaCaT keratinocytes. J. Ginseng Res. 2022, 46, 115–125. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Kang, K.A.; Lee, J.H.; Chae, S.; Zhang, R.; Piao, M.J.; Kim, H.S.; You, H.J.; Hyun, J.W. Butin decreases oxidative stress-induced 8-hydroxy-2′-deoxyguanosine levels via activation of oxoguanine glycosylase 1. Chem. Interact. 2009, 181, 338–342. [Google Scholar] [CrossRef]
- Dhénaut, A.; Boiteux, S.; Radicella, J. Characterization of the hOGG1 promoter and its expression during the cell cycle. Mutat. Res. Repair 2000, 461, 109–118. [Google Scholar] [CrossRef]
- Huang, H.-C.; Nguyen, T.; Pickett, C.B. Phosphorylation of Nrf2 at Ser-40 by Protein Kinase C Regulates Antioxidant Response Element-mediated Transcription. J. Biol. Chem. 2002, 277, 42769–42774. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, M.; Zhang, L.-J. Keratin 6, 16 and 17—Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, W.; Chen, B.; Pan, H.; Zhu, M.; Pang, X.; Zhang, Q. Deep eutectic solvents in the extraction of active compounds from Eucommia Ulmoides Oliv. leaves. J. Food Meas. Charact. 2022, 16, 3410–3422. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Yang, M.; Cao, J.; Khan, A.; Cheng, G. UHPLC-ESI-HRMS/MS analysis on phenolic compositions of different E Se tea extracts and their antioxidant and cytoprotective activities. Food Chem. 2020, 318, 126512. [Google Scholar] [CrossRef]
- Santos, P.H.; Kammers, J.C.; Silva, A.P.; Oliveira, J.V.; Hense, H. Antioxidant and antibacterial compounds from feijoa leaf extracts obtained by pressurized liquid extraction and supercritical fluid extraction. Food Chem. 2021, 344, 128620. [Google Scholar] [CrossRef]
- Deetae, P.; Parichanon, P.; Trakunleewatthana, P.; Chanseetis, C.; Lertsiri, S. Antioxidant and anti-glycation properties of Thai herbal teas in comparison with conventional teas. Food Chem. 2012, 133, 953–959. [Google Scholar] [CrossRef]
- Franke, S.; Ckless, K.; Silveira, J.; Rubensam, G.; Brendel, M.; Erdtmann, B.; Henriques, J. Study of antioxidant and mutagenic activity of different orange juices. Food Chem. 2004, 88, 45–55. [Google Scholar] [CrossRef]
- Wang, X.-S.; Peng, M.-J.; He, C.-T. The antihypertensive effects of Eucommia ulmoides leaf water/ethanol extracts are chlorogenic acid dependent. J. Funct. Foods 2022, 94, 105129. [Google Scholar] [CrossRef]
- Duan, J.H.; Li, Q.; Du, H.Y.; Du, L.Y.; He, J.J. Determination of geniposidic acid, chlorogenic acid and geniposide in Eucommia ulmoides Oliver male flower tea by HPLC. Chin. Tradit. Herb. Drugs 2009, 40, 71–72. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, K.-M.; Park, J.; Kwak, J.-H.; Kim, Y.S.; Lee, S.-M. Geniposidic acid protects against d-galactosamine and lipopolysaccharide-induced hepatic failure in mice. J. Ethnopharmacol. 2013, 146, 271–277. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Liu, Y.; Disasa, D.; Akira, M.; Xiang, L.; Qi, J. A New Geniposidic Acid Derivative Exerts Antiaging Effects through Antioxidative Stress and Autophagy Induction. Antioxidants 2021, 10, 987. [Google Scholar] [CrossRef]
- Singh, B.; Chatterjee, A.; Ronghe, A.M.; Bhat, N.K.; Bhat, H.K. Antioxidant-mediated up-regulation of OGG1 via NRF2 induction is associated with inhibition of oxidative DNA damage in estrogen-induced breast cancer. BMC Cancer 2013, 13, 253–259. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Z.; Misra, B.R.; Zhang, L.; Cao, Z.; Yamamoto, M.; Trush, M.A.; Misra, H.P.; Li, Y. Nuclear Factor E2-Related Factor 2-Dependent Myocardiac Cytoprotection Against Oxidative and Electrophilic Stress. Cardiovasc. Toxicol. 2008, 8, 71–85. [Google Scholar] [CrossRef]
- Jung, K.-A.; Kwak, M.-K. The Nrf2 System as a Potential Target for the Development of Indirect Antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef]
- Föller, M.; Harris, I.S.; Elia, A.; John, R.; Lang, F.; Kavanagh, T.J.; Mak, T.W. Functional significance of glutamate–cysteine ligase modifier for erythrocyte survival in vitro and in vivo. Cell Death Differ. 2013, 20, 1350–1358. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wu, S.-B.; Hong, C.-H.; Yu, H.-S.; Wei, Y.-H. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef]
- Akino, N.; Wada-Hiraike, O.; Terao, H.; Honjoh, H.; Isono, W.; Fu, H.; Hirano, M.; Miyamoto, Y.; Tanikawa, M.; Harada, M.; et al. Activation of Nrf2 might reduce oxidative stress in human granulosa cells. Mol. Cell. Endocrinol. 2018, 470, 96–104. [Google Scholar] [CrossRef]
- Piao, M.J.; Kim, K.C.; Kang, K.A.; Fernando, P.D.S.M.; Herath, H.M.U.L.; Hyun, J.W. Phloroglucinol Attenuates Ultraviolet B-Induced 8-Oxoguanine Formation in Human HaCaT Keratinocytes through Akt and Erk Mediated Nrf2/Ogg1 Signaling Pathways. Biomol. Ther. 2021, 29, 90–97. [Google Scholar] [CrossRef]
- Liu, T.; Lv, Y.-F.; Zhao, J.-L.; You, Q.-D.; Jiang, Z.-Y. Regulation of Nrf2 by phosphorylation: Consequences for biological function and therapeutic implications. Free. Radic. Biol. Med. 2021, 168, 129–141. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Kim, H.-E.; Lee, S.-G. Caffeoylserotonin Protects Human Keratinocyte HaCaT Cells against H2O2-Induced Oxidative Stress and Apoptosis through Upregulation of HO-1 Expression via Activation of the PI3K/Akt/Nrf2 Pathway. Phytother. Res. 2013, 27, 1810–1818. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.C.; Wu, J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]



| Sample | TPC | TFC | FRAP | DPPH | ABTS | ||
|---|---|---|---|---|---|---|---|
| mg GAE/g | mg RE/g | mg Trolox/g | IC50 μg/mL | mg Trolox/g | IC50 μg/mL | mg Trolox/g | |
| CE | 126 ± 7.94 b | 124.57 ± 3.56 b | 108.87 ± 2.2 b | 59.71 ± 1.78 | 58.97 ± 6.29 b | 42.78 ± 1.17 | 284.27 ± 45.38 b |
| CHF | 78.36 ± 4.13 c | 85.3 ± 4.12 c | 47.95 ± 1.32 d | 131.68 ± 7.72 | 26.83 ± 3.60 c | 56.52 ± 1.85 | 215.27 ± 35.58 b |
| n-BUF | 272 ± 6.52 a | 286.92 ± 2.56 a | 286.45 ± 11.04 a | 24.45 ± 0.74 | 144.04 ± 15.26 a | 17.25 ± 0.04 | 703.21 ± 94.90 a |
| RWF | 74.13 ± 3.12 c | 84.24 ± 2.64 c | 70.42 ± 0.77 c | 101.19 ± 11.23 | 34.81 ± 1.45 c | 82.94 ± 0.29 | 146.31 ± 19.92 b |
| TPC | TFC | FRAP | DPPH | ABTS | IC50 | ||
|---|---|---|---|---|---|---|---|
| DPPH | ABTS | ||||||
| TPC | 1 | 0.998 | 0.994 | 0.997 | 0.994 | −0.893 | −0.893 |
| TFC | 1 | 0.996 | 0.996 | 0.994 | −0.873 | −0.871 | |
| Compounds | Molecular Formula | CE (mg/g) | CHF (mg/g) | n-BUF (mg/g) | RWF (mg/g) |
|---|---|---|---|---|---|
| Geniposidic acid | C16H22O10 | 60.65 ± 0.01 | - | 149.0 ± 3.83 | 37.75 ± 0.92 |
| Chlorogenic acid | C16H18O9 | 41.69 ± 7.67 | 1.55 ± 0.61 | 52.41 ± 0.63 | 5.53 ± 1.45 |
| Catechin | C15H14O6 | 5.09 ± 0.3 | - | 13.34 ± 0.31 | 1.03 ± 0.26 |
| Caffeic acid | C9H8O4 | 0.29 ± 0.01 | - | 2.4 ± 0.2 | - |
| Geniposide | C17H24O10 | 23.19 ± 0.19 | 1.67 ± 0.23 | 84.10 ± 4.9 | 3.18 ± 0.25 |
| Rutin | C27H30O16 | 4.003 ± 0.03 | 0.25 ± 0.03 | 12.66 ± 0.64 | 0.43 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Jia, H.; Zhang, Y.; Zhou, J.; Chen, X.; Wu, L.; Wang, J. Geniposidic Acid from Eucommia ulmoides Oliver Staminate Flower Tea Mitigates Cellular Oxidative Stress via Activating AKT/NRF2 Signaling. Molecules 2022, 27, 8568. https://doi.org/10.3390/molecules27238568
Cheng S, Jia H, Zhang Y, Zhou J, Chen X, Wu L, Wang J. Geniposidic Acid from Eucommia ulmoides Oliver Staminate Flower Tea Mitigates Cellular Oxidative Stress via Activating AKT/NRF2 Signaling. Molecules. 2022; 27(23):8568. https://doi.org/10.3390/molecules27238568
Chicago/Turabian StyleCheng, Shuo, Huiling Jia, Yisen Zhang, Juanjuan Zhou, Xue Chen, Lifang Wu, and Jun Wang. 2022. "Geniposidic Acid from Eucommia ulmoides Oliver Staminate Flower Tea Mitigates Cellular Oxidative Stress via Activating AKT/NRF2 Signaling" Molecules 27, no. 23: 8568. https://doi.org/10.3390/molecules27238568
APA StyleCheng, S., Jia, H., Zhang, Y., Zhou, J., Chen, X., Wu, L., & Wang, J. (2022). Geniposidic Acid from Eucommia ulmoides Oliver Staminate Flower Tea Mitigates Cellular Oxidative Stress via Activating AKT/NRF2 Signaling. Molecules, 27(23), 8568. https://doi.org/10.3390/molecules27238568






