Advanced Photoelectrochemical Hydrogen Generation by CdO-g-C3N4 in Aqueous Medium under Visible Light
Abstract
1. Introduction
2. Results and Discussion
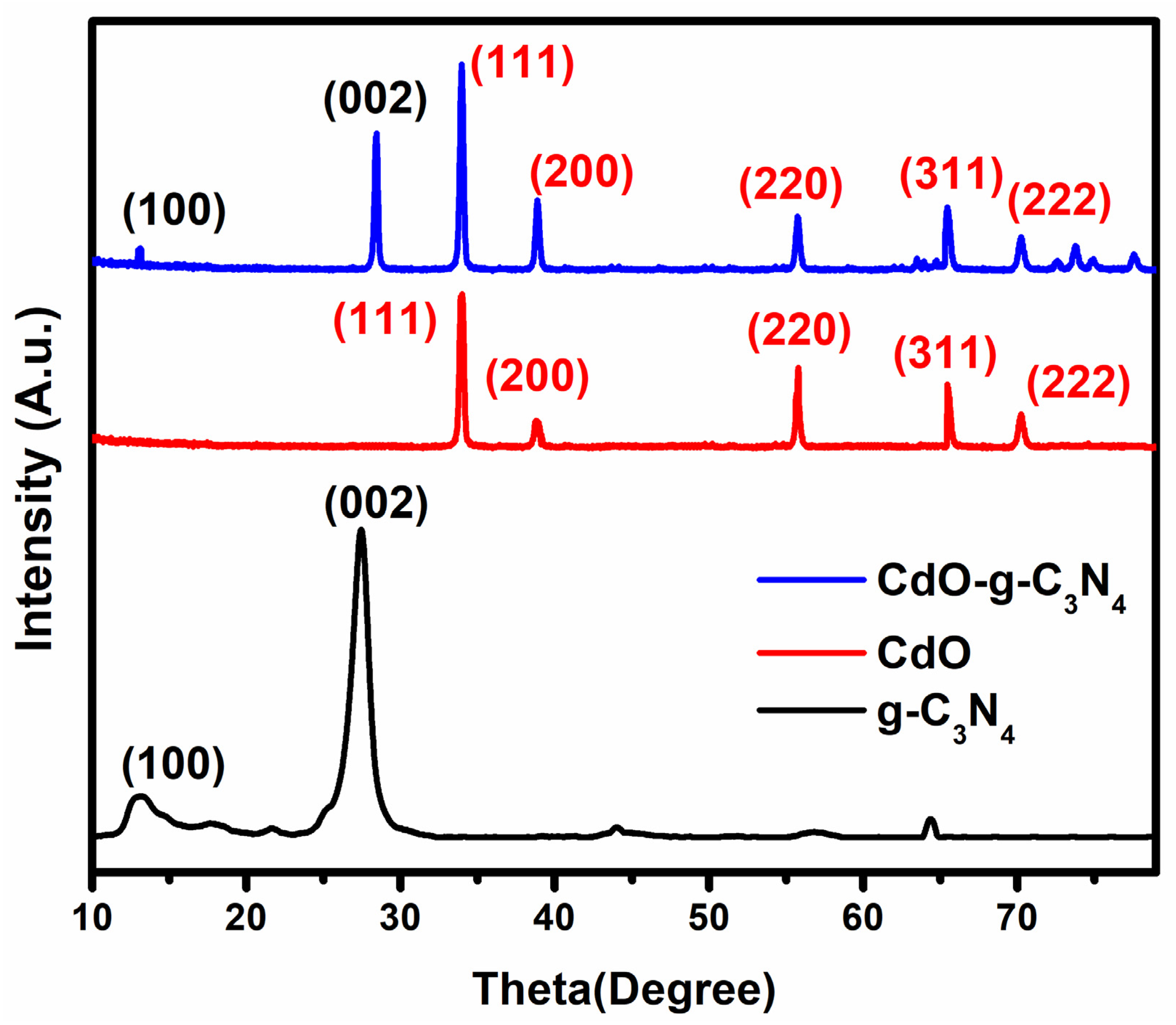
2.1. X-ray Diffraction (XRD) Analysis
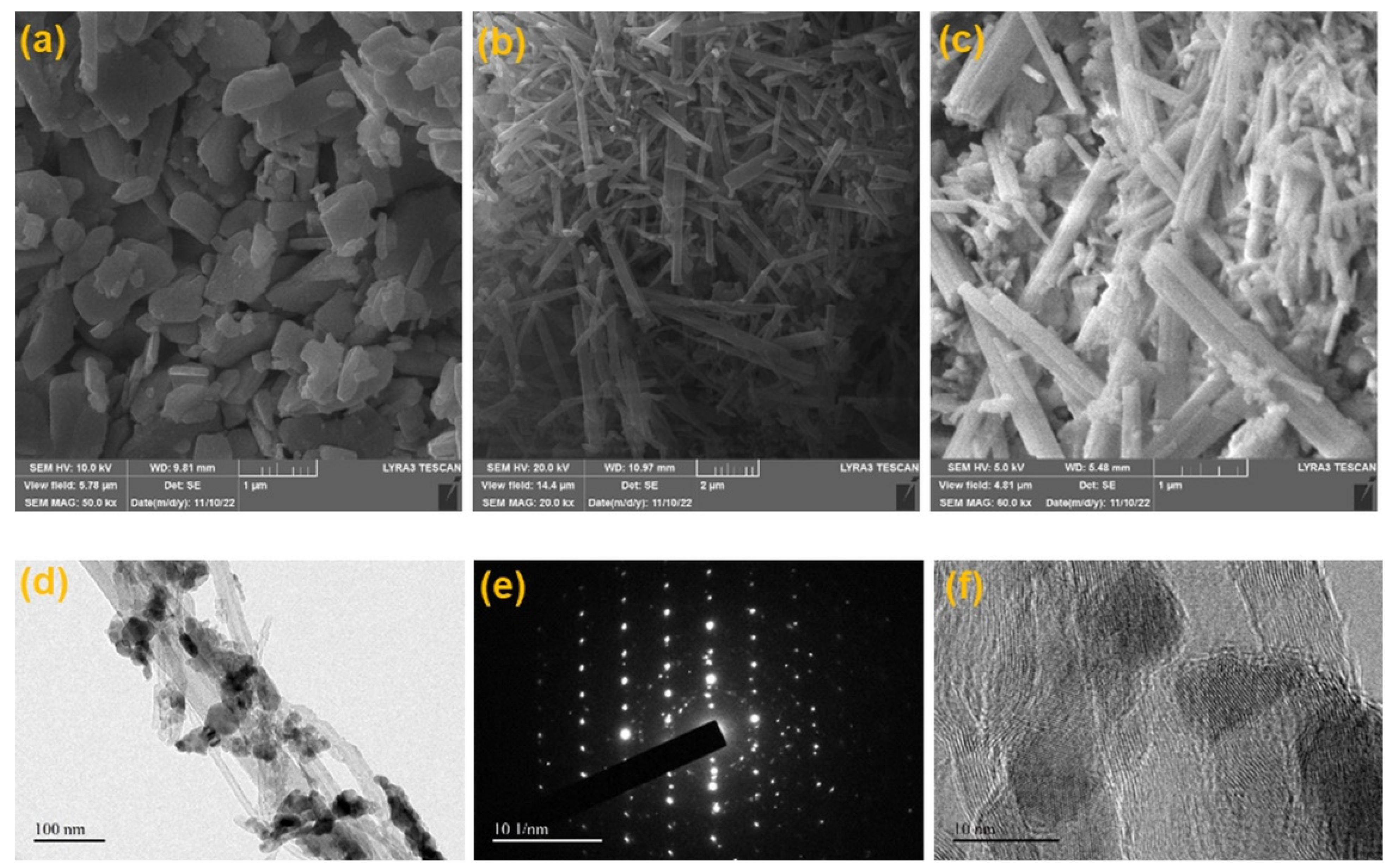
2.2. Scaning Electron Microscopy (SEM) and High Resolution Transmission Electron Microscopy (HR-TEM)
2.3. FTIR-Spectroscopic Analysis
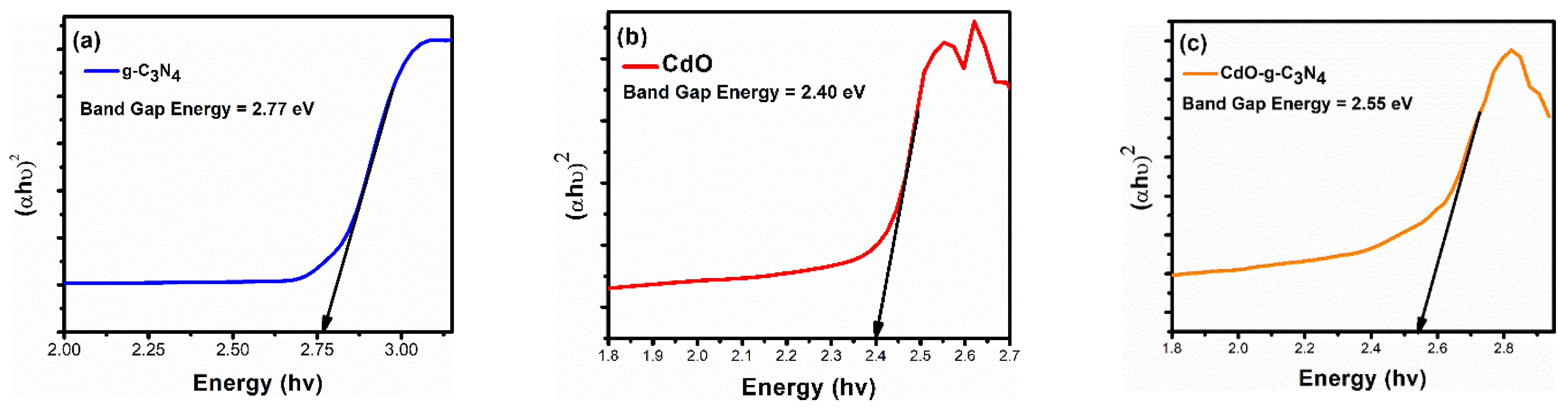
2.4. UV-Visible Analysis
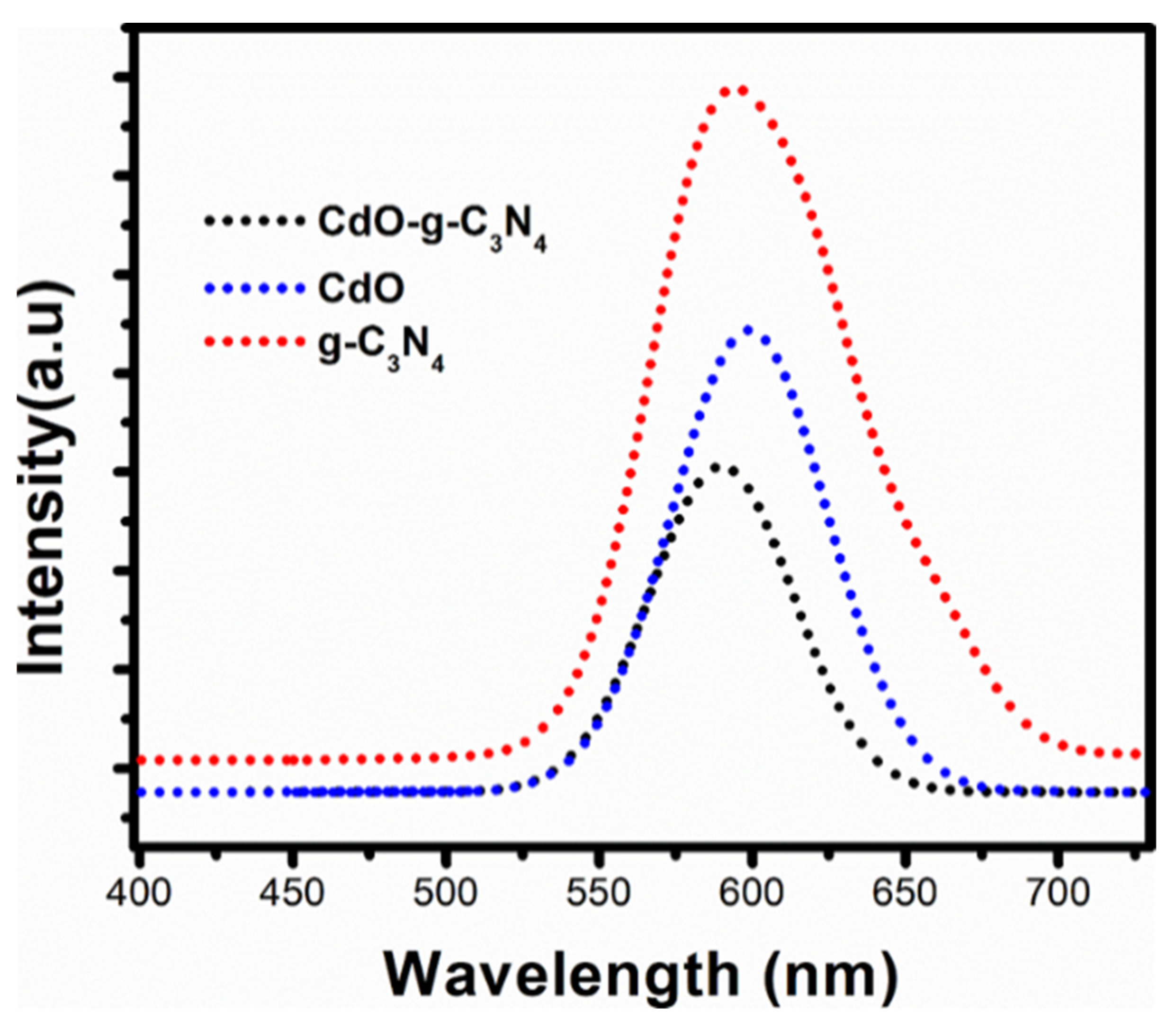
2.5. Photoluminesence (PL) Spectroscopy
3. Photoelectrochemical (PEC) Measurements
3.1. Chronoamperometric Analysis (CA)
3.2. Linear Sweep Voltammetry (LSV)
3.3. Electrochemical Impedance Spectroscopic (EIS) Analysis
3.4. Charge Transfer Mechanism
4. Experimental Strategies
4.1. Material and Chemicals
4.2. Synthesis of Graphitic-Carbon Nitride (g-C3N4)
4.3. Synthesis of Cadmium Oxide (CdO)
4.4. Synthesis of Cadmium Oxide Dopped Graphitic Carbon Nitride (CdO-g-C3N4) Nanocomposite
5. Characterization
6. Fabrication of g-C3N4/FTO, CdO/FTO, and CdO-g-C3N4/FTO Photoanodes for Photoelectrochemical (PEC) Studies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sivula, K.; van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- He, F.; Chen, G.; Zhou, Y.; Yu, Y.; Li, L.; Hao, S.; Liu, B. ZIF-8 derived carbon (C-ZIF) as a bifunctional electron acceptor and HER cocatalyst for g-C3N4: Construction of a metal-free, all carbon-based photocatalytic system for efficient hydrogen evolution. J. Mater. Chem. A 2016, 4, 3822–3827. [Google Scholar] [CrossRef]
- Kuang, Y.; Jia, Q.; Nishiyama, H.; Yamada, T.; Kudo, A.; Domen, K. A Front-Illuminated Nanostructured Transparent BiVO4 Photoanode for >2% Efficient Water Splitting. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Lai, C.W. Surface Morphology and Growth of Anodic Titania Nanotubes Films: Photoelectrochemical Water Splitting Studies. J. Nanomater. 2015, 2015, 7. [Google Scholar] [CrossRef]
- Appavoo, K.; Liu, M.; Black, C.T.; Sfeir, M.Y. Quantifying Bulk and Surface Recombination Processes in Nanostructured Water Splitting Photocatalysts via In Situ Ultrafast Spectroscopy. Nano Lett. 2015, 15, 1076–1082. [Google Scholar] [CrossRef]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Uemura, J.; Kazoe, Y.; Kosar, S.; Makita, K.; Sugaya, T.; Matsui, T.; Fujita, D.; et al. Photocatalytic generation of hydrogen by core-shell WO3/BiVO4 nanorods with ultimate water splitting efficiency. Sci. Rep. 2015, 5, 11141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kuai, L.; Liu, Y.; Wang, P.; Arandiyan, H.; Cao, S.; Zhang, J.; Li, F.; Wang, Q.; Geng, B.; et al. Well-Constructed Single-Layer Molybdenum Disulfide Nanorose Cross-Linked by Three Dimensional-Reduced Graphene Oxide Network for Superior Water Splitting and Lithium Storage Property. Sci. Rep. 2015, 5, 8722. [Google Scholar] [CrossRef]
- Ai, G.; Mo, R.; Li, H.; Zhong, J. Cobalt phosphate modified TiO2 nanowire arrays as co-catalysts for solar water splitting. Nanoscale 2015, 7, 6722–6728. [Google Scholar] [CrossRef]
- Qurashi, A.; Zhang, Z.; Asif, M.; Yamazaki, T. Template-less surfactant-free hydrothermal synthesis NiO nanoflowers and their photoelectrochemical hydrogen production. Int. J. Hydrogen Energy 2015, 40, 15801–15805. [Google Scholar] [CrossRef]
- Avasare, V.; Zhang, Z.; Avasare, D.; Khan, I.; Qurashi, A. Room-temperature synthesis of TiO2 nanospheres and their solar driven photoelectrochemical hydrogen production. Int. J. Energy Res. 2015, 39, 1714–1719. [Google Scholar] [CrossRef]
- Kanan, M.W.; Surendranath, Y.; Nocera, D.G. Cobalt-phosphate oxygen-evolving compound. Chem. Soc. Rev. 2009, 38, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Momirlan, M.; Veziroglu, T.N. The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet. Int. J. Hydrogen Energy 2005, 30, 795–802. [Google Scholar] [CrossRef]
- Iqbal, N. Ultrasonically anchored MoO3-g-C3N4 photocatalyst for enhanced solar driven hydrogen generation and environmental remediation. J. Photochem. Photobiol. A Chem. 2022, 427, 113813. [Google Scholar] [CrossRef]
- Song, H.; Luo, S.; Huang, H.; Deng, B.; Ye, J. Solar-Driven Hydrogen Production: Recent Advances, Challenges, and Future Perspectives. ACS Energy Lett. 2022, 7, 1043–1065. [Google Scholar] [CrossRef]
- Iqbal, N.; Afzal, A.; Khan, I.; Khan, M.S.; Qurashi, A. Molybdenum impregnated g-C3N4 nanotubes as potentially active photocatalyst for renewable energy applications. Sci. Rep. 2021, 11, 16886. [Google Scholar] [CrossRef]
- Sher, M.; Javed, M.; Shahid, S.; Iqbal, S.; Qamar, M.A.; Bahadur, A.; Qayyum, M.A. The controlled synthesis of g-C3N4/Cd-doped ZnO nanocomposites as potential photocatalysts for the disinfection and degradation of organic pollutants under visible light irradiation. RSC Adv. 2021, 11, 2025–2039. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, I.; Yamani, Z.H.; Qurashi, A. Sonochemical Assisted Solvothermal Synthesis of Gallium Oxynitride Nanosheets and their Solar-Driven Photoelectrochemical Water-Splitting Applications. Sci. Rep. 2016, 6, 32319. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Khaselev, O.; Turner, J.A. A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting. Science 1998, 280, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.; Cowan, A.J.; Pendlebury, S.R.; Grätzel, M.; Klug, D.R.; Durrant, J.R. The Role of Cobalt Phosphate in Enhancing the Photocatalytic Activity of α-Fe2O3 toward Water Oxidation. J. Am. Chem. Soc. 2011, 133, 14868–14871. [Google Scholar] [CrossRef]
- Wang, J.; Osterloh, F.E. Limiting factors for photochemical charge separation in BiVO4/Co3O4, a highly active photocatalyst for water oxidation in sunlight. J. Mater. Chem. A 2014, 2, 9405–9411. [Google Scholar] [CrossRef]
- Osterloh, F.E.; Parkinson, B.A. Recent developments in solar water-splitting photocatalysis. MRS Bull. 2011, 36, 17–22. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic materials as catalysts for photochemical splitting of water. Chem. Mater. 2007, 20, 35–54. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Liao, J.; Sa, B.; Zhou, J.; Ahuja, R.; Sun, Z. Design of High-Efficiency Visible-Light Photocatalysts for Water Splitting: MoS2/AlN(GaN) Heterostructures. J. Phys. Chem. C 2014, 118, 17594–17599. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, X.; Yin, J.; Peng, G.; Cui, P.; Xu, X. MoS2 nanoflowers consisting of nanosheets with a controllable interlayer distance as high-performance lithium ion battery anodes. RSC Adv. 2015, 5, 7938–7943. [Google Scholar] [CrossRef]
- Mak, K.F.; He, K.; Shan, J.; Heinz, T.F. Control of valley polarization in monolayer MoS2 by optical helicity. Nat. Nano. 2012, 7, 494–498. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic Effect of MoS2 and Graphene as Cocatalysts for Enhanced Photocatalytic H2 Production Activity of TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Han, C.; Xu, Y.-J. Insight into the Effect of Highly Dispersed MoS2 versus Layer-Structured MoS2 on the Photocorrosion and Photoactivity of CdS in Graphene–CdS–MoS2 Composites. J. Phys. Chem. C 2015, 119, 27234–27246. [Google Scholar] [CrossRef]
- Zeng, Y.-X.; Zhong, X.-W.; Liu, Z.-Q.; Chen, S.; Li, N. Preparation and enhancement of thermal conductivity of heat transfer oil-based MoS 2 nanofluids. J. Nanomater. 2013, 2013, 3. [Google Scholar] [CrossRef]
- Gulino, A.; Tabbì, G. CdO thin films: A study of their electronic structure by electron spin resonance spectroscopy. Appl. Surf. Sci. 2005, 245, 322–327. [Google Scholar] [CrossRef]
- Mahdi, R.R.; Makki, S.A. Synthesis and Properties of Cadmium Oxide Thin Films Prepared By Simple Chemical Method. Energy Procedia 2019, 157, 261–269. [Google Scholar] [CrossRef]
- Chu, T.L.; Chu, S.S. Degenerate cadmium oxide films for electronic devices. J. Electron. Mater. 1990, 19, 1003–1005. [Google Scholar] [CrossRef]
- Ishihara, K.; Kubota, M.; Kurihara, H.; Yamamoto, H. Scandium Trifluoromethanesulfonate as an Extremely Active Acylation Catalyst. J. Am. Chem. Soc. 1995, 117, 4413–4414. [Google Scholar] [CrossRef]
- Saravanan, P.; Singh, V.K. An efficient method for acylation reactions. Tetrahedron Lett. 1999, 40, 2611–2614. [Google Scholar] [CrossRef]
- Munusamy, T.D.; Yee, C.S.; Khan, M.M.R. Construction of hybrid g-C3N4/CdO nanocomposite with improved photodegradation activity of RhB dye under visible light irradiation. Adv. Powder Technol. 2020, 31, 2921–2931. [Google Scholar] [CrossRef]
- Iqbal, N. Tailoring g-C3N4 with Lanthanum and Cobalt Oxides for Enhanced Photoelectrochemical and Photocatalytic Activity. Catalysts 2022, 12, 15. [Google Scholar] [CrossRef]
- Wang, L.; Si, W.; Tong, Y.; Hou, F.; Pergolesi, D.; Hou, J.; Lippert, T.; Dou, S.X.; Liang, J. Graphitic carbon nitride (g-C3N4)-based nanosized heteroarrays: Promising materials for photoelectrochemical water splitting. Carbon Energy 2020, 2, 223–250. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Imani, M.; Kerdari, H. Experimental design to optimize the synthesis of CdO cauliflower-like nanostructure and high performance in photodegradation of toxic azo dyes. Mater. Res. Bull. 2013, 48, 935–942. [Google Scholar] [CrossRef]
- Venkata Reddy, C.; Bandaru, N.; Shim, J.; Vattikuti, S.V.P. Synthesis of CdO/ZnS heterojunction for photodegradation of organic dye molecules. Appl. Phys. A 2017, 123, 396. [Google Scholar] [CrossRef]
- Dhanalekshmi, K.I.; Magesan, P.; Umapathy, M.J.; Zhang, X.; Srinivasan, N.; Jayamoorthy, K. Enhanced photocatalytic and photodynamic activity of chitosan and garlic loaded CdO–TiO2 hybrid bionanomaterials. Sci. Rep. 2021, 11, 20790. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, M.; Xie, S.; Zhai, T.; Yu, M.; Liang, C.; Ouyang, X.; Lu, X.; Li, H.; Tong, Y. Improving the photoelectrochemical and photocatalytic performance of CdO nanorods with CdS decoration. CrystEngComm 2013, 15, 4212–4216. [Google Scholar] [CrossRef]
- Saravanan, R.; Shankar, H.; Prakash, T.; Narayanan, V.; Stephen, A. ZnO/CdO composite nanorods for photocatalytic degradation of methylene blue under visible light. Mater. Chem. Phys. 2011, 125, 277–280. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, H.; Wang, Z.; Wang, H.; He, Y.; Tian, Q.; Luo, Q.; Xu, P.J.E.S.; Research, P. Facile synthesis of cadmium-doped graphite carbon nitride for photocatalytic degradation of tetracycline under visible light irradiation. Environ. Sci. Pollut. Res. 2022, 29, 74062–74080. [Google Scholar] [CrossRef]
- Abu Hanif, M.; Akter, J.; Akherul Islam, M.; Sapkota, K.P.; Hahn, J.R. Visible-light-driven enhanced photocatalytic performance using cadmium-doping of tungsten (VI) oxide and nanocomposite formation with graphitic carbon nitride disks. Appl. Surf. Sci. 2021, 565, 150541. [Google Scholar] [CrossRef]
- Karimi, M.A.; Atashkadi, M.; Ranjbar, M.; Habibi-Yangjeh, A. Novel visible-light-driven photocatalyst of NiO/Cd/g-C3N4 for enhanced degradation of methylene blue. Arab. J. Chem. 2020, 13, 5810–5820. [Google Scholar] [CrossRef]
- Lu, H.B.; Liao, L.; Li, H.; Tian, Y.; Wang, D.F.; Li, J.C.; Fu, Q.; Zhu, B.P.; Wu, Y. Fabrication of CdO nanotubes via simple thermal evaporation. Mater. Lett. 2008, 62, 3928–3930. [Google Scholar] [CrossRef]
- Yang, Z.-x.; Zhong, W.; Yin, Y.-x.; Du, X.; Deng, Y.; Au, C.; Du, Y.-w. Controllable Synthesis of Single-Crystalline CdO and Cd(OH)2Nanowires by a Simple Hydrothermal Approach. Nanoscale Res. Lett. 2010, 5, 961. [Google Scholar] [CrossRef]
- Yin, L.; Ding, X.; Wei, W.; Wang, Y.; Zhu, Z.; Xu, K.; Zhao, Z.; Zhao, H.; Yu, T.; Yang, T. Improving catalysis for electrochemical water splitting using a phosphosulphide surface. Inorg. Chem. Front. 2020, 7, 2388–2395. [Google Scholar] [CrossRef]
- Przezdziecka, E.; Wierzbicka, A.; Dluzewski, P.; Sankowska, I.; Morawiec, K.; Pietrzyk, M.; Kozanecki, A.J. CdO/MgO superlattices grown by MBE as cubic CdMgO quasi-alloys. arXiv 2020, arXiv:2003.04704. [Google Scholar]
- Elshafie, M.; Younis, S.A.; Serp, P.; Gad, E.A.M. Preparation characterization and non-isothermal decomposition kinetics of different carbon nitride sheets. Egypt. J. Pet. 2020, 29, 21–29. [Google Scholar] [CrossRef]
- Prakash, T.; Thirugnanasambantham, A.; Raj, D.; Rajan, J. Surfactant-liaised Variation in CdO Nanocomposites Morphology. Phys. Procedia 2013, 49, 36–43. [Google Scholar] [CrossRef]
- Dharma, J.; Pisal, A.; Shelton, C.J.A.N.S. CT: PerkinElmer, Simple Method of Measuring the Band Gap Energy Value of TiO2 in the Powder Form Using a UV/Vis/NIR Spectrometer. Appl. Note Shelton CT: PerkinElmer. 2009, pp. 1–4. Available online: https://www.perkinelmer.com/libraries/APP_UVVISNIRMeasureBandGapEnergyValue (accessed on 20 October 2022).
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Davis, E.A.; Mott, N.F. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1970, 22, 0903–0922. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, B.; Mao, L.; Cheng, C.; Hu, Y.; Wang, H.; Li, G.; Jing, D.; Liang, X. MoO3/g-C3N4 Z-scheme (S-scheme) system derived from MoS2/melamine dual precursors for enhanced photocatalytic H2 evolution driven by visible light. Int. J. Hydrogen Energy 2021, 46, 2927–2935. [Google Scholar] [CrossRef]
- Pawade, V.; Salame, P.H.; Bhanvase, B.A. Multifunctional Nanostructured Metal Oxides for Energy Harvesting and Storage Devices; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Wrede, S.; Tian, H. Towards sustainable and efficient p-type metal oxide semiconductor materials in dye-sensitised photocathodes for solar energy conversion. Phys. Chem. Chem. Phys. 2020, 22, 13850–13861. [Google Scholar] [CrossRef]
- Rehman, A.; Ehsan, M.A.; Afzal, A.; Ali, A.; Iqbal, N. Aerosol-assisted nanostructuring of nickel/cobalt oxide thin films for viable electrochemical hydrazine sensing. Analyst 2021, 146, 3317–3327. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, B.; Zhang, J.; Wang, R.; Liu, H. Rational construction of a direct Z-scheme g-C3N4/CdS photocatalyst with enhanced visible light photocatalytic activity and degradation of erythromycin and tetracycline. Appl. Surf. Sci. 2019, 478, 1056–1064. [Google Scholar] [CrossRef]
- Kumar, S.; Ahmed, B.; Ojha, A.K.; Das, J.; Kumar, A. Facile synthesis of CdO nanorods and exploiting its properties towards supercapacitor electrode materials and low power UV irradiation driven photocatalysis against methylene blue dye. Mater. Res. Bull. 2017, 90, 224–231. [Google Scholar] [CrossRef]





| # | Photocatalyst | Synthetic Strategy | Photoelectrochemical/ Photodegradation Study | Band Gap Energy (eV) | Current Density/Hydrogen/Oxygen Generation | Ref. |
|---|---|---|---|---|---|---|
| 1 | CdO cauliflower | Mechanochemical process followed by heating treatment | 95%, 91.5%, and 98% photodegradation Congo red, Malachite green and Crystal violet | 2.22 | _ | [41] |
| 2 | CdS/CdO | Co-precipitation | 92% Photodegradation of the RhB | 2.9 | _ | [42] |
| 3 | CdO–TiO2 | sol–gel method | >70% Methylene blue (MB), Methyl orange (MO) and Rhodamine B (Rh-B) degradation efficiency | 3.12–3.19 | _ | [43] |
| 4 | CdO–CdS | Electrochemical deposition | 71.1% of photodegradation efficiency of MB. | 2.25–2.29 | 2.6 mA/cm2 | [44] |
| 5 | ZnO/CdO | Thermal decomposition | 97.8% degradation efficiency for MB | 2.99 | _ | [45] |
| 6 | g-C3N4/CdO | Chemical precipitation & self-assembly | 96% rhodamine B(RhB) dye removal efficiency. | 2.35 | _ | [38] |
| 7 | Cd-g-C3N4 | Thermal polymerization | 98.1% tetracycline (TC) degradation efficiency. | _ | _ | [46] |
| 8 | Cd-WO3 (CWC) and Cd-doped WO3@g-C3N4 (CWCC) | Ultrasonication, stirring & thermolysis | 95.98% Photodegradation Efficiency | 2.06 & 1.85 | _ | [47] |
| 9 | NiO/Cd/g-C3N4 | Microwave-assisted | 81.8% MB degradation efficiency | 3.6 | [48] | |
| 10 | Cd-ZnO/g-C3N4 | Co-precipitation & hydrothermal | >90% MB degradation efficiency | 2.50 | [17] | |
| 11 | CdO-g-C3N4 | Precipitation and Hydrothermal method | 92% efficiency of MB degradation | 2.55 | >5 mA/cm2/ H2 generation | Current work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, N.; Khan, M.S.; Zubair, M.; Khan, S.A.; Ali, A.; Aldhafeeri, N.; Alsahli, S.; Alanzi, M.; Enazi, A.; Alroyle, T.; et al. Advanced Photoelectrochemical Hydrogen Generation by CdO-g-C3N4 in Aqueous Medium under Visible Light. Molecules 2022, 27, 8646. https://doi.org/10.3390/molecules27248646
Iqbal N, Khan MS, Zubair M, Khan SA, Ali A, Aldhafeeri N, Alsahli S, Alanzi M, Enazi A, Alroyle T, et al. Advanced Photoelectrochemical Hydrogen Generation by CdO-g-C3N4 in Aqueous Medium under Visible Light. Molecules. 2022; 27(24):8646. https://doi.org/10.3390/molecules27248646
Chicago/Turabian StyleIqbal, Naseer, Muhammad Shahzeb Khan, Muhammad Zubair, Safyan Akram Khan, Asghar Ali, Naif Aldhafeeri, Saud Alsahli, Misheal Alanzi, Abdelazeez Enazi, Talal Alroyle, and et al. 2022. "Advanced Photoelectrochemical Hydrogen Generation by CdO-g-C3N4 in Aqueous Medium under Visible Light" Molecules 27, no. 24: 8646. https://doi.org/10.3390/molecules27248646
APA StyleIqbal, N., Khan, M. S., Zubair, M., Khan, S. A., Ali, A., Aldhafeeri, N., Alsahli, S., Alanzi, M., Enazi, A., Alroyle, T., & Alrashidi, A. (2022). Advanced Photoelectrochemical Hydrogen Generation by CdO-g-C3N4 in Aqueous Medium under Visible Light. Molecules, 27(24), 8646. https://doi.org/10.3390/molecules27248646







