Donor-Acceptor Dyads and Triads Employing Core-Substituted Naphthalene Diimides: A Synthetic and Spectro (Electrochemical) Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Dyads and Triads
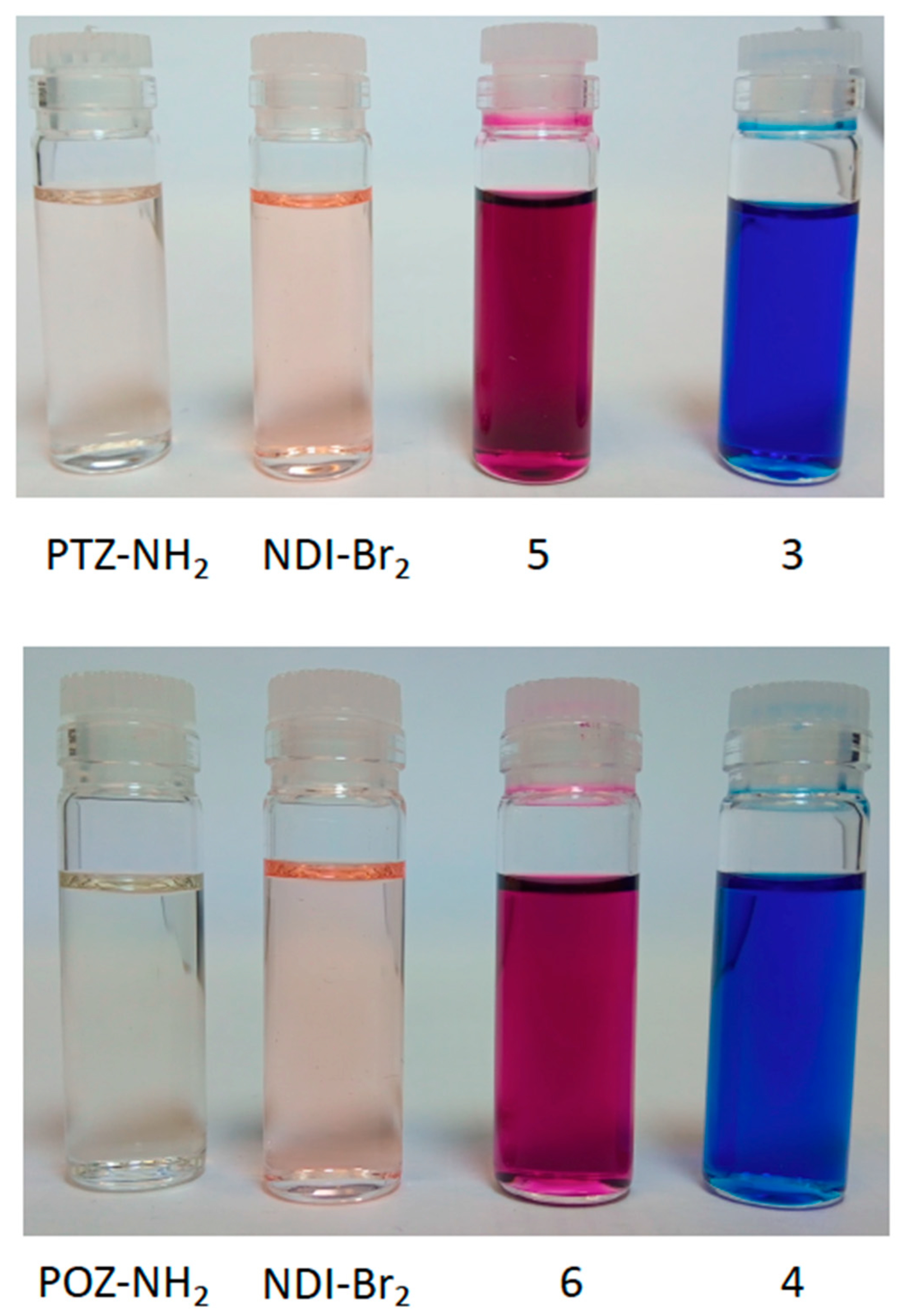
2.2. Investigation of Optical and Electrochemical Properties
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pearce, N.; Davies, E.S.; Horvath, R.; Pfeiffer, C.R.; Sun, X.-Z.; Lewis, W.; McMaster, J.; George, M.W.; Champness, N.R. Thionated naphthalene diimides: Tuneable chromophores for applications in photoactive dyads. Phys. Chem. Chem. Phys. 2018, 20, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.; Davies, E.S.; Pfeiffer, C.R.; Lewis, W.; McMaster, J.; Champness, N.R. Core-Substituted Naphthalene Diimides: Influence of Substituent Conformation on Strong Visible Absorption. ChemPlusChem 2017, 82, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.; Davies, E.S.; Lewis, W.; Champness, N.R. Thionated Perylene Diimide–Phenothiazine Dyad: Synthesis, Structure, and Electrochemical Studies. ACS Omega 2018, 3, 14236–14244. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.; Reynolds, K.E.A.; Kayal, S.; Sun, X.Z.; Davies, E.S.; Malagreca, F.; Schürmann, C.J.; Ito, S.; Yamano, A.; Argent, S.P.; et al. Selective photoinduced charge separation in perylenediimide-pillar[5]arene rotaxanes. Nat. Commun. 2022, 13, 415. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.V.; al Kobaisi, M.; Jadhav, R.W.; Morajkar, P.P.; Jones, L.A.; George, S. Naphthalene diimides: Perspectives and promise. Chem. Soc. Rev. 2021, 50, 9845–9998. [Google Scholar] [CrossRef]
- Ha, Y.H.; Oh, J.G.; Park, S.; Kwon, S.-K.; An, T.K.; Jang, J.; Kim, Y.-H. Novel naphthalene-diimide-based small molecule with a bithiophene linker for use in organic field-effect transistors. Org. Electron. 2018, 63, 250–256. [Google Scholar] [CrossRef]
- Sommer, M.J. Conjugated polymers based on naphthalene diimide for organic electronics. Mater. Chem. C 2014, 2, 3088–3098. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Y.; Liu, Y. 25th Anniversary Article: Recent Advances in n-Type and Ambipolar Organic Field-Effect Transistors. Adv. Mater. 2013, 25, 5372–5391. [Google Scholar] [CrossRef]
- Zhou, N.; Facchetti, A. Naphthalenediimide (NDI) polymers for all-polymer photovoltaics. Mater. Today 2018, 21, 377–390. [Google Scholar] [CrossRef]
- Valero, S.; Cabrera-Espinoza, A.; Collavini, S.; Pascual, J.; Marinova, N.; Kosta, I.; Delgado, J.L. Naphthalene Diimide-Based Molecules for Efficient and Stable Perovskite Solar Cells. Eur. J. Org. 2020, 33, 5329–5339. [Google Scholar] [CrossRef]
- Liu, G.; Xiao, C.; Negri, F.; Li, Y.; Wang, Z. Dodecatwistarene Imides with Zigzag-Twisted Conformation for Organic Electronics. Angew. Chem. Int. Ed. 2020, 59, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, G.; Zhang, L.; Wang, Z. Integrating pyracylene and naphthalenediimides into planar structures: Synthesis and characterization. Dyes Pigments 2019, 168, 295–299. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, C.-H.; She, N.-Z.; Juan, C.-Y.; Chang, B.; Li, M.-H.; Wang, H.-C.; Cheng, H.-W.; Yabushita, A.; Yang, Y.; et al. Twisted-graphene-like perylene diimide with dangling functional chromophores as tunable small-molecule acceptors in binary-blend active layers of organic photovoltaics. J. Mater. Chem. A 2021, 9, 20510–20517. [Google Scholar] [CrossRef]
- Lin, Y.-C.; She, N.-Z.; Chen, C.-H.; Yabushita, A.; Lin, H.; Li, M.-H.; Chang, B.; Hsueh, T.-F.; Tsai, B.-S.; Chen, P.-T.; et al. Perylene Diimide-Fused Dithiophenepyrroles with Different End Groups as Acceptors for Organic Photovoltaics. ACS Appl. Mater. Interfaces 2022, 14, 37990–38003. [Google Scholar] [CrossRef] [PubMed]
- Macreadie, L.K.; Gilchrist, A.M.; McNaughton, D.A.; Ryder, W.G.; Fares, M.; Gale, P.A. Progress in anion receptor chemistry. Chem 2022, 8, 46–118. [Google Scholar] [CrossRef]
- Hein, R.; Beer, P.D. Halogen bonding and chalcogen bonding mediated sensing. Chem. Sci. 2022, 13, 7098–7125. [Google Scholar] [CrossRef]
- Luo, N.; Ao, Y.-F.; Wang, D.-X.; Wang, Q.-Q. Putting Anion-π Interactions at Work for Catalysis. Chem. Eur. J. 2022, 28, e2021033. [Google Scholar] [CrossRef]
- Ling, Q.-H.; Zhu, J.-L.; Qin, Y.; Xu, L. Naphthalene diimide- and perylene diimide-based supramolecular cages. Mater. Chem. Front. 2020, 4, 3176–3189. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, L. Recent advances in naphthalenediimide-based metal-organic frameworks: Structures and applications. Coord. Chem. Rev. 2021, 430, 213665. [Google Scholar] [CrossRef]
- Sweetman, A.M.; Jarvis, S.; Sang, H.; Lekkas, I.; Rahe, P.; Wang, Y.; Wang, J.; Champness, N.R.; Kantorovich, L.; Moriarty, P.J. Mapping the force field of a hydrogen-bonded assembly. Nat. Commun. 2014, 5, 3931. [Google Scholar] [CrossRef]
- Palma, C.-A.; Bjork, J.; Bonini, M.; Dyer, M.S.; Llanes-Pallas, A.; Bonifazi, D.; Persson, M.; Samori, P. Tailoring Bicomponent Supramolecular Nanoporous Networks: Phase Segregation, Polymorphism, and Glasses at the Solid−Liquid Interface. J. Am. Chem. Soc. 2009, 131, 13062–13071. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.; Perez-Velasco, A.; Ravikumar, V.; Kishore, R.S.K.; Kel, O.; Gomez-Casado, A.; Jonkheijm, P.; Huskens, J.; Maroni, P.; Borkovec, M.; et al. Topologically Matching Supramolecular n/p-Heterojunction Architectures. Angew. Chem. Int. Ed. 2009, 48, 6461–6464. [Google Scholar] [CrossRef] [PubMed]
- Kishore, R.S.K.; Kel, O.; Banerji, N.; Emery, D.; Bollot, G.; Mareda, J.; Gomez-Casado, A.; Jonkheijm, P.; Huskens, J.; Maroni, P.; et al. Ordered and Oriented Supramolecular n/p-Heterojunction Surface Architectures: Completion of the Primary Color Collection. J. Am. Chem. Soc. 2009, 131, 11106–11116. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Sukhanov, A.A.; Zhao, J.; Yang, W.; Wang, Z.; Liu, Q.; Voronkova, V.K.; di Donato, M.; Escudero, D.; Jacquemin, D. Red Thermally Activated Delayed Fluorescence and the Intersystem Crossing Mechanisms in Compact Naphthalimide–Phenothiazine Electron Donor/Acceptor Dyads. J. Phys. Chem. C 2019, 123, 30171–30186. [Google Scholar] [CrossRef]
- Ye, K.; Cao, L.; van Raamsdonk, D.M.E.; Wang, Z.; Zhao, J.; Escudero, D.; Jacquemin, D. Naphthalimide-phenothiazine dyads: Effect of conformational flexibility and matching of the energy of the charge-transfer state and the localized triplet excited state on the thermally activated delayed fluorescence. Beilstein J. Org. Chem. 2022, 18, 1435–1453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Taddei, M.; Bussotti, L.; Kurganskii, I.; Li, M.; Jiang, X.; Xing, L.; Ji, S.; Huo, Y.; et al. Red Light-Emitting Thermally-Activated Delayed Fluorescence of Naphthalimide-Phenoxazine Electron Donor-Acceptor Dyad: Time-Resolved Optical and Magnetic Spectroscopic Studies. Chem. Eur. J. 2022, 28, e202200510. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.A.; Ahrens, M.J.; Sinks, L.E.; Gusev, A.V.; Ratner, M.A.; Wasielewski, M.R. Making a Molecular Wire: Charge and Spin Transport through para-Phenylene Oligomers. J. Am. Chem. Soc. 2004, 126, 5577–5584. [Google Scholar] [CrossRef]
- Damaceanu, M.-D.; Constantin, C.-P.; Bejan, A.-E.; Mihaila, M.; Kusko, M.; Diaconuc, C.; Mihalache, I.; Pascu, R. Heteroatom-mediated performance of dye-sensitized solar cells based on T-shaped molecules. Dyes Pigm. 2019, 166, 15–31. [Google Scholar] [CrossRef]
- Suseela, Y.V.; Sasikumar, M.; Govindaraju, T. An effective and regioselective bromination of 1,4,5,8-naphthalenetetracarboxylic dianhydride using tribromoisocyanuric acid. Tetrahedron Lett. 2013, 54, 6314–6318. [Google Scholar] [CrossRef]
- Sridhar, M.A.; Ramegowda, M.; Lokanath, N.K.; Prasad, J.S.; Gowda, G.B.E.; Thimmaiah, K.N. Structural Studies of Some Phenoxazine Derivatives. Mol. Cryst. Liq. Cryst. 1999, 326, 189–214. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, M.Z. The crystal structure of 10-(3,5-di(pyridin-4-yl)phenyl)-10H-phenoxazine dihydrate, C28H23N3O3. Z. Krist.–New Cryst. St. 2021, 236, 605–607. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K. and Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]








| Compound | 1st Ox E1/2/V | 1st Red E1/2/V | 2nd Red E1/2/V |
|---|---|---|---|
| 1 | +0.33 (0.07) | −1.06 (0.07) | −1.48 (0.07) |
| 2 | +0.36 (0.07) | −1.04 (0.07) | −1.48 (0.07) |
| 3 | +0.31 (0.08) | −1.14 (0.08) | −1.52 (0.08) |
| 4 | +0.33 (0.08) | −1.12 (0.07) | −1.51 (0.07) |
| 7 | +0.32 (0.08) | −1.12 (0.07) | −1.51 (0.07) |
| 8 | +0.31 (0.07) | −1.15 (0.07) | −1.56 (0.07) |
| 9 | +0.33 (0.07) | −1.14 (0.07) | −1.54 (0.07) |
| POZ-NH2 | +0.25 (0.08) | ||
| PTZ-NH2 | +0.21 (0.07) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinn, S.; Davies, E.S.; Pearce, N.; Rosenberg, C.; Pfeiffer, C.R.; Orton, G.R.F.; Champness, N.R. Donor-Acceptor Dyads and Triads Employing Core-Substituted Naphthalene Diimides: A Synthetic and Spectro (Electrochemical) Study. Molecules 2022, 27, 8671. https://doi.org/10.3390/molecules27248671
Quinn S, Davies ES, Pearce N, Rosenberg C, Pfeiffer CR, Orton GRF, Champness NR. Donor-Acceptor Dyads and Triads Employing Core-Substituted Naphthalene Diimides: A Synthetic and Spectro (Electrochemical) Study. Molecules. 2022; 27(24):8671. https://doi.org/10.3390/molecules27248671
Chicago/Turabian StyleQuinn, Samuel, E. Stephen Davies, Nicholas Pearce, Callum Rosenberg, Constance R. Pfeiffer, Georgia R. F. Orton, and Neil R. Champness. 2022. "Donor-Acceptor Dyads and Triads Employing Core-Substituted Naphthalene Diimides: A Synthetic and Spectro (Electrochemical) Study" Molecules 27, no. 24: 8671. https://doi.org/10.3390/molecules27248671
APA StyleQuinn, S., Davies, E. S., Pearce, N., Rosenberg, C., Pfeiffer, C. R., Orton, G. R. F., & Champness, N. R. (2022). Donor-Acceptor Dyads and Triads Employing Core-Substituted Naphthalene Diimides: A Synthetic and Spectro (Electrochemical) Study. Molecules, 27(24), 8671. https://doi.org/10.3390/molecules27248671






