The Biomedical Importance of the Missing Pathway for Farnesol and Geranylgeraniol Salvage
Abstract
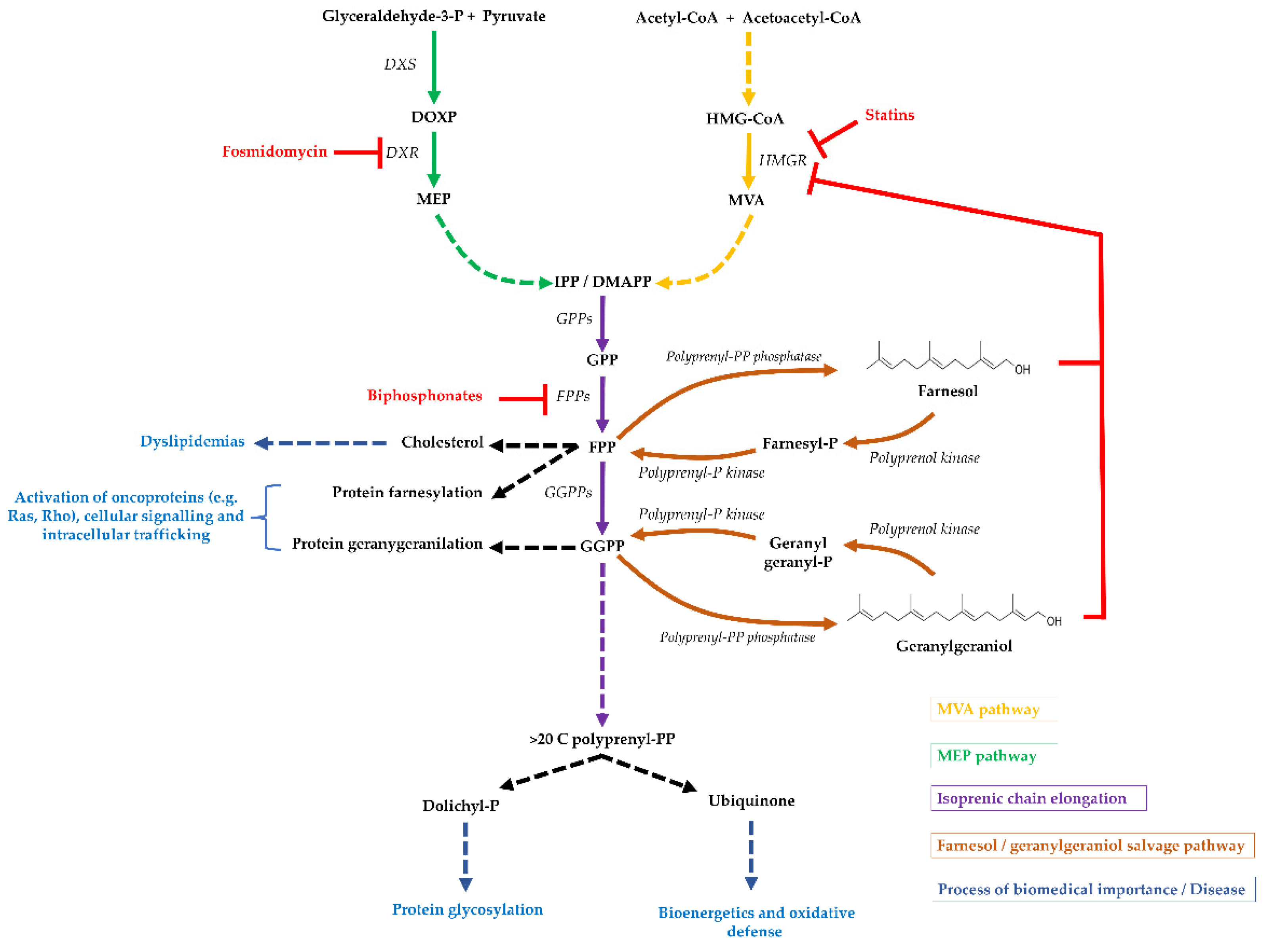
1. Isoprenoid Biosynthesis and Distribution
2. The Origin of Geranylgeraniol and Farnesol
3. Geranylgeraniol and Farnesol Are Incorporated into Cellular Components
4. The Geranylgeraniol and Farnesol Salvage Pathway
5. The Few Polyprenol/Polyprenyl-P Kinases Discovered
6. Biomedical Importance of the Geranylgeraniol and Farnesol Salvage Pathway
6.1. Oncology
6.2. Infectious Diseases
6.3. Isoprenoid Deficiencies
6.4. Dyslipidemias
6.5. Rare Diseases
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| Acetyl-CoA | Acetyl-coenzyme A |
| ATP | Adenosine triphosphate |
| CoA | Coenzyme A |
| CDP | Cytidine diphosphate |
| CTP | Cytidine triphosphate |
| DMAPP | Dimethylallyl diphosphate |
| DOXP | 1-deoxy-D-xylulose 5-phosphate |
| DXR | 1-Deoxy-d-xylulose 5-phosphate reductase |
| DXS | 1-Deoxy-d-xylulose 5-phosphate synthase |
| FOH | Farnesol |
| FP | Farnesyl monophosphate |
| FPP | Farnesyl pyrophosphate |
| FPPs | farnesyl pyrophosphate synthase |
| GGPP | Geranylgeranyl pyrophosphate |
| GGPPs | Geranylgeranyl pyrophosphate synthase |
| GGOH | Geranylgeraniol |
| GPP | Geranyl pyrophosphate |
| GPPs | Geranyl pyrophosphate synthase |
| HMBPP | Hydroxymethylbutenyl diphosphate |
| HMG | 3-Hydroxy-3-methyl-glutaryl |
| HMG-CoA | 3-Hydroxy-3-methyl-glutaryl- coenzyme A |
| HMGR | 3-Hydroxy-3-methyl-glutaryl- coenzyme A reductase |
| IPP | Isopentenyl diphosphate |
| LytB | Hydroxymethylbutenyl diphosphate reductase |
| MEP | Methyl erythritol phosphate |
| MVA | Mevalonate |
| UTP | Uridine triphosphate |
References
- Nakatani, Y.; Ribeiro, N.; Streiff, S.; Gotoh, M.; Pozzi, G.; Désaubry, L.; Milon, A. Search for the most ‘primitive’ membranes and their reinforcers: A review of the polyprenyl phosphates theory. Orig. Life Evol. Biosph. 2014, 44, 197–208. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Non-mevalonate isoprenoid biosynthesis: Enzymes, genes and inhibitors. Biochem. Soc. Trans. 2000, 28, 785–789. [Google Scholar] [CrossRef]
- Miziorko, H.M. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch. Biochem. Biophys. 2011, 505, 131–143. [Google Scholar] [CrossRef]
- Suga, T.; Hirata, T.; Shishibori, T.; Tange, K. The first proof of the biosynthesis of isoprenoid from amino acid in higher plant. The incorporation of L-Leucine into linalool. Chem. Lett. 1974, 3, 189–192. [Google Scholar] [CrossRef]
- Ginger, M.L.; Chance, M.L.; Sadler, I.H.; Goad, L.J. The biosynthetic incorporation of the intact leucine skeleton into sterol by the trypanosomatid Leishmania mexicana. J. Biol. Chem. 2001, 276, 11674–11682. [Google Scholar] [CrossRef]
- Verdaguer, I.B.; Zafra, C.A.; Crispim, M.; Sussmann, R.A.; Kimura, E.A.; Katzin, A.M. Prenylquinones in human parasitic protozoa: Biosynthesis, physiological functions, and potential as chemotherapeutic targets. Molecules 2019, 24, 3721. [Google Scholar] [CrossRef]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef]
- Jackson, E.R.; Dowd, C.S. Inhibition of 1-deoxy-D-xylulose-5-phosphate reductoisomerase (Dxr): A review of the synthesis and biological evaluation of recent inhibitors. Curr. Top. Med. Chem. 2012, 12, 706–728. [Google Scholar] [CrossRef]
- Lell, B.; Ruangweerayut, R.; Wiesner, J.; Missinou, M.A.; Schindler, A.; Baranek, T.; Kremsner, P.G. Fosmidomycin, a novel chemotherapeutic agent for malaria. Antimicrob. Agents Chemother. 2003, 47, 735–738. [Google Scholar] [CrossRef]
- Kakimoto, T. Biosynthesis of cytokinins. J. Plant Res. 2003, 116, 233–239. [Google Scholar] [CrossRef]
- Kellogg, B.A.; Poulter, C.D. Chain elongation in the isoprenoid biosynthetic pathway. Curr. Opin. Chem. Biol. 1997, 1, 570–578. [Google Scholar] [CrossRef]
- Yamashita, S.; Takahashi, S. Molecular mechanisms of natural rubber biosynthesis. Annu. Rev. Biochem. 2020, 89, 821–851. [Google Scholar] [CrossRef] [PubMed]
- Gutbrod, K.; Romer, J.; Dörmann, P. Phytol metabolism in plants. Prog. Lipid Res. 2019, 74, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Skorupinska-Tudek, K.; Wojcik, J.; Swiezewska, E. Polyisoprenoid alcohols—Recent results of structural studies. Chem. Rec. 2008, 8, 33–45. [Google Scholar] [CrossRef]
- Burda, P.; Aebi, M. The dolichol pathway of N-linked glycosylation. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 1999, 1426, 239–257. [Google Scholar] [CrossRef]
- Glomset, J.A.; Gelb, M.H.; Farnsworth, C.C. Prenyl proteins in eukaryotic cells: A new type of membrane anchor. Trends Biochem. Sci. 1990, 15, 139–142. [Google Scholar] [CrossRef]
- McTaggart, S.J. Isoprenylated proteins. Cell. Mol. Life Sci. CMLS 2006, 63, 255–267. [Google Scholar] [CrossRef]
- Gutkowska, M.; Bieńkowski, T.; Hung, V.S.; Wanke, M.; Hertel, J.; Danikiewicz, W.; Swiezewska, E. Proteins are polyisoprenylated in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2004, 322, 998–1004. [Google Scholar] [CrossRef]
- D’Alexandri, F.L.; Kimura, E.A.; Peres, V.J.; Katzin, A.M. Protein dolichylation in Plasmodium falciparum. FEBS Lett. 2006, 580, 6343–6348. [Google Scholar] [CrossRef]
- Hjertman, M.; Wejde, J.; Dricu, A.; Carlberg, M.; Griffiths, W.J.; Sjövall, J.; Larsson, O. Evidence for protein dolichylation. FEBS Lett. 1997, 416, 235–238. [Google Scholar] [CrossRef]
- Hartley, M.D.; Imperiali, B. At the membrane frontier: A prospectus on the remarkable evolutionary conservation of polyprenols and polyprenyl-phosphates. Arch. Biochem. Biophys. 2012, 517, 83–97. [Google Scholar] [CrossRef][Green Version]
- Van Gelder, K.; Rea, K.A.; Virta, L.; Whitnell, K.L.; Osborn, M.; Vatta, M.; Khozin, A.; Skorupinska-Tudek, K.; Surmacz, L.; Akhtar, T.A. Medium-Chain Polyprenols Influence Chloroplast Membrane Dynamics in Solanum lycopersicum. Plant Cell Physiol. 2018, 59, 2350–2365. [Google Scholar] [CrossRef]
- Swiezewska, E.; Danikiewicz, W. Polyisoprenoids: Structure, biosynthesis and function. Prog. Lipid Res. 2005, 44, 235–258. [Google Scholar] [CrossRef]
- Bentley, R.; Meganathan, R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol. Rev. 1982, 46, 241–280. [Google Scholar] [CrossRef]
- Russell, D.W. Cholesterol biosynthesis and metabolism. Cardiovasc. Drugs Ther. 1992, 6, 103–110. [Google Scholar] [CrossRef]
- Kawamukai, M. Biosynthesis, bioproduction and novel roles of ubiquinone. J. Biosci. Bioeng. 2002, 94, 511–517. [Google Scholar] [CrossRef]
- Nowicka, B.; Kruk, J. Occurrence, biosynthesis and function of isoprenoid quinones. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 1587–1605. [Google Scholar] [CrossRef]
- Fleisch, H.; Reszka, A.; Rodan, G.; Rogers, M. Bisphosphonates: Mechanisms of action. Princ. Bone Biol. 2002, 1, 1361-XLIII. [Google Scholar]
- Rogers, M.J.; Watts, D.J.; Russell RG, G. Overview of bisphosphonates. Cancer: Interdiscip. Int. J. Am. Cancer Soc. 1997, 80, 1652–1660. [Google Scholar] [CrossRef]
- Wiesner, J.; Jomaa, H. Isoprenoid biosynthesis of the apicoplast as drug target. Curr. Drug Targets 2007, 8, 3–13. [Google Scholar] [CrossRef]
- Schroepfer, G.J., Jr.; Gore, I.Y. Chromatographic separation of allylic alcohols on silicic acid columns: Analysis of the nonsaponifiable lipids of an ascites tumor derived from a benzpyrene-induced sarcoma. J. Lipid Res. 1963, 4, 266–269. [Google Scholar] [CrossRef]
- Endo, S.; Matsunaga, T.; Ohta, C.; Soda, M.; Kanamori, A.; Kitade, Y.; Hara, A. Roles of rat and human aldo–keto reductases in metabolism of farnesol and geranylgeraniol. Chem. Biol. Interact. 2011, 191, 261–268. [Google Scholar] [CrossRef]
- Caple, M.B.; Chow, H.C.; Strouse, C.E. Photosynthetic pigments of green sulfur bacteria. The esterifying alcohols of bacteriochlorophylls c from Chlorobium limicola. J. Biol. Chem. 1978, 253, 6730–6737. [Google Scholar] [CrossRef]
- Huchelmann, A.; Brahim, M.S.; Gerber, E.; Tritsch, D.; Bach, T.J.; Hemmerlin, A. Farnesol-mediated shift in the metabolic origin of prenyl groups used for protein prenylation in plants. Biochimie 2016, 127, 95–102. [Google Scholar] [CrossRef]
- Malizia, R.A.; Cardell, D.A.; Molli, J.S.; Grau, R.J. Volatile constituents of Acacia caven (Mol.) Mol. flower concrete from species growing in Argentina. J. Essent. Oil Res. 2002, 14, 132–134. [Google Scholar] [CrossRef]
- Silva, E.K.; Zabot, G.L.; Meireles, M.A.A. Ultrasound-assisted encapsulation of annatto seed oil: Retention and release of a bioactive compound with functional activities. Food Res. Int. 2015, 78, 159–168. [Google Scholar] [CrossRef]
- Chaberlain, W.J.; Severson, R.F.; Chortyk, O.T.; Sisson, V.E. Determination of solanesol in tobacco by capillary gas chromatography. J. Chromatogr. A 1990, 513, 55–60. [Google Scholar] [CrossRef]
- Vanaga, I.; Gubernator, J.; Nakurte, I.; Kletnieks, U.; Muceniece, R.; Jansone, B. Identification of Abies sibirica L. polyprenols and characterisation of polyprenol-containing liposomes. Molecules 2020, 25, 1801. [Google Scholar] [CrossRef]
- Surmacz, L.; Swiezewska, E. Polyisoprenoids–secondary metabolites or physiologically important superlipids? Biochem. Biophys. Res. Commun. 2011, 407, 627–632. [Google Scholar] [CrossRef]
- Yagi, K.J.; Konz, K.G.; Stay, B.; Tobe, S.S. Production and utilization of farnesoic acid in the juvenile hormone biosynthetic pathway by corpora allata of larval Diploptera punctata. Gen. Comp. Endocrinol. 1991, 81, 284–294. [Google Scholar] [CrossRef]
- Wierzbicki, A.S.; Mayne, P.D.; Lloyd, M.D.; Burston, D.; Mei, G.; Sidey, M.C.; Gibberd, F.B. Metabolism of phytanic acid and 3-methyl-adipic acid excretion in patients with adult Refsum disease. J. Lipid Res. 2003, 44, 1481–1488. [Google Scholar] [CrossRef]
- Endo, S.; Matsunaga, T.; Mamiya, H.; Ohta, C.; Soda, M.; Kitade, Y.; Tajima, K.; Zhao, H.T.; El-Kabbani, O.; Hara, A. Kinetic studies of AKR1B10, human aldose reductase-like protein: Endogenous substrates and inhibition by steroids. Arch. Biochem. 2009, 487, 1–9. [Google Scholar] [CrossRef]
- Meigs, T.E.; Roseman, D.S.; Simoni, R.D. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase degradation by the nonsterol mevalonate metabolite farnesol in vivo. J. Biol. Chem. 1996, 271, 7916–7922. [Google Scholar] [CrossRef]
- Fernandes, N.V.; Yeganehjoo, H.; Katuru, R.; DeBose-Boyd, R.A.; Morris, L.L.; Michon, R.; Mo, H. Geranylgeraniol suppresses the viability of human DU145 prostate carcinoma cells and the level of HMG CoA reductase. Exp. Biol. Med. 2013, 238, 1265–1274. [Google Scholar] [CrossRef]
- Miriyala, S.; Subramanian, T.; Panchatcharam, M.; Ren, H.; McDermott, M.I.; Sunkara, M.; Morris, A.J. Functional characterization of the atypical integral membrane lipid phosphatase PDP1/PPAPDC2 identifies a pathway for interconversion of isoprenols and isoprenoid phosphates in mammalian cells. J. Biol. Chem. 2010, 285, 13918–13929. [Google Scholar] [CrossRef]
- Elsabrouty, R.; Jo, Y.; Hwang, S.; Jun, D.-J.; DeBose-Boyd, R.A. Type 1 polyisoprenoid diphosphate phosphatase modulates geranylgeranyl-mediated control of HMG CoA reductase and UBIAD1. Elife 2021, 10, e64688. [Google Scholar] [CrossRef]
- Bansal, V.S.; Vaidya, S. Characterization of 2 Distinct Allyl Pyrophosphatase Activities from Rat-Liver Microsomes. Arch. Biochem. Biophys. 1994, 315, 393–3994. [Google Scholar] [CrossRef]
- Goodman, D.S.; Popjak, G. Studies on the biosynthesis of cholesterol: XII. synthesis of allyl pyrophosphates from mevalonate and their conversion into squalene with liver enzymes. J. Lipid Res. 1960, 1, 286–300. [Google Scholar] [CrossRef]
- Nualkaew, N.; De-Eknamkul, W.; Kutchan, T.M.; Zenk, M.H. Membrane-bound geranylgeranyl diphosphate phosphatases: Purification and characterization from Croton stellatopilosus leaves. Phytochemistry 2006, 67, 1613–1620. [Google Scholar] [CrossRef]
- Nyati, P.; Nouzova, M.; Rivera-Perez, C.; Clifton, M.E.; Mayoral, J.G.; Noriega, F.G. Farnesyl phosphatase, a Corpora allata enzyme involved in juvenile hormone biosynthesis in Aedes aegypti. PLoS ONE 2013, 8, e71967. [Google Scholar] [CrossRef]
- de Wolf, E.; Abdullah, M.I.; Jones, S.M.; Menezes, K.; Moss, D.M.; Drijfhout, F.P.; Richardson, A. Dietary geranylgeraniol can limit the activity of pitavastatin as a potential treatment for drug-resistant ovarian cancer. Sci. Rep. 2017, 7, 1–10. [Google Scholar]
- Jawad, M.J.; Ibrahim, S.; Kumar, M.; Burgert, C.; Li, W.W.; Richardson, A. Identification of foods that affect the anti-cancer activity of pitavastatin in cells. Oncol. Lett. 2022, 23, 1–8. [Google Scholar] [CrossRef]
- Popjak, G.; Edmond, J.; Clifford, K.; Williams, V. Biosynthesis and structure of a new intermediate between farnesyl pyrophosphate and squalene. J. Biol. Chem. 1969, 244, 1897–1918. [Google Scholar] [CrossRef]
- Togashi, N.; Inoue, Y.; Hamashima, H.; Takano, A. Effects of two terpene alcohols on the antibacterial activity and the mode of action of farnesol against Staphylococcus aureus. Molecules 2008, 13, 3069–3076. [Google Scholar] [CrossRef]
- Burke, Y.D.; Stark, M.J.; Roach, S.L.; Sen, S.E.; Crowell, P.L. Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol. Lipids 1997, 32, 151. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Hwang, S.T.; Sethi, G.; Fan, L.; Arfuso, F.; Ahn, K.S. Potential anti-inflammatory and anti-cancer properties of farnesol. Molecules 2018, 23, 2827. [Google Scholar] [CrossRef]
- Kotti, T.J.; Ramirez, D.M.; Pfeiffer, B.E.; Huber, K.M.; Russell, D.W. Brain cholesterol turnover required for geranylgeraniol production and learning in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 3869–3874. [Google Scholar] [CrossRef]
- Kotti, T.; Head, D.D.; McKenna, C.E.; Russell, D.W. Biphasic requirement for geranylgeraniol in hippocampal long-term potentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 11394–11399. [Google Scholar] [CrossRef]
- Ho, H.J.; Shirakawa, H.; Giriwono, P.E.; Ito, A.; Komai, M. A novel function of geranylgeraniol in regulating testosterone production. Biosci. Biotechnol. Biochem. 2018, 82, 956–962. [Google Scholar] [CrossRef]
- Irwin, J.C.; Fenning, A.S.; Vella, R.K. Geranylgeraniol prevents statin-induced skeletal muscle fatigue without causing adverse effects in cardiac or vascular smooth muscle performance. Transl. Res. 2020, 215, 17–30. [Google Scholar] [CrossRef]
- Rattanawonsakul, K.; Bullock, G.; Bolt, R.; Claeyssens, F.; Atkins, S.; Hearnden, V. In vitro Effect of Geranylgeraniol (GGOH) on Bisphosphonate-Induced Cytotoxicity of Oral Mucosa Cells. Front. Oral Health 2022, 3, 892615. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Coates, D.E.; Cullinan, M.P.; Drummond, B.K.; Milne, T.; Seymour, G.J. Zoledronic acid and geranylgeraniol regulate cellular behaviour and angiogenic gene expression in human gingival fibroblasts. J. Oral Pathol. Med. 2014, 43, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, A.; Pontillo, A.; Leo, L.D.; Tommasini, A.; Decorti, G.; Not, T.; Ventura, A. Natural isoprenoids are able to reduce inflammation in a mouse model of mevalonate kinase deficiency. Pediatr. Res. 2008, 64, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, A.; Piscianz, E.; Zweyer, M.; Bortul, R.; Loganes, C.; Girardelli, M.; Celeghini, C. Geranylgeraniol and neurological impairment: Involvement of apoptosis and mitochondrial morphology. Int. J. Mol. Sci. 2016, 17, 365. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.N.; Ye, J.; Hao, R.; DeBose-Boyd, R.; Ye, J. Sufficient production of geranylgeraniol is required to maintain endotoxin tolerance in macrophages. J. Lipid Res. 2013, 54, 3430–3437. [Google Scholar] [CrossRef]
- Campia, I.; Lussiana, C.; Pescarmona, G.; Ghigo, D.; Bosia, A.; Riganti, C. Geranylgeraniol prevents the cytotoxic effects of mevastatin in THP-1 cells, without decreasing the beneficial effects on cholesterol synthesis. Br. J. Pharmacol. 2009, 158, 1777–1786. [Google Scholar] [CrossRef]
- Crick, D.C.; Waechter, C.J.; Andres, D.A. Utilization of geranylgeraniol for protein isoprenylation in C6 glial cells. Biochem. Biophys. Res. Commun. 1994, 205, 955–961. [Google Scholar] [CrossRef]
- Andres, D.A.; Crick, D.C.; Finlin, B.S.; Waechter, C.J. Rapid Identification of Cysteine-Linked Isoprenyl Groups by Metabolic Labeling with [3H] Farnesol and [3H] Geranylgeraniol. Protein Lipidation Protoc. 1998, 116, 107–123. [Google Scholar]
- Moura, I.C.; Wunderlich, G.; Uhrig, M.L.; Couto, A.S.; Peres, V.J.; Katzin, A.M.; Kimura, E.A. Limonene arrests parasite development and inhibits isoprenylation of proteins in Plasmodium falciparum. Antimicrob. Agents Chemother. 2001, 45, 2553–2558. [Google Scholar] [CrossRef]
- Thai, L.; Rush, J.S.; Maul, J.E.; Devarenne, T.; Rodgers, D.L.; Chappell, J.; Waechter, C.J. Farnesol is utilized for isoprenoid biosynthesis in plant cells via farnesyl pyrophosphate formed by successive monophosphorylation reactions. Proc. Natl. Acad. Sci. USA 1999, 96, 13080–13085. [Google Scholar] [CrossRef]
- Crick, D.C.; Andres, D.A.; Waechter, C.J. Farnesol is utilized for protein isoprenylation and the biosynthesis of cholesterol in mammalian cells. Biochem. Biophys. Res. Commun. 1995, 211, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Crick, D.C.; Andres, D.A.; Waechter, C.J. Novel salvage pathway utilizing farnesol and geranylgeraniol for protein isoprenylation. Biochem. Biophys. Res. Commun. 1997, 237, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.A.; Bach, T.J. Incorporation of all-trans-farnesol into sterols and ubiquinone in Nicotiana tabacum L. cv Bright Yellow-2 cell cultures. Tetrahedron Lett. 2001, 42, 655–657. [Google Scholar] [CrossRef]
- Rodrigues Goulart, H.; Kimura, E.A.; Peres, V.J.; Couto, A.S.; Aquino Duarte, F.A.; Katzin, A.M. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob. Agents Chemother. 2004, 48, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Fliesler, S.J.; Keller, R.K. Metabolism of [3H] farnesol to cholesterol and cholesterogenic intermediates in the living rat eye. Biochem. Biophys. Res. Commun. 1995, 210, 695–702. [Google Scholar] [CrossRef]
- Callegari, S.; McKinnon, R.A.; Andrews, S.; de Barros Lopes, M.A. Atorvastatin-induced cell toxicity in yeast is linked to disruption of protein isoprenylation. FEMS Yeast Res. 2010, 10, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.; Cobbold, S.A.; Hanssen, E.; Birnbaum, J.; Spillman, N.J.; McHugh, E.; Ralph, S.A. Delayed death in the malaria parasite Plasmodium falciparum is caused by disruption of prenylation-dependent intracellular trafficking. PLoS Biol. 2019, 17, e3000376. [Google Scholar] [CrossRef]
- Crick, D.C.; Andres, D.A.; Waechter, C.J. Geranylgeraniol promotes entry of UT-2 cells into the cell cycle in the absence of mevalonate. Exp. Cell Res. 1997, 231, 302–307. [Google Scholar] [CrossRef]
- Hartley, M.D.; Schneggenburger, P.E.; Imperiali, B. Lipid bilayer nanodisc platform for investigating polyprenol-dependent enzyme interactions and activities. Proc. Natl. Acad. Sci. USA 2013, 110, 20863–20870. [Google Scholar] [CrossRef]
- Inoue, H.; Korenaga, T.; Sagami, H.; Koyama, T.; Ogura, K. Phosphorylation of farnesol by a cell-free system from Botryococcus braunii. Biochem. Biophys. Res. Commun. 1994, 200, 1036–1041. [Google Scholar] [CrossRef]
- Ohnuma, S.I.; Watanabe, M.; Nishino, T. Identification and characterization of geranylgeraniol kinase and geranylgeranyl phosphate kinase from the Archaebacterium Sulfolobus acidocaldariu. J. Biochem. 1996, 119, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Bentinger, M.; Grünler, J.; Peterson, E.; Swiezewska, E.; Dallner, G. Phosphorylation of farnesol in rat liver microsomes: Properties of farnesol kinase and farnesyl phosphate kinase. Arch. Biochem. Biophys. 1998, 353, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Westfall, D.; Aboushadi, N.; Shackelford, J.E.; Krisans, S.K. Metabolism of farnesol: Phosphorylation of farnesol by rat liver microsomal and peroxisomal fractions. Biochem. Biophys. Res. Commun. 1997, 230, 562–568. [Google Scholar] [CrossRef]
- Ischebeck, T.; Zbierzak, A.M.; Kanwischer, M.; Dörmann, P. A salvage pathway for phytol metabolism in Arabidopsis. J. Biol. Chem. 2006, 281, 2470–2477. [Google Scholar] [CrossRef] [PubMed]
- Sussmann, R.A.; Gabriel, H.B.; Ríos, A.G.; Menchaca Vega, D.S.; Yamaguchi, L.F.; Doménech-Carbó, A.; Katzin, A.M. Presence of Phylloquinone in the Intraerythrocytic Stages of Plasmodium falciparum. Front. Cell. Infect. Microbiol. 2022, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Azevedo, M.; Spicher, L.; Glauser, G.; vom Dorp, K.; Guyer, L.; del Valle Carranza, A.; Asis, R.; de Souza, A.P.; Buckeridge, M.; et al. Down-regulation of tomato PHYTOL KINASE strongly impairs tocopherol biosynthesis and affects prenyllipid metabolism in an organ-specific manner. J. Exp. Bot. 2016, 67, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Valentin, H.E.; Lincoln, K.; Moshiri, F.; Jensen, P.K.; Qi, Q.; Venkatesh, T.V.; Last, R.L. The Arabidopsis vitamin E pathway gene5-1 mutant reveals a critical role for phytol kinase in seed tocopherol biosynthesis. Plant Cell 2006, 18, 212–224. [Google Scholar] [CrossRef]
- Fitzpatrick, A.H.; Bhandari, J.; Crowell, D.N. Farnesol kinase is involved in farnesol metabolism, ABA signaling and flower development in Arabidopsis. Plant J. 2011, 66, 1078–1088. [Google Scholar] [CrossRef]
- Spicher, L.; Almeida, J.; Gutbrod, K.; Pipitone, R.; Dörmann, P.; Glauser, G.; Kessler, F. Essential role for phytol kinase and tocopherol in tolerance to combined light and temperature stress in tomato. J. Exp. Bot. 2017, 68, 5845–5856. [Google Scholar] [CrossRef]
- Vom Dorp, K.; Hölzl, G.; Plohmann, C.; Eisenhut, M.; Abraham, M.; Weber, A.P.; Dörmann, P. Remobilization of phytol from chlorophyll degradation is essential for tocopherol synthesis and growth of Arabidopsis. Plant Cell 2015, 27, 2846–2859. [Google Scholar] [CrossRef]
- Seaver, S.M.; Gerdes, S.; Frelin, O.; Lerma-Ortiz, C.; Bradbury, L.M.; Zallot, R.; Hasnain, G.; Niehaus, T.D.; El Yacoubi, B.; Pasternak, S.; et al. High-throughput comparison, functional annotation, and metabolic modeling of plant genomes using the PlantSEED resource. Proc. Natl. Acad. Sci. USA 2014, 111, 9645–9650. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ekeuku, S.O.; Trias, A. The Role of Geranylgeraniol in Managing Bisphosphonate-Related Osteonecrosis of the Jaw. Front. Pharmacol. 2022, 13, 878556. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Ivanova, K.; Fiandra, L.; Mantecca, P.; Catelani, T.; Natan, M.; Banin, E.; Jacobi, G.; Tzanov, T. Antibacterial, Antibiofilm, and Antiviral Farnesol-Containing Nanoparticles Prevent Staphylococcus aureus from Drug Resistance Development. Int. J. Mol. Sci. 2022, 23, 7527. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Jeter, R.; Bachmann, A.; Yount, S.T.; Shen, C.L.; Yeganehjoo, H. The Potential of Isoprenoids in Adjuvant Cancer Therapy to Reduce Adverse Effects of Statins. Front. Pharmacol. 2019, 9, 1515. [Google Scholar] [CrossRef]
- Prior, I.A.; Lewis, P.D.; Mattos, C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012, 72, 2457–2467. [Google Scholar] [CrossRef]
- Healy, F.M.; Prior, I.A.; MacEwan, D.J. The importance of Ras in drug resistance in cancer. Br. J. Pharmacol. 2022, 179, 2844–2867. [Google Scholar] [CrossRef]
- Berndt, N.; Hamilton, A.D.; Sebti, S.M. Targeting protein prenylation for cancer therapy. Nat. Rev. Cancer 2011, 11, 775. [Google Scholar] [CrossRef]
- Marcuzzi, A.; Zanin, V.; Piscianz, E.; Tricarico, P.M.; Vuch, J.; Girardelli, M.; Crovella, S. Lovastatin-induced apoptosis is modulated by geranylgeraniol in a neuroblastoma cell line. Int. J. Dev. Neurosci. 2012, 30, 451–456. [Google Scholar] [CrossRef]
- Matusewicz, L.; Meissner, J.; Toporkiewicz, M.; Sikorski, A.F. The effect of statins on cancer cells. Tumor Biol. 2015, 36, 4889–4904. [Google Scholar] [CrossRef]
- Elson, C.E.; Yu, S.G. The chemoprevention of cancer by mevalonate-derived constituents of fruits and vegetables. J Nutr. 1994, 124, 607–614. [Google Scholar] [CrossRef]
- Yilmaz Öztürk, B.; Feyzullazade, N.; Dağ, İ.; Şengel, T. The investigation of in vitro effects of farnesol at different cancer cell lines. Microsc. Res. Tech. 2022, 85, 2760–2775. [Google Scholar] [CrossRef] [PubMed]
- Balaraman, G.; Sundaram, J.; Mari, A.; Krishnan, P.; Salam, S.; Subramaniam, N.; Thiruvengadam, D. Farnesol alleviates diethyl nitrosamine induced inflammation and protects experimental rat hepatocellular carcinoma. Environ. Toxicol. 2021, 36, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, N.; Pallerla, D.S.; Kaur, P.K.; Kishore Babu, N.; Singh, S. Leishmania donovani 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) as a potential drug target by biochemical, biophysical and inhibition studies. Microb. Pathog. 2014, 66, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Parihar, S.P.; Guler, R.; Brombacher, F. Statins: A viable candidate for host-directed therapy against infectious diseases. Nat. Rev. Immunol. 2019, 19, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Balanco, J.M.F.; Sussmann, R.A.; Verdaguer, I.B.; Gabriel, H.B.; Kimura, E.A.; Katzin, A.M. Tocopherol biosynthesis in Leishmania (L.) amazonensis promastigotes. FEBS Open Bio. 2019, 9, 743–754. [Google Scholar] [CrossRef]
- Gabriel, H.; Sussmann, R.; Kimura, E.; Marin Rodriguez, A.; Bofill Verdaguer, I.; Leite, G.; Katzin, A. Terpenes as Potential Antimalarial Drugs; Intechopen: London, UK, 2018. [Google Scholar]
- Porta, E.O.; Verdaguer, I.B.; Perez, C.; Banchio, C.; de Azevedo, M.F.; Katzin, A.M.; Labadie, G.R. Repositioning Salirasib as a new antimalarial agent. MedChemComm 2019, 10, 1599–1605. [Google Scholar] [CrossRef]
- Verdaguer, I.B.; Crispim, M.; Zafra, C.A.; Sussmann RA, C.; Buriticá, N.L.; Melo, H.R.; Katzin, A.M. Exploring ubiquinone biosynthesis inhibition as a strategy for improving atovaquone efficacy in malaria. Antimicrob. Agents Chemother. 2021, 65, e01516–e01520. [Google Scholar] [CrossRef]
- Li, Z.H.; Ramakrishnan, S.; Striepen, B.; Moreno, S.N. Toxoplasma gondii relies on both host and parasite isoprenoids and can be rendered sensitive to atorvastatin. PLoS Pathog. 2013, 9, e1003665. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Luo, X.; Yu, L.; Nie, Z.; Liu, Q.; He, L. Inhibitory effects of fosmidomycin against Babesia microti in vitro. Front. Cell Dev. Biol. 2020, 8, 247. [Google Scholar] [CrossRef]
- Brown, A.C.; Parish, T. Dxr is essential in Mycobacterium tuberculosis and fosmidomycin resistance is due to a lack of uptake. BMC Microbiol. 2008, 8, 1–9. [Google Scholar] [CrossRef]
- Fernandes, J.F.; Lell, B.; Agnandji, S.T.; Obiang, R.M.; Bassat, Q.; Kremsner, P.G.; Grobusch, M.P. Fosmidomycin as an antimalarial drug: A meta-analysis of clinical trials. Future Microbiol. 2015, 10, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Watts, K.M.; Hodge, D.; Kemp, L.M.; Hunstad, D.A.; Hicks, L.M.; Odom, A.R. A second target of the antimalarial and antibacterial agent fosmidomycin revealed by cellular metabolic profiling. Biochemistry 2011, 50, 3570–3577. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, P.; Luo, X.; Li, M.; Yu, L.; Guo, J.; Zhao, J. The MEP pathway in Babesia orientalis apicoplast, a potential target for anti-babesiosis drug development. Parasites Vectors 2018, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.; Shieh, A. Polypharmacy in Osteoporosis Treatment. Clin. Geriatr. Med. 2022, 38, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Ziebart, T.; Koch, F.; Klein, M.O.; Guth, J.; Adler, J.; Pabst, A.; Walter, C. Geranylgeraniol—A new potential therapeutic approach to bisphosphonate associated osteonecrosis of the jaw. Oral Oncol. 2011, 47, 195–201. [Google Scholar] [CrossRef]
- Koneski, F.; Popovic-Monevska, D.; Gjorgoski, I.; Krajoska, J.; Popovska, M.; Muratovska, I.; Popovski, V. In vivo effects of geranylgeraniol on the development of bisphosphonate-related osteonecrosis of the jaws. J. Cranio-Maxillofac. Surg. 2018, 46, 230–236. [Google Scholar] [CrossRef]
- Golomb, B.A.; Evans, M.A. Statin adverse effects. Am. J. Cardiovasc. Drugs 2008, 8, 373–418. [Google Scholar] [CrossRef]
- Batista, M.A.; de Lima Teixeira dos Santos, A.V.T.; do Nascimento, A.L.; Moreira, L.F.; Souza IR, S.; da Silva, H.R.; Carvalho, J.C.T. Potential of the Compounds from Bixa orellana Purified Annatto Oil and Its Granules (Chronic®) against Dyslipidemia and Inflammatory Diseases: In Silico Studies with Geranylgeraniol and Tocotrienols. Molecules 2022, 27, 1584. [Google Scholar] [CrossRef]
- Jaśkiewicz, A.; Pająk, B.; Litwiniuk, A.; Urbańska, K.; Orzechowski, A. Geranylgeraniol prevents statin-dependent myotoxicity in C2C12 muscle cells through RAP1 GTPase prenylation and cytoprotective autophagy. Oxidative Med. Cell. Longev. 2018, 2018, 6463807. [Google Scholar] [CrossRef]
- Zhang, Y.; Åberg, F.; Appelkvist, E.L.; Dallner, G.; Ernster, L. Uptake of dietary coenzyme Q supplement is limited in rats. J. Nutr. 1995, 125, 446–453. [Google Scholar]
- Young, J.M.; Florkowski, C.M.; Molyneux, S.L.; McEwan, R.G.; Frampton, C.M.; George, P.M.; Scott, R.S. Effect of coenzyme Q10 supplementation on simvastatin-induced myalgia. Am. J. Cardiol. 2007, 100, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, Z.; Zhu, L.; Meng, L.; Liu, H.; Wang, K.; Chen, J.; Li, P.; Yang, H. Geranylgeranyl pyrophosphate depletion by statins compromises skeletal muscle insulin sensitivity. J. Cachexia Sarcopenia Muscle 2022. advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, L.; Zheng, Z.; Meng, L.; Liu, H.; Wang, K.; Chen, J.; Li, P.; Yang, H. Mevalonate pathway orchestrates insulin signaling via RAB14 geranylgeranylation-mediated phosphorylation of AKT to regulate hepatic glucose metabolism. Metab. Clin. Exp. 2022, 128, 155120. [Google Scholar] [CrossRef] [PubMed]
- Abukhalil, M.H.; Hussein, O.E.; Bin-Jumah, M.; Saghir, S.A.; Germoush, M.O.; Elgebaly, H.A.; Mahmoud, A.M. Farnesol attenuates oxidative stress and liver injury and modulates fatty acid synthase and acetyl-CoA carboxylase in high cholesterol-fed rats. Environ. Sci. Pollut. Res. 2020, 27, 30118–30132. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Sashidhara, K.V. Lipid lowering agents of natural origin: An account of some promising chemotypes. Eur. J. Med. Chem. 2017, 140, 331–348. [Google Scholar] [CrossRef]
- Favier, L.A.; Schulert, G.S. Mevalonate kinase deficiency: Current perspectives. Appl. Clin. Genet. 2016, 9, 101. [Google Scholar]
- Frenkel, J.; Rijkers, G.T.; Mandey, S.H.; Buurman, S.W.; Houten, S.M.; Wanders, R.J.; Waterham, H.R.; Kuis, W. Lack of isoprenoid products raises ex vivo interleukin-1beta secretion in hyperimmunoglobulinemia D and periodic fever syndrome. Arthritis Rheum. 2002, 46, 2794–2803. [Google Scholar] [CrossRef]
- Jurczyluk, J.; Munoz, M.A.; Skinner, O.P.; Chai, R.C.; Ali, N.; Palendira, U.; Rogers, M.J. Mevalonate kinase deficiency leads to decreased prenylation of Rab GTPases. Immunol. Cell Biol. 2016, 94, 994–999. [Google Scholar] [CrossRef]
- Saputra, W.D.; Shono, H.; Ohsaki, Y.; Sultana, H.; Komai, M.; Shirakawa, H. Geranylgeraniol Inhibits Lipopolysaccharide-Induced Inflammation in Mouse-Derived MG6 Microglial Cells via NF-κB Signaling Modulation. Int. J. Mol. Sci. 2021, 22, 10543. [Google Scholar] [CrossRef]
- Giriwono, P.E.; Shirakawa, H.; Ohsaki, Y.; Hata, S.; Kuriyama, H.; Sato, S.; Komai, M. Dietary supplementation with geranylgeraniol suppresses lipopolysaccharide-induced inflammation via inhibition of nuclear factor-κB activation in rats. Eur. J. Nutr. 2013, 52, 1191–1199. [Google Scholar] [CrossRef]
- Souza, D.S.; Barreto, T.O.; Menezes-Filho, J.; Heimfarth, L.; Rhana, P.; Rabelo, T.K.; Santana, M.; Durço, A.O.; Conceição, M.; Quintans-Júnior, L.J.; et al. Myocardial hypertrophy is prevented by farnesol through oxidative stress and ERK1/2 signaling pathways. Eur. J. Pharmacol. 2020, 887, 173583. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdaguer, I.B.; Crispim, M.; Hernández, A.; Katzin, A.M. The Biomedical Importance of the Missing Pathway for Farnesol and Geranylgeraniol Salvage. Molecules 2022, 27, 8691. https://doi.org/10.3390/molecules27248691
Verdaguer IB, Crispim M, Hernández A, Katzin AM. The Biomedical Importance of the Missing Pathway for Farnesol and Geranylgeraniol Salvage. Molecules. 2022; 27(24):8691. https://doi.org/10.3390/molecules27248691
Chicago/Turabian StyleVerdaguer, Ignasi Bofill, Marcell Crispim, Agustín Hernández, and Alejandro Miguel Katzin. 2022. "The Biomedical Importance of the Missing Pathway for Farnesol and Geranylgeraniol Salvage" Molecules 27, no. 24: 8691. https://doi.org/10.3390/molecules27248691
APA StyleVerdaguer, I. B., Crispim, M., Hernández, A., & Katzin, A. M. (2022). The Biomedical Importance of the Missing Pathway for Farnesol and Geranylgeraniol Salvage. Molecules, 27(24), 8691. https://doi.org/10.3390/molecules27248691








