Highly Sensitive Sub-ppm CH3COOH Detection by Improved Assembly of Sn3O4-RGO Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
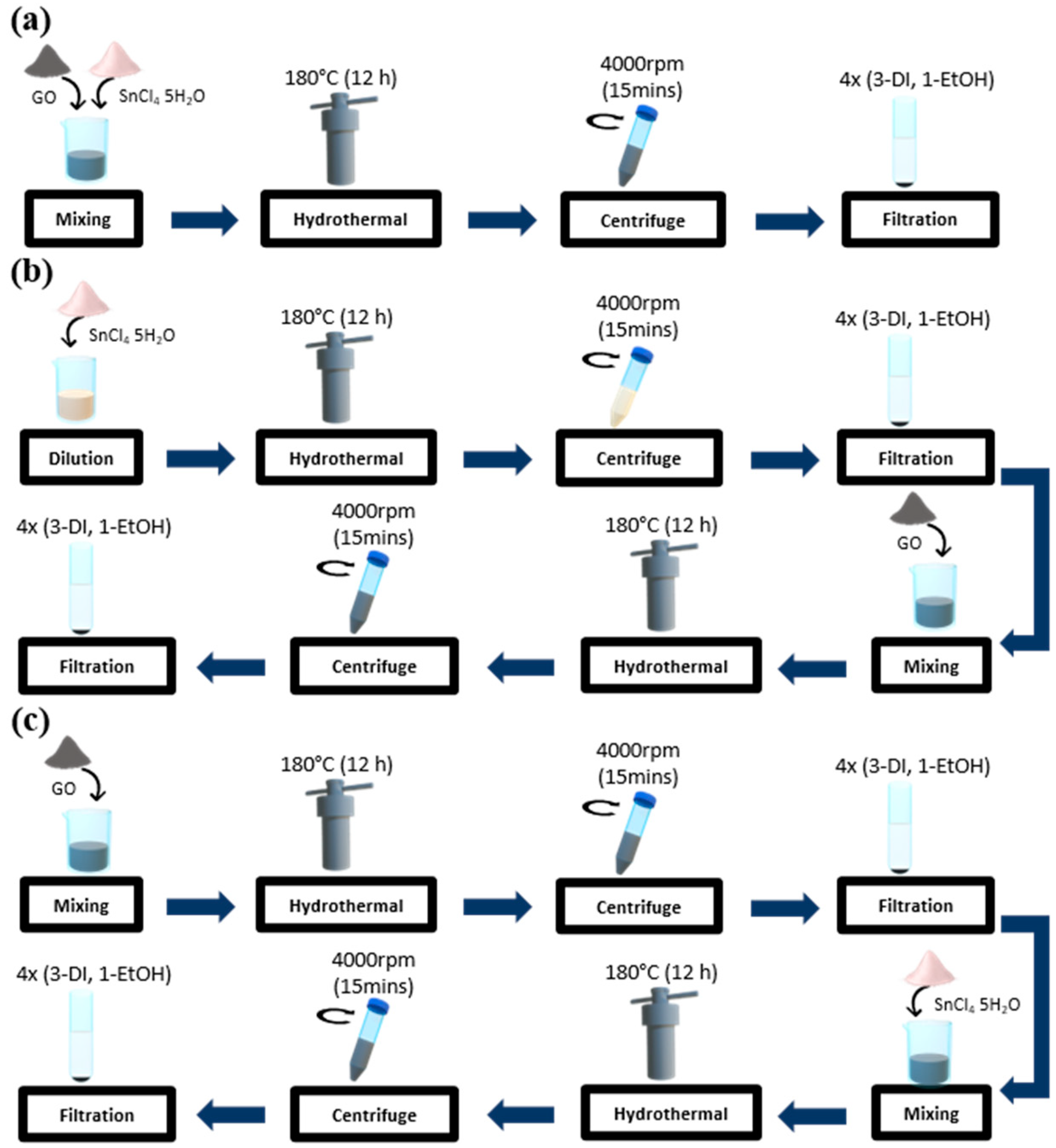
2.1.1. Preparation of One-Step Sn3O4-RGO Nanocomposite (OS Nanocomposite)
2.1.2. Preparation of Cast-On Sn3O4-RGO Nanocomposite (CO Nanocomposite)
2.1.3. Preparation of Pre-Reduced Sn3O4-RGO Nanocomposite (RS Nanocomposite)
2.2. Material Characterization
2.3. Sensor Fabrication and Measurement Set-Up
3. Results and Discussion
3.1. Sn3O4-RGO Nanocomposites Formation Mechanism
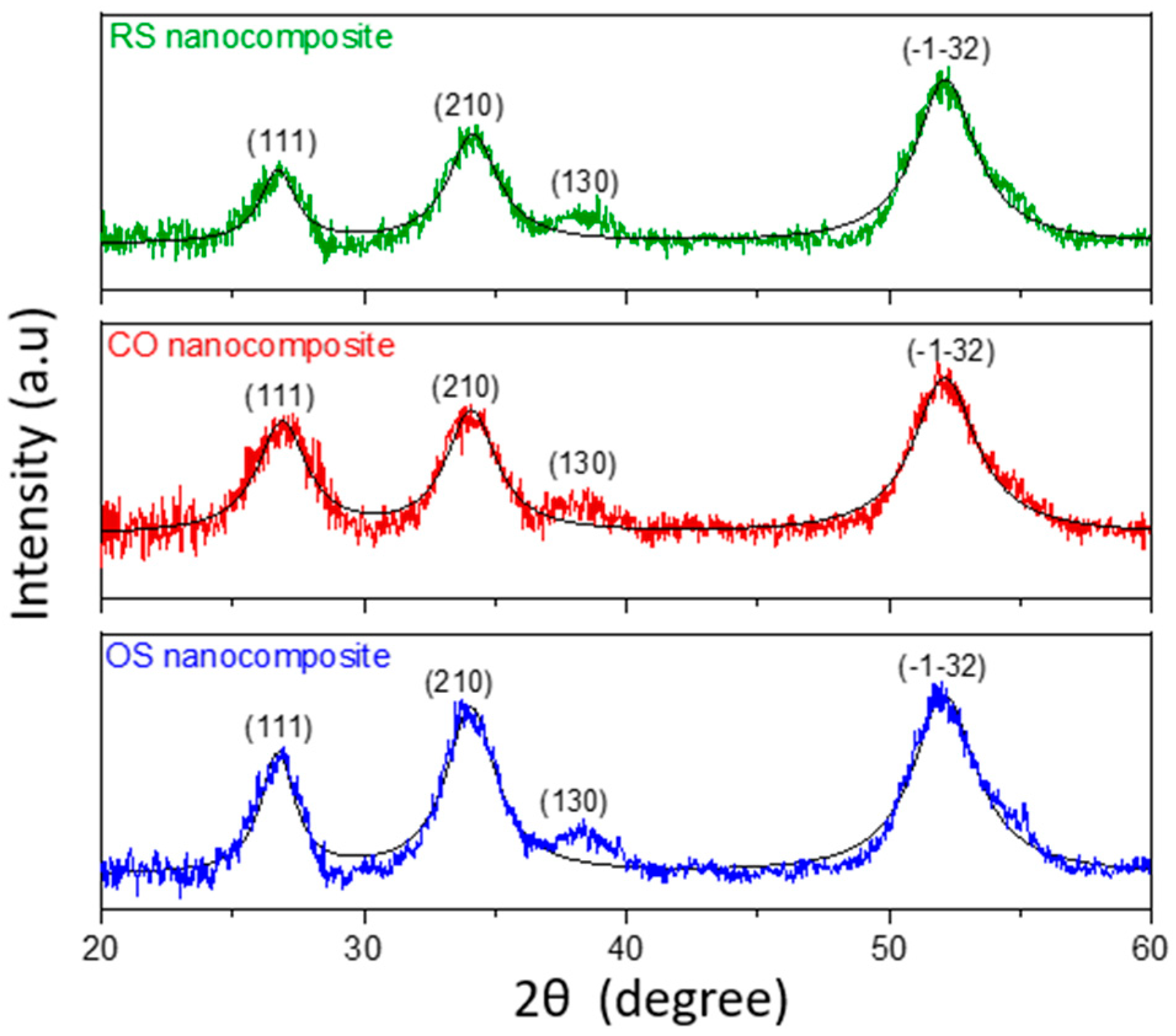
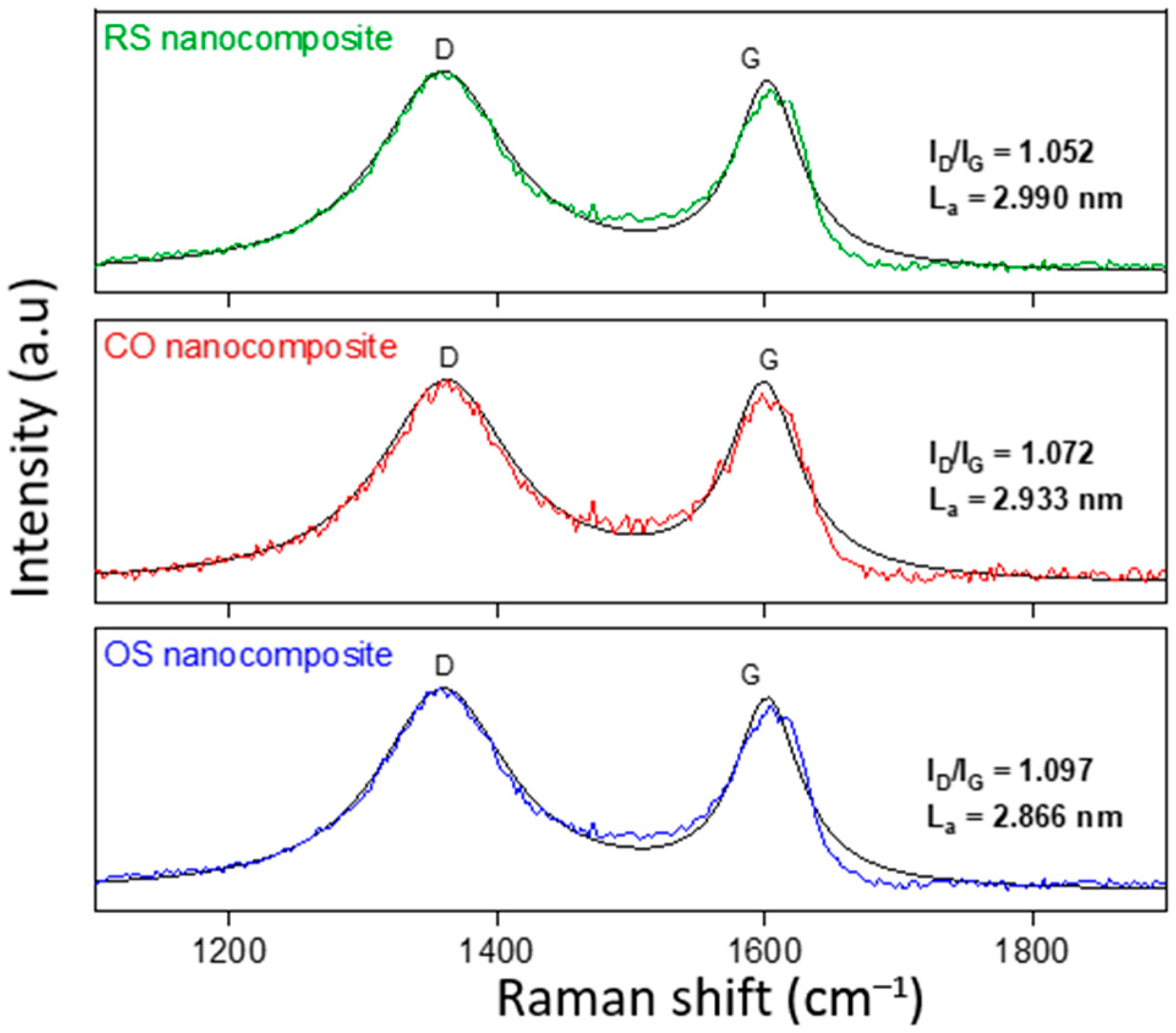
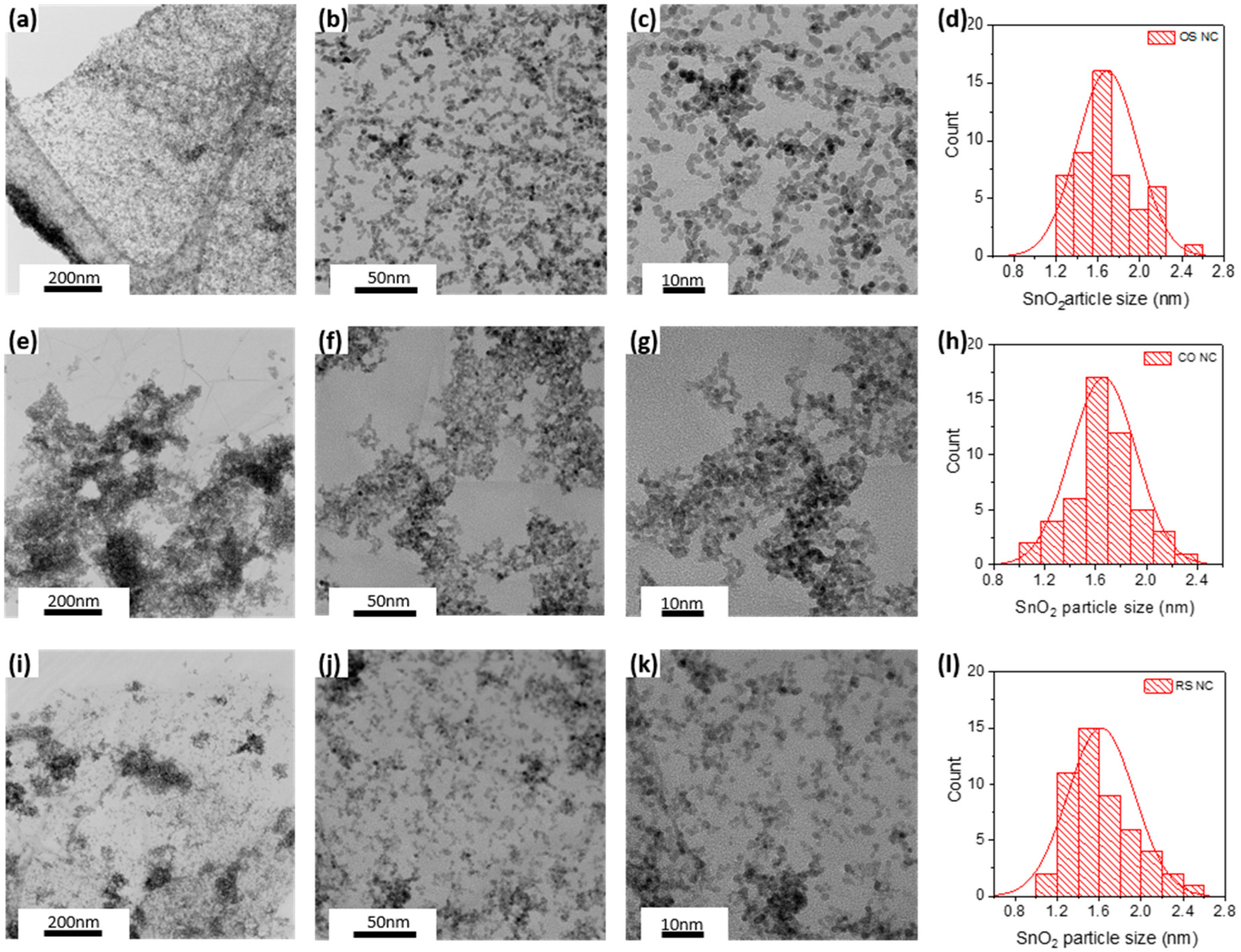
3.2. Structural and Morphological Analysis
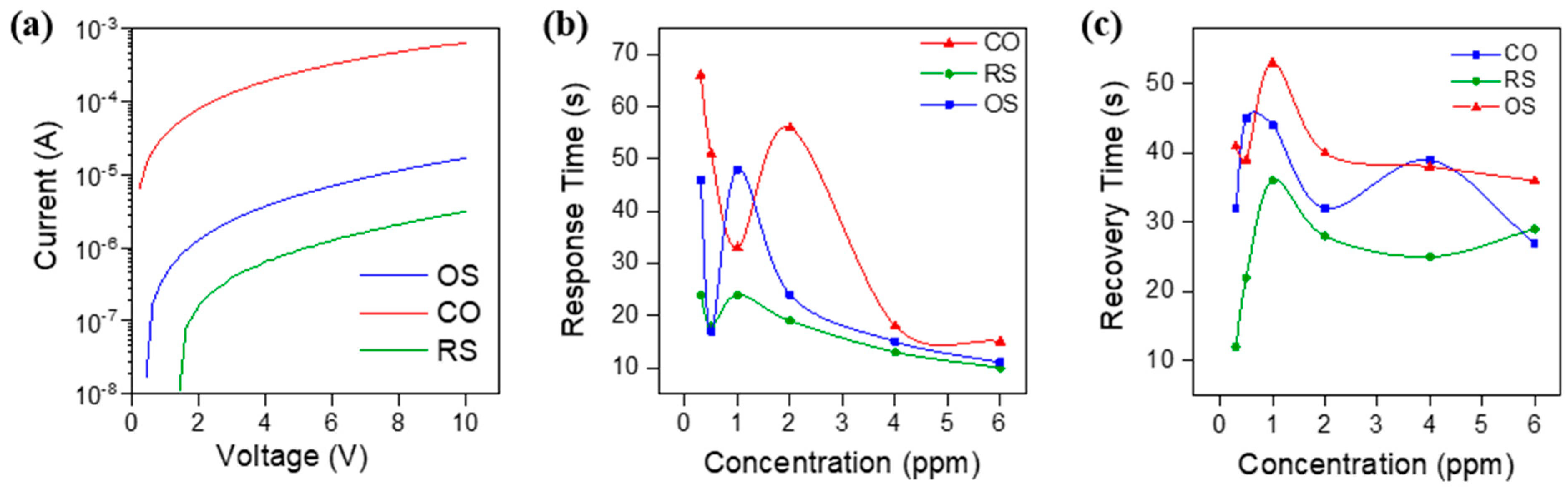
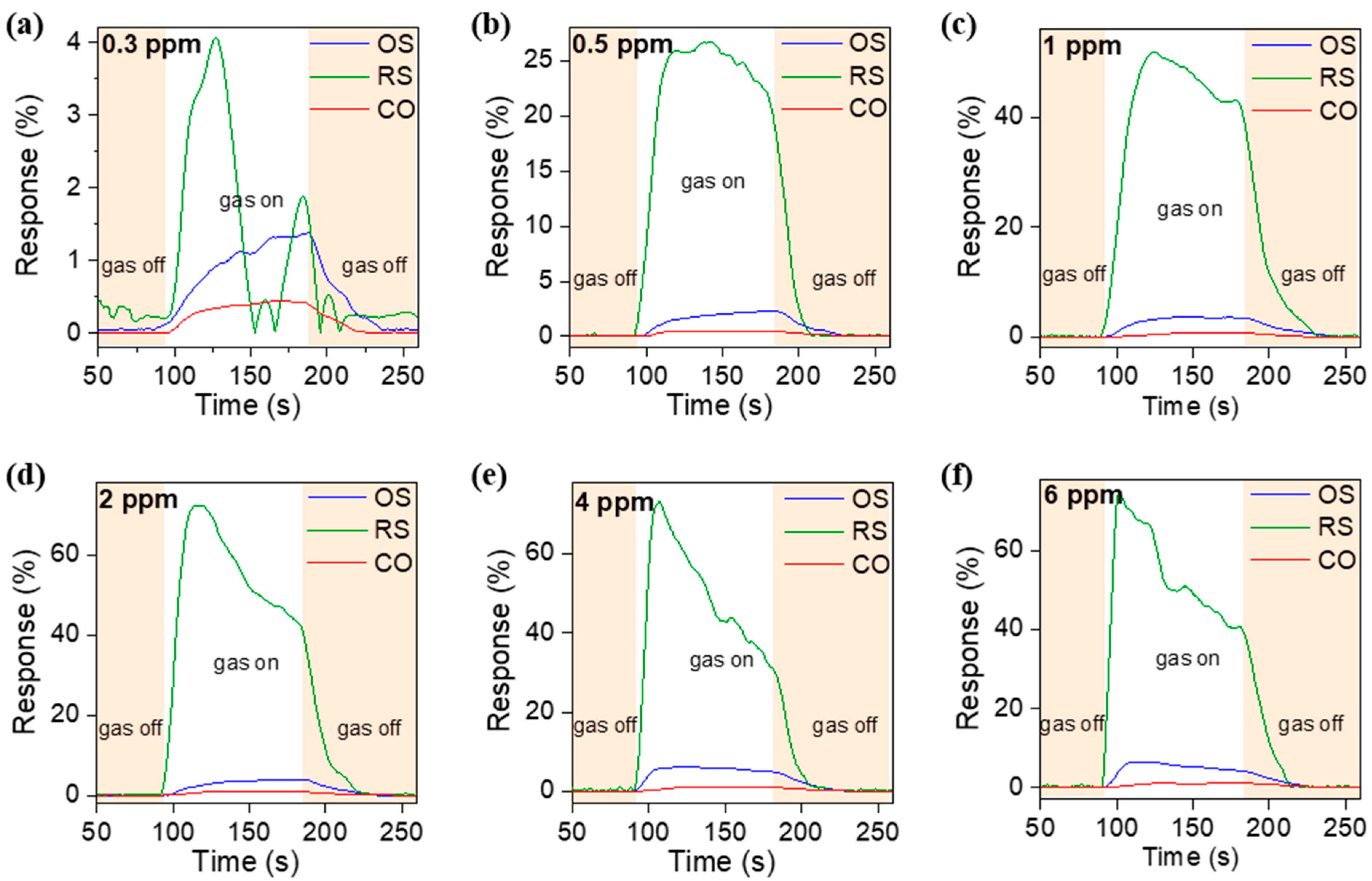
3.3. Sensing Response
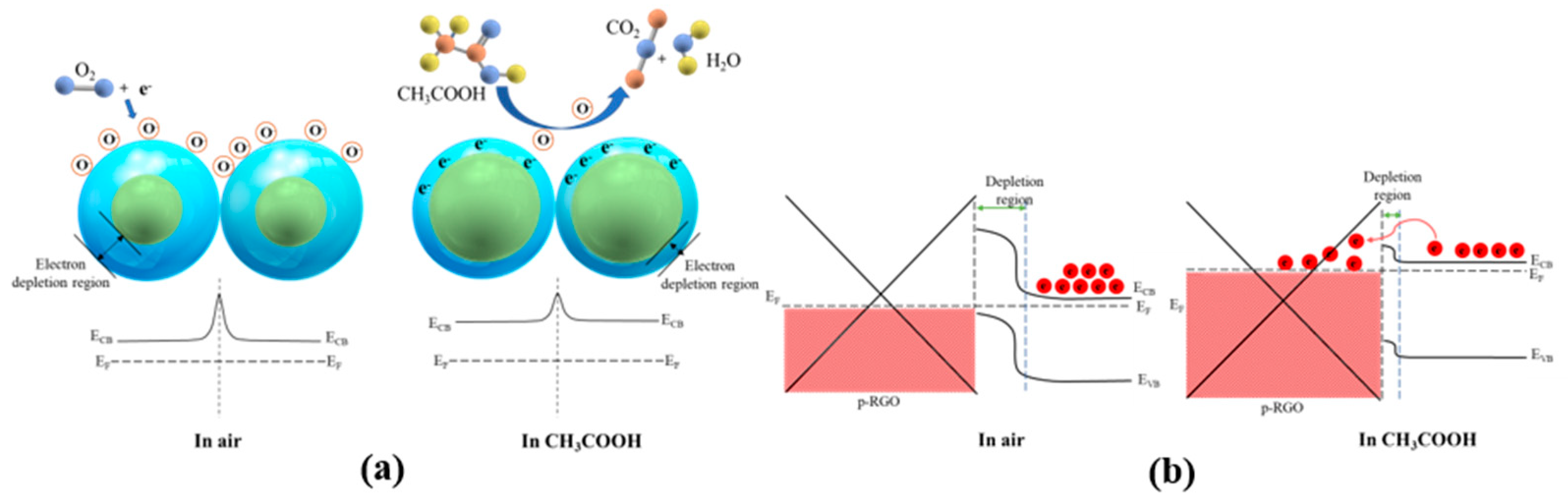
3.4. Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.H.; Rao, M.V.; Li, Q. Recent Advances in Electrochemical Sensors for Detecting Toxic Gases: NO2, SO2 and H2S. Sensors 2019, 4, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kus, F.; Altinkok, C.; Zayim, E.; Erdemir, S.; Tasaltin, C.; Gurol, I. Surface acoustic wave (SAW) sensor for volatile organic compounds (VOCs) detection with calix[4]arene functionalized Gold nanorods (AuNRs) and silver nanocubes (AgNCs). Sens. Actuators B Chem. 2021, 330, 129402. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, L.; Zheng, Q.L.; Zhang, Y.X.; Wen, L. On-chip readout plasmonic mid-IR gas sensor. Opto-Electron. Adv. 2020, 3, 190040. [Google Scholar] [CrossRef]
- Duffy, E.; Cauven, E.; Morrin, A. Colorimetric Sensing of Volatile Organic Compounds Produced from Heated Cooking Oils. ACS Omega 2021, 11, 7394–7401. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Cao, X.; Peng, Y.; Liu, Y.; Zhang, R. Cataluminescence sensor for gaseous acetic acid using a thin film of In2O3. Microchim. Acta 2012, 176, 485–491. [Google Scholar] [CrossRef]
- Hosaini, P.N.; Khan, M.F.; Mustaffa, N.I.H.; Amil, N.; Mohamad, N.; Jaafar, S.A.; Nadzir, M.S.M.; Latif, M.T. Concentration and source apportionment of volatile organic compounds (VOCs) in the ambient air of Kuala Lumpur, Malaysia. Nat. Hazards 2017, 85, 437–452. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Sun, Z.-S.; Wang, S.-Z.; Wang, S.-Y.; Cai, S.-X.; Huang, X.-Y.; Li, K.; Chi, Z.-T.; Pan, S.-D.; Xie, W.-F. Sub-ppm acetic acid gas sensor based on In2O3 nanofibers. J. Mater. Sci. 2019, 54, 14055–14063. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Pang, A.L.; Ardani, M.R.; Pung, S.-Y.; Ooi, P.C.; Hamzah, A.A.; Wee, M.M.R.; Haniff, M.A.S.M.; Dee, C.F.; Mahmoudi, E.; et al. Detection Of Breath Acetone By Semiconductor Metal Oxide Nanostructures-Based Gas Sensors: A Review. Mater. Sci. Semicond. Process. 2022, 149, 106897. [Google Scholar] [CrossRef]
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 082001. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructuresbased gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Tee, T.S.; Hui, T.C.; Yi, C.W.; Chin, Y.C.; Umar, A.A.; Titian, G.R.; Beng, L.H.; Sing, L.K.; Yahaya, M.; Salleh, M.M. Microwave-Assisted Hydrolysis Preparation Of Highly Crystalline Zno Nanorod Array For Room Temperature Photoluminescence-Based Co Gas Sensor. Sens. Actuators B Chem. 2016, 227, 304–312. [Google Scholar]
- Sopiha, K.V.; Oleksandr, I.; Malyi, O.I.; Persson, C.; Wu, P. Chemistry of Oxygen Ionosorption on SnO2 Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 33664–33676. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Y.; Zeng, H.; Feng, Y.; Yang, X.; Zhang, S.; Xu, Y.; Wang, G.; Wang, Y.; Zhang, Z. Rational Design of SnO2 Hollow Microspheres Functionalized with Derivatives of Pt Loaded MOFs for Superior Formaldehyde Detection. Nanomaterials 2022, 12, 1881. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, T.; Wang, L.; Lou, Z.; Deng, J.; Zhang, T. The synthesis and fast ethanol sensing properties of core–shell SnO2@ZnO composite nanospheres using carbon spheres as templates. New J. Chem. 2016, 40, 6796–6802. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, Y.; Mei, J.; Tang, C.; Luo, K.; Li, S.; Zhan, H.; He, Z. Hierarchical SnO2–Sn3O4 heterostructural gas sensor with high sensitivity and selectivity to NO2. Sens. Actuators B Chem. 2019, 127010. [Google Scholar] [CrossRef]
- Shanmugasundaram, A.; Basak, P.; Satyanarayana, L.; Manorama, S.V. Hierarchical SnO/SnO2 nanocomposites: Formation of in situ p–n junctions and enhanced H2 sensing. Sens. Actuators B Chem. 2013, 185, 265–273. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Chen, W. Fabrication of SnO2-SnO nanocomposites with p-n heterojunctions for the low-temperature sensing of NO2 gas. Nanoscale 2015, 7, 12133–12142. [Google Scholar] [CrossRef]
- Yu, H.; Yang, T.; Wang, Z.; Li, Z.; Zhao, Q.; Zhang, M. p-N heterostructural sensor with SnO-SnO2 for fast NO2 sensing response properties at room temperature. Sens. Actuators B Chem. 2018, 258, 517–526. [Google Scholar] [CrossRef]
- Mäkijaskari, M.A.; Rantala, T.T. Possible structures of nonstoichiometric tin oxide: The composition Sn2O3. Model. Simul. Mat. Sci. Eng. 2004, 12, 33–41. [Google Scholar] [CrossRef]
- Berengue, O.M.; Simon, R.A.; Chiquito, A.J.; Dalmaschio, C.J.; Leite, E.R.; Guerreiro, H.A.; Guimarães, F.E.G. Semiconducting Sn3O4 nanobelts: Growth and electronic structure. J. Appl. Phys. 2010, 107, 033717. [Google Scholar] [CrossRef]
- Ma, X.; Shen, J.; Hu, D.; Sun, L.; Chen, Y.; Liu, M.; Li, C.; Ruan, S. Preparation of three-dimensional Ce-doped Sn3O4 hierarchical microsphere and its application on formaldehyde gas sensor. J. Alloys Compd. 2017, 726, 1092–1100. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Yang, Q.; Gao, Y.; Zhou, X.; Liang, X.; Sun, P.; Lu, G. Hydrothermal synthesis and gas-sensing properties of flower-like Sn3O4. Sens. Actuators B Chem. 2016, 224, 128–133. [Google Scholar] [CrossRef]
- Wang, J.; Umezawa, N.; Hosono, H. Mixed Valence Tin Oxides as Novel van der Waals Materials: Theoretical Predictions and Potential Application. Adv. Energy Mater. 2016, 6, 1501190. [Google Scholar] [CrossRef]
- Hoa, N.D.; Duy, N.V.; El-Safty, S.A.; Hieu, N.V. Meso-/Nanoporous semiconducting metal oxides for gas sensor applications. J. Nanomater. 2015, 16, 186. [Google Scholar] [CrossRef] [Green Version]
- Fu, D.C.; Yeop Majlis, B.; Hamzah, A.A.; Choon, O.P.; Mohamed, M.A.; Yaw, T.T. Ultraviolet Light-Assisted Copper Oxide Nanowires Hydrogen Gas Sensor. Nanoscale Res. Lett. 2018, 13, 150–156. [Google Scholar]
- Xu, Y.; Zheng, L.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Oxygen Vacancies Enabled Porous SnO2 Thin Films for Highly Sensitive Detection of Triethylamine at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 20704–20713. [Google Scholar] [CrossRef]
- Norizan, M.N.; Abdullah, N.; Halim, N.A.; Demon, S.Z.N.; Mohamad, I.S. Heterojunctions of rGO/Metal Oxide Nanocomposites as Promising Gas-Sensing Materials—A Review. Nanomaterials 2022, 12, 2278. [Google Scholar] [CrossRef]
- Hashtroudi, H.; Yu, A.; Juodkazis, S.; Shafiei, M. Two-Dimensional Dy2O3-Pd-PDA/rGO Heterojunction Nanocomposite: Synergistic Effects of Hybridisation, UV Illumination and Relative Humidity on Hydrogen Gas Sensing. Chemosensors 2022, 10, 78. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Meng, F. Metal Oxide Semiconductor Sensors for Triethylamine Detection: Sensing Performance and Improvements. Chemosensors 2022, 10, 231. [Google Scholar] [CrossRef]
- Lei, G.; Pan, H.; Mei, H.; Liu, X.; Lu, G.; Lou, C.; Li, Z.; Zhang, J. Emerging single atom catalysts in gas sensors. Chem. Soc. Rev. 2022, 51, 7260–7280. [Google Scholar] [CrossRef]
- Ho, K.C.; Teow, Y.H.; Mohammad, A.W.; Ang, W.L.; Lee, P.H. Development of graphene oxide (GO)/multi-walled carbon nanotubes (MWCNTs) nanocomposite conductive membranes for electrically enhanced fouling mitigation. J. Membr. Sci. 2018, 552, 189–201. [Google Scholar] [CrossRef]
- Allaedini, G.; Mahmoudi, E.; Aminayi, P.; Tasirin, S.M.; Mohammad, A.W. Optical investigation of reduced graphene oxide and reduced graphene oxide/CNTs grown via simple CVD method. Synth. Met. 2016, 220, 72–77. [Google Scholar] [CrossRef]
- Gupta, M.; Hawari, H.F.; Kumar, P.; Burhanudin, Z.A. Copper Oxide/Functionalized Graphene Hybrid Nanostructures for Room Temperature Gas Sensing Applications. Crystals 2022, 12, 264. [Google Scholar] [CrossRef]
- Lee, Z.Y.; Hawari, H.F.B.; Djaswadi, G.W.B.; Kamarudin, K. A Highly Sensitive Room Temperature CO2 Gas Sensor Based on SnO2-rGO Hybrid Composite. Materials 2021, 14, 522. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Chen, W.; Zhang, H.; Xiao, G.; Yu, C.; Zhou, Q. Characterization of Reduced Graphene Oxide (rGO)-Loaded SnO2 Nanocomposite and Applications in C2H2 Gas Detection. Appl. Sci. 2017, 7, 19. [Google Scholar] [CrossRef]
- Zhang, H.; Cen, Y.; Du, Y.; Ruan, S. Enhanced Acetone Sensing Characteristics of ZnO/Graphene Composites. Sensors 2016, 16, 1876. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.; Lee, D.; Yoon, J.; Yin, Y.; Lee, Y.N.; Uprety, S.; Yoon, Y.S.; Kim, D.-J. Enhanced Gas-Sensing Performance of GO/TiO2 Composite by Photocatalysis. Sensors 2018, 18, 3334. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Han, T.; Fei, T.; Liu, S.; Zhang, T. Investigation of Microstructure Effect on NO2 Sensors Based on SnO2 Nanoparticles/Reduced Graphene Oxide Hybrids. ACS Appl. Mater. Interfaces 2018, 48, 41773–41783. [Google Scholar] [CrossRef]
- Suman, P.H.; Felix, A.A.; Tuller, H.L.; Varela, J.A.; Orlandi, M.O. Comparative gas sensor response of SnO2, SnO and Sn3O4 nanobelts to NO2 and potential interferents. Sens. Actuators B Chem. 2015, 208, 122–127. [Google Scholar] [CrossRef]
- Suman, P.H.; Longo, E.; Varela, J.A.; Orlandi, M.O. Controlled synthesis of layered Sn3O4 nanobelts by carbothermal reduction method and their gas sensor properties. J. Nanosci. Nanotechnol. 2014, 14, 6662–6668. [Google Scholar] [CrossRef]
- He, L.; Gao, C.; Yang, L.; Zhang, K.; Chu, X.; Liang, S.; Zeng, D. Facile synthesis of MgGa2O4/graphene composites for room temperature acetic acid gas sensing. Sens. Actuators B Chem. 2020, 306, 127453. [Google Scholar] [CrossRef]
- Gong, Y.; Li, H.; Pei, W.; Fan, J.; Umar, A.; Al-Assiri, M.S.; Wang, Y.; Rooija, N.F.; Zhou, G. Assembly with copper(ii) ions and D–π–A molecules on a graphene surface for ultra-fast acetic acid sensing at room temperature. RSC Adv. 2019, 9, 30432–30438. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Li, Y.; Yue, W.; Gao, S.; Zhang, C.; Chen, Z. Sn3O4/rGO heterostructure as a material for formaldehyde gas sensor with a wide detecting range and low operating temperature. Sens. Actuators B Chem. 2020, 312, 127954. [Google Scholar] [CrossRef]
- Pham, V.T.; Trung, H.L.; Tran, N.K.; Manh, H.C.; Duc, H.N.; Quynh, H.T.T.; Pham, T.H. Hydrothermal synthesis, structure, and photocatalytic properties of SnO2/rGO nanocomposites with different GO concentrations. Mater. Res. Express 2018, 5, 095506. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Han, T.; Fei, T.; Liu, S.; Lu, G. Oxygen vacancy engineering for enhanced sensing performances: A case of SnO2 nanoparticles-reduced graphene oxide hybrids for ultrasensitive ppb-level room-temperature NO2 sensing. Sens. Actuators B Chem. 2018, 226, 812–822. [Google Scholar] [CrossRef]
- Jin, W.; Ma, S.; Tie, Z.; Li, W.; Luo, J.; Cheng, L.; Xu, X.; Wang, T.; Jiang, X.; Mao, Y. Synthesis of hierarchical SnO2 nanoflowers with enhanced acetic acid gas sensing properties. Appl. Surf. Sci. 2015, 353, 71–78. [Google Scholar] [CrossRef]
- Wang, T.T.; Ma, S.Y.; Cheng, L.; Xu, X.L.; Luo, J.; Jiang, X.H.; Li, W.Q.; Jin, W.X.; Sun, X.X. Performance of 3D SnO2 microstructure with porous nanosheets for acetic acid sensing. Mater. Lett. 2015, 142, 141–144. [Google Scholar] [CrossRef]
- Khorramshahi, V.; Karamdel, J.; Yousefi, R. Acetic acid sensing of Mg-doped ZnO thin films fabricated by the sol–gel method. J. Mater. Sci. Mater. Electron. 2018, 29, 14679–14688. [Google Scholar] [CrossRef]
- Wang, S.; Ma, A.; Sun, R.; Qin, F.; Yang, X.; Li, F.; Li, X.; Yang, X. Characterization of electrospun Pr-doped ZnO nanostructure for acetic acid sensor. Sens. Actuators B Chem. 2014, 193, 326–333. [Google Scholar] [CrossRef]
- Jie, Z.; Fan, L.; Yan, Z.; Sun, J.; Shao, M. Visible-light-enhanced gas sensing of CdSxSe1−x nanoribbons for acetic acid at room temperature. Sens. Actuators B Chem. 2015, 215, 497–503. [Google Scholar]
- Geng, W.; Ma, Z.; Yang, J.; Duan, L.; Li, F.; Zhang, Q. Pore size dependent acetic acid gas sensing performance of mesoporous CuO. Sens Actuators B Chem. 2021, 334, 129639. [Google Scholar] [CrossRef]
- Abdullah, M.F. Defect Repair of Thermally Reduced Graphene Oxide by Gold Nanoparticles as a p-Type Transparent Conductor. J. Electron. Mater. 2021, 50, 6795–6803. [Google Scholar] [CrossRef]



| Samples | Sn (At.%) | C (At.%) | O (At.%) | O* (At.%) | O** (At.%) | Sn:O** [Sn3O4] | C:O* [RGO] | Ratio Sn3O4:RGO |
|---|---|---|---|---|---|---|---|---|
| OS nanocomposite | 29.15 | 19.58 | 51.27 | 12.95 | 38.32 | 0.76 | 1.51 | 2.07 |
| CO nanocomposite | 36.1 | 4.72 | 59.19 | 13.07 | 46.12 | 0.78 | 0.36 | 4.62 |
| RS nanocomposite | 33.89 | 8.88 | 57.23 | 12.85 | 44.38 | 0.76 | 0.70 | 3.60 |
| Samples | Carbon Bonding | Binding Energy (eV) | At. (%) | Relative Percentage (%) |
|---|---|---|---|---|
| OS nanocomposite | C-C | 284.6 | 5.35 | 27.33 |
| C-O | 285.2 | 6.67 | 34.05 | |
| C=O | 287.8 | 7.55 | 38.60 | |
| CO nanocomposite | C-C | 284.6 | 1.68 | 34.93 |
| C-O | 285.5 | 1.57 | 33.25 | |
| C=O | 289.2 | 1.50 | 31.81 | |
| RS nanocomposite | C-C | 284.6 | 3.69 | 41.61 |
| C-O | 285.6 | 2.18 | 24.62 | |
| C=O | 289.1 | 2.99 | 33.76 |
| Samples | Oxygen Species | Binding Energy (eV) | At (%) | Relative Percentage (%) |
|---|---|---|---|---|
| OS nanocomposite | OL (Sn-O) | 530.8 | 8.85 | 17.26 |
| Ov (vacancy) | 531.3 | 14.58 | 28.44 | |
| Oc (chemisorbed) | 531.8 | 14.87 | 29.01 | |
| C=O | 532.6 | 12.96 | 25.27 | |
| CO nanocomposite | OL (Sn-O) | 530.7 | 10.65 | 17.99 |
| Ov (vacancy) | 531.2 | 17.41 | 29.41 | |
| Oc (chemisorbed) | 531.7 | 18.06 | 30.51 | |
| C=O | 532.4 | 13.07 | 22.07 | |
| RS nanocomposite | OL (Sn-O) | 530.7 | 10.52 | 18.38 |
| Ov (vacancy) | 531.2 | 16.85 | 29.45 | |
| Oc (chemisorbed) | 531.7 | 17.0 | 29.70 | |
| C=O | 532.4 | 12.85 | 22.46 |
| Material | Operating Temp. (oC) | Concentration (ppm) | Response (%) | Response Time, tres (s) | Recovery Time, trec (s) | Ref. |
|---|---|---|---|---|---|---|
| Hierarchical SnO2 nanoflowers | 260 | 100 | 47.7 | 18 | 11 | [46] |
| Porous flower-like SnO2 | 340 | 20 | 5.0 | 11 | 6 | [47] |
| Mg-doped ZnO/rGO composites | 250 | 100 | 200 | 60 | 35 | [48] |
| Pr-doped ZnO nanofibers | 380 | 400 | 7.38 | 51 | 40 | [49] |
| CdSxSe1−x nanoribbons | 200 | 100 | 5.7 | 80 | 50 | [50] |
| Mesoporous CuO | 200 | 10 | 5.6 | 79 | 53 | [51] |
| MgGa2O4/graphene composites | RT | 100 | 363 | 50 | 35 | [39] |
| 4HQ-rGO/Cu composite | RT | 500 | 1.75 | 5 | 5 | [40] |
| Sn3O4-RGO-RS nanocomposite | RT | 2 | 74 | 15 | 36 | This work |
| Sn3O4-RGO-RS nanocomposite | RT | 0.3 | 4 | 25 | 11 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, N.A.; Abdullah, M.F.; Badaruddin, S.A.M.; Hussin, M.R.M.; Hashim, A.M. Highly Sensitive Sub-ppm CH3COOH Detection by Improved Assembly of Sn3O4-RGO Nanocomposite. Molecules 2022, 27, 8707. https://doi.org/10.3390/molecules27248707
Aziz NA, Abdullah MF, Badaruddin SAM, Hussin MRM, Hashim AM. Highly Sensitive Sub-ppm CH3COOH Detection by Improved Assembly of Sn3O4-RGO Nanocomposite. Molecules. 2022; 27(24):8707. https://doi.org/10.3390/molecules27248707
Chicago/Turabian StyleAziz, Norazreen Abd, Mohd Faizol Abdullah, Siti Aishah Mohamad Badaruddin, Mohd Rofei Mat Hussin, and Abdul Manaf Hashim. 2022. "Highly Sensitive Sub-ppm CH3COOH Detection by Improved Assembly of Sn3O4-RGO Nanocomposite" Molecules 27, no. 24: 8707. https://doi.org/10.3390/molecules27248707
APA StyleAziz, N. A., Abdullah, M. F., Badaruddin, S. A. M., Hussin, M. R. M., & Hashim, A. M. (2022). Highly Sensitive Sub-ppm CH3COOH Detection by Improved Assembly of Sn3O4-RGO Nanocomposite. Molecules, 27(24), 8707. https://doi.org/10.3390/molecules27248707







