PEEK and Hyaluronan-Based 3D Printed Structures: Promising Combination to Improve Bone Regeneration
Abstract
1. Introduction
2. Results
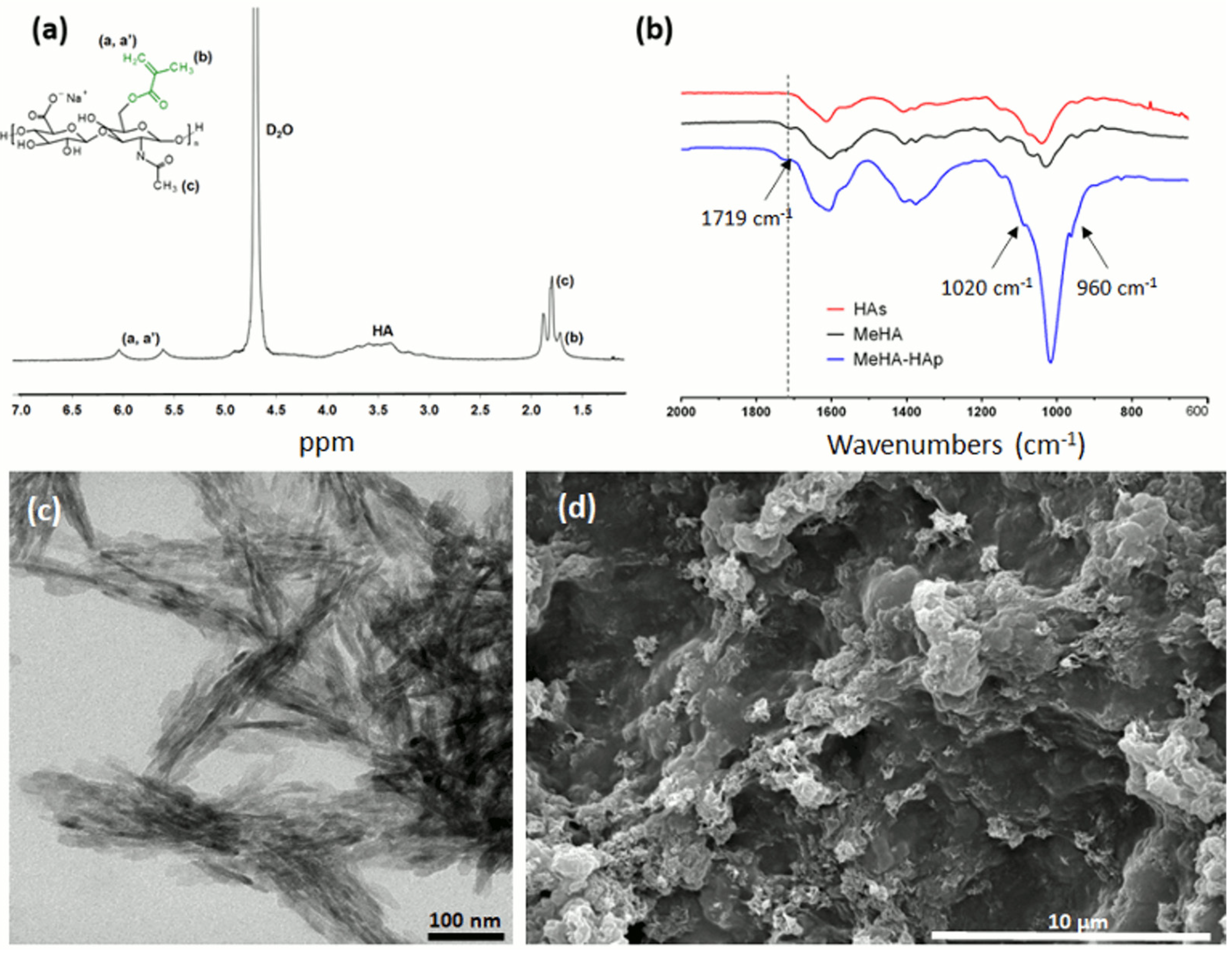
2.1. Hyaluronic Acid Methacrylation and Composite Characterization
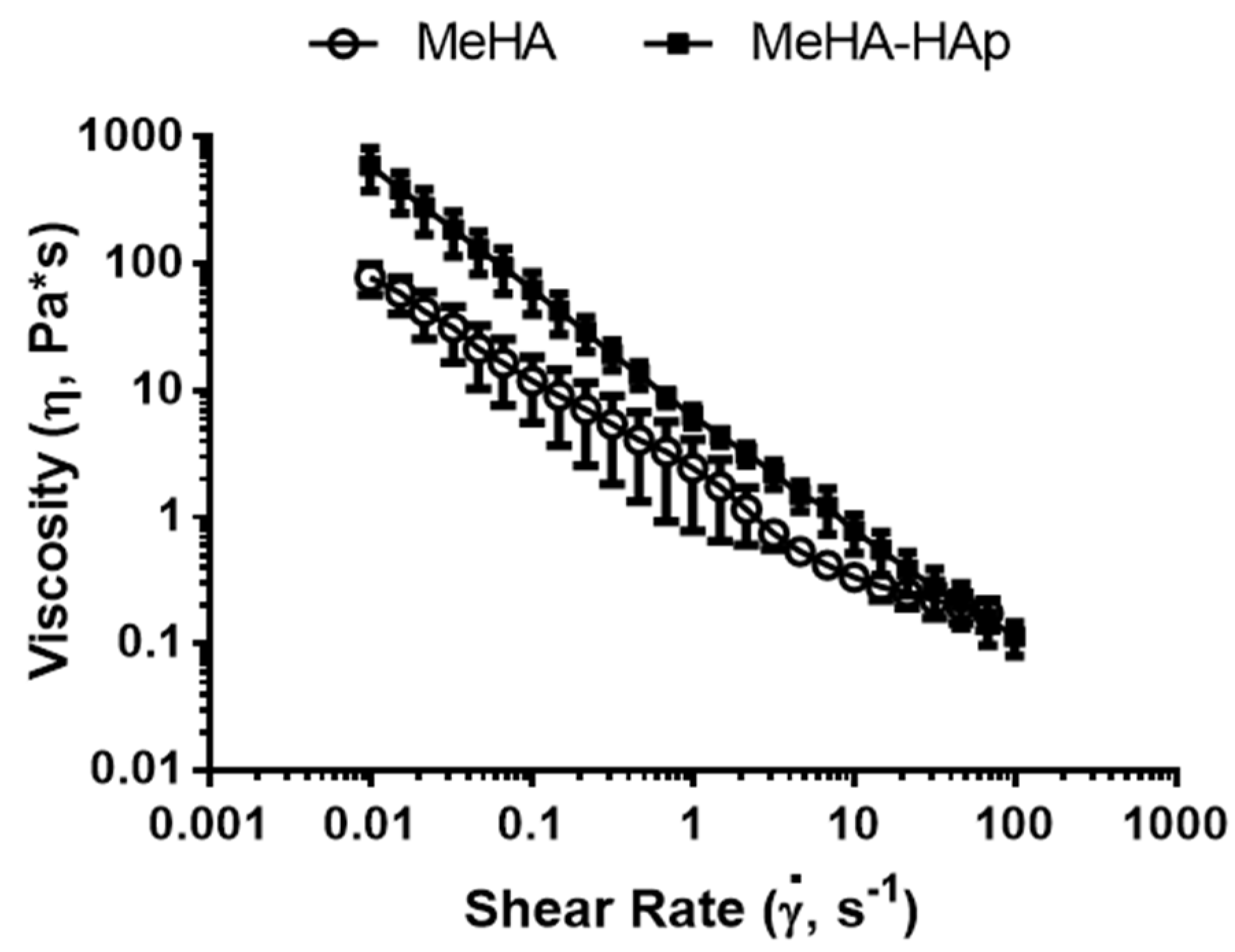
2.2. PEEK-MeHA-HAp Bone Substitute: Preparation and Characterization
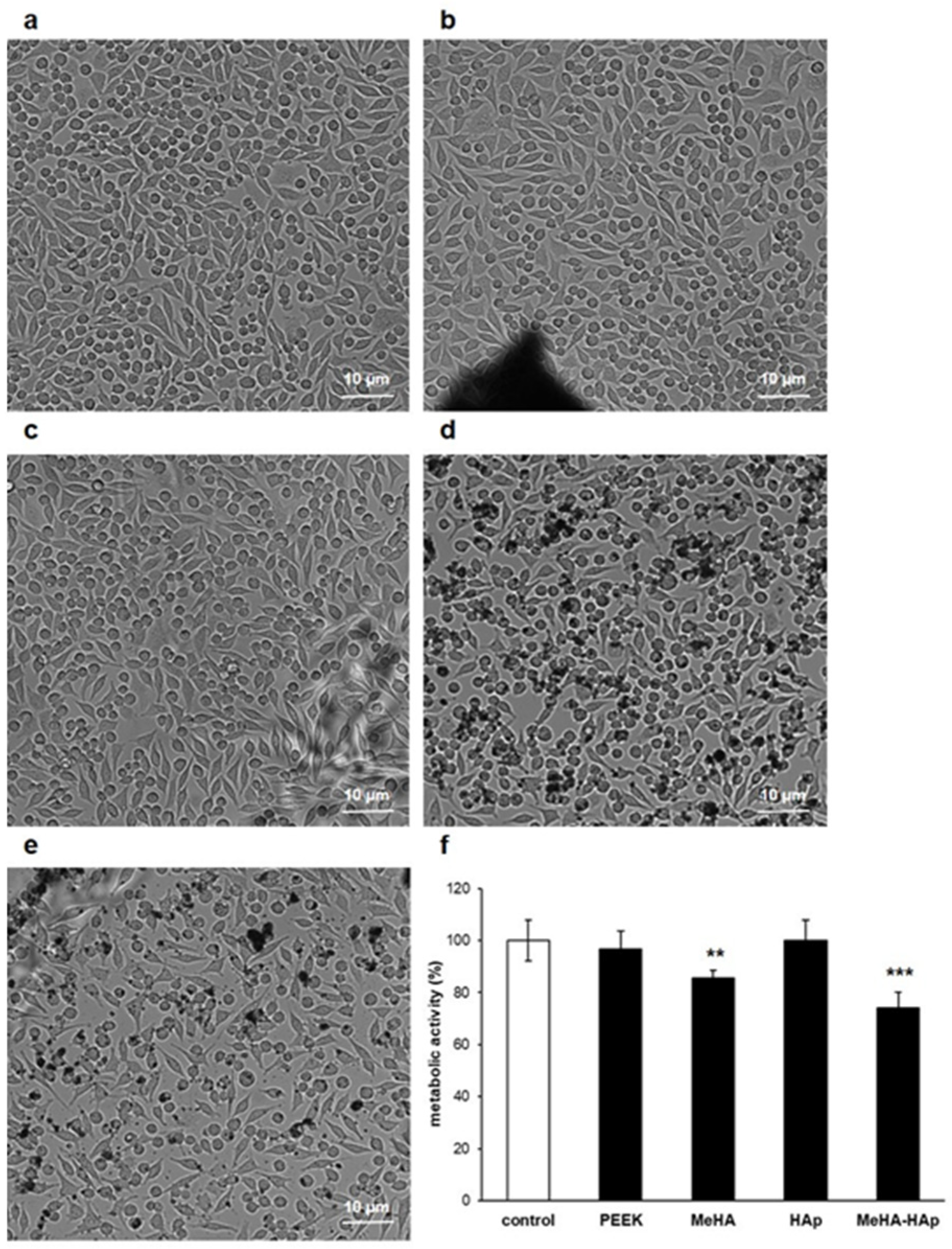
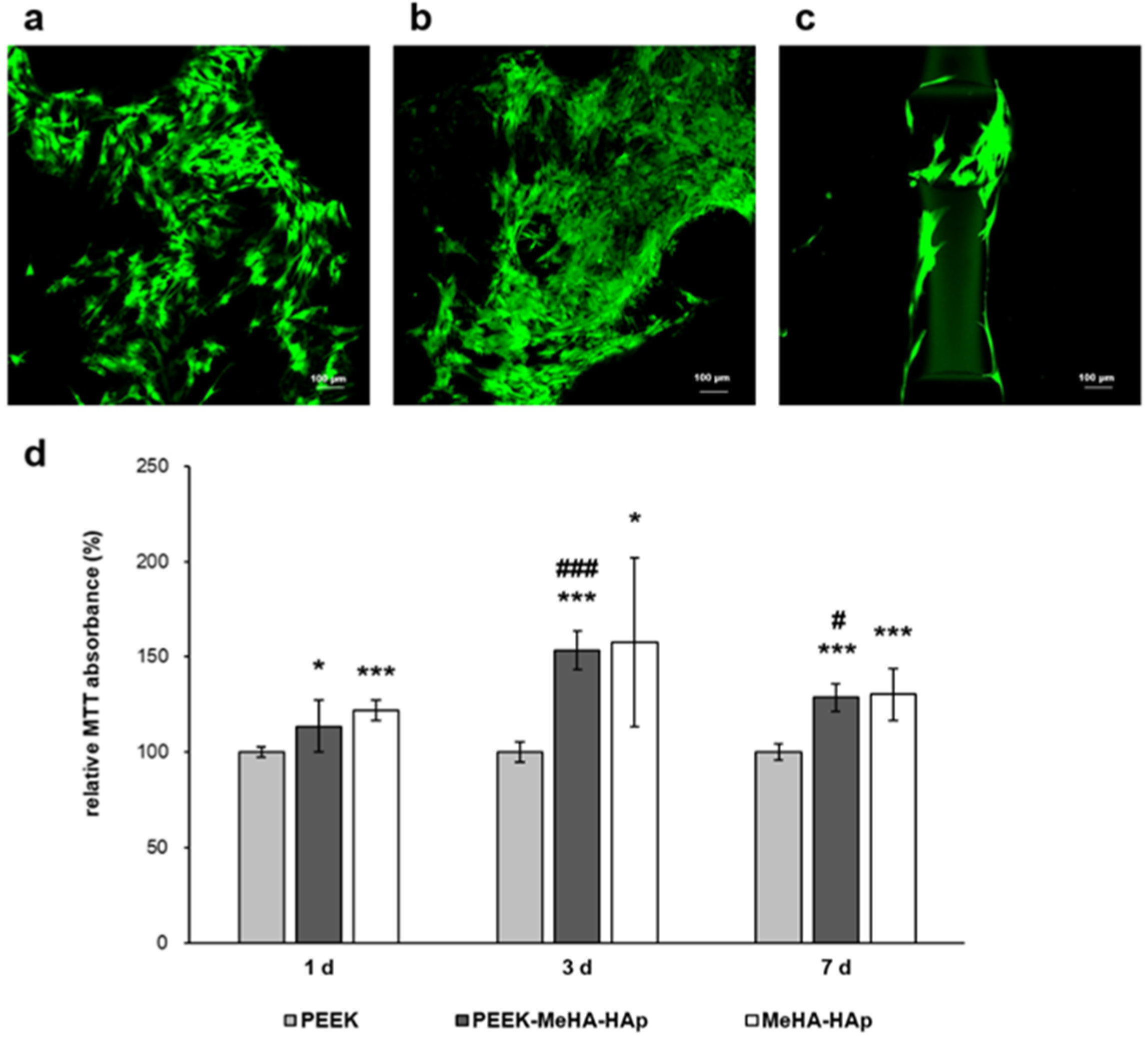
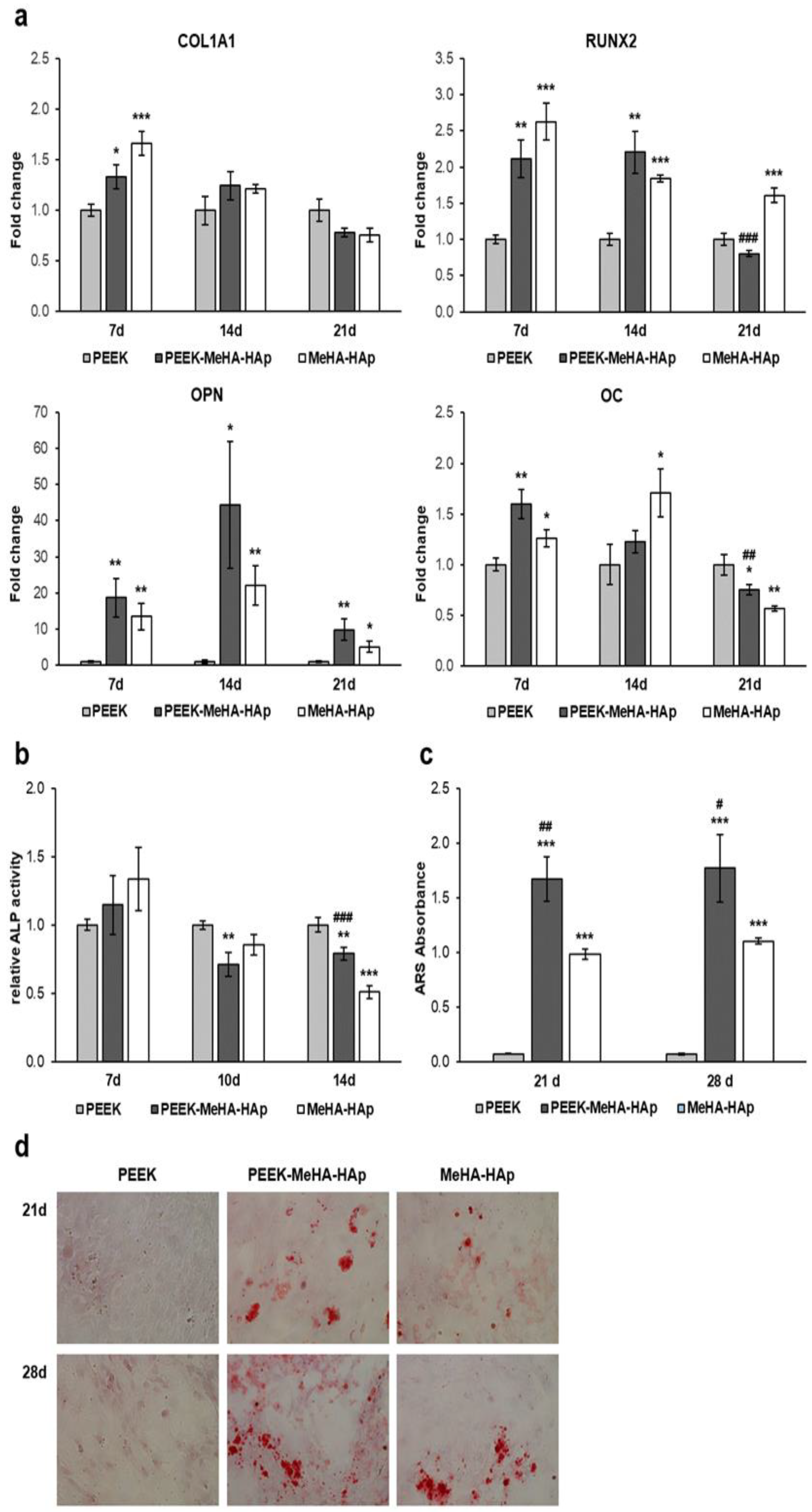
2.3. PEEK-MeHA-HAp Bone Substitute: Bioactivity
3. Discussion
4. Materials and Methods
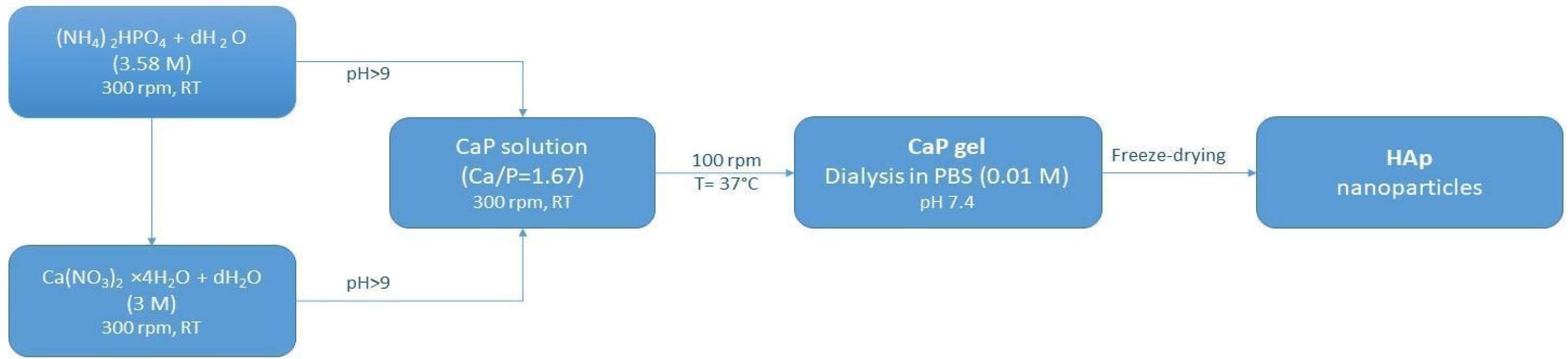
4.1. Synthesis and Characterization of MeHA-HAp
4.2. Production and Characterization of PEEK-MeHA-HAp
4.3. Cell Seeding, Adhesion and Proliferation Assay
4.4. Real-Time PCR
4.5. ARS Staining and Quantification
4.6. ALP Activity
4.7. Statistical Analysis
5. Conclusions
- -
- The presence of a MeHA-HAp coating did not influence the mechanical properties of the neat scaffold. Conversely, it synergically improved the hydrophilicity of the PEEK surface and the biological behavior thanks to the polymer MeHA and the bioactive molecule HAp, respectively.
- -
- MeHA-HAp coated PEEK scaffold supports a significant increase in cell adhesion and proliferation than bare PEEK.
- -
- The MeHA-HAp coating on PEEK promotes osteogenic differentiation thanks to the increased expression of osteogenesis-related genes and the increased ALP activity after 7 days, promoting greater mineralization of the extracellular matrix already at 21 days.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivolella, S.; Stellini, E.; Brunello, G.; Gardin, C.; Ferroni, L.; Bressan, E.; Zavan, B. Silver Nanoparticles in Alveolar Bone Surgery Devices. J. Nanomater. 2012, 2012, 15. [Google Scholar] [CrossRef]
- Bressan, E.; Sbricoli, L.; Guazzo, R.; Tocco, I.; Roman, M.; Vindigni, V.; Stellini, E.; Gardin, C.; Ferroni, L.; Sivolella, S. Nanostructured Surfaces of Dental Implants. Int. J. Mol. Sci. 2013, 14, 1918–1931. [Google Scholar] [CrossRef] [PubMed]
- Brunello, G.; Brun, P.; Gardin, C.; Ferroni, L.; Bressan, E.; Meneghello, R.; Zavan, B.; Sivolella, S. Biocompatibility and Antibacterial Properties of Zirconium Nitride Coating on Titanium Abutments: An in Vitro Study. PLoS ONE 2018, 13, e0199591. [Google Scholar] [CrossRef] [PubMed]
- Ghensi, P.; Bressan, E.; Gardin, C.; Ferroni, L.; Soldini, M.C.; Mandelli, F.; Soldini, C.; Zavan, B. The Biological Properties of OGI Surfaces Positively Act on Osteogenic and Angiogenic Commitment of Mesenchymal Stem Cells. Materials 2017, 10, 1321. [Google Scholar] [CrossRef]
- Ghensi, P.; Bressan, E.; Gardin, C.; Ferroni, L.; Ruffato, L.; Caberlotto, M.; Soldini, C.; Zavan, B. Osteo Growth Induction Titanium Surface Treatment Reduces ROS Production of Mesenchymal Stem Cells Increasing Their Osteogenic Commitment. Mater. Sci. Eng. C 2017, 74, 389–398. [Google Scholar] [CrossRef]
- Gardin, C.; Ferroni, L.; Erdoğan, Y.K.; Zanotti, F.; De Francesco, F.; Trentini, M.; Brunello, G.; Ercan, B.; Zavan, B. Nanostructured Modifications of Titanium Surfaces Improve Vascular Regenerative Properties of Exosomes Derived from Mesenchymal Stem Cells: Preliminary in Vitro Results. Nanomaterials 2021, 11, 3452. [Google Scholar] [CrossRef]
- Mijiritsky, E.; Ferroni, L.; Gardin, C.; Peleg, O.; Gultekin, A.; Saglanmak, A.; Delogu, L.G.; Mitrecic, D.; Piattelli, A.; Tatullo, M. Presence of ROS in Inflammatory Environment of Peri-Implantitis Tissue: In Vitro and in Vivo Human Evidence. J. Clin. Med. 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Bhatnagar, D.; Bansal, D.; Batra, H.; Goyal, N. Recent Advancements in Nanomaterials for Biomedical Implants. Biomed. Eng. Adv. 2022, 3, 100029. [Google Scholar] [CrossRef]
- Han, X.; Yang, D.; Yang, C.; Spintzyk, S.; Scheideler, L.; Li, P.; Li, D.; Geis-Gerstorfer, J.; Rupp, F. Carbon Fiber Reinforced PEEK Composites Based on 3D-Printing Technology for Orthopedic and Dental Applications. J. Clin. Med. 2019, 8, 240. [Google Scholar] [CrossRef]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef]
- Phan, K.; Hogan, J.A.; Assem, Y.; Mobbs, R.J. PEEK-Halo Effect in Interbody Fusion. J. Clin. Neurosci. 2016, 24, 138–140. [Google Scholar] [CrossRef]
- Buck, E.; Li, H.; Cerruti, M. Surface Modification Strategies to Improve the Osseointegration of Poly (Etheretherketone) and Its Composites. Macromol. Biosci. 2020, 20, 1900271. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; Bucchieri, F.; Fucarino, A.; Ronca, A.; D’Amora, U. Three-Dimensional Bioprinting for Cartilage Tissue Engineering: Insights into Naturally-Derived Bioinks from Land and Marine Sources. J. Funct. Biomater. 2022, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- El-Habashy, S.E.; El-Kamel, A.H.; Essawy, M.M.; Abdelfattah, E.-Z.A.; Eltaher, H.M. Engineering 3D-Printed Core–Shell Hydrogel Scaffolds Reinforced with Hybrid Hydroxyapatite/Polycaprolactone Nanoparticles for in Vivo Bone Regeneration. Biomater. Sci. 2021, 9, 4019–4039. [Google Scholar] [CrossRef]
- Raucci, M.G.; D’Amora, U.; Ronca, A.; Ambrosio, L. Injectable Functional Biomaterials for Minimally Invasive Surgery. Adv. Healthc. Mater. 2020, 9, 2000349. [Google Scholar] [CrossRef]
- Zavan, B.; Giorgi, C.; Bagnara, G.; Vindigni, V.; Abatangelo, G.; Cortivo, R. Osteogenic and Chondrogenic Differentiation: Comparison of Human and Rat Bone Marrow Mesenchymal Stem Cells Cultured into Polymeric Scaffolds. Eur. J. Histochem. 2007, 51, 1. [Google Scholar] [PubMed]
- Petta, D.; D’amora, U.; Ambrosio, L.; Grijpma, D.; Eglin, D.; D’este, M. Hyaluronic Acid as a Bioink for Extrusion-Based 3D Printing. Biofabrication 2020, 12, 032001. [Google Scholar] [CrossRef] [PubMed]
- D’Amora, U.; Ronca, A.; Raucci, M.; Dozio, S.; Lin, H.; Fan, Y.; Zhang, X.; Ambrosio, L. In Situ Sol-Gel Synthesis of Hyaluronan Derivatives Bio-Nanocomposite Hydrogels. Regen. Biomater. 2019, 6, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Jiang, M.; Zhang, M.; Cui, L.; Yang, X.; Wang, X.; Liu, G.; Ding, J.; Chen, X. Biofunctionalized Composite Scaffold to Potentiate Osteoconduction, Angiogenesis, and Favorable Metabolic Microenvironment for Osteonecrosis Therapy. Bioact. Mater. 2022, 9, 446–460. [Google Scholar] [CrossRef]
- Guarino, V.; Veronesi, F.; Marrese, M.; Giavaresi, G.; Ronca, A.; Sandri, M.; Tampieri, A.; Fini, M.; Ambrosio, L. Needle-like Ion-Doped Hydroxyapatite Crystals Influence Osteogenic Properties of PCL Composite Scaffolds. Biomed. Mater. 2016, 11, 015018. [Google Scholar] [CrossRef]
- D’Amora, U.; Ronca, A.; Raucci, M.G.; Lin, H.; Soriente, A.; Fan, Y.; Zhang, X.; Ambrosio, L. Bioactive Composites Based on Double Network Approach with Tailored Mechanical, Physico-chemical, and Biological Features. J. Biomed. Mater. Res. A 2018, 106, 3079–3089. [Google Scholar] [CrossRef] [PubMed]
- ISO I 10993-5; 2009 Biological Evaluation of Medical Devices—Part 5: Tests for in vitro Cytotoxicity. International Organization for Standardization: Geneva, Swizerland, 2009.
- Li, H.; Qi, Z.; Zheng, S.; Chang, Y.; Kong, W.; Fu, C.; Yu, Z.; Yang, X.; Pan, S. The Application of Hyaluronic Acid-Based Hydrogels in Bone and Cartilage Tissue Engineering. Adv. Mater. Sci. Eng. 2019, 2019, 3027303. [Google Scholar] [CrossRef]
- Spearman, B.S.; Agrawal, N.K.; Rubiano, A.; Simmons, C.S.; Mobini, S.; Schmidt, C.E. Tunable Methacrylated Hyaluronic Acid-based Hydrogels as Scaffolds for Soft Tissue Engineering Applications. J. Biomed. Mater. Res. A 2020, 108, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic Acid-Based Scaffolds for Tissue Engineering. Rom. J. Morphol. Embryol. 2018, 59, 71–76. [Google Scholar] [PubMed]
- Monslow, J.; Govindaraju, P.; Puré, E. Hyaluronan–a Functional and Structural Sweet Spot in the Tissue Microenvironment. Front. Immunol. 2015, 6, 231. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Cortivo, R.; Zavan, B.; Vecchiato, N.; Abatangelo, G. In Vitro Reconstructed Tissues on Hyaluronan-Based Temporary Scaffolding. J. Mater. Sci. Mater. Med. 1999, 10, 683–688. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; Sivolella, S.; Brunello, G.; Berengo, M.; Piattelli, A.; Bressan, E.; Zavan, B. A Hyaluronan-Based Scaffold for the in Vitro Construction of Dental Pulp-like Tissue. Int. J. Mol. Sci. 2015, 16, 4666–4681. [Google Scholar] [CrossRef]
- Gardin, C.; Vindigni, V.; Bressan, E.; Ferroni, L.; Nalesso, E.; Puppa, A.D.; D’Avella, D.; Lops, D.; Pinton, P.; Zavan, B. Hyaluronan and Fibrin Biomaterial as Scaffolds for Neuronal Differentiation of Adult Stem Cells Derived from Adipose Tissue and Skin. Int. J. Mol. Sci. 2011, 12, 6749–6764. [Google Scholar] [CrossRef]
- Pandis, L.; Zavan, B.; Bassetto, F.; Ferroni, L.; Iacobellis, L.; Abatangelo, G.; Lepidi, S.; Cortivo, R.; Vindigni, V. Hyaluronic Acid Biodegradable Material for Reconstruction of Vascular Wall: A Preliminary Study in Rats. Microsurgery 2011, 31, 138–145. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical Modifications of Hyaluronic Acid for the Synthesis of Derivatives for a Broad Range of Biomedical Applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Ronca, A.; D’Amora, U.; Raucci, M.G.; Lin, H.; Fan, Y.; Zhang, X.; Ambrosio, L. A Combined Approach of Double Network Hydrogel and Nanocomposites Based on Hyaluronic Acid and Poly (Ethylene Glycol) Diacrylate Blend. Materials 2018, 11, 2454. [Google Scholar] [CrossRef] [PubMed]
- Noh, I.; Kim, N.; Tran, H.N.; Lee, J.; Lee, C. 3D Printable Hyaluronic Acid-Based Hydrogel for Its Potential Application as a Bioink in Tissue Engineering. Biomater. Res. 2019, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Wenz, A.; Borchers, K.; Tovar, G.E.M.; Kluger, P.J. Bone Matrix Production in Hydroxyapatite-Modified Hydrogels Suitable for Bone Bioprinting. Biofabrication 2017, 9, 44103. [Google Scholar] [CrossRef] [PubMed]
- Kapat, K.; Srivas, P.K.; Rameshbabu, A.P.; Maity, P.P.; Jana, S.; Dutta, J.; Majumdar, P.; Chakrabarti, D.; Dhara, S. Influence of Porosity and Pore-Size Distribution in Ti6Al4 V Foam on Physicomechanical Properties, Osteogenesis, and Quantitative Validation of Bone Ingrowth by Micro-Computed Tomography. ACS Appl. Mater. Interfaces 2017, 9, 39235–39248. [Google Scholar] [CrossRef] [PubMed]
- Hannink, G.; Arts, J.J.C. Bioresorbability, Porosity and Mechanical Strength of Bone Substitutes: What Is Optimal for Bone Regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Feng, F.; Liu, X.; Leoreanu-Fotea, V.; Jun, Y.B. Soft Sets and Soft Rough Sets. Inf. Sci. 2011, 181, 1125–1137. [Google Scholar] [CrossRef]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.-P.; Schrooten, J. The Effect of Pore Geometry on the in Vitro Biological Behavior of Human Periosteum-Derived Cells Seeded on Selective Laser-Melted Ti6Al4V Bone Scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef]
- Zhao, P.; Gu, H.; Mi, H.; Rao, C.; Fu, J.; Turng, L. Fabrication of Scaffolds in Tissue Engineering: A Review. Front. Mech. Eng. 2018, 13, 107–119. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, L.; Xiao, L.; Zhang, Q.; Liu, Y. Enhanced Osteogenic Activity of Phosphorylated Polyetheretherketone via Surface-Initiated Grafting Polymerization of Vinylphosphonic Acid. Colloids Surf. B Biointerfaces 2019, 173, 591–598. [Google Scholar] [CrossRef]
- Li, L.; Qian, Y.; Jiang, C.; Lv, Y.; Liu, W.; Zhong, L.; Cai, K.; Li, S.; Yang, L. The Use of Hyaluronan to Regulate Protein Adsorption and Cell Infiltration in Nanofibrous Scaffolds. Biomaterials 2012, 33, 3428–3445. [Google Scholar] [CrossRef] [PubMed]
- Gardin, C.; Bressan, E.; Ferroni, L.; Nalesso, E.; Vindigni, V.; Stellini, E.; Pinton, P.; Sivolella, S.; Zavan, B. In Vitro Concurrent Endothelial and Osteogenic Commitment of Adipose-Derived Stem Cells and Their Genomical Analyses through Comparative Genomic Hybridization Array: Novel Strategies to Increase the Successful Engraftment of Tissue-Engineered Bone Grafts. Stem Cells Dev. 2012, 21, 767–777. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium Phosphate Ceramics in Bone Tissue Engineering: A Review of Properties and Their Influence on Cell Behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Sartuqui, J.; Gardin, C.; Ferroni, L.; Zavan, B.; Messina, P.V. Nanostructured Hydroxyapatite Networks: Synergy of Physical and Chemical Cues to Induce an Osteogenic Fate in an Additive-Free Medium. Mater. Today Commun. 2018, 16, 152–163. [Google Scholar] [CrossRef]
- Ruijtenberg, S.; van den Heuvel, S. Coordinating Cell Proliferation and Differentiation: Antagonism between Cell Cycle Regulators and Cell Type-Specific Gene Expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; He, X.; Xie, K.; Xie, L.; Deng, Y. Dual Therapy Coating on Micro/Nanoscale Porous Polyetheretherketone to Eradicate Biofilms and Accelerate Bone Tissue Repair. Macromol. Biosci. 2019, 19, 1800376. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhang, H.; Lan, A.; Yang, S.; Zhang, J.; Wang, H.; Zhou, Z.; Zhou, Y.; Zhao, J.; Jiang, Z. Enhanced Bioactivity and Osteogenic Property of Carbon Fiber Reinforced Polyetheretherketone Composites Modified with Amino Groups. Colloids Surf. B Biointerfaces 2020, 193, 111098. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zheng, Y.; Li, H.; Lin, H.; Chen, Z.; Tian, Y.; Chen, H.; Zhang, P.; Xu, X.; Shen, Y. The Toll-like Receptor Ligand, CpG Oligodeoxynucleotides, Regulate Proliferation and Osteogenic Differentiation of Osteoblast. J. Orthop. Surg. 2020, 15, 327. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; D’Amora, U.; Calzà, L.; Ronca, A.; Tremoli, E.; Ambrosio, L.; Zavan, B. Exosomes of Mesenchymal Stem Cells Delivered from Methacrylated Hyaluronic Acid Patch Improve the Regenerative Properties of Endothelial and Dermal Cells. Biomater. Adv. 2022, 139, 213000. [Google Scholar] [CrossRef]
- Fiocco, L.; Elsayed, H.; Ferroni, L.; Gardin, C.; Zavan, B.; Bernardo, E. Bioactive Wollastonite-Diopside Foams from Preceramic Polymers and Reactive Oxide Fillers. Materials 2015, 8, 2480–2494. [Google Scholar] [CrossRef]
- Gardin, C.; Bosco, G.; Ferroni, L.; Quartesan, S.; Rizzato, A.; Tatullo, M.; Zavan, B. Hyperbaric Oxygen Therapy Improves the Osteogenic and Vasculogenic Properties of Mesenchymal Stem Cells in the Presence of Inflammation in Vitro. Int. J. Mol. Sci. 2020, 21, 1452. [Google Scholar] [CrossRef] [PubMed]
- Cecchinato, F.; Karlsson, J.; Ferroni, L.; Gardin, C.; Galli, S.; Wennerberg, A.; Zavan, B.; Andersson, M.; Jimbo, R. Osteogenic Potential of Human Adipose-Derived Stromal Cells on 3-Dimensional Mesoporous TiO2 Coating with Magnesium Impregnation. Mater. Sci. Eng. C 2015, 52, 225–234. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferroni, L.; D’Amora, U.; Leo, S.; Tremoli, E.; Raucci, M.G.; Ronca, A.; Ambrosio, L.; Zavan, B. PEEK and Hyaluronan-Based 3D Printed Structures: Promising Combination to Improve Bone Regeneration. Molecules 2022, 27, 8749. https://doi.org/10.3390/molecules27248749
Ferroni L, D’Amora U, Leo S, Tremoli E, Raucci MG, Ronca A, Ambrosio L, Zavan B. PEEK and Hyaluronan-Based 3D Printed Structures: Promising Combination to Improve Bone Regeneration. Molecules. 2022; 27(24):8749. https://doi.org/10.3390/molecules27248749
Chicago/Turabian StyleFerroni, Letizia, Ugo D’Amora, Sara Leo, Elena Tremoli, Maria Grazia Raucci, Alfredo Ronca, Luigi Ambrosio, and Barbara Zavan. 2022. "PEEK and Hyaluronan-Based 3D Printed Structures: Promising Combination to Improve Bone Regeneration" Molecules 27, no. 24: 8749. https://doi.org/10.3390/molecules27248749
APA StyleFerroni, L., D’Amora, U., Leo, S., Tremoli, E., Raucci, M. G., Ronca, A., Ambrosio, L., & Zavan, B. (2022). PEEK and Hyaluronan-Based 3D Printed Structures: Promising Combination to Improve Bone Regeneration. Molecules, 27(24), 8749. https://doi.org/10.3390/molecules27248749








