Sodium Succinate as a Corrosion Inhibitor for Carbon Steel Rebars in Simulated Concrete Pore Solution
Abstract
1. Introduction
2. Results and Discussion
2.1. Electrochemical Results
2.1.1. Potentiodynamic Polarization Curves
2.1.2. Electrochemical Impedance Spectroscopy Measurements (EIS)
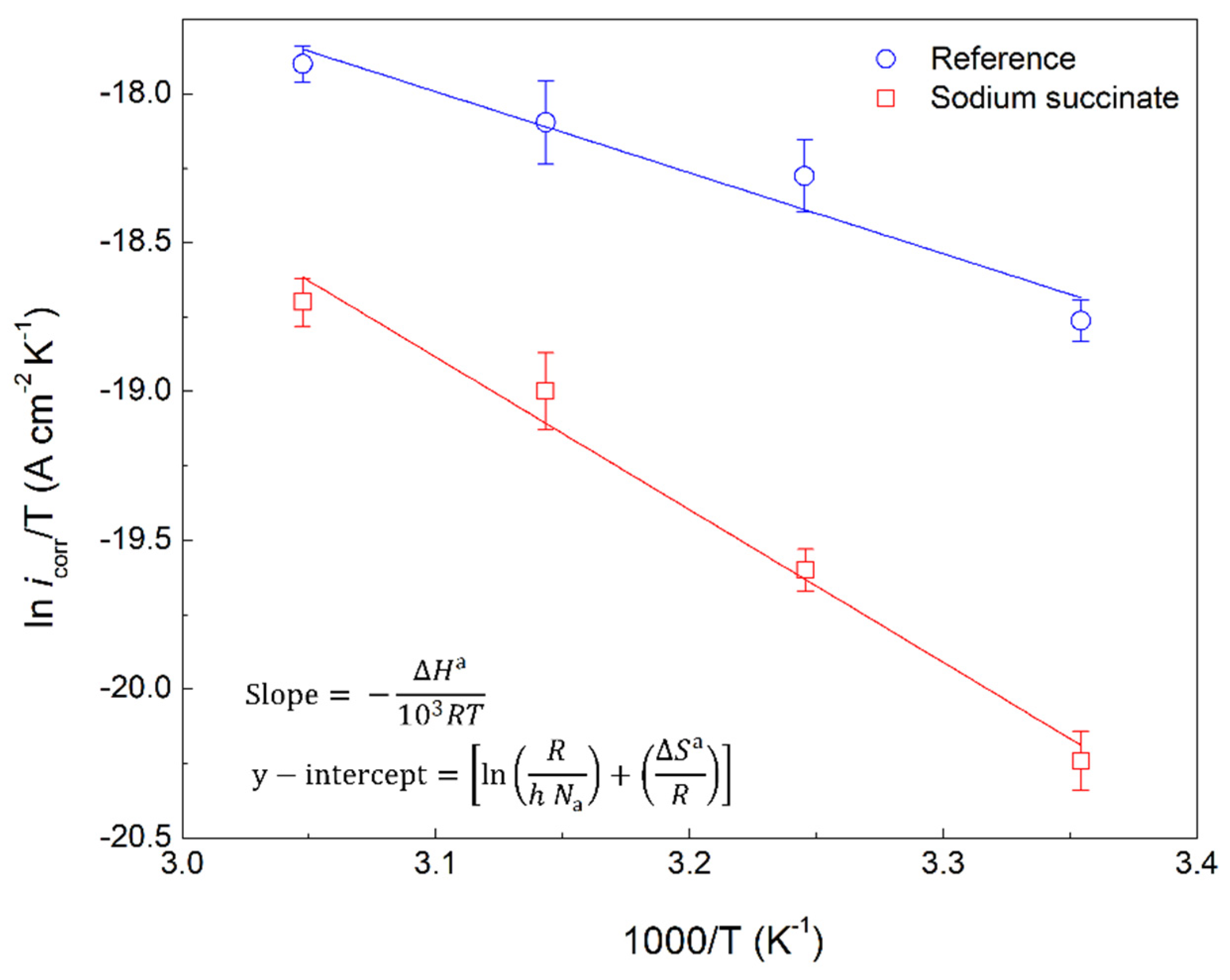
2.2. Activation Thermodynamic Parameters of the Corrosion Process

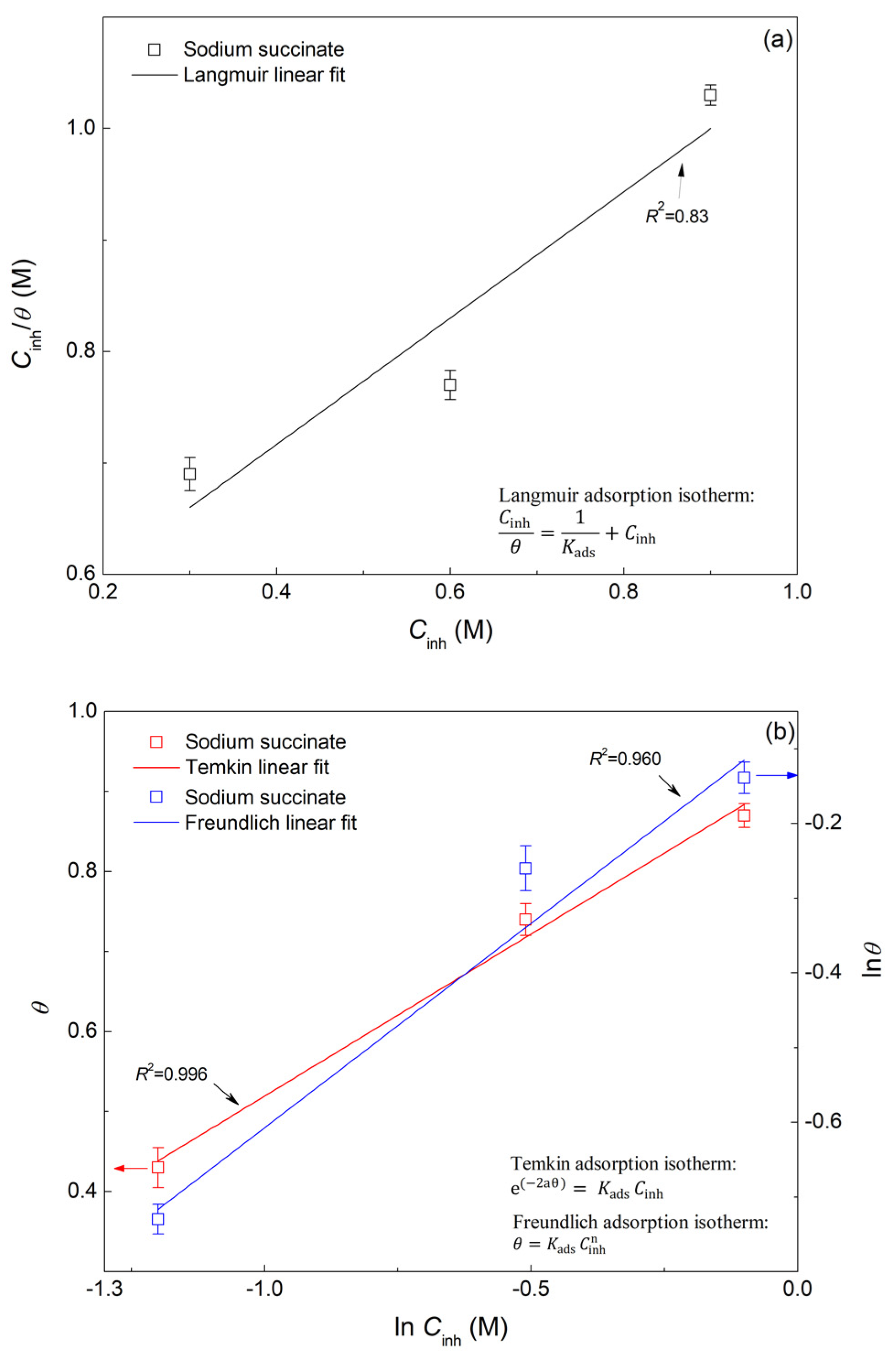
2.3. Adsorption Isotherm
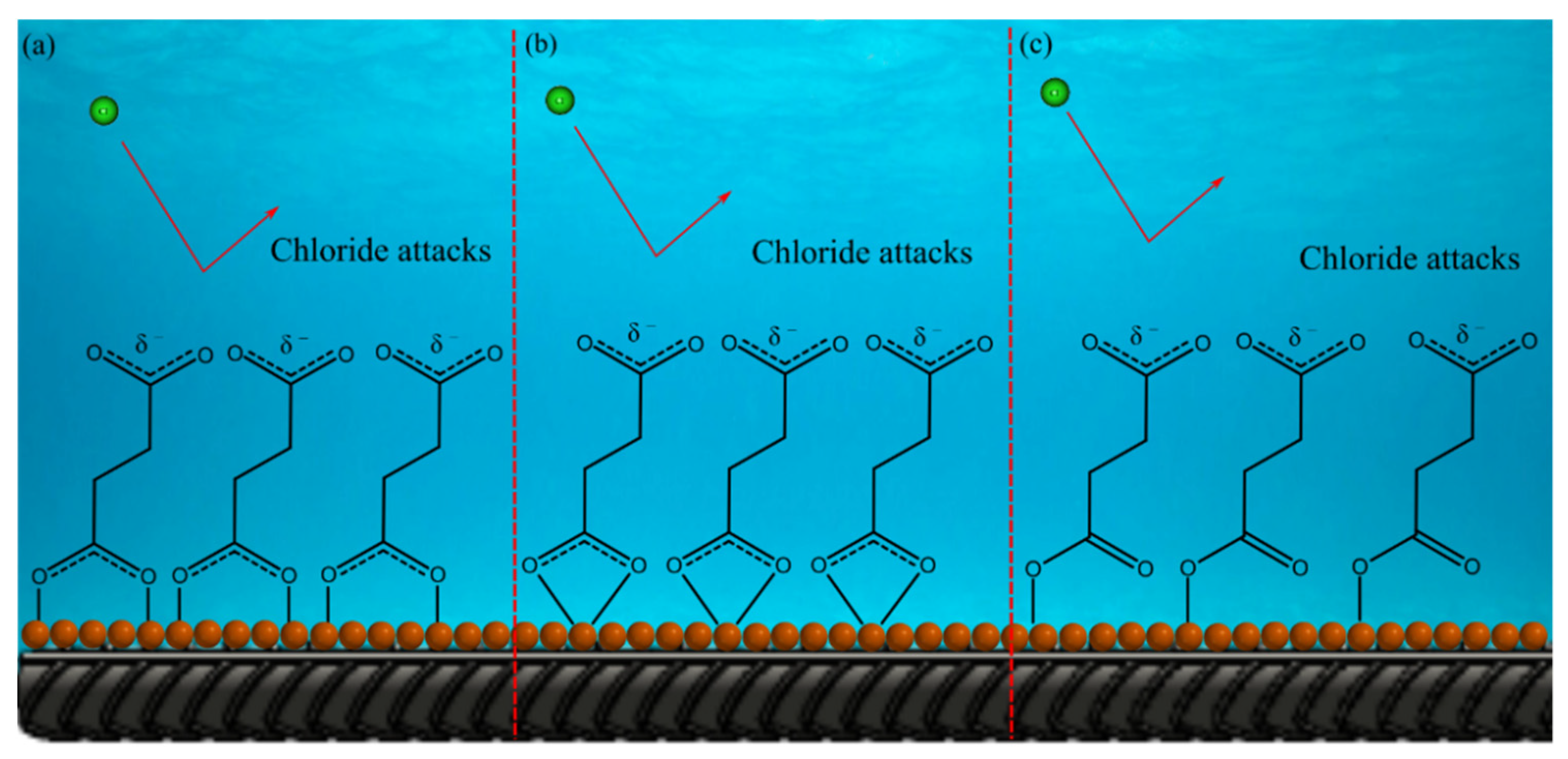
2.4. Surface Analysis
2.4.1. SEM/EDX Analysis
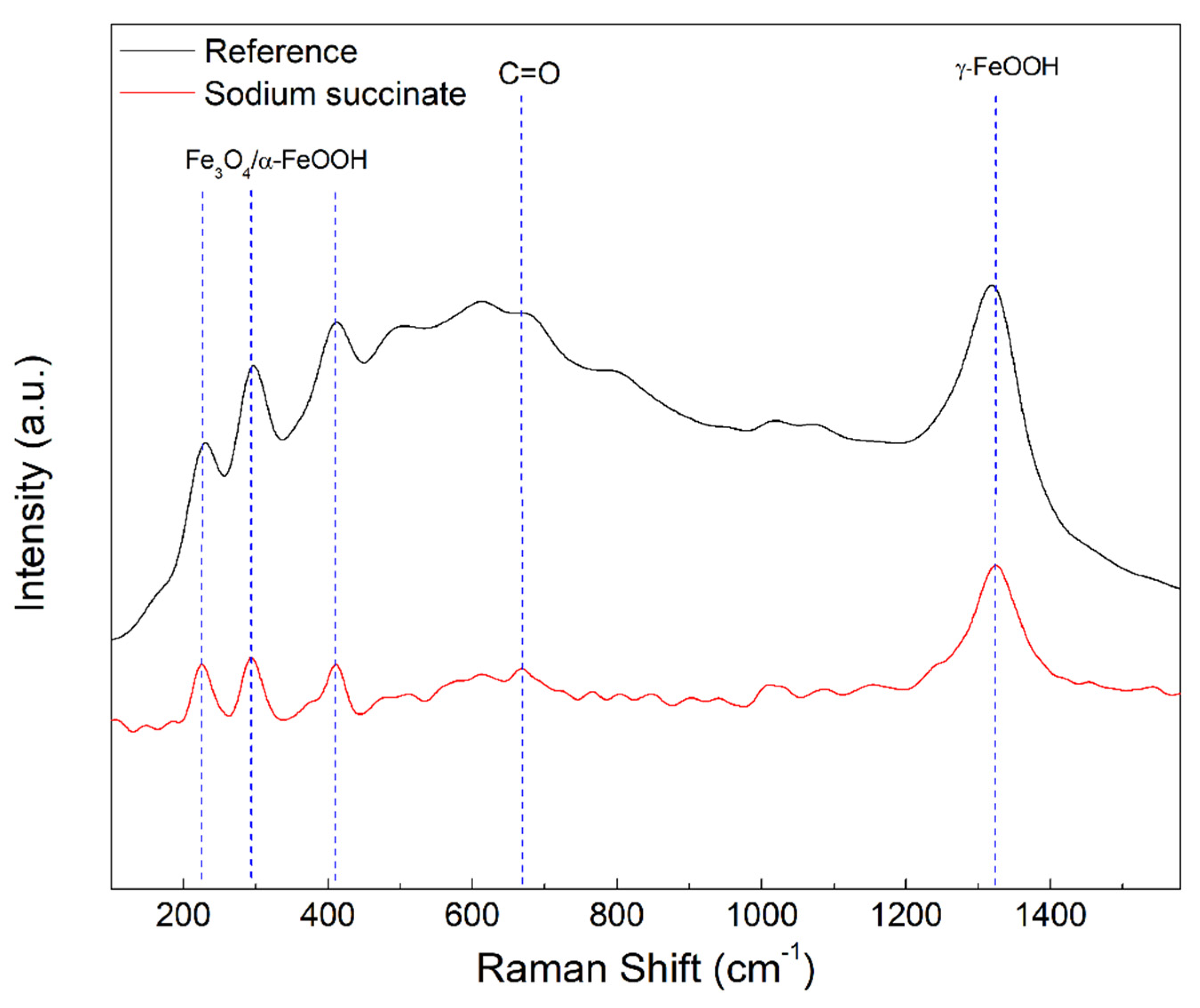
2.4.2. Micro-Raman Spectroscopy
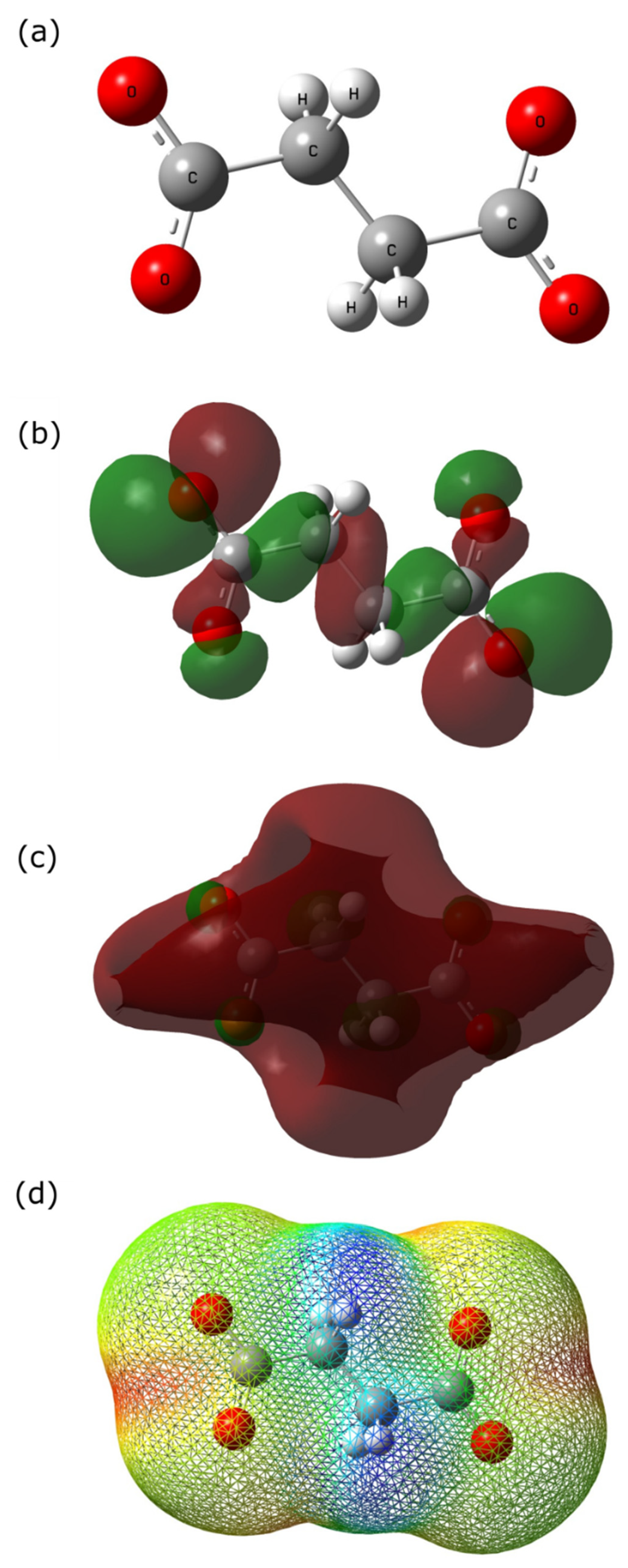
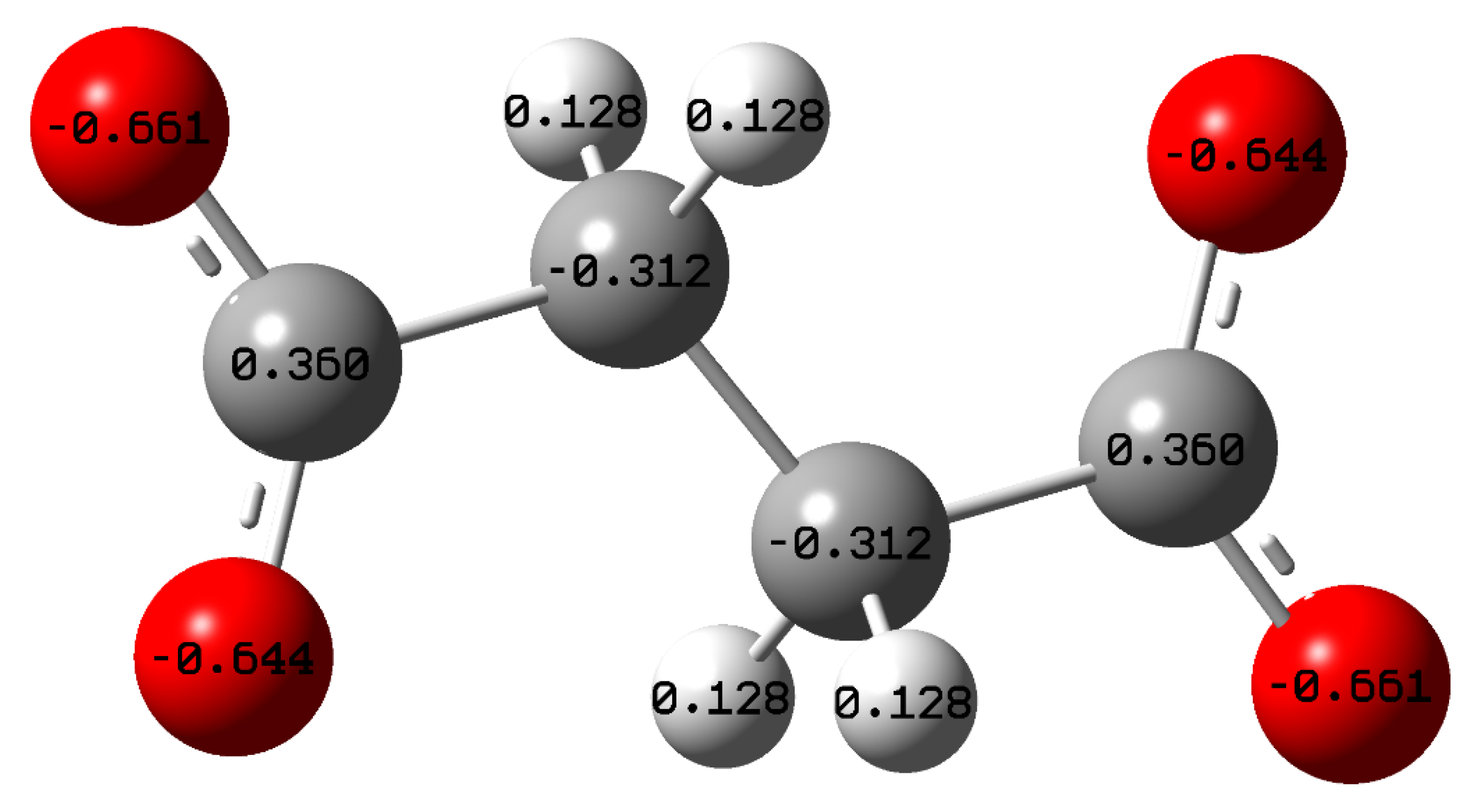
2.5. Quantum Chemical Calculations
3. Materials and Methods
4. Conclusions
- Sodium succinate, an environmentally friendly organic compound, possesses physicochemical properties that inhibit corrosion for carbon steel rebars in 0.6 M Cl− contaminated SCPS. Na2C4H4O4 creates an organic adsorption film on the surface of the rebar, by forming complexes with ferrous ions that protect the rebar from Cl− induced corrosion.
- The IE of Na2C4H4O4 according to PDP curves were 77, 69, 59, and 54% at 25, 35, 45, and 55 °C, respectively. The decrease in IE with temperature is attributed to the increased corrosion kinetics and desorption of Na2C4H4O4 on the surface of the rebar.
- The IE of sodium succinate according to EIS were 83.6, 71.2, 65.0, and 59.0% for 25, 35, 45, and 55 °C, respectively, corroborating PDP curves. The Ceff,dl was calculated at each temperature and was found to be lower than the reference indicating a decrease in the local dielectric constant and/or increase in the thickness of the electrochemical double layer suggesting that the inhibition process is attributed to surface adsorption. The film thickness increased in the presence of sodium succinate at every temperature, due to the formation of R–COO–Fe complexes.
- The activation energy (Ea) is greater in the presence of the inhibitor compared to the reference. This is attributed to the adsorption of the inhibitor on the surface of the carbon steel rebar, making corrosion harder to initiate. Enthalpy of activation (ΔHa) is positive signifying the endothermic nature of the steel dissolution process. Entropy of activation (ΔSa) in the presence of the inhibitor is greater than the reference due to disorder from the displacement of water molecules by the adsorbed sodium succinate.
- Sodium succinate follows the Temkin adsorption isotherm. The ΔG0ads was found to be −32.75 kJ/mol, indicating a combined physicochemical adsorption process.
- Different quantum chemical parameters were calculated to elucidate the experimental results. HOMO energies were found concentrated at the end of the carboxylic group, while LUMO energies were found at the C–C bonds, indicating that the corrosion inhibitor can donate and accept electrons from the metal surface. Finally, the electrostatic potential map shows that the terminal carboxyl groups act as an active site for the adsorption process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, M.; Beushausen, H. Durability, service life prediction, and modelling for reinforced concrete structures—Review and critique. Cem. Concr. Res. 2019, 122, 17–29. [Google Scholar] [CrossRef]
- Söylev, T.A.; Richardson, M.G. Corrosion inhibitors for steel in concrete: State-of-the-art report. Constr. Build. Mater. 2008, 22, 609–622. [Google Scholar] [CrossRef]
- Lee, H.-S.; Saraswathy, V.; Kwon, S.-J.; Karthick, S. Corrosion inhibitors for reinforced concrete: A review. In Corrosion Inhibitors, Principles and Recent Applications; Intech: Vienna, Austria, 2018; pp. 95–120. [Google Scholar]
- Garcés, P.; Saura, P.; Méndez, A.; Zornoza, E.; Andrade, C. Effect of nitrite in corrosion of reinforcing steel in neutral and acid solutions simulating the electrolytic environments of micropores of concrete in the propagation period. Corros. Sci. 2008, 50, 498–509. [Google Scholar] [CrossRef]
- Bolzoni, F.; Brenna, A.; Ormellese, M. Recent advances in the use of inhibitors to prevent chloride-induced corrosion in reinforced concrete. Cem. Concr. Res. 2022, 154, 106719. [Google Scholar] [CrossRef]
- Lin, B.; Zuo, Y. Corrosion inhibition of carboxylate inhibitors with different alkylene chain lengths on carbon steel in an alkaline solution. RSC Adv. 2019, 9, 7065–7077. [Google Scholar] [CrossRef]
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Almobarak, N.A.; El-Naggar, M.M.; Al-Mufraj, R.S.; Al-Zoghbi, O.A. Carboxylic acids: Pitting corrosion inhibitors for carbon steel in alkaline medium and in the presence of chlorides. Chem. Technol. Fuels Oils 2014, 50, 170–178. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. In Handbook of Vibrational Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Mohamed, A.; Visco, D.P.; Bastidas, D.M. Significance of π-electrons in the design of corrosion inhibitors for carbon steel in simulated concrete pore solution. Corrosion 2021, 77, 976–990. [Google Scholar] [CrossRef]
- Fazayel, A.S.; Khorasani, M.; Sarabi, A.A. The effect of functionalized polycarboxylate structures as corrosion inhibitors in a simulated concrete pore solution. Appl. Surf. Sci. 2018, 441, 895–913. [Google Scholar] [CrossRef]
- Noor, E.A. The inhibition of mild steel corrosion in phosphoric acid solutions by some N-heterocyclic compounds in the salt form. Corros. Sci. 2005, 47, 33–55. [Google Scholar] [CrossRef]
- Amin, M.A.; Abd El-Rehim, S.S.; El-Sherbini, E.E.F.; Bayoumi, R.S. The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid: Part I. Weight loss, polarization, EIS, PZC, EDX and SEM studies. Electrochim. Acta 2007, 52, 3588–3600. [Google Scholar] [CrossRef]
- Deyab, M.A.; El-Rehim, S.S.A. Effect of succinic acid on carbon steel corrosion in produced water of crude oil. J. Taiwan Inst. Chem. Eng. 2014, 45, 1065–1072. [Google Scholar] [CrossRef]
- Parashar, K.; kumar Dubey, D.R.; Padmaja, M.; Phasinam, K.; Tracy Tina Angelina, D.J.; Gupta, P. Effect of succinic acid on compression strength concrete material. Mater. Today Proc. 2022, 51, 314–318. [Google Scholar] [CrossRef]
- Ormellese, M.; Lazzari, L.; Goidanich, S.; Fumagalli, G.; Brenna, A. A study of organic substances as inhibitors for chloride-induced corrosion in concrete. Corros. Sci. 2009, 51, 2959–2968. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quraishi, M.A. Inhibition of mild steel corrosion in acid solution by Pheniramine drug: Experimental and theoretical study. Corros. Sci. 2010, 52, 3033–3041. [Google Scholar] [CrossRef]
- Abd-Elaal, A.A.; Elbasiony, N.M.; Shaban, S.M.; Zaki, E.G. Studying the corrosion inhibition of some prepared nonionic surfactants based on 3-(4-hydroxyphenyl) propanoic acid and estimating the influence of silver nanoparticles on the surface parameters. J. Mol. Liq. 2018, 249, 304–317. [Google Scholar] [CrossRef]
- Mohamed, A.; Visco, D.P.; Bastidas, D.M. Effect of cations on the activity coefficient of NO2−/NO3− corrosion inhibitors in simulated concrete pore solution: An electrochemical thermodynamics study. Corros. Sci. 2022, 206, 110476. [Google Scholar] [CrossRef]
- Macdonald, D.D. Some advantages and pitfalls of electrochemical impedance spectroscopy. Corrosion 1990, 46, 229–242. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Berdimurodov, E.; Kholikov, A.; Akbarov, K.; Guo, L.; Kaya, S.; Katin, K.P.; Verma, D.K.; Rbaa, M.; Dagdag, O.; Haldhar, R. Novel gossypol–indole modification as a green corrosion inhibitor for low–carbon steel in aggressive alkaline–saline solution. Colloids Surf. A 2022, 637, 128207. [Google Scholar] [CrossRef]
- Mandal, S.; Singh, J.K.; Mallapur, S.; Lee, D.-E.; Park, T. Effect of triethanolamine and sodium hexametaphosphate on formation, growth and breakdown of passive layer in concrete pore solution. J. Build. Eng. 2022, 59, 105113. [Google Scholar] [CrossRef]
- Petrović, Ž.; Metikoš-Huković, M.; Babić, R. The electrochemical transfer reactions and the structure of the iron|oxide layer|electrolyte interface. Electrochim. Acta 2012, 75, 406–413. [Google Scholar] [CrossRef]
- Wang, D.; Ming, J.; Shi, J. Enhanced corrosion resistance of rebar in carbonated concrete pore solutions by Na2HPO4 and benzotriazole. Corros. Sci. 2020, 174, 108830. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J. Corrosion resistance of carbon steel in alkaline concrete pore solutions containing phytate and chloride ions. Corros. Sci. 2022, 205, 110451. [Google Scholar] [CrossRef]
- Teymouri, F.; Allahkaram, S.R.; Shekarchi, M.; Azamian, I.; Johari, M. A comprehensive study on the inhibition behaviour of four carboxylate-based corrosion inhibitors focusing on efficiency drop after the optimum concentration for carbon steel in the simulated concrete pore solution. Constr. Build. Mater. 2021, 296, 123702. [Google Scholar] [CrossRef]
- Mohamed, A.; Martin, U.; Bastidas, D.M. Adsorption and surface analysis of sodium phosphate corrosion inhibitor on carbon steel in simulated concrete pore solution. Materials 2022, 15, 7429. [Google Scholar] [CrossRef]
- Bosch, J.; Martin, U.; Aperador, W.; Bastidas, J.M.; Ress, J.; Bastidas, D.M. Corrosion behavior of high-Mn austenitic Fe–Mn–Al–Cr–C steels in NaCl and NaOH solutions. Materials 2021, 14, 425. [Google Scholar] [CrossRef]
- Bastidas, D.M. Interpretation of impedance data for porous electrodes and diffusion processes. Corrosion 2007, 63, 515–521. [Google Scholar] [CrossRef]
- Perez, N. Electrochemistry and Corrosion Science; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Berdimurodov, E.; Kholikov, A.; Akbarov, K.; Guo, L.; Kaya, S.; Katin, K.P.; Verma, D.K.; Rbaa, M.; Dagdag, O. Novel cucurbit[6]uril-based [3]rotaxane supramolecular ionic liquid as a green and excellent corrosion inhibitor for the chemical industry. Colloids Surf. A 2022, 633, 127837. [Google Scholar] [CrossRef]
- Aiad, I.; El-Sukkary, M.M.; Soliman, E.A.; El-Awady, M.Y.; Shaban, S.M. Inhibition of mild steel corrosion in acidic medium by some cationic surfactants. J. Ind. Eng. Chem. 2014, 20, 3524–3535. [Google Scholar] [CrossRef]
- Popova, A. Temperature effect on mild steel corrosion in acid media in presence of azoles. Corros. Sci. 2007, 49, 2144–2158. [Google Scholar] [CrossRef]
- Issaadi, S.; Douadi, T.; Chafaa, S. Adsorption and inhibitive properties of a new heterocyclic furan Schiff base on corrosion of copper in HCl 1M: Experimental and theoretical investigation. Appl. Surf. Sci. 2014, 316, 582–589. [Google Scholar] [CrossRef]
- Venkatachalam, C.S.; Rajagopalan, S.R.; Sastry, M.V.C. Mechanism of inhibition of electrode reactions at high surface coverages—II. Electrochim. Acta 1981, 26, 1219–1224. [Google Scholar] [CrossRef]
- Gomma, G.K.; Wahdan, M.H. Schiff bases as corrosion inhibitors for aluminium in hydrochloric acid solution. Mater. Chem. Phys. 1995, 39, 209–213. [Google Scholar] [CrossRef]
- Fusco, M.A.; Ay, Y.; Casey, A.H.M.; Bourham, M.A.; Winfrey, A.L. Corrosion of single layer thin film protective coatings on steel substrates for high level waste containers. Prog. Nucl. Energy 2016, 89, 159–169. [Google Scholar] [CrossRef]
- Fernine, Y.; Ech-chihbi, E.; Arrousse, N.; El Hajjaji, F.; Bousraf, F.; Ebn Touhami, M.; Rais, Z.; Taleb, M. Ocimum basilicium seeds extract as an environmentally friendly antioxidant and corrosion inhibitor for aluminium alloy 2024 -T3 corrosion in 3 wt% NaCl medium. Colloids Surf. A 2021, 627, 127232. [Google Scholar] [CrossRef]
- Benabdellah, M.; Aouniti, A.; Dafali, A.; Hammouti, B.; Benkaddour, M.; Yahyi, A.; Ettouhami, A. Investigation of the inhibitive effect of triphenyltin 2-thiophene carboxylate on corrosion of steel in 2M H3PO4 solutions. Appl. Surf. Sci. 2006, 252, 8341–8347. [Google Scholar] [CrossRef]
- Kosari, A.; Momeni, M.; Parvizi, R.; Zakeri, M.; Moayed, M.H.; Davoodi, A.; Eshghi, H. Theoretical and electrochemical assessment of inhibitive behavior of some thiophenol derivatives on mild steel in HCl. Corros. Sci. 2011, 53, 3058–3067. [Google Scholar] [CrossRef]
- Mandal, S.; Singh, J.K.; Lee, D.-E.; Park, T. Ammonium phosphate as inhibitor to mitigate the corrosion of steel rebar in chloride contaminated concrete pore solution. Molecules 2020, 25, 3785. [Google Scholar] [CrossRef]
- Hermoso-Diaz, I.A.; Lopez-Cecenes, R.; Rios, J.P.F.-D.l.; Landeros-Martínez, L.L.; Sarmiento-Bustos, E.; Uruchurtu-Chavarin, J.; Gonzalez-Rodriguez, J.G. Experimental and theoretical studies of α-linolenic acid as green corrosion inhibitor for carbon steel in 0.5 M sulfuric acid. Molecules 2021, 26, 6169. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Dahlous, K.A.; AL Othman, Z.A.; Al-Lohedan, H.A.; El-Mahdy, G.A. sym-Trisubstituted 1,3,5-triazine derivatives as promising organic corrosion inhibitors for steel in acidic solution. Molecules 2016, 21, 436. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Niu, X.; Cui, Y.; Wang, Z.; Wang, J.; Wang, R. Study on the film forming mechanism, corrosion inhibition effect and synergistic action of two different inhibitors on copper surface chemical mechanical polishing for GLSI. Appl. Surf. Sci. 2020, 505, 144507. [Google Scholar] [CrossRef]
- El Basiony, N.M.; Badr, E.E.; Baker, S.A.; El-Tabei, A.S. Experimental and theoretical (DFT&MC) studies for the adsorption of the synthesized Gemini cationic surfactant based on hydrazide moiety as X-65 steel acid corrosion inhibitor. Appl. Surf. Sci. 2021, 539, 148246. [Google Scholar] [CrossRef]
- Azamian, I.; Allahkaram, S.R.; Johari, M.; Teymouri, F. Interfacial interaction study of EDTA with the defect structure of Fe3−δO4 passive film in an aggressive alkaline medium based on the lattice theory of point defects. RSC Adv. 2022, 12, 3524–3541. [Google Scholar] [CrossRef]
- Mennucci, M.M.; Banczek, E.P.; Rodrigues, P.R.P.; Costa, I. Evaluation of benzotriazole as corrosion inhibitor for carbon steel in simulated pore solution. Cem. Concr. Compos. 2009, 31, 418–424. [Google Scholar] [CrossRef]
- Gartner, N.; Kosec, T.; Legat, A. The efficiency of a corrosion inhibitor on steel in a simulated concrete environment. Mater. Chem. Phys. 2016, 184, 31–40. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, K.; Dong, C.; Wu, J.; Li, X.; Huang, Y. In situ Raman spectroscopy study of corrosion products on the surface of carbon steel in solution containing Cl− and SO42−. Eng. Fail. Anal. 2011, 18, 1981–1989. [Google Scholar] [CrossRef]
- Calderón, J.A.; Vásquez, F.A.; Carreño, J.A. Adsorption and performance of the 2-mercaptobenzimidazole as a carbon steel corrosion inhibitor in EDTA solutions. Mater. Chem. Phys. 2017, 185, 218–226. [Google Scholar] [CrossRef]
- Obot, I.B.; Macdonald, D.D.; Gasem, Z.M. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Amin, M.A.; Mersal, G.A.M.; El-Hendawy, M.M.; Shaltout, A.A.; Badawi, A.; Boman, J.; Gobouri, A.A.; Saracoglu, M.; Kandemirli, F.; Boukherroub, R.; et al. Synthesis of cyano-benzylidene xanthene synthons using a diprotic Brøsnsted acid catalyst, and their application as efficient inhibitors of aluminum corrosion in alkaline solutions. Molecules 2022, 27, 5733. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, S.; Li, W.; Guo, L.; Xu, S.; Feng, L.; Madkour, L.H. Experimental and theoretical investigations of some pyrazolo-pyrimidine derivatives as corrosion inhibitors on copper in sulfuric acid solution. Appl. Surf. Sci. 2018, 459, 612–620. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- El Basiony, N.M.; Elgendy, A.; Nady, H.; Migahed, M.A.; Zaki, E.G. Adsorption characteristics and inhibition effect of two Schiff base compounds on corrosion of mild steel in 0.5 M HCl solution: Experimental, DFT studies, and Monte Carlo simulation. RSC Adv. 2019, 9, 10473–10485. [Google Scholar] [CrossRef]
- Elgendy, A.; Nady, H.; El-Rabiei, M.M.; Elhenawy, A.A. Understanding the adsorption performance of two glycine derivatives as novel and environmentally safe anti-corrosion agents for copper in chloride solutions: Experimental, DFT, and MC studies. RSC Adv. 2019, 9, 42120–42131. [Google Scholar] [CrossRef]
- Nghiem, N.P.; Kleff, S.; Schwegmann, S. Succinic acid: Technology development and commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Schlackl, K.; Herchl, R.; Samhaber, W. Nanofiltration of succinic acid in strong alkaline conditions. Membranes 2019, 9, 147. [Google Scholar] [CrossRef]
- G106-89; Standard Practice for Verification of Algorithm and Equipment for Electrochemical Impedance Measurements. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- G61-86; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys. ASTM International: West Conshohocken, PA, USA, 2009. [CrossRef]
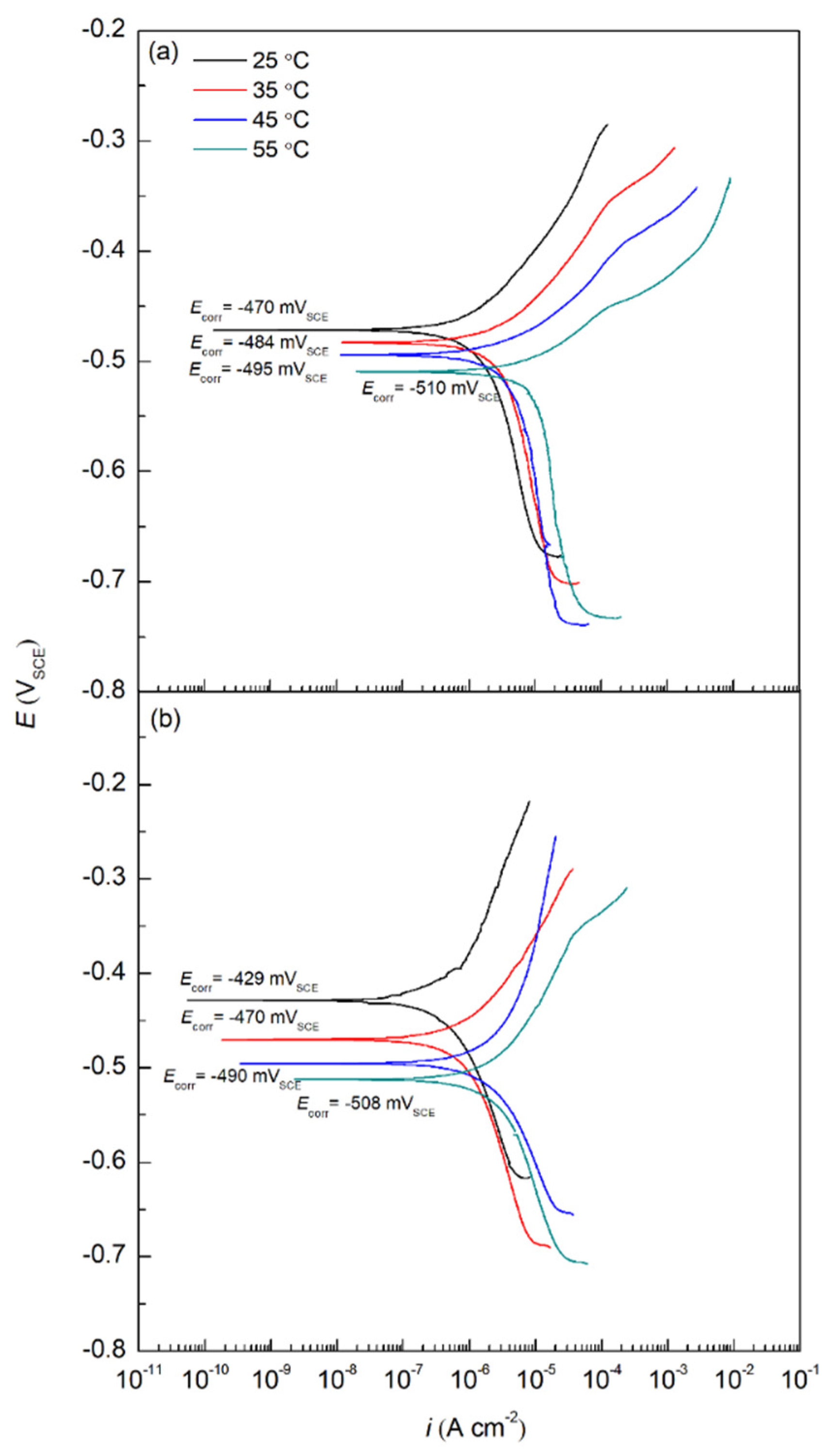
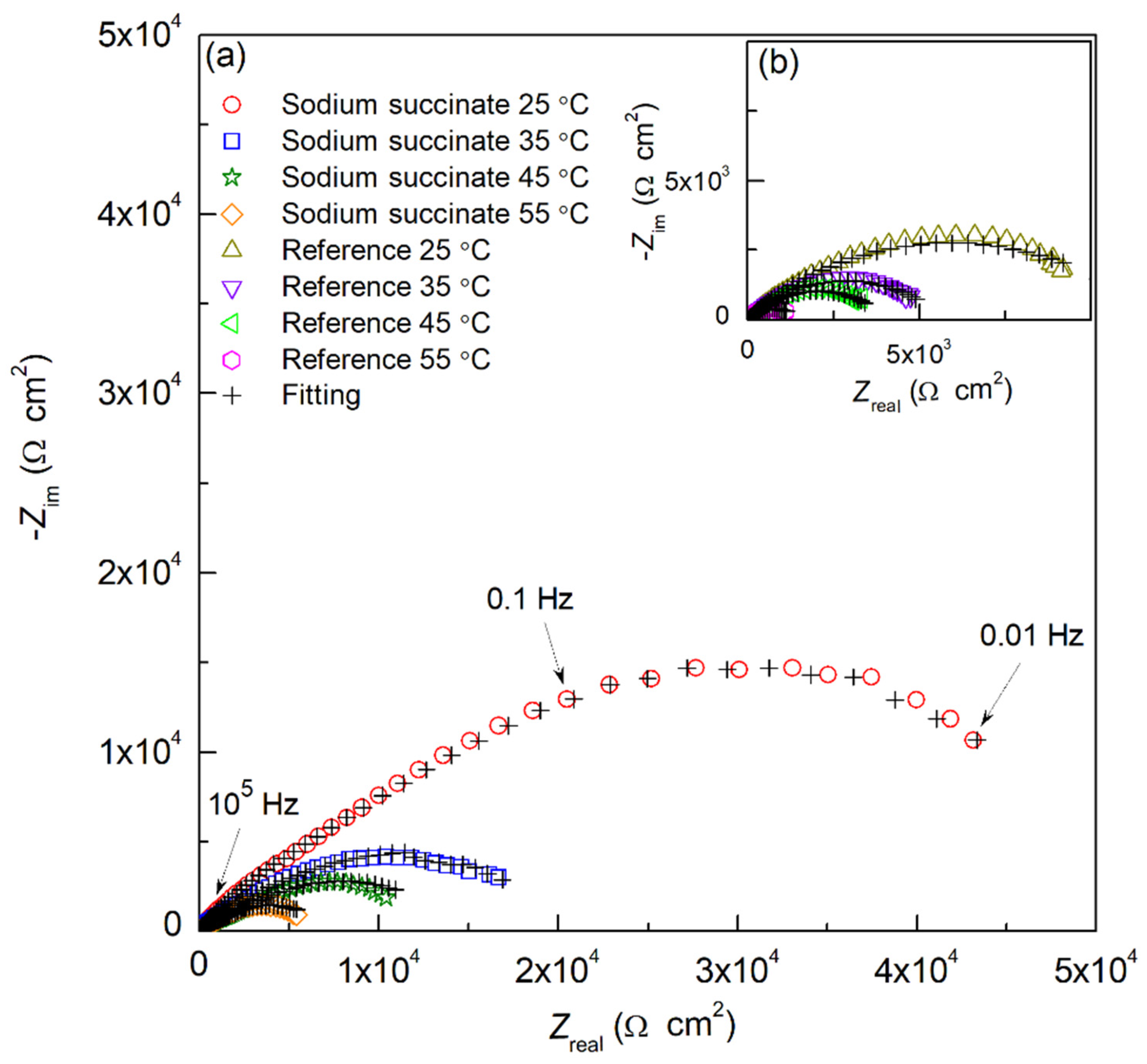
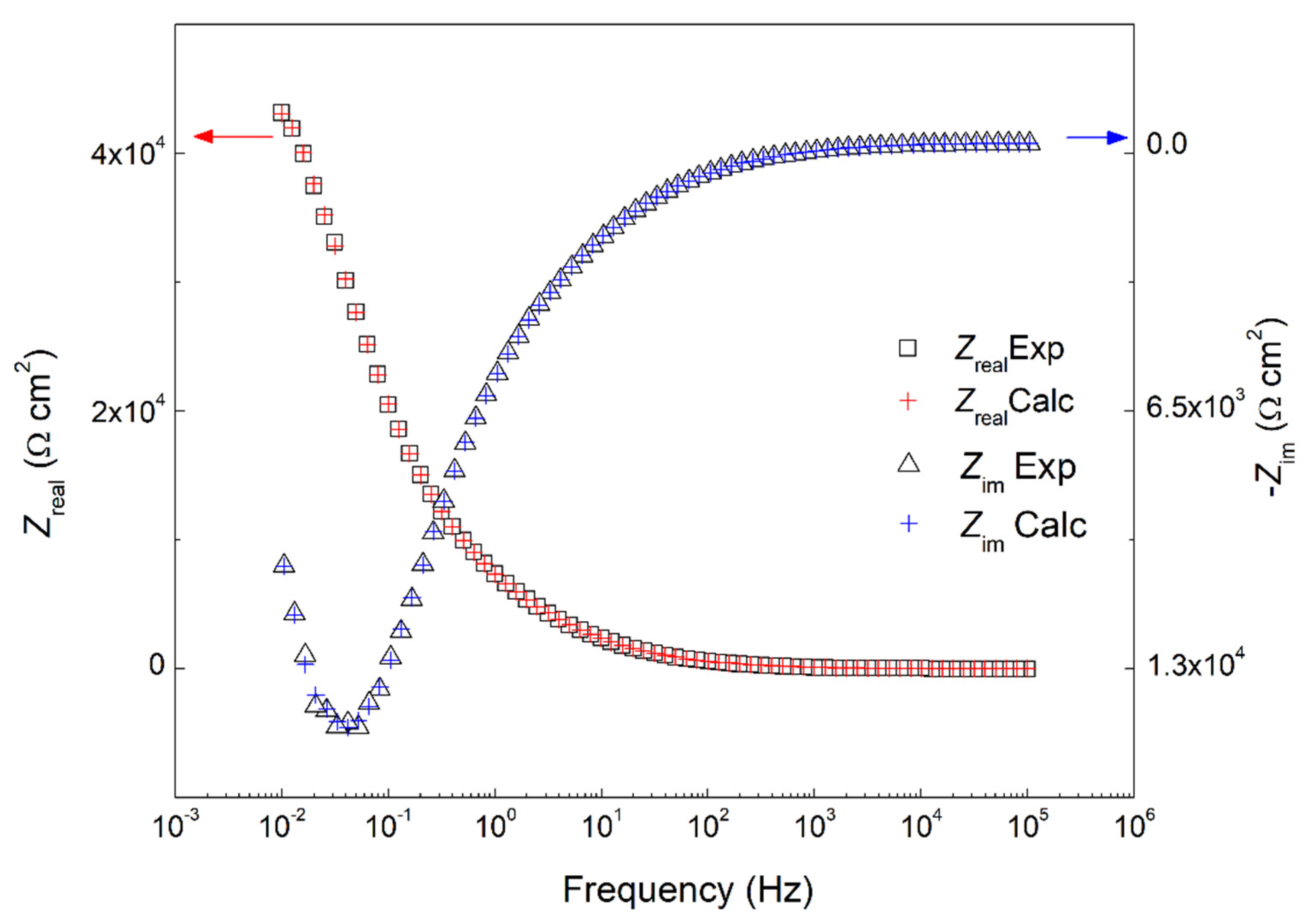
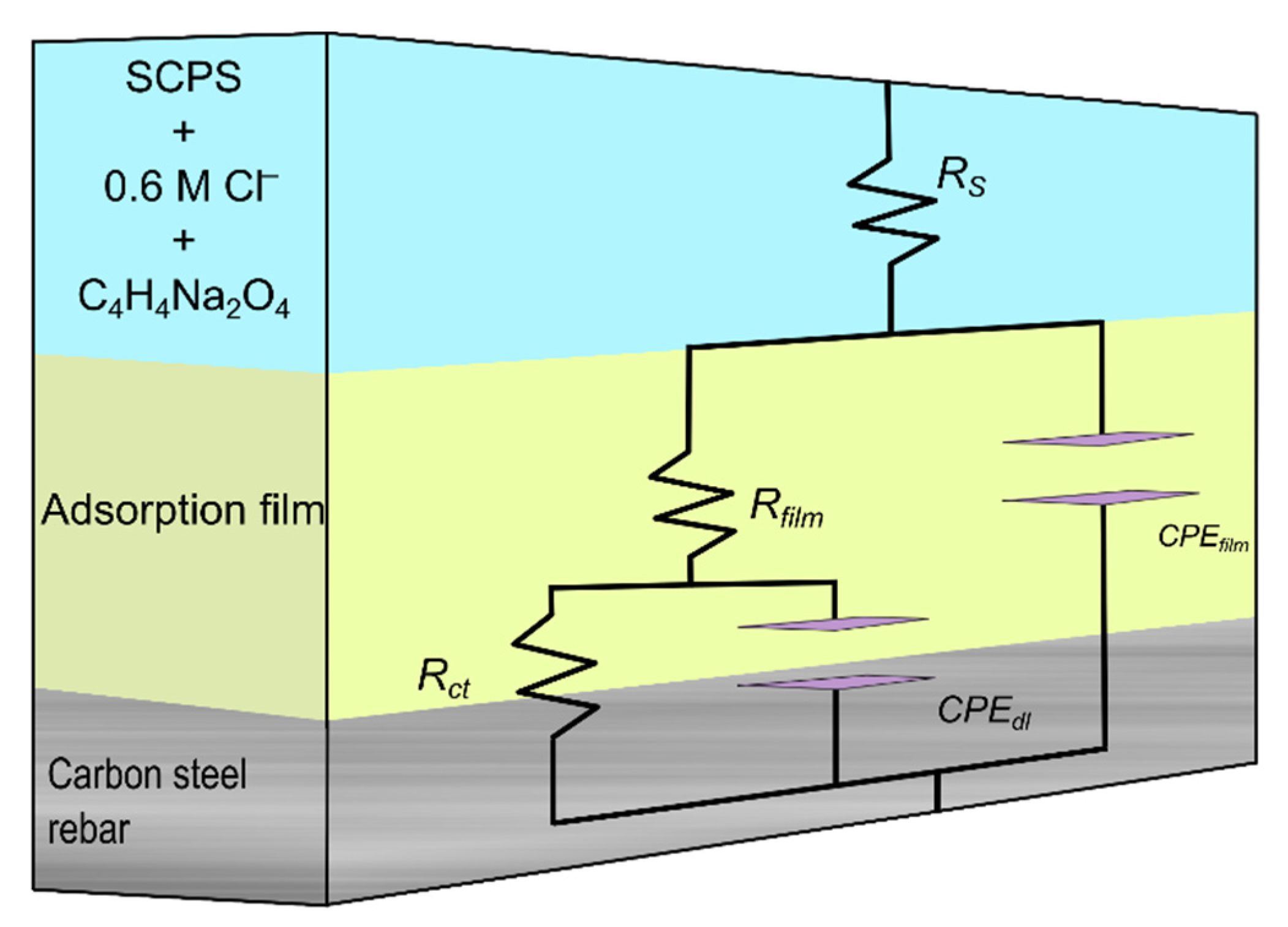



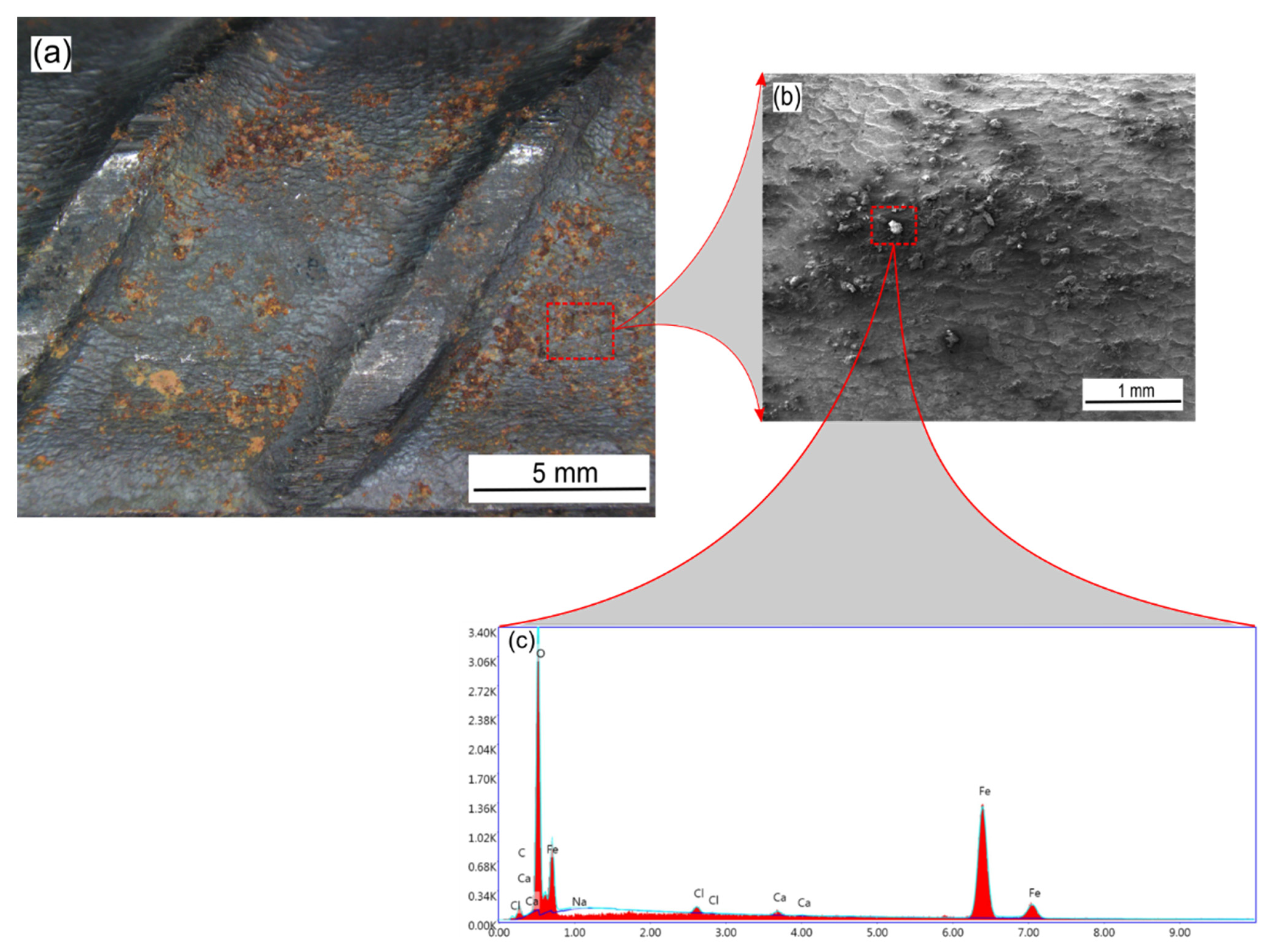

| Sample | Temperature (°C) | Ecorr (mVSCE) | icorr (µA cm−2) | IE (%) | βc (mV/dec) | βa (mV/dec) |
|---|---|---|---|---|---|---|
| Reference | 25 | –470 | 2.12 | – | 288 | 103 |
| 35 | –484 | 3.56 | – | 309 | 77 | |
| 45 | –495 | 4.40 | – | 334 | 56 | |
| 55 | –510 | 5.50 | – | 445 | 54 | |
| Sodium succinate | 25 | –429 | 0.48 | 77 | 250 | 222 |
| 35 | –470 | 1.10 | 69 | 275 | 120 | |
| 45 | –490 | 1.80 | 59 | 287 | 127 | |
| 55 | −508 | 2.53 | 54 | 294 | 132 |
| Sample | Temperature (°C) | Rs (Ω cm2) | Rfilm (Ω cm2) | Rct (Ω cm2) | Yfilm (S cm−2 snf) | nfilm | Ydl (S cm−2 sndl) | ndl | IE (%) | χ2 (*) |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | 25 | 15.66 | 2.47 × 103 | 1.28 × 104 | 3.51 × 10−6 | 0.71 | 7.21 × 10−5 | 0.71 | – | 1.16 × 10−4 |
| 35 | 11.49 | 1.98 × 103 | 5.62 × 103 | 5.11 × 10−6 | 0.72 | 9.14 × 10−5 | 0.73 | – | 2.51 × 10−4 | |
| 45 | 11.97 | 1.23 × 103 | 3.96 × 103 | 7.86 × 10−6 | 0.71 | 3.95 × 10−4 | 0.73 | – | 4.44 × 10−4 | |
| 55 | 13.94 | 8.46 × 102 | 1.37 × 103 | 1.50 × 10−5 | 0.77 | 7.84 × 10−4 | 0.78 | – | 5.25 × 10−4 | |
| Sodium succinate | 25 | 11.38 | 6.48 × 103 | 6.96 × 104 | 1.11 × 10−6 | 0.74 | 8.07 × 10−6 | 0.72 | 81.6 | 3.60 × 10−4 |
| 35 | 14.36 | 4.24 × 103 | 2.19 × 104 | 1.96 × 10−6 | 0.79 | 3.42 × 10−5 | 0.80 | 74.3 | 3.31 × 10−4 | |
| 45 | 17.73 | 3.00 × 103 | 1.15 × 104 | 3.16 × 10−6 | 0.82 | 8.22 × 10−5 | 0.86 | 65.6 | 2.00 × 10−4 | |
| 55 | 12.68 | 1.80 × 103 | 3.36 × 103 | 5.13 × 10−6 | 0.85 | 2.17 × 10−4 | 0.80 | 59.1 | 3.58 × 10−4 |
| Sample | Temperature (°C) | Ceff,dl (F cm−2) | Ceff,film (F cm−2) | deff (nm) |
|---|---|---|---|---|
| Reference | 25 | 4.51 × 10−6 | 6.37 × 10−6 | 4.16 |
| 35 | 7.24 × 10−6 | 7.53 × 10−6 | 3.52 | |
| 45 | 5.24 × 10−5 | 1.17 × 10−5 | 2.27 | |
| 55 | 2.20 × 10−4 | 2.17 × 10−5 | 1.22 | |
| Sodium succinate | 25 | 2.17 × 10−7 | 2.59 × 10−6 | 10.2 |
| 35 | 5.08 × 10−6 | 3.35 × 10−6 | 7.92 | |
| 45 | 2.84 × 10−5 | 5.20 × 10−6 | 5.10 | |
| 55 | 4.97 × 10−5 | 7.34 × 10−6 | 3.61 |
| Sample | R2 | Ea (kJ/mol) | ΔHa (kJ/mol) | ΔSa (J/mol K) | Ea − ΔHa (kJ/mol) |
|---|---|---|---|---|---|
| Reference | 0.92 | 25.35 | 22.44 | –276.93 | 2.91 |
| Sodium succinate | 0.95 | 44.73 | 42.50 | −222.48 | 2.23 |
| Sample | [Na2C4H4O4]/[Cl−] | Ecorr (mVSCE) | icorr (µA cm−2) | IE (%) | Θ | βc (mV/dec) | βa (mV/dec) |
|---|---|---|---|---|---|---|---|
| Reference | – | −470 | 2.12 | – | – | 288 | 103 |
| Sodium succinate | 0.5 | −540 | 1.20 | 48 | 0.48 | 313 | 354 |
| 1.0 | −429 | 0.48 | 77 | 0.77 | 250 | 222 | |
| 1.5 | −360 | 0.27 | 87 | 0.87 | 184 | 149 |
| Quantum Parameter | C4H4O42− |
|---|---|
| EHOMO (eV) | −4.64 |
| ELUMO (eV) | 2.37 |
| ΔEgap (eV) | 7.01 |
| η (eV) | 3.50 |
| χ (eV) | 1.13 |
| ΔN | 0.83 |
| C | Mn | P | S | Si | Cu | Ni | Cr | Mo | V | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.28 | 1.08 | 0.019 | 0.043 | 0.20 | 0.37 | 0.16 | 0.16 | 0.050 | 0.0379 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.; Visco, D.P., Jr.; Bastidas, D.M. Sodium Succinate as a Corrosion Inhibitor for Carbon Steel Rebars in Simulated Concrete Pore Solution. Molecules 2022, 27, 8776. https://doi.org/10.3390/molecules27248776
Mohamed A, Visco DP Jr., Bastidas DM. Sodium Succinate as a Corrosion Inhibitor for Carbon Steel Rebars in Simulated Concrete Pore Solution. Molecules. 2022; 27(24):8776. https://doi.org/10.3390/molecules27248776
Chicago/Turabian StyleMohamed, Ahmed, Donald P. Visco, Jr., and David M. Bastidas. 2022. "Sodium Succinate as a Corrosion Inhibitor for Carbon Steel Rebars in Simulated Concrete Pore Solution" Molecules 27, no. 24: 8776. https://doi.org/10.3390/molecules27248776
APA StyleMohamed, A., Visco, D. P., Jr., & Bastidas, D. M. (2022). Sodium Succinate as a Corrosion Inhibitor for Carbon Steel Rebars in Simulated Concrete Pore Solution. Molecules, 27(24), 8776. https://doi.org/10.3390/molecules27248776







