Dihydrochalcones in Sweet Tea: Biosynthesis, Distribution and Neuroprotection Function
Abstract
:1. Introduction
2. Physio-Biochemical Characteristics of the Sweet Tea
2.1. Morphological Characteristics
2.2. Concentration of the DHCs and Influence Factors
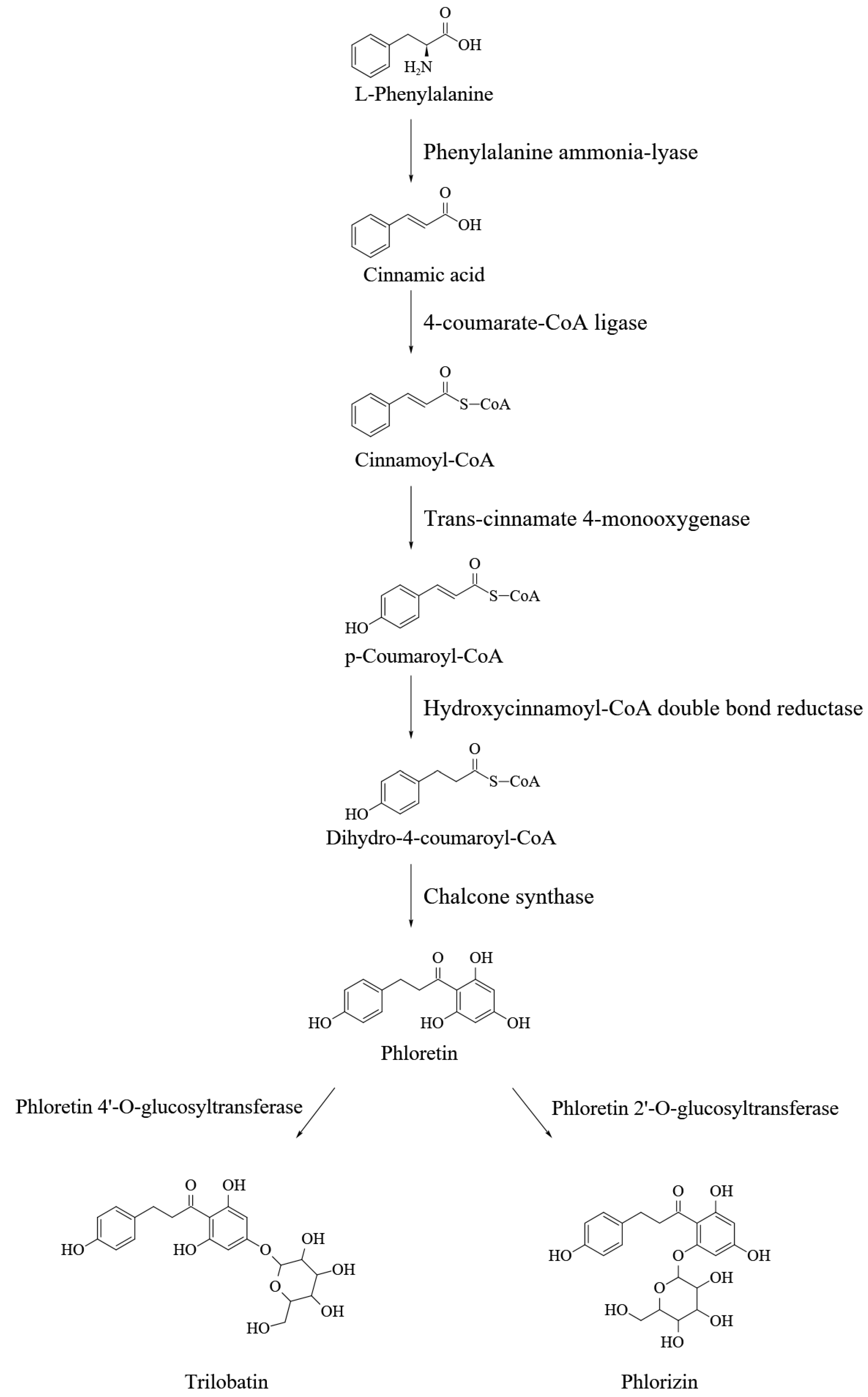
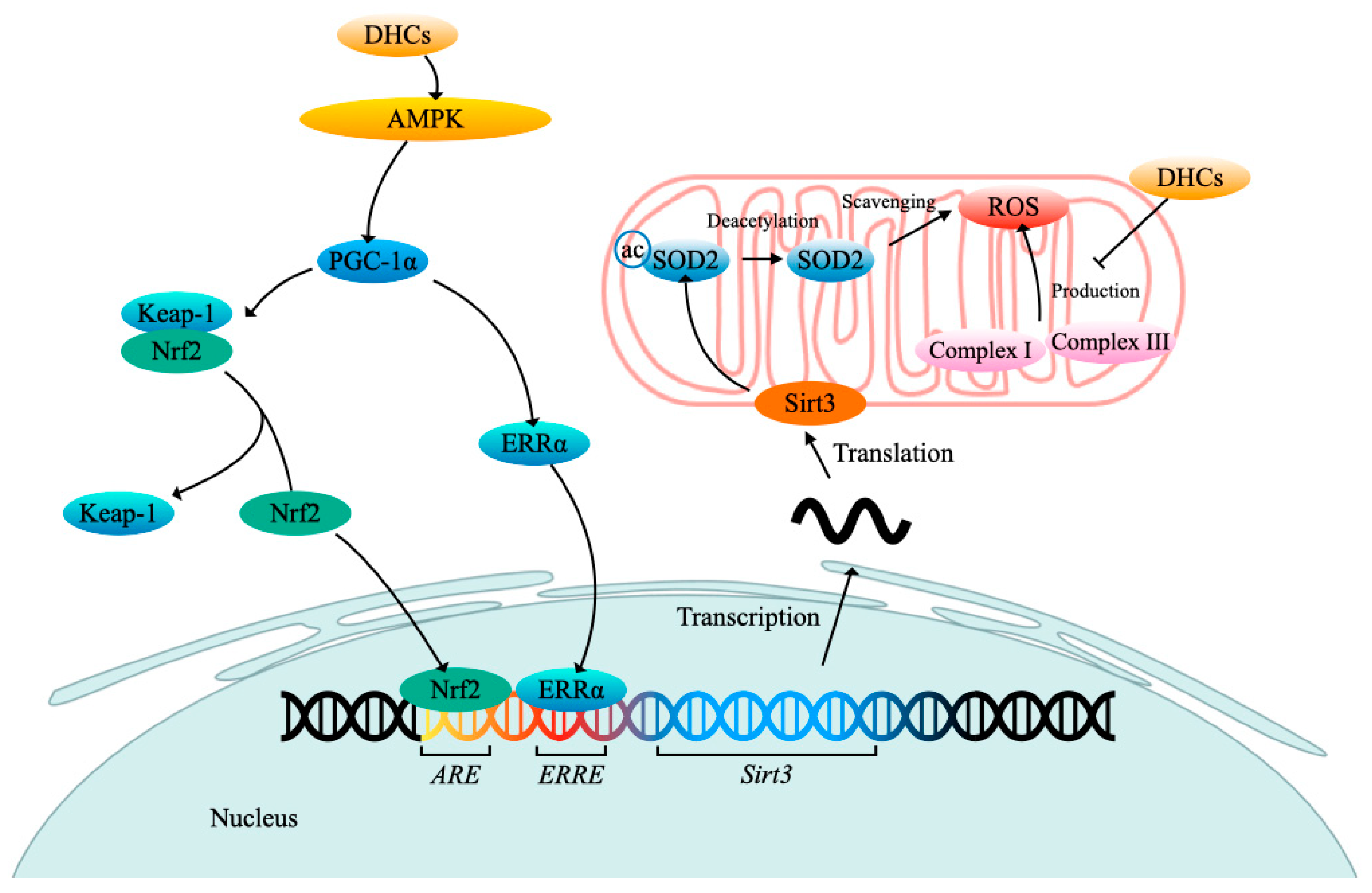
3. DHCs Biosynthesis and Its Regulation
4. Neuroprotective Effects of the DHCs
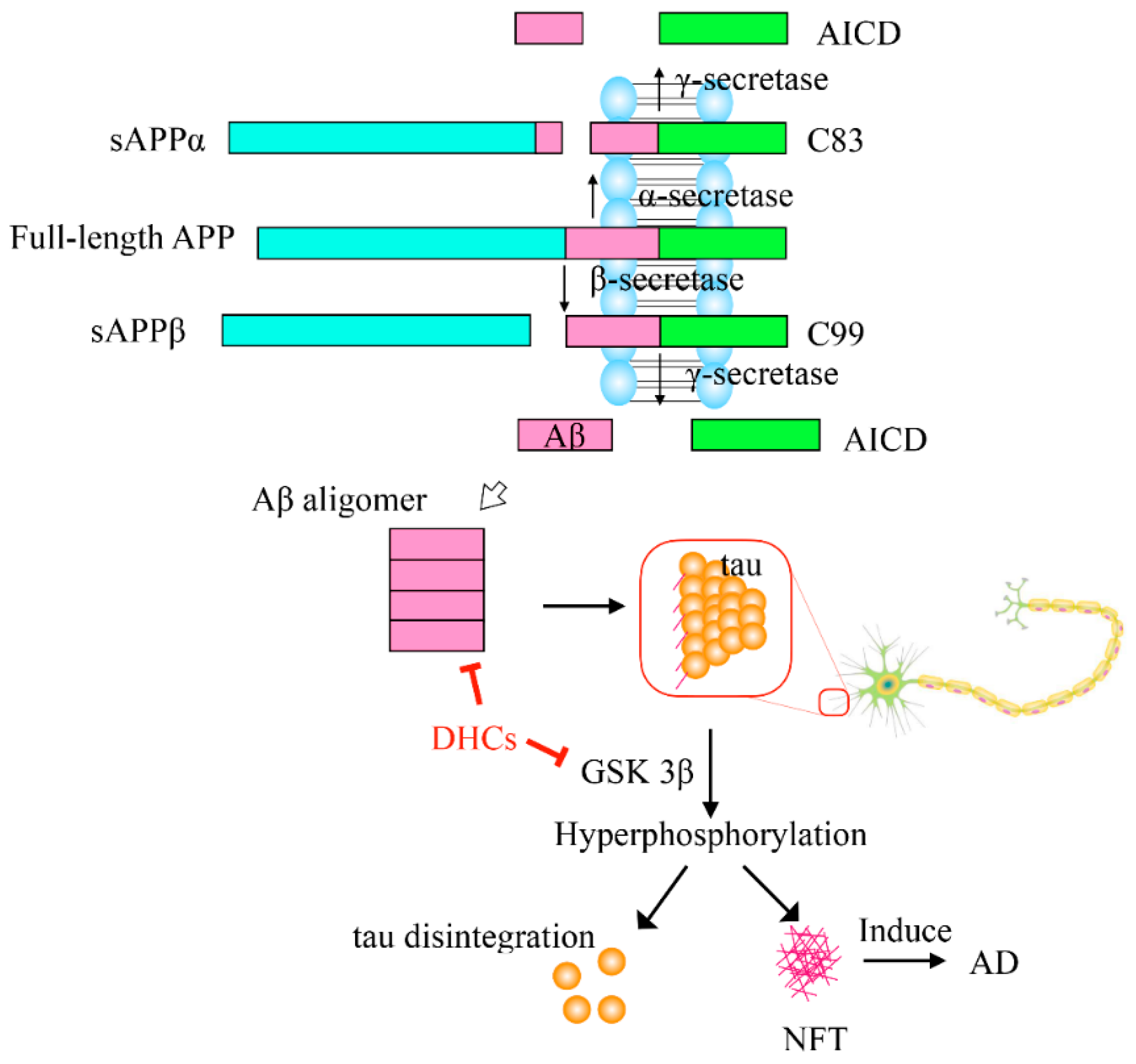
4.1. Prevention and Treatment of Neurological Diseases
4.2. Antioxidative Effect
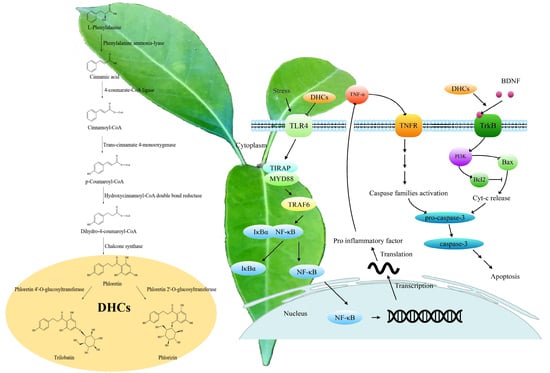
4.3. Anti-Neuroinflammation and Anti-Apoptosis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gosch, C.; Halbwirth, H.; Kuhn, J.; Miosic, S.; Stich, K. Biosynthesis of phloridzin in apple (Malus × domestica Borkh.). Plant Sci. 2009, 176, 223–231. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glycosylated derivatives present in apple and kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D. Antioxidant structure-activity relationship analysis of five dihydroc, halcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.; Huang, W.; Liou, C. Evaluation of the anti-inflammatory effects of phloretin and phlorizin in lipopolysaccharide-stimulated mouse macrophages. Food Chem. 2012, 134, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, Y.; Dong, H.; Wang, B.; Ji, H.; Liu, X. Trilobatin attenuates the LPS-mediated inflammatory response by suppressing the NF-κB signaling pathway. Food Chem. 2015, 166, 609–615. [Google Scholar] [CrossRef]
- Dong, H.; Li, M.; Zhu, F.; Liu, F.; Huang, J. Inhibitory potential of trilobatin from Lithocarpus polystachyus Rehd against α-glucosidase and α-amylase linked to type 2 diabetes. Food Chem. 2012, 130, 261–266. [Google Scholar] [CrossRef]
- Londzin, P.; Siudak, S.; Cegiela, U.; Pytlik, M.; Janas, A.; Waligora, A.; Folwarczna, J. Phloridzin, an apple polyphenol, exerted unfavorable effects on bone and muscle in an experimental model of type 2 diabetes in rats. Nutrients 2018, 10, 1701. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wang, L.; Dong, S.; Hong, Y.; Zhou, X.; Zheng, W.; Zheng, C. Phlorizin exerts direct protective effects on palmitic acid (PA)-induced endothelial dysfunction by activating the PI3K/AKT/eNOS signaling pathway and increasing the levels of nitric oxide (NO). Med. Sci. Monit. Basic Res. 2018, 24, 1. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, J.; Chen, S.; Wang, B.; Yuan, H.; Li, C.; Wu, Y.; Wu, Y.; Shi, X.; Gao, J.; et al. Phloridzin alleviate colitis in mice by protecting the intestinal brush border and improving the expression of sodium glycogen transporter 1. J. Funct. Foods 2018, 45, 348–354. [Google Scholar] [CrossRef]
- Zuo, A.; Yu, Y.; Shu, Q.; Zheng, L.; Wang, X.; Peng, S.; Xie, Y.; Cao, S. Hepatoprotective effects and antioxidant, antityrosinase activities of phloretin and phloretin isonicotinyl hydrazone. J. Chin. Med. Assoc. 2014, 77, 290–301. [Google Scholar] [CrossRef]
- Yang, K.; Tsai, C.; Wang, Y.; Wei, P.; Lee, C.; Chen, J.; Wu, C.; Ho, Y. Apple polyphenol phloretin potentiates the anticancer actions of paclitaxel through induction of apoptosis in human hep G2 cells. Mol. Carcinog. 2009, 48, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, S.; Xu, F.; Liu, Y.; Lv, C.; Deng, Y.; Shi, J.; Gong, Q. Trilobatin protects against oxidative injury in neuronal PC12 cells through regulating mitochondrial ROS homeostasis mediated by AMPK/Nrf2/Sirt3 signaling pathway. Front. Mol. Neurosci. 2018, 11, 267. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, B.; Zhong, G.; Brown, S. Genetic diversity of dihydrochalcone content in Malus germplasm. Genet. Resour. Crop Evol. 2018, 65, 1485–1502. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, L.; Long, Y.; Guo, H.; Wang, Z.; Cui, M.; Huang, J.; Xing, Z. Comprehensive transcriptome analysis revealed the effects of the light quality, light intensity, and photoperiod on phlorizin accumulation in Lithocarpus polystachyus Rehd. Forests 2019, 10, 995. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Lyu, L.; Shen, Y.; Li, K.; Liu, Z.; Wang, W.; Xie, L. Population structure and genetic diversity of Lithocarpus litseifolius (Fagaceae) assessed using microsatellite markers. Nord. J. Bot. 2016, 34, 752–760. [Google Scholar] [CrossRef]
- Wang, H.; Ning, R.; Shen, Y.; Chen, Z.; Li, J.; Zhang, R.; Leng, Y.; Zhao, W. Lithocarpic acids A-N, 3,4-seco-cycloartane derivatives from the cupules of Lithocarpus polystachyus. J. Nat. Prod. 2014, 77, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Ning, R.; Wang, H.; Shen, Y.; Chen, Z.; Zhang, R.; Leng, Y.; Zhao, W. Lithocarpic acids O-S, five homo-cycloartane derivatives from the cupules of Lithocarpus polystachyus. Bioorg. Med. Chem. Lett. 2014, 24, 5395–5398. [Google Scholar] [CrossRef]
- Liu, L.; Peng, J.; Shi, S.; Li, K.; Xiong, P.; Cai, W. Characterization of flavonoid constituents in stems of Lithocarpus litseifolius (Hance) Chun by UHPLC-Q-exactive orbitrap MS. Curr. Anal. Chem. 2021, 17, 521–527. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, F.; Wang, C.; Hwang, T.; Tsai, Y.; Yen, C.; Wang, H.; Tseng, Y.; Chien, C.; Chen, Y.; et al. Bioactive triterpenoids from the leaves and twigs of Lithocarpus litseifolius and L. corneus. Planta Med. 2018, 84, 49–58. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Li, M.; Mai, Y.; Guo, H.; Wadood, S.; Raza, A.; Wang, Y.; Zhang, J.; Li, H.; et al. The chemical, sensory, and volatile characteristics of instant sweet tea (Lithocarpus litseifolius [Hance] Chun) using electronic nose and GC-MS-baced metabolomics analysis. LWT-Food Sci. Technol. 2022, 163, 113518. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Zeng, X.; Huang, S.; Hou, S.; Lai, X. Characterization of phenolic constituents in Lithocarpus polystachyus. Anal. Methods 2014, 6, 1359–1363. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Z.; Wang, Y. Active component content in different Lithocarpus litseifolius populations related to meteorologic and soil factors. J. Cent. South Univ. For. Technol. 2021, 41, 34–41. (In Chinese) [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Yang, Z.; Zhou, C.; Hu, X. Identification and quantitative evaluation of major sweet ingredients in sweet tea (Lithocarpus polystachyus Rehd.) based upon location, harvesting time, leaf age. J. Chem. Soc. Pak. 2018, 40, 158–164. [Google Scholar]
- Wei, M.; Tuo, Y.; Zhang, Y.; Deng, Q.; Shi, C.; Chen, X.; Zhang, X. Evaluation of two parts of Lithocarpus polystachyus Rehd. from different Chinese areas by multicomponent content determination and pattern recognition. J. Anal. Methods Chem. 2020, 2020, 8837526. [Google Scholar] [CrossRef]
- He, C.; Peng, Y.; Xiao, W.; Hu, Y.; Xiao, P. Quick determination of five sweet constituents in Duosuike Tiancha by RSLC. China J. Chin. Mater. Med. 2012, 37, 961–965. (In Chinese) [Google Scholar]
- Huang, X.; Liang, W.; Li, B.; Wang, K.; Chen, J.; Li, K. On leaf morphological and venation of Lithocarpus polystachyus from different provenances. J. Beihua. Univ. Nat. Sci. 2019, 20, 237–243. (In Chinese) [Google Scholar]
- Huang, X.; Wang, K.; Li, B.; Liang, W.; Chen, J.; Lan, J.; Li, K. Variation comparison of seedling growth and physiological characteristics of different provenances of Lithocarpus polystachyus. Guangxi For. Sci. 2018, 47, 409–414. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, K.; Hu, L.; Li, Y.; Chen, X.; Zhang, Z.; Liu, B.; Li, P.; Gong, X.; Ma, F. MdUGT88F1-mediated phloridzin biosynthesis regulates apple development and Valsa canker resistance. Plant Physiol. 2019, 180, 2290–2305. [Google Scholar] [CrossRef] [Green Version]
- Dare, A.; Yauk, Y.; Tomes, S.; McGhie, T.; Rebstock, R.; Cooney, J.; Atkinson, R. Silencing a phloretin-specific glycosyltransferase perturbs both general phenylpropanoid biosynthesis and plant development. Plant J. 2017, 91, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Hu, L.; Yue, H.; Zhang, Z.; Zhang, J.; Gong, X.; Ma, F. MdUGT88F1-mediated phloridzin biosynthesis coordinates carbon and nitrogen accumulation in apple. J. Exp. Bot. 2021, 73, 886–902. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, J.; Wang, Y.; Yao, X.; Lv, L. Effects of different processing methods on active ingredients in the sweet tea. Spec. Wild Econ. Anim. Plant Res. 2021, 43, 75–82+92. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Y.; Huang, W.; Li, C.; Liu, A.; Wang, T.; Tang, T. Active components and hypoglycemic activities of the whole fermentation tea of Lithocarpus litseifolius. Food Ferment. Ind. 2020, 46, 53–60. (In Chinese) [Google Scholar] [CrossRef]
- Sun, Z.; Liu, J.; Wang, H.; Yang, C. The influence on the yield of phlorizin in Lithocarpus polysachyus Rehd fermented by Saussurea bacteria. Guangzhou Chem. Ind. 2014, 42, 116–118. (In Chinese) [Google Scholar]
- Liu, H.; Liu, Y.; Mai, Y.; Guo, H.; He, X.; Xia, Y.; Li, H.; Zhuang, Q.; Gan, R. Phenolic content, main flavonoids, and antioxidant capacity of instant sweet tea (Lithocarpus litseifolius Hance Chun) prepared with different raw materials and drying methods. Foods 2021, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, W.; Liu, Z. Preparative isolation, quantification and antioxidant activity of dihydrochalcones from Sweet Tea (Lithocarpus polystachyus Rehd.). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1002, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, K.; Chen, J.; Huang, J.; Ma, J. Determination and variation trends of main active constituents in wild Lithocarpus ploystachyus. Non-Wood For. Res. 2016, 34, 96–100+122. (In Chinese) [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef]
- Ibdah, M.; Berim, A.; Martens, S.; Valderrama, A.; Palmieri, L.; Lewinsohn, E.; Gang, D. Identification and cloning of an NADPH-dependent hydroxycinnamoyl-CoA double bond reductase involved in dihydrochalcone formation in Malus × domestica Borkh. Phytochemistry 2014, 107, 24–31. [Google Scholar] [CrossRef]
- Feinbaum, R.; Ausubel, F. Transcriptional regulation of the Arabidopsis thaliana chalcone synthase gene. Mol. Cell Biol. 1988, 8, 1985–1992. [Google Scholar]
- Yahyaa, M.; Davidovich-Rikanati, R.; Eyal, Y.; Sheachter, A.; Marzouk, S.; Lewinsohn, E.; Ibdah, M. Identification and characterization of UDP-glucose:Phloretin 4’-0-glycosyltransferase from Malus × domestica Borkh. Phytochemistry 2016, 130, 47–55. [Google Scholar] [CrossRef]
- Jugdé, H.; Nguy, D.; Moller, I.; Cooney, J.; Atkinson, R. Isolation and characterization of a novel glycosyltransferase that converts phloretin to phlorizin, a potent antioxidant in apple. FEBS J. 2008, 275, 3804–3814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liang, J.; Wang, P.; Xu, Y.; Wang, Y.; Wei, X.; Fan, M. Purification and characterization of a novel phloretin-2’-O-glycosyltransferase favoring phloridzin biosynthesis. Sci. Rep. 2016, 6, 35274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosch, C.; Flachowsky, H.; Halbwirth, H.; Thill, J.; Mjka-Wittmann, R.; Treutter, D.; Richter, K.; Hanke, M.; Stich, K. Substrate specificity and contribution of the glycosyltransferase UGT71A15 to phloridzin biosynthesis. Trees-Struct. Funct. 2012, 26, 259–271. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Schneider, B.; Hoelscher, D.; Stich, K. Cloning and heterologous expression of glycosyltransferases from Malus × domestica and Pyrus communis, which convert phloretin to phloretin 2’-O-glucoside (phloridzin). Plant Sci. 2010, 178, 299–306. [Google Scholar] [CrossRef]
- Nawade, B.; Yahyaa, M.; Davidovich-Rikanati, R.; Lewinsohn, E.; Ibdah, M. Optimization of culture conditions for the efficient biosynthesis of trilobatin from phloretin by engineered Escherichia coil harboring the apple phloretin-4 ’-O-glycosyltransferase. J. Agric. Food Chem. 2020, 68, 14212–14220. [Google Scholar] [CrossRef]
- Lei, L.; Hu, B.; Liu, A.; Lu, Y.; Zhou, J.; Zhang, J.; Wong, W. Enzymatic production of natural sweetener trilobatin from citrus flavanone naringin using immobilised α-L-rhamnosidase as the catalyst. Int. J. Food Sci. Technol. 2018, 53, 2097–2103. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Z.; Wang, Z.; Zhang, Y.; Huang, J.; Xing, Z. Cloning and expression of LAR gene and its correlation with phloridzin content in Lithocarpus polystachyus. Chin. Traditional. Herb. Drugs 2020, 51, 3292–3297. (In Chinese) [Google Scholar]
- Lin, L.; Long, Y.; Feng, R.; Yin, F.; Huang, J.; Xing, Z. Cloning and bioinformatic analysis of chalcone isomerase gene in Lithocarpus polystachyus. Chin. Traditional. Herb. Drugs 2017, 48, 5080–5084. (In Chinese) [Google Scholar]
- Yin, F.; Long, Y.; Feng, R.; Lin, L.; Huang, J.; Xing, Z. Cloning of flavanone 3-hydroxylase gene from Lithocarpus polystachyus and its sequence analysis. Chin. Traditional. Herb. Drugs 2017, 48, 5085–5089. (In Chinese) [Google Scholar]
- Xing, Z.; Feng, R.; Wang, Z.; Zhang, Y.; Wang, Z.; Huang, J.; Long, Y. Cloning and expression analysis on 4-coumarate-CoA ligase gene in Lithocarpus polystachyus. Non-Wood For. Res. 2019, 37, 16–21. (In Chinese) [Google Scholar] [CrossRef]
- Chen, N.; Wang, J.; He, Y.; Xu, Y.; Zhang, Y.; Gong, Q.; Yu, C.; Gao, J. Trilobatin protects against Aβ25–35-induced hippocampal HT22 cells apoptosis through mediating ROS/p38/Caspase 3-dependent pathway. Front. Pharmacol. 2020, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Huang, J.; Yin, D.; Liu, T.; Ren, Z.; Hu, S.; Ye, Y.; Le, C.; Zhao, N.; Zhou, H.; et al. Trilobatin alleviates cognitive deficits and pathologies in an Alzheimer’s Disease mouse model. Oxid. Med. Cell. Longev. 2021, 2021, 3298400. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, N.; Li, N.; Xu, F.; Wang, W.; Lei, Y.; Shi, J.; Gong, Q. Neuroprotective effects of trilobatin, a novel naturally occurring Sirt3 agonist from Lithocarpus polystachyus Rehd., mitigate cerebral ischemia/reperfusion injury: Involvement of TLR4/NF-kappa B and Nrf2/Keap-1 signaling. Antioxid. Redox Signal. 2020, 33, 117–143. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Shang, Y.; Wu, Q.; Ren, H. The protective effect of trilobatin against isoflurane-induced neurotoxicity in mouse hippocampal neuronal HT22 cells involves the Nrf2/ARE pathway. Toxicology 2020, 442, 152537. [Google Scholar] [CrossRef] [PubMed]
- Ghumatkar, P.; Peshattiwar, V.; Patil, S.; Muke, S.; Whitfield, D.; Howlett, D.; Francis, P.; Sathaye, S. The effect of phloretin on synaptic proteins and adult hippocampal neurogenesis in Aβ1–42-injected male Wistar rats. J. Pharm. Pharmacol. 2018, 70, 1022–1030. [Google Scholar] [CrossRef]
- Ghumatkar, P.; Patil, S.; Peshattiwar, V.; Vijaykumar, T.; Dighe, V.; Vanage, G.; Sathaye, S. The modulatory role of phloretin in Aβ25–35 induced sporadic Alzheimer’s disease in rat model. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 327–339. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, G.; Liu, J. Phloretin attenuates behavior deficits and neuroinflammatory response in MPTP induced Parkinson’s disease in mice. Life Sci. 2019, 232, 116600. [Google Scholar] [CrossRef]
- Ghumatkar, P.; Patil, S.; Jain, P.; Tambe, R.; Sathaye, S. Nootropic, neuroprotective and neurotrophic effects of phloretin in scopolamine induced amnesia in mice. Pharmacol. Biochem. Behav. 2015, 135, 182–191. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Liang, J. Activation of the Nrf2 defense pathway contributes to neuroprotective effects of phloretin on oxidative stress injury after cerebral ischemia/reperfusion in rats. J. Neurol. Sci. 2015, 351, 88–92. [Google Scholar] [CrossRef]
- Kamdi, S.; Raval, A.; Nakhate, K. Phloridzin attenuates lipopolysaccharide-induced cognitive impairment via antioxidant, anti-inflammatory and neuromodulatory activities. Cytokine 2021, 139, 155408. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, Y. Research progress on amyloid β-protein aggregation and its regulation. CIESC J. 2022, 73, 2381–2396. (In Chinese) [Google Scholar]
- Barreca, D.; Curro, M.; Bellocco, E.; Ficarra, S.; Lagana, G.; Tellone, E.; Giunta, M.; Visalli, G.; Caccamo, D.; Galtieri, A.; et al. Neuroprotective effects of phloretin and its glycosylated derivative on rotenone-induced toxicity in human SH-SY5Y neuronal-like cells. Biofactors 2017, 43, 549–557. [Google Scholar] [CrossRef]
- Wang, K.; Ren, J. Research progress of brain-derived neurotrophic factor in treatment of cerebral infarction. Med. Recapitul. 2020, 26, 2151–2155. (In Chinese) [Google Scholar]
- Wu, H.; Li, T.; Gao, F.; Qian, Y.; Wang, X. Advances of acetylcholinesterase and its traditional Chinese medicine inhibitors. Pharm. Biotechnol. 2015, 22, 362–365. (In Chinese) [Google Scholar] [CrossRef]
- Yu, F.; Xu, Y. Research progress of caspase-3. Chin. J. Cell Biol. 2020, 42, 2072–2078. (In Chinese) [Google Scholar]

| Influencing Factors | Content (mg/g) | Reference | ||
|---|---|---|---|---|
| Trilobatin | Phloridzin | Phloretin | ||
| Picking time | ||||
| April | 257.52–279.74 | 11.44–22.34 | 0.13–0.55 | [24,26] |
| November | 30.94–33.59 | 143.12–208.31 | 0.11–0.44 | |
| Leaf maturity | ||||
| Tender leaf | 82.90–279.74 | 9.30–57.40 | 1.90–2.50 | [24,36,37] |
| Old leaf | 19.30–128.80 | 144.60–208.31 | 3.60–4.30 | |
| Location | ||||
| Sichuan | 73.32–278.15 | 4.89–62.87 | 0.08–1.25 | [24,25,26,33,36,37] |
| Chongqing | 41.87–133.98 | 14.90–31.69 | 0.10–1.39 | |
| Guangxi | 0.90–198.70 | 4.80–144.60 | 4.98 | |
| Hunan | 171.95–272.35 | 8.35–19.25 | 0.18–1.01 | |
| Jiangxi | 69.56–183.84 | 11.58–49.84 | 0.27–4.16 | |
| Guizhou | 161.42–176.90 | 5.26–7.46 | 0.36–0.38 | |
| Yunnan | 4.87–264.60 | 0.61–208.29 | 0.27–1.55 | |
| Guangdong | 0.44–60.62 | 20.29–57.33 | Not mentioned | |
| Fujian | 185.47–244.56 | 7.83–19.24 | 0.48–1.19 | |
| Compound and Effects | Treatment | Model | Reference |
|---|---|---|---|
| Trilobatin | |||
| Decrease the phosphorylation of p38 | 12.5, 25, and 50 µM | Aβ25–35-induced HT22 cells | [52] |
| Reduce the production of Aβ by decreasing the BACE1 levels | 10 or 20 mg/kg | 3 × FAD AD model mice | [53] |
| Activate the AMPK signaling pathway to respond the oxidative stress | 15, 30 and 60 µM | H2O2-induced injury PC12 cells | [12] |
| Increase Sirt3 expression and activity | 5, 10 and 20 mg/kg | MCAO-induced focal cerebral ischemia rats | [54] |
| Activate the Nrf2/ARE pathway | 10, 20, and 40 µM | Isoflurane-induced HT22 cells | [55] |
| Phloretin | |||
| Protect synaptophysin and improve neuron cells | 5 mg/kg | Aβ1-42-injected male Wistar rats | [56] |
| Inhibit the Aβ accumulation through antioxidation and anti-inflammation | 2.5 and 5 mg/kg | Aβ25–35-induced sporadic Alzheimer’s disease rats | [57] |
| Inhibit the activation of microglia and astrocytes | 5 mg/kg | MPTP-induced Parkinson’s disease mice | [58] |
| Improve the activity of neuron cells via normalizing the AChE activity and alleviating reactive gliosis | 2.5, 5 and 10 mg/kg | Scopolamine induced amnesia mice | [59] |
| Up-regulate the transcription and translation of Nrf2 | 40 and 80 mg/kg | Cerebral ischemia/reperfusion rats | [60] |
| Phloridzin | |||
| Normalize neural signaling and exhibit anti-inflammatory effect | 10 or 20 mg/kg | Lipopolysaccharide-induced cognitive impairment mice | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-K.; Hu, S.-Y.; Xiao, F.-Y.; Dong, Z.-B.; Ye, J.-H.; Zheng, X.-Q.; Liang, Y.-R.; Lu, J.-L. Dihydrochalcones in Sweet Tea: Biosynthesis, Distribution and Neuroprotection Function. Molecules 2022, 27, 8794. https://doi.org/10.3390/molecules27248794
Wang Y-K, Hu S-Y, Xiao F-Y, Dong Z-B, Ye J-H, Zheng X-Q, Liang Y-R, Lu J-L. Dihydrochalcones in Sweet Tea: Biosynthesis, Distribution and Neuroprotection Function. Molecules. 2022; 27(24):8794. https://doi.org/10.3390/molecules27248794
Chicago/Turabian StyleWang, Yong-Kang, Si-Yi Hu, Feng-Yi Xiao, Zhan-Bo Dong, Jian-Hui Ye, Xin-Qiang Zheng, Yue-Rong Liang, and Jian-Liang Lu. 2022. "Dihydrochalcones in Sweet Tea: Biosynthesis, Distribution and Neuroprotection Function" Molecules 27, no. 24: 8794. https://doi.org/10.3390/molecules27248794