Venom Collection by Electrical Stimulation in the Invasive Species Polistes dominula Reared Using a Vespiculture Regime
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
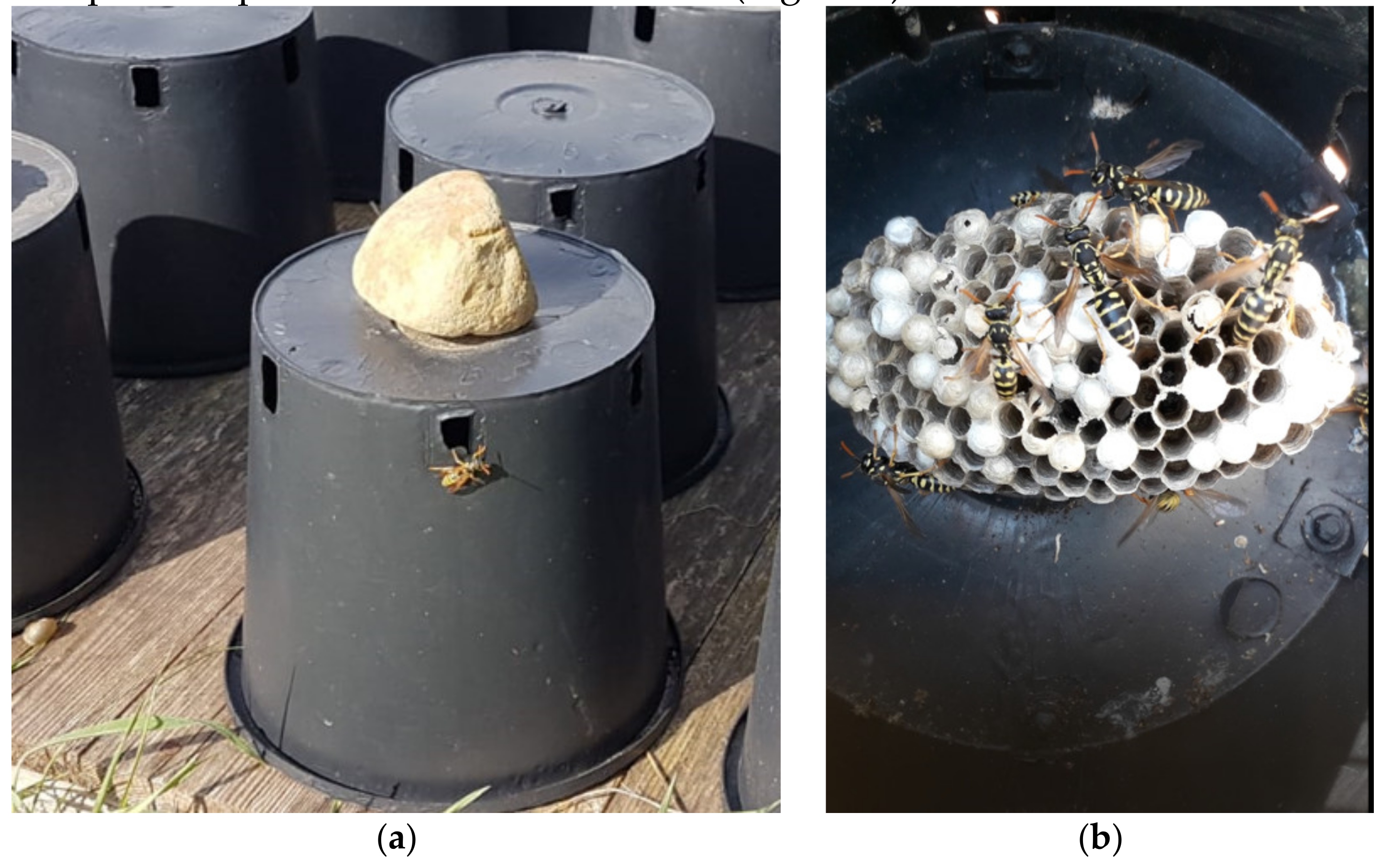
3.1. Vespiculture
3.2. Collection of Venom
3.3. Chemical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Golden, D.B.K.; Valentine, M.D.; Kagey-Sobotka, A.; Lichtenstein, L.M. Regimens of Hymenoptera venom immunotherapy. Ann. Intern. Med. 1980, 92, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Bilò, B.M.; Bonifazi, F. Hymenoptera venom immunotherapy. Future Med. Immunother. Rev. 2011, 3, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, C.; Mauro, M.; Pravettoni, V.; Pucci, S. Hypersensitivity to Hymenoptera Venom: Advances in Diagnosis and Implications for Treatment. Recent Patents on Inflammation & Allergy Drug Discovery 2011, 5, 128–135. [Google Scholar]
- Sturm, G.J.; Varga, E.M.; Roberts, G.; Mosbech, H.; Bilò, M.B.; Akdis, C.A.; Antolín-Amérigo, D.; Cichocka-Jarosz, E.; Gawlik, R.; Jakob, T.; et al. EAACI Guidelines on Allergen Immunotherapy: Hymenoptera venom allergy. Allergy 2018, 73, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Luzar, D.; Korošec, P.; Košnik, M.; Zidarn, M.; Rijavec, M. Hymenoptera Venom Immunotherapy: Immune Mechanisms of Induced Protection and Tolerance. Cells 2021, 10, 1575. [Google Scholar] [CrossRef]
- Bonifazi, F.; Jutel, M.; Bilò, B.M.; Birnbaum, J.; Muller, U.; the EAACI Interest Group on Insect Venom Hypersensitivity. Prevention and treatment of Hymenoptera venom allergy: Guidelines for clinical practice. Allergy 2005, 60, 1459–1470. [Google Scholar] [CrossRef]
- Feás, X.; Vidal, C.; Vázquez-Tato, M.P.; Seijas, J.A. Asian Hornet, Vespa velutina Lepeletier 1836 (Hym.: Vespidae), Venom Obtention Based on an Electric Stimulation Protocol. Molecules 2022, 27, 138. [Google Scholar] [CrossRef]
- Forister, M.L.; Pelton, E.M.; Black, H. Declines in insect abundance and diversity: We know enough to act now. Conserv. Sci. Pract. 2019, 1, e80. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Hoag, H. Wasp Venom Can Save Lives. But the Supply Chain Is Shaky. 2020. Available online: https://undark.org/2020/11/16/wasp-venom-shaky-supply-chain/ (accessed on 15 March 2020).
- Aleccia, J.N. Bee, Wasp Venom Shortage Could Be Dangerous for Those with Allergies. 2017. Available online: https://edition.cnn.com/2017/06/27/health/insect-venom-shortage-partner/index.html (accessed on 15 March 2020).
- Van Itterbeeck, J.; Feng, Y.; Zhao, M.; Wang, C.; Tan, K.; Saga, T.; Nonaka, K.; Jung, C. Rearing techniques for hornets with emphasis on Vespa velutina (Hymenoptera: Vespidae): A review. J. Asia-Pac. Entomol. 2021, 24, 103–117. [Google Scholar] [CrossRef]
- Hoffmann, D.R. Hymenoptera venom allergens. Clin. Rev. Allergy Immunol. 2006, 30, 109–128. [Google Scholar] [CrossRef]
- Benton, A.W.; Morse, R.A.; Stuart, J.D. Venom collection from Honey Bees. Science 1963, 142, 228–230. [Google Scholar] [CrossRef]
- Mueller, U.; Reisman, R.J.; Wypych, W.E.; Steger, B.F.; Walsh, M.S.; Arbesman, M.D. Comparison of vespid venoms collected by electrostimulation and by venom sac extraction. J. Allergy Clin. Immunol. 1981, 68, 254–261. [Google Scholar] [CrossRef]
- Littler, S.; Wypych, J.I.; Noble, R.E.; Reisman, R.E. Allergenic components of bald-faced hornet (V. maculata) venom. J. Allergy Clin. Immunol. 1983, 71, 120. [Google Scholar] [CrossRef]
- Hoffmann, D.R. Allergens in Hymenoptera venom XIII: Isolation and purification of protein components from three species of vespid venoms. J. Allergy Clin. Immunol. 1985, 75, 599–605. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Fang, Y.; Han, B.; Lu, X.; Zhou, T.; Feng, M.; Li, J. Proteome and phosphoproteome analysis of honeybee (Apis mellifera) venom collected from electrical stimulation and manual extraction of the venom gland. BMC Genom. 2013, 14, 766. [Google Scholar] [CrossRef]
- Eskridge, E.M.; Elliott, W.B.; Elliott, A.H.; Eskridge, P.B.; Doer, J.C.; Schneller, N.; Reisman, R.E. Adaptation of the electrical stimulation procedure for the collection of vespid venoms. Toxicon 1981, 6, 893–897. [Google Scholar] [CrossRef]
- Simon, R.P.; Benton, A.W. A method for mass collection of wasp venoms. Ann. Entomol. Soc. Am. 1969, 62, 277–278. [Google Scholar] [CrossRef]
- Gillaspy, J.E.; Grant, J.A. Mass collection of Polistes wasp venom by electrical stimulation. Southwest Entomol. 1979, 4, 96–101. [Google Scholar]
- Hoffman, D.R.; Jacobson, R.S. Allergens in Hymenoptera venom XII: How much protein is in a sting. Ann. Allergy 1984, 52, 276–278. [Google Scholar]
- Pantera, B.; Hoffman, D.R.; Carresi, L.; Cappugi, G.; Turillazzi, S.; Manao, G.; Severino, M.; Spadolini, I.; Orsomando, G.; Moneti, G.; et al. Characterization of the major allergens purified from the venom of the paper wasp Polistes gallicus. Biochim. Biophys. Acta Gen. Subj. 2003, 1623, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Bruschini, C.; Cervo, R.; Turillazzi, S. Evidence of alarm pheromones in the venom of Polistes dominulus workers (Hymenoptera: Vespidae). Physiol. Entomol. 2006, 31, 286–293. [Google Scholar] [CrossRef]
- Bilò, B.M.; Jakob, T.; Ollert, M.; Blank, S. Vespid venom allergy. Mol. Allergol. Clin. Pract. 2022, B21. [Google Scholar]
- Turillazzi, S.; Mastrobuoni, G.; Dani, F.R.; Moneti, G.; Pieraccini, G.; La Marca, G.; Bartolucci, G.; Perito, B.; Lambardi, D.; Cavallini, V.; et al. Dominulin A and B: Two new antibacterial peptides identified on the cuticle and in the venom of the social paper wasp Polistes dominulus using MALDI-TOF, MALDI-TOF/TOF, and ESI-ION trap. J. Am. Soc. Mass. Spectrom. 2006, 17, 376–383. [Google Scholar] [CrossRef]
- Dani, F.R.; Pieraccini, G. Chapter Four—Proteomics of arthropod soluble olfactory proteins. Methods Enzymol. 2020, 642, 81–102. [Google Scholar] [CrossRef]
- Brock, R.E.; Cini, A.; Sumner, S. Ecosystem services provided by aculeate wasps. Biol. Rev. 2021, 96, 1645–1675. [Google Scholar] [CrossRef]


| Accession | Score | Mass | Num. of Significant Matches | Num. of Significant Sequences | emPAI | Description |
|---|---|---|---|---|---|---|
| P83377 | 2297 | 23138 | 138 | 9 | 4.82 | Venom allergen 5 OS = Polistes gallicus OX = 34730 PE = 1 SV = 1 |
| Q7Z269 | 1952 | 30784 | 69 | 10 | 4.17 | Venom serine protease OS = Polistes dominula OX = 743375 PE = 2 SV = 1 |
| Q9U6V9 | 1767 | 42992 | 105 | 9 | 1.26 | Hyaluronidase (Fragment) OS = Polistes annularis OX = 27505 PE = 1 SV = 1 |
| Q6Q251 | 1706 | 35037 | 84 | 18 | 6.29 | Phospholipase A1 2 (Fragment) OS=Polistes dominula OX = 743375 PE = 2 SV = 1 |
| Q6Q250 | 1185 | 34997 | 73 | 18 | 5.7 | Phospholipase A1 3 (Fragment) OS = Polistes dominula OX = 743375 PE = 2 SV = 1 |
| Q9U6W0 | 45 | 33462 | 6 | 3 | 0.33 | Phospholipase A1 OS = Polistes annularis OX = 27505 PE = 2 SV = 1 |
| P0C1M6 | 142 | 1855 | 9 | 2 | 9.28 | Dominulin-A OS = Polistes dominula OX = 743375 PE = 1 SV = 1 |
| B1A4F7 | 107 | 88868 | 2 | 1 | 0.04 | Venom dipeptidyl peptidase 4 OS = Vespula vulgaris OX = 7454 PE = 1 SV = 1 |
| P0C1M7 | 94 | 1910 | 8 | 2 | 9.28 | Dominulin-B OS = Polistes dominula OX = 743375 PE = 1 SV = 1 |
| P85873 | 83 | 1358 | 13 | 2 | 83.54 | Wasp kinin PMM1 OS = Polistes major OX = 91420 PE = 1 SV = 1 |
| Accession | Score | Mass | Num. of Significant Matches | Num. of Significant Sequences | emPAI | Description |
|---|---|---|---|---|---|---|
| Q6Q249 | 2073 | 35001 | 95 | 17 | 9.55 | Phospholipase A1 4 (Fragment) OS = Polistes dominula OX = 743375 PE = 2 SV = 1 |
| Q6Q252 | 1768 | 37535 | 82 | 16 | 7.29 | Phospholipase A1 1 OS=Polistes dominula OX = 743375 PE = 1 SV = 1 |
| P81656 | 1426 | 25430 | 64 | 10 | 7.16 | Venom allergen 5 OS = Polistes dominula OX = 743375 PE = 1 SV = 2 |
| Q7Z269 | 1070 | 30784 | 36 | 8 | 3.21 | Venom serine protease OS = Polistes dominula OX = 743375 PE = 2 SV = 1 |
| Q9U6V9 | 607 | 42992 | 23 | 5 | 0.56 | Hyaluronidase (Fragment) OS = Polistes annularis OX = 27505 PE = 1 SV = 1 |
| P0C1M6 | 139 | 1855 | 7 | 1 | 2.21 | Dominulin-A OS = Polistes dominula OX = 743375 PE = 1 SV = 1 |
| P85873 | 111 | 1358 | 19 | 2 | 83.54 | Wasp kinin PMM1 OS = Polistes major OX = 91420 PE = 1 SV = 1 |
| B1A4F7 | 93 | 88868 | 2 | 1 | 0.04 | Venom dipeptidyl peptidase 4 OS = Vespula vulgaris OX = 7454 PE = 1 SV = 1 |
| P0C1M7 | 82 | 1910 | 4 | 1 | 2.21 | Dominulin-B OS = Polistes dominula OX = 743375 PE = 1 SV = 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turillazzi, F.; Pieraccini, G.; Turillazzi, S.; Orsi Battaglini, N.; Severino, M. Venom Collection by Electrical Stimulation in the Invasive Species Polistes dominula Reared Using a Vespiculture Regime. Molecules 2022, 27, 8821. https://doi.org/10.3390/molecules27248821
Turillazzi F, Pieraccini G, Turillazzi S, Orsi Battaglini N, Severino M. Venom Collection by Electrical Stimulation in the Invasive Species Polistes dominula Reared Using a Vespiculture Regime. Molecules. 2022; 27(24):8821. https://doi.org/10.3390/molecules27248821
Chicago/Turabian StyleTurillazzi, Francesco, Giuseppe Pieraccini, Stefano Turillazzi, Neri Orsi Battaglini, and Maurizio Severino. 2022. "Venom Collection by Electrical Stimulation in the Invasive Species Polistes dominula Reared Using a Vespiculture Regime" Molecules 27, no. 24: 8821. https://doi.org/10.3390/molecules27248821
APA StyleTurillazzi, F., Pieraccini, G., Turillazzi, S., Orsi Battaglini, N., & Severino, M. (2022). Venom Collection by Electrical Stimulation in the Invasive Species Polistes dominula Reared Using a Vespiculture Regime. Molecules, 27(24), 8821. https://doi.org/10.3390/molecules27248821






