Antiproliferative Evaluation of Novel 4-Imidazolidinone Derivatives as Anticancer Agent Which Triggers ROS-Dependent Apoptosis in Colorectal Cancer Cell
Abstract
:1. Introduction
2. Results
2.1. Anticancer Activity Evaluation of Compounds 9
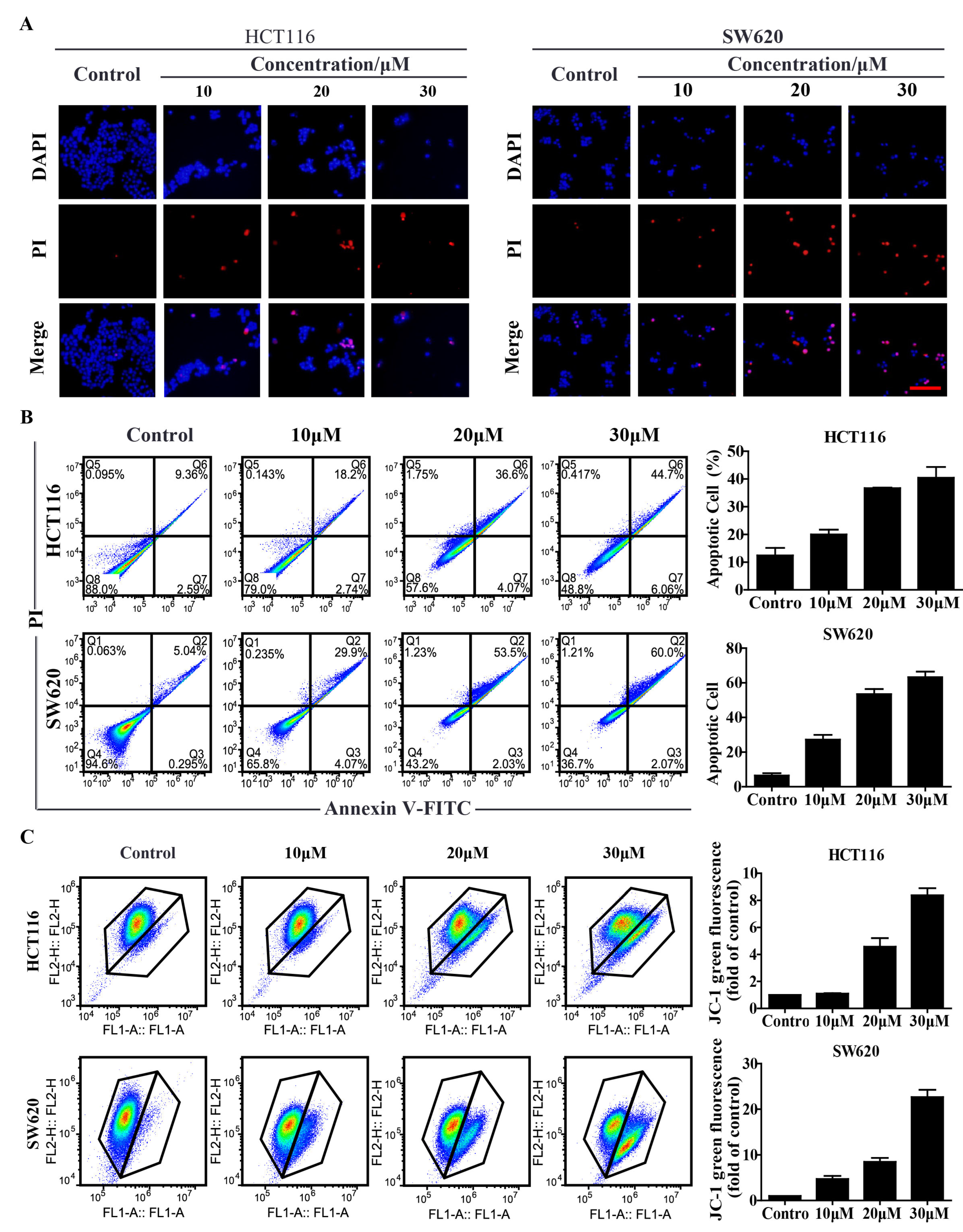
2.2. Compound 9r Induces Apoptosis
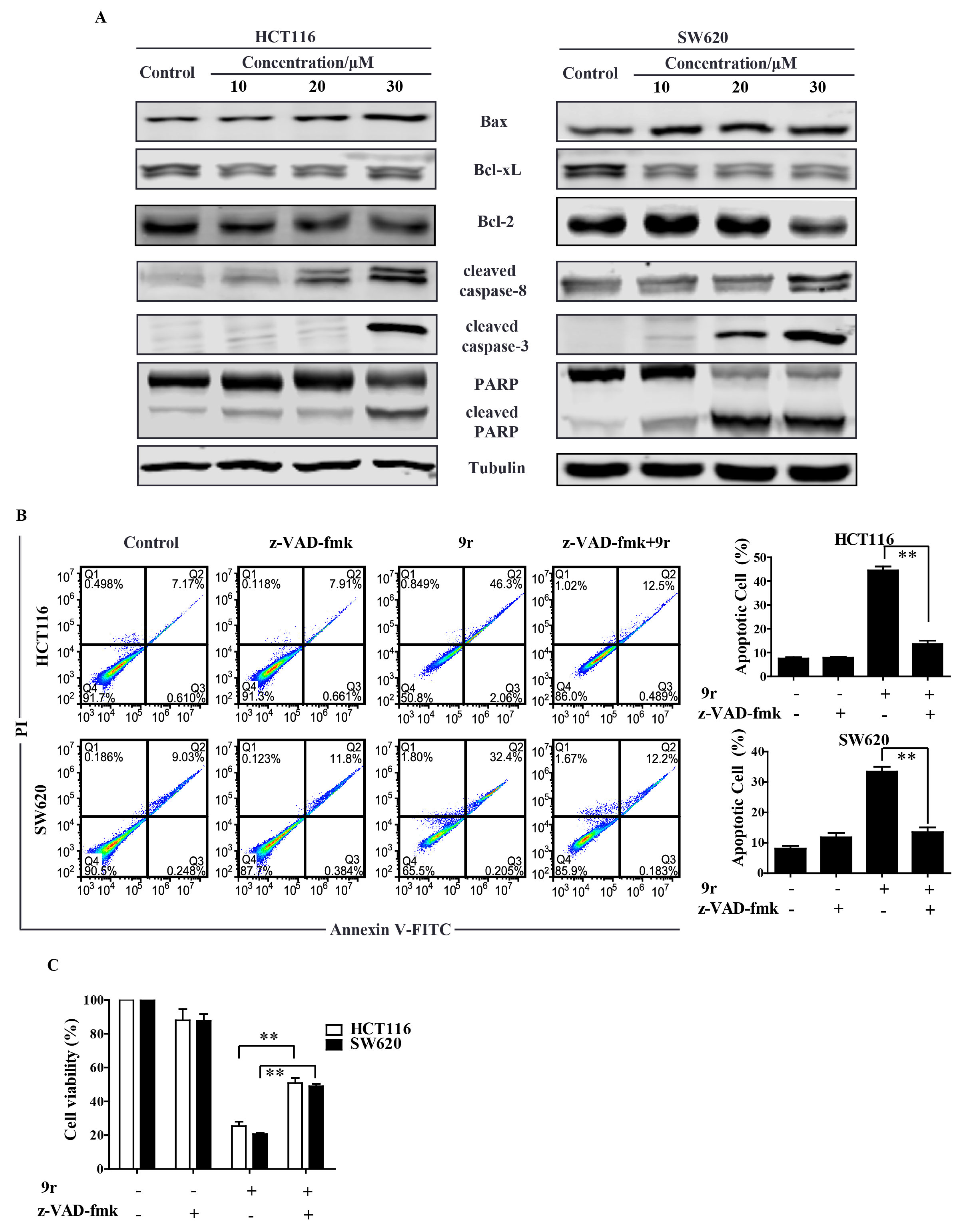
2.3. Compound 9r Triggers Mitochondrial Apoptosis
2.4. Compound 9r Induces Caspase-Dependent Apoptosis
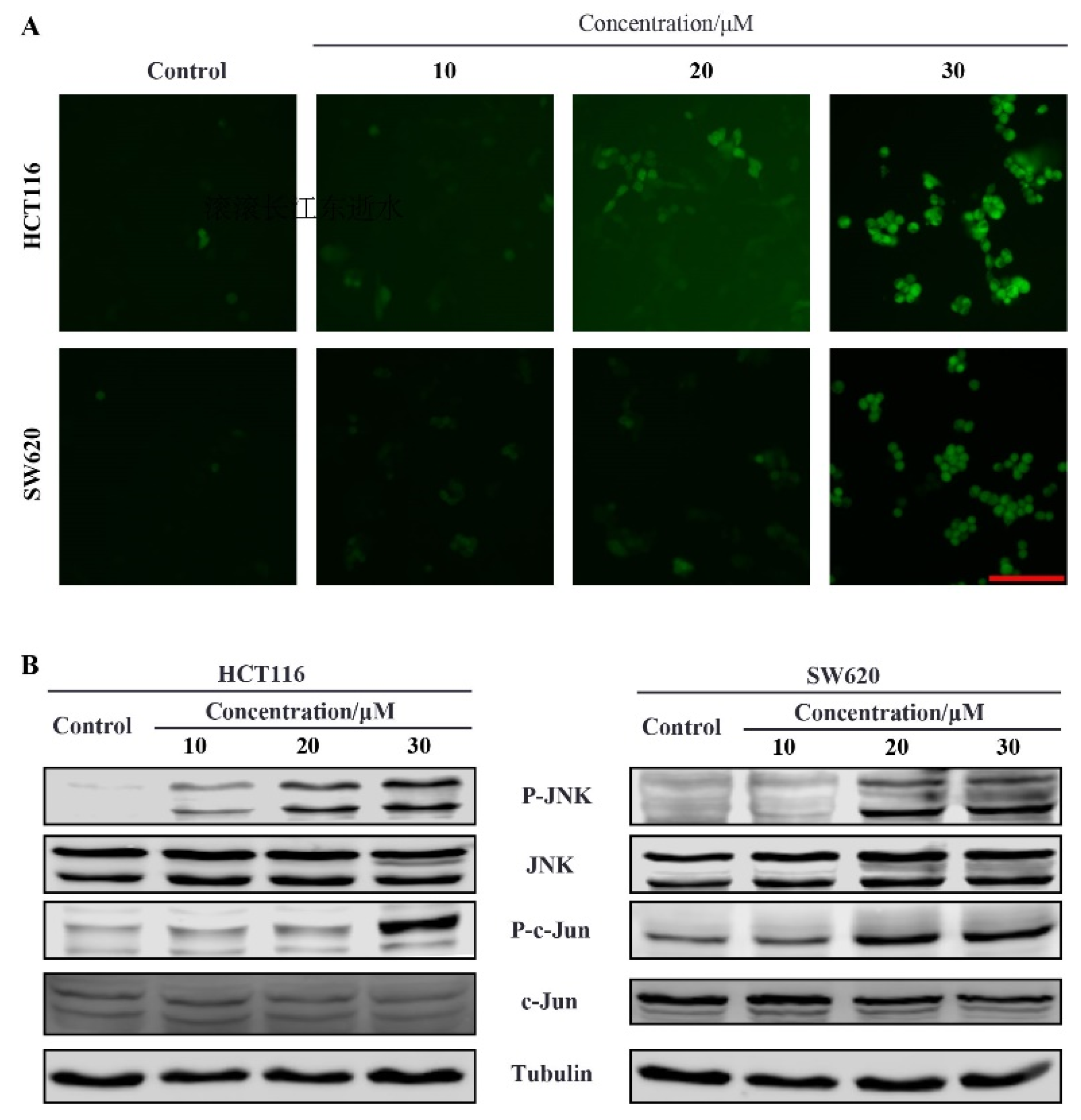
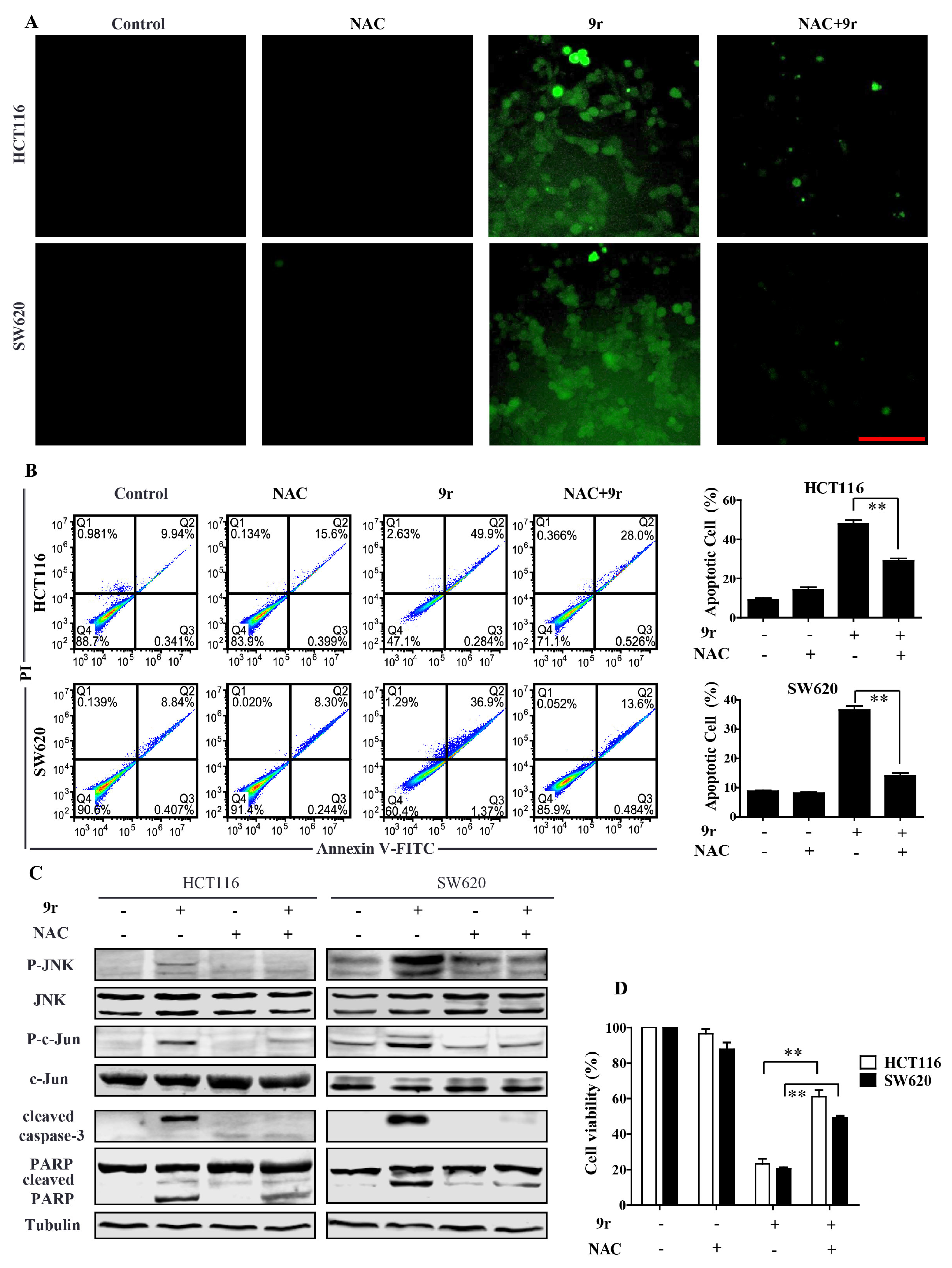
2.5. Compound 9r Induces ROS Production
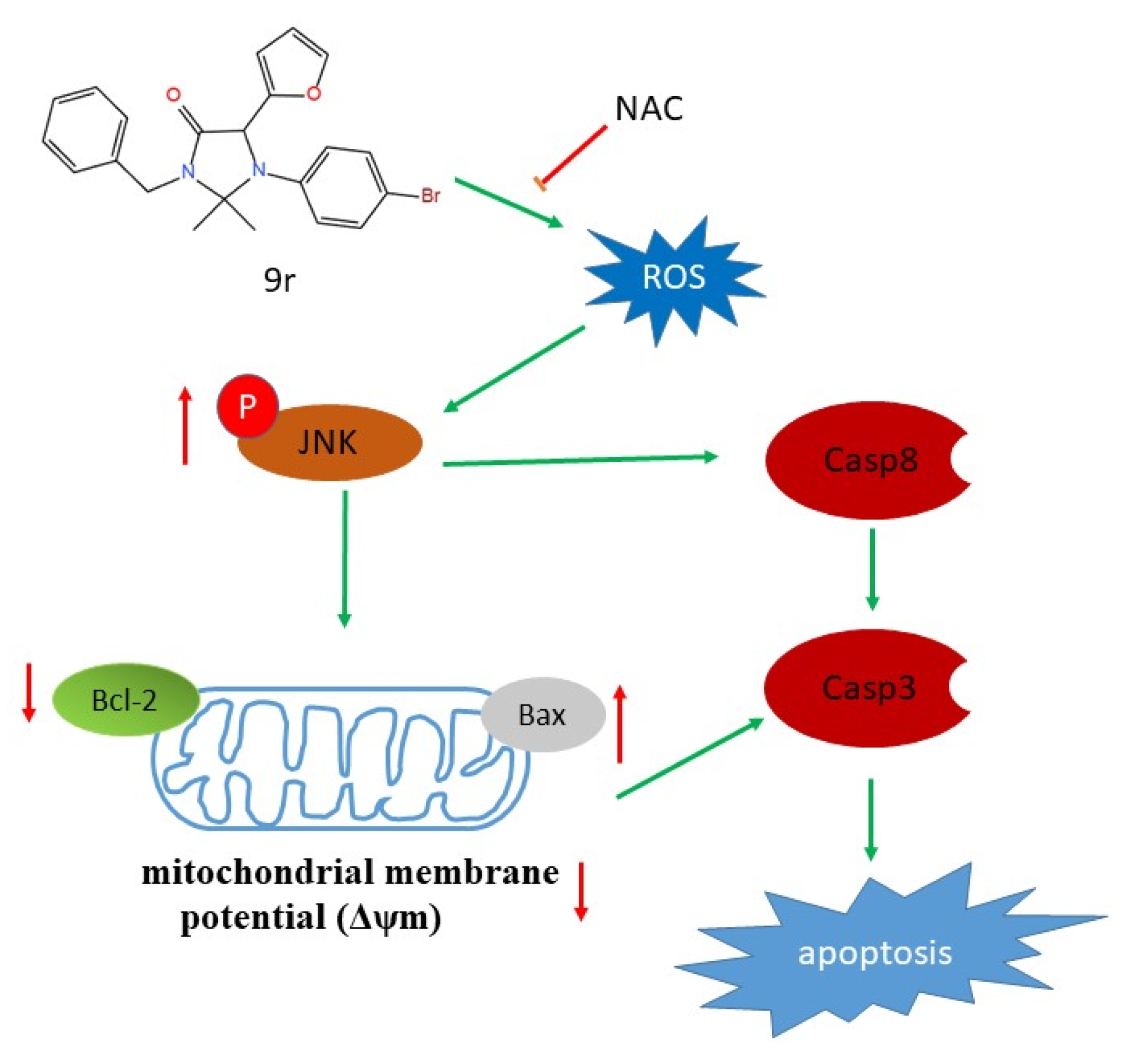
2.6. Compound 9r Induces Apoptosis through ROS Generation Which Activates JNK Pathway
3. Discussion
4. Materials and Methods
4.1. Chemical Synthesis of Compounds 9
4.2. Cell Culture
4.3. Reagents and Antibodies
4.4. MTT Assay
4.5. Colony Formation Assay
4.6. DAPI/PI Staining Assay
4.7. Apoptosis Analysis
4.8. Mitochondrial Membrane Potential Assay
4.9. Measurement of Intracellular ROS
4.10. Western Blotting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, I.S.; Jung, Y.H. Metronomic chemotherapy in metastatic colorectal cancer. Cancer Lett. 2017, 400, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Chau, I.; Cunningham, D. Chemotherapy in colorectal cancer: New options and new challenges. Br. Med. Bull. 2002, 64, 159–180. [Google Scholar] [CrossRef]
- Geng, F.; Wang, Z.; Yin, H.; Yu, J.; Cao, B. Molecular Targeted Drugs and Treatment of Colorectal Cancer: Recent Progress and Future Perspectives. Cancer Biother. Radiopharm. 2017, 32, 149–160. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signore, M.; Ricci-Vitiani, L.; De Maria, R. Targeting apoptosis pathways in cancer stem cells. Cancer Lett. 2013, 332, 374–382. [Google Scholar] [CrossRef]
- Moccia, M.; Yang, D.; Lakkaniga, N.R.; Frett, B.; McConnell, N.; Zhang, L.; Brescia, A.; Federico, G.; Zhang, L.; Salerno, P.; et al. Targeted activity of the small molecule kinase inhibitor Pz-1 towards RET and TRK kinases. Sci. Rep. 2021, 11, 16103. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yang, D.L.; Qin, H.X.; He, L.J.; Huang, J.H.; Tang, D.Y.; Xu, Z.G.; Chen, Z.Z.; Li, Y. DMAPT-D6 induces death-receptor-mediated apoptosis to inhibit glioblastoma cell oncogenesis via induction of DNA damage through accumulation of intracellular ROS. Oncol. Rep. 2021, 45, 1261–1272. [Google Scholar] [CrossRef]
- Ijzendoorn, D.R.; Botman, P.N.; Blaauw, R.H. Diastereoselective cationic tandem cyclizations to N-heterocyclic scaffolds: Total synthesis of (-)-dysibetaine PP. Org. Lett. 2006, 8, 239–242. [Google Scholar] [CrossRef]
- Aboul-Enein, M.N.; El-Azzouny, A.A.; Saleh, O.A.; Amin, K.M.; Maklad, Y.A.; Hassan, R.M. Synthesis and Anticonvulsant Activity of Substituted-1,3-diazaspiro[4.5]decan-4-ones. Arch. Pharm. 2015, 348, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.J.; Bom, J.; Capela, R.; Casimiro, C.; Chambel, P.; Gomes, P.; Iley, J.; Lopes, F.; Morais, J.; Moreira, R.; et al. Imidazolidin-4-one derivatives of primaquine as novel transmission-blocking antimalarials. J. Med. Chem. 2005, 48, 888–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramu, D.; Jain, R.; Kumar, R.R.; Sharma, V.; Garg, S.; Ayana, R.; Luthra, T.; Yadav, P.; Sen, S.; Singh, S. Design and synthesis of imidazolidinone derivatives as potent anti-leishmanial agents by bioisosterism. Arch. Pharm. 2019, 352, e1800290. [Google Scholar] [CrossRef] [PubMed]
- Song, G.-T.; Qu, C.-H.; Lei, J.; Yan, W.; Tang, D.-Y.; Li, H.-Y.; Chen, Z.-Z.; Xu, Z.-G. A Decarboxylative C(sp3)−N Bond Forming Reaction to Construct 4-Imidazolidinones via Post-Ugi Cascade Sequence in One Pot. Adv. Synth. Catal. 2020, 362, 4084–4091. [Google Scholar] [CrossRef]
- Montenegro, M.F.; Moral-Naranjo, M.T.; Munoz-Delgado, E.; Campoy, F.J.; Vidal, C.J. Hydrolysis of acetylthiocoline, o-nitroacetanilide and o-nitrotrifluoroacetanilide by fetal bovine serum acetylcholinesterase. FEBS J. 2009, 276, 2074–2083. [Google Scholar] [CrossRef]
- Swain, S.P.; Mohanty, S. Imidazolidinones and Imidazolidine-2,4-diones as Antiviral Agents. ChemMedChem 2019, 14, 291–302. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.; El-Azab, A.S.; Ekinci, D.; Senturk, M.; Supuran, C.T. Investigation of arenesulfonyl-2-imidazolidinones as potent carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2015, 30, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Dorokhov, V.S.; Golovanov, I.S.; Tartakovsky, V.A.; Sukhorukov, A.Y.; Ioffe, S.L. Diastereoselective synthesis and profiling of bicyclic imidazolidinone derivatives bearing a difluoromethylated catechol unit as potent phosphodiesterase 4 inhibitors. Org. Biomol. Chem. 2018, 16, 6900–6908. [Google Scholar] [CrossRef]
- Matos-Rocha, T.J.; Lima, M.C.A.; Veras, D.L.; Santos, A.F.; Silva, A.L.; Almeida Junior, A.S.A.; Pitta-Galdino, M.R.; Pitta, I.R.; Pitta, M.G.R.; Alves, L.C.; et al. In vivo study of schistosomicidal action of (Z)-1-(2-chloro-6-fluoro-benzyl)-5-thioxo-4-(2,4,6-trimethoxy-benzylidene)-imidaz olidin-2-one. Braz. J. Biol. 2020, 80, 187–189. [Google Scholar] [CrossRef] [Green Version]
- El-Sharief, M.; Abbas, S.Y.; El-Sharief, A.M.S.; Sabry, N.M.; Moussa, Z.; El-Messery, S.M.; Elsheakh, A.R.; Hassan, G.S.; El Sayed, M.T. 5-Thioxoimidazolidine-2-one derivatives: Synthesis, anti-inflammatory activity, analgesic activity, COX inhibition assay and molecular modelling study. Bioorg. Chem. 2019, 87, 679–687. [Google Scholar] [CrossRef]
- Lei, H.; Jiang, N.; Miao, X.; Xing, L.; Guo, M.; Liu, Y.; Xu, H.; Gong, P.; Zuo, D.; Zhai, X. Discovery of novel mutant-combating ALK and ROS1 dual inhibitors bearing imidazolidin-2-one moiety with reasonable PK properties. Eur. J. Med. Chem. 2019, 171, 297–309. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Dou, D.; Wei, L.; Alliston, K.R.; Groutas, W.C. Inhibitors of human neutrophil elastase based on a highly functionalized N-amino-4-imidazolidinone scaffold. Eur. J. Med. Chem. 2010, 45, 4280–4287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chern, J.H.; Shia, K.S.; Chang, C.M.; Lee, C.C.; Lee, Y.C.; Tai, C.L.; Lin, Y.T.; Chang, C.S.; Tseng, H.Y. Synthesis and in vitro cytotoxicity of 5-substituted 2-cyanoimino-4-imidazodinone and 2-cyanoimino-4-pyrimidinone derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Mao, Y.; Zhang, H.; Xu, Y.; An, J.; Huang, Z. The chemical biology of apoptosis: Revisited after 17 years. Eur. J. Med. Chem. 2019, 177, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Medema, J.P. BCL-2 family deregulation in colorectal cancer: Potential for BH3 mimetics in therapy. Apoptosis Int. J. Program. Cell Death 2020, 25, 305–320. [Google Scholar] [CrossRef] [Green Version]
- Knight, T.; Luedtke, D.; Edwards, H.; Taub, J.W.; Ge, Y. A delicate balance—The BCL-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics. Biochem. Pharmacol. 2019, 162, 250–261. [Google Scholar] [CrossRef]
- Croce, C.M.; Reed, J.C. Finally, An Apoptosis-Targeting Therapeutic for Cancer. Cancer Res. 2016, 76, 5914–5920. [Google Scholar] [CrossRef] [Green Version]
- Dong, D.; Dong, Y.; Fu, J.; Lu, S.; Yuan, C.; Xia, M.; Sun, L. Bcl2 inhibitor ABT737 reverses the Warburg effect via the Sirt3-HIF1alpha axis to promote oxidative stress-induced apoptosis in ovarian cancer cells. Life Sci. 2020, 255, 117846. [Google Scholar] [CrossRef]
- Yang, Y.; Karakhanova, S.; Werner, J.; Bazhin, A.V. Reactive oxygen species in cancer biology and anticancer therapy. Curr. Med. Chem. 2013, 20, 3677–3692. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, Y.; Zamyatnin, A.A., Jr.; Werner, J.; Bazhin, A.V. Reactive oxygen species and colorectal cancer. J. Cell. Physiol. 2018, 233, 5119–5132. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.H.; Wang, J.L.; Song, G.T.; Tang, D.Y.; Yao, F.; Lin, H.K.; Yan, W.; Li, H.Y.; Xu, Z.G.; et al. Diversity-Oriented Synthesis of Imidazo-Dipyridines with Anticancer Activity via the Groebke-Blackburn-Bienayme and TBAB-Mediated Cascade Reaction in One Pot. J. Org. Chem. 2019, 84, 12632–12638. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Wang, J.; Song, G.; Hu, C.; Xu, Z.; Chen, Z.; Xu, C.; Yang, D. Antiproliferative Evaluation of Novel 4-Imidazolidinone Derivatives as Anticancer Agent Which Triggers ROS-Dependent Apoptosis in Colorectal Cancer Cell. Molecules 2022, 27, 8844. https://doi.org/10.3390/molecules27248844
Huang J, Wang J, Song G, Hu C, Xu Z, Chen Z, Xu C, Yang D. Antiproliferative Evaluation of Novel 4-Imidazolidinone Derivatives as Anticancer Agent Which Triggers ROS-Dependent Apoptosis in Colorectal Cancer Cell. Molecules. 2022; 27(24):8844. https://doi.org/10.3390/molecules27248844
Chicago/Turabian StyleHuang, Jiuhong, Juanli Wang, Guiting Song, Chunsheng Hu, Zhigang Xu, Zhongzhu Chen, Chuan Xu, and Donglin Yang. 2022. "Antiproliferative Evaluation of Novel 4-Imidazolidinone Derivatives as Anticancer Agent Which Triggers ROS-Dependent Apoptosis in Colorectal Cancer Cell" Molecules 27, no. 24: 8844. https://doi.org/10.3390/molecules27248844
APA StyleHuang, J., Wang, J., Song, G., Hu, C., Xu, Z., Chen, Z., Xu, C., & Yang, D. (2022). Antiproliferative Evaluation of Novel 4-Imidazolidinone Derivatives as Anticancer Agent Which Triggers ROS-Dependent Apoptosis in Colorectal Cancer Cell. Molecules, 27(24), 8844. https://doi.org/10.3390/molecules27248844




