Effects of Rhapontigenin as a Novel Quorum-Sensing Inhibitor on Exoenzymes and Biofilm Formation of Pectobacterium carotovorum subsp. carotovorum and Its Application in Vegetables
Abstract
:1. Introduction
2. Results and Discussion
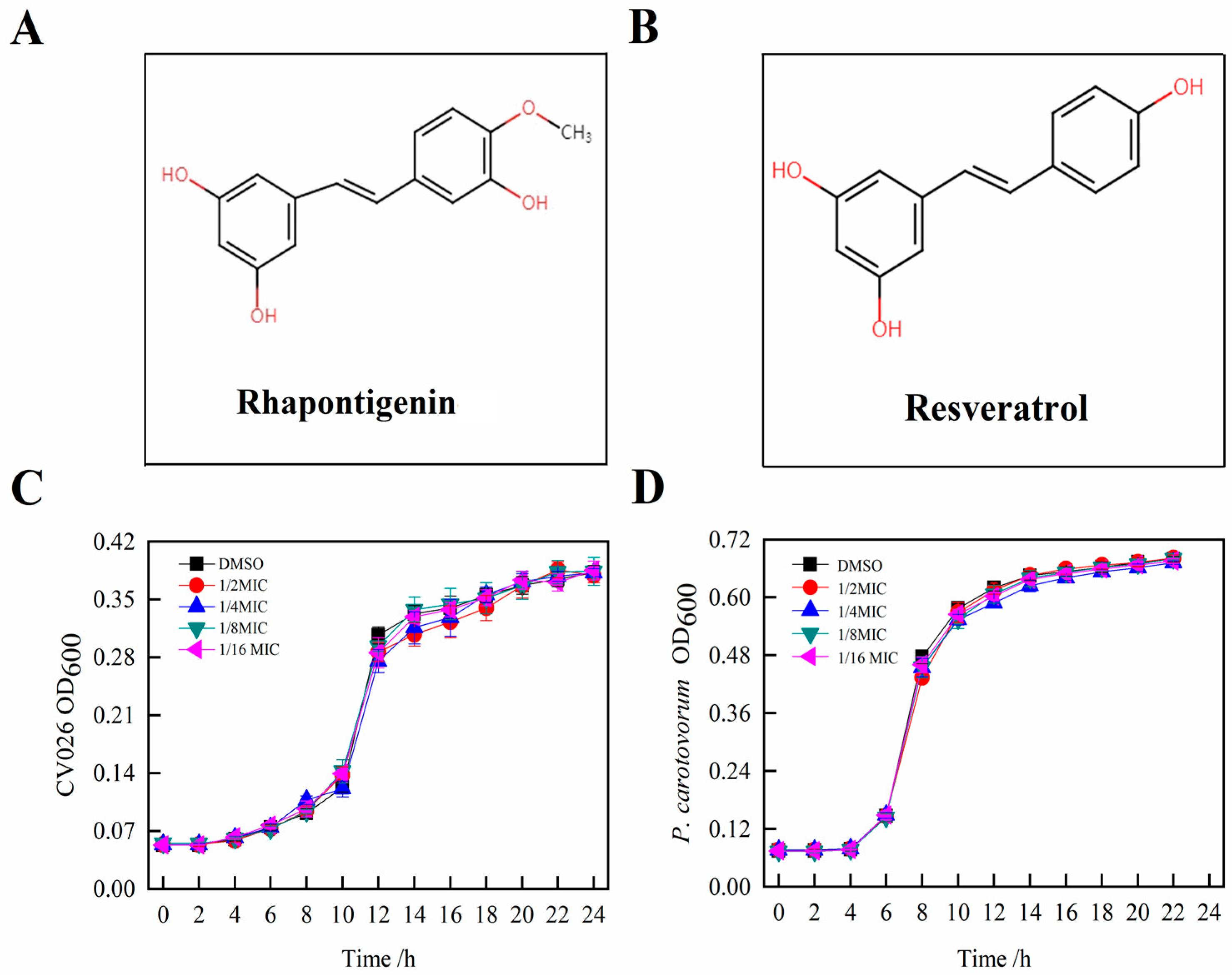
2.1. Evaluation of Quorum-Sensing Activity of Rhapontigenin
2.1.1. Screening of Rhapontigenin at Minimum Inhibitory Concentration (MIC)
2.1.2. Quorum-Sensing Inhibition of CV026
2.2. Evaluation of Antibiofilm Capacity of Rhapontigenin against P. carotovorum
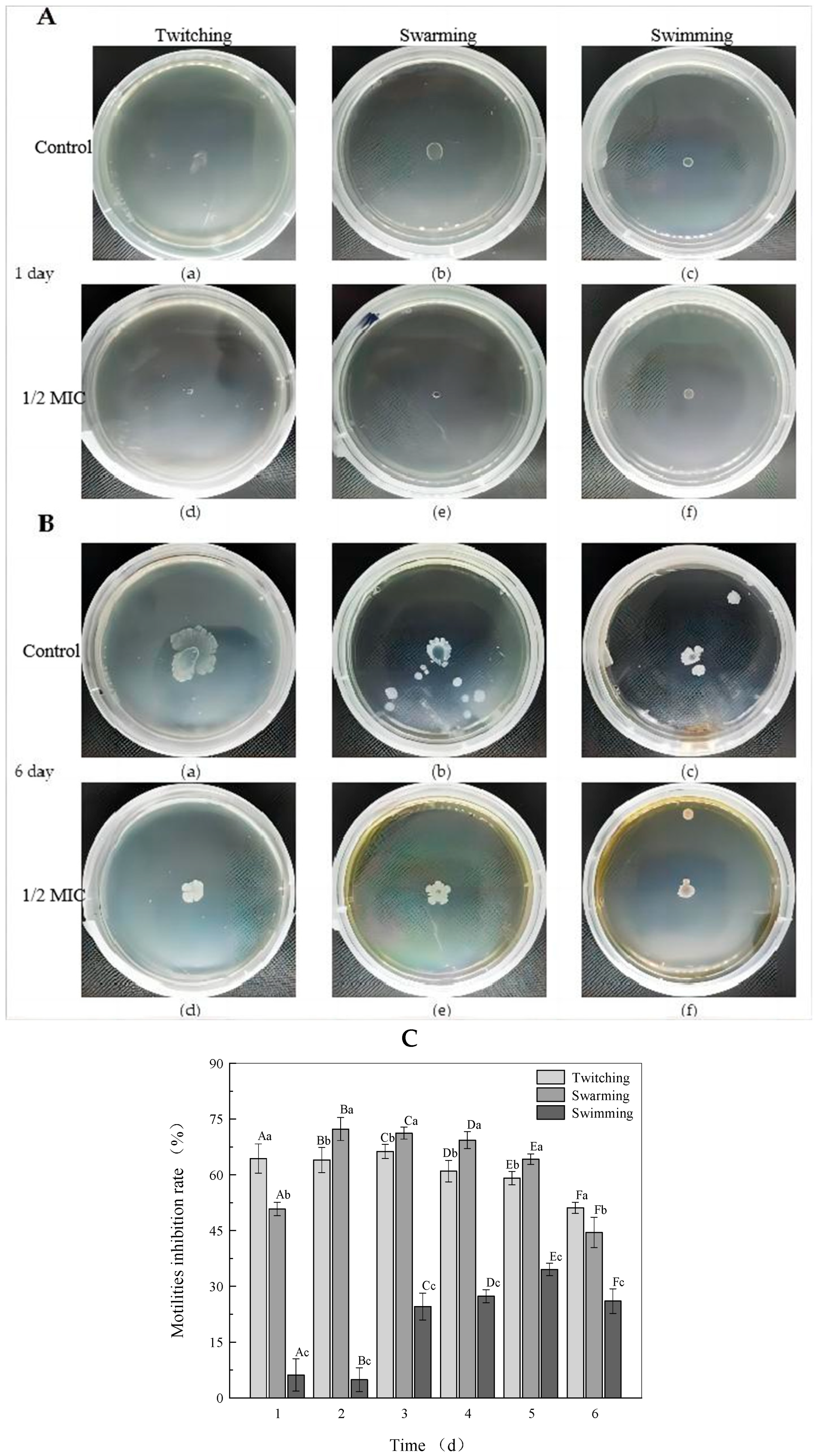
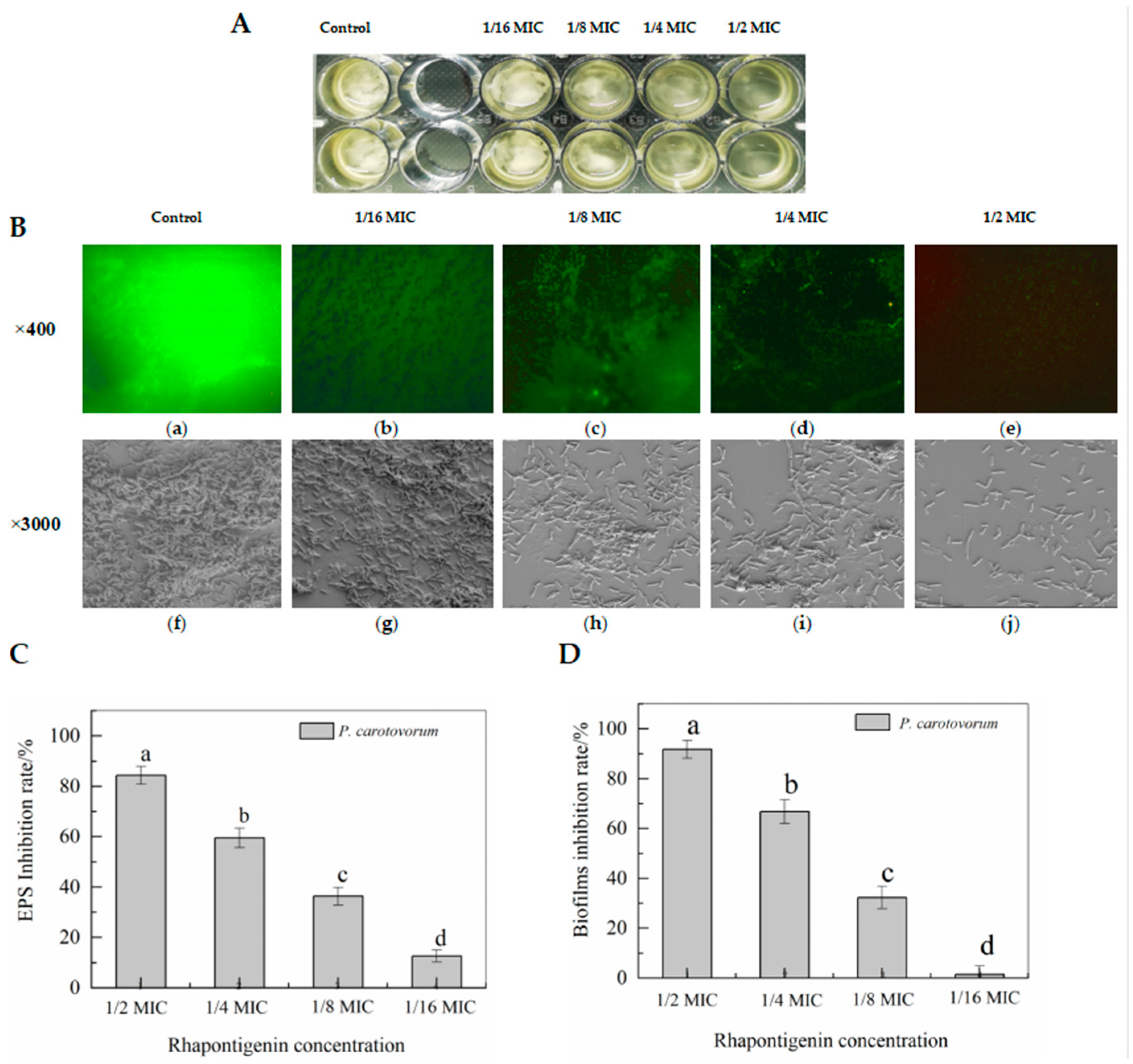
2.2.1. Effects of Rhapontigenin on Motility, EPS Production and Biofilm Formation of P. carotovorum
2.2.2. Effects of Rhapontigenin on Biofilm Formation of P. carotovorum Observed Using Fluorescence Microscopy and Scanning Electron Microscopy (SEM)
2.3. Effects of Rhapontigenin on AHL Synthesis of P. carotovorum
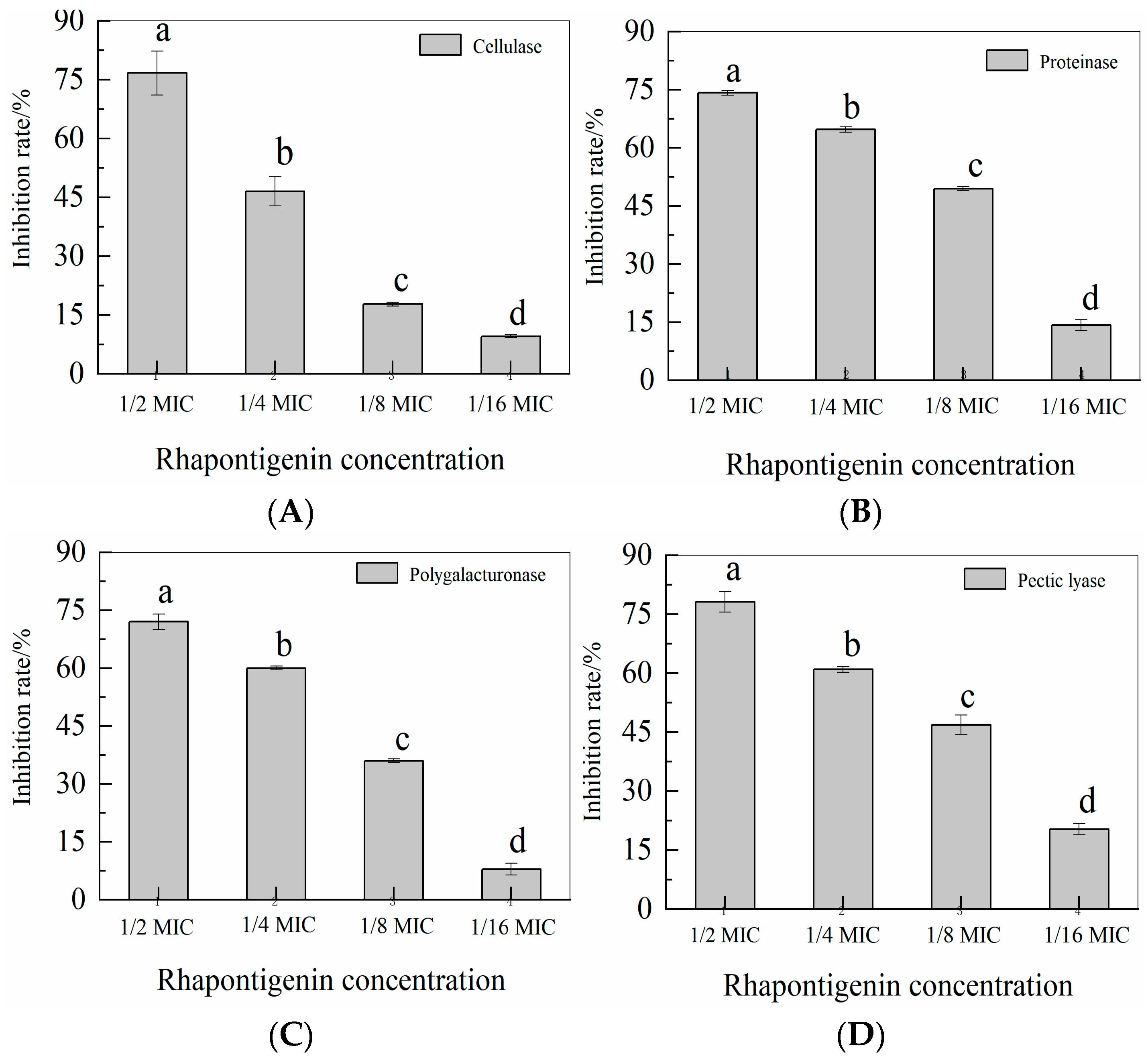
2.4. Effects of Rhapontigenin on Exoenzyme Activities of P. carotovorum
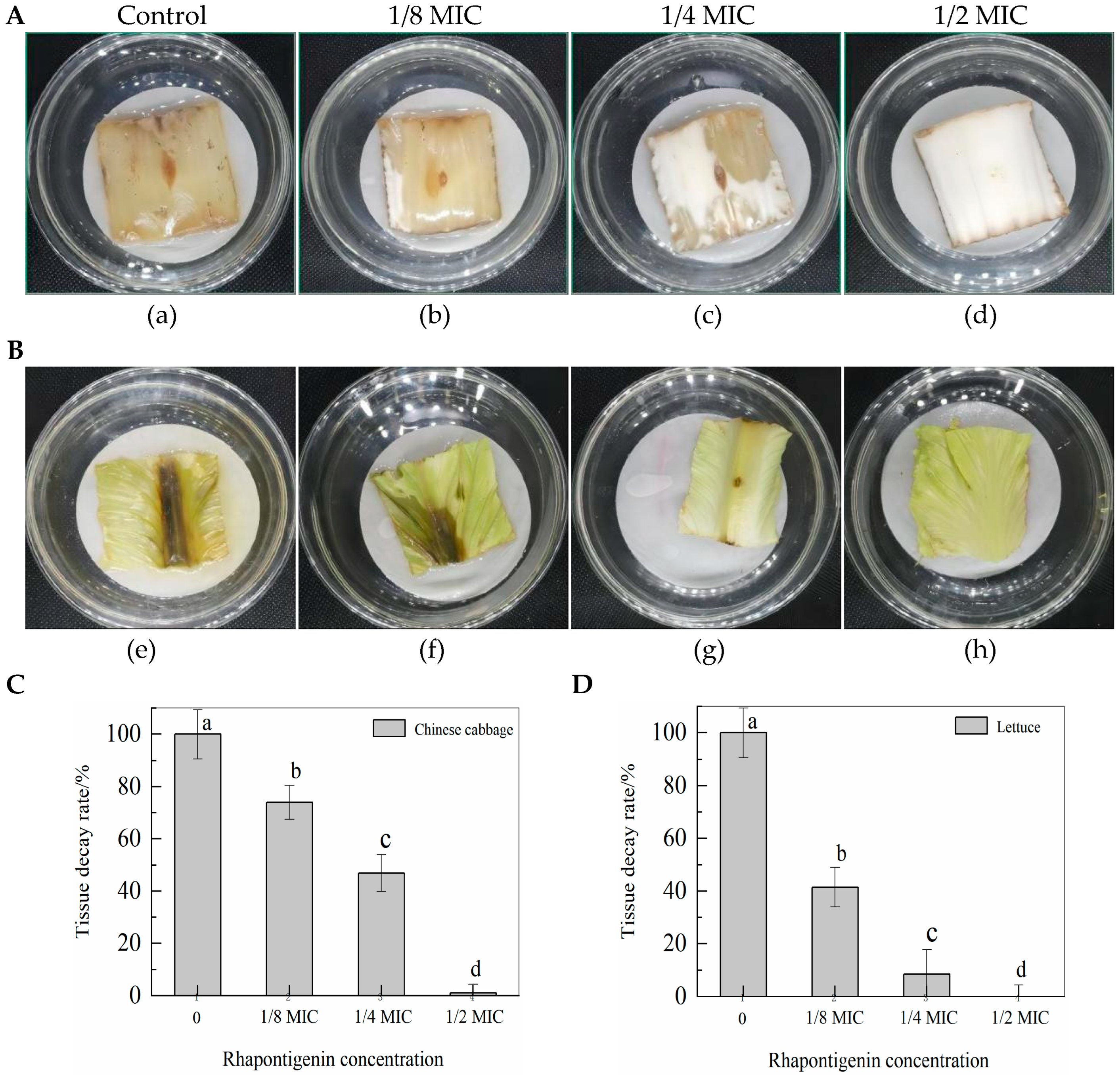
2.5. Effects of QSI Application of Rhapontigenin on P. carotovorum in Vegetables
3. Materials and Methods
3.1. Microbial Isolates and Growth Conditions
3.2. Determination of Minimum Inhibitory Concentration (MIC) of Rhapontigenin
3.3. Quorum-Sensing Inhibition (QSI) Assays
3.4. Quantification of Violacein Production
3.5. Detection of Motility, EPS Production and Biofilm Formation
3.6. Observation of Biofilms Using Fluorescence Microscopy and Scanning Electron Microscopy
3.7. LC–MS/MS Analysis of AHLs Produced
3.8. Detection of Rhapontigenin on Exoenzymes
3.9. QSI Application of Rhapontigenin in Vegetables
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, M.; Kim, S.J.; Lee, J.Y.; Yoon, S.R.; Kim, S.H.; Ha, J.H. Inactivation of Pectobacterium carotovorum subsp. carotovorum on Chinese cabbage (Brassica rapa L. subsp. pekinensis) by wash treatments with phenolic compounds. LWT 2018, 93, 229–236. [Google Scholar] [CrossRef]
- Toth, I.K.; Sullivan, L.; Brierley, J.L.; Avrova, A.O.; Hyman, L.J.; Holeva, M.; Broadfoot, L.; Pérombelon, M.C.M.; McNicol, J. Relationship between potato seed tuber contamination by Erwinia carotovora ssp. atroseptica, blackleg disease development and progeny tuber contamination. Plant Pathol. 2003, 52, 119–126. [Google Scholar] [CrossRef]
- Prigent-Combaret, C.; Zghidi-Abouzid, O.; Effantin, G.; Lejeune, P.; Reverchon, S.; Nasser, W. The nucleoid-associated protein Fis directly modulates the synthesis of cellulose, an essential component of pellicle–biofilms in the phytopathogenic bacterium Dickeya dadantii. Mol. Microbiol. 2012, 86, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-the, C.; Carlin, F. The microbiology of minimally processed fresh fruits and vegetables. Crit. Rev. Food Sci. Nutr. 1994, 34, 371–401. [Google Scholar] [CrossRef]
- Petersen, L.M.; Tisa, L.S. Friend or foe? A review of the mechanisms that drive Serratia towards diverse lifestyles. Can. J. Microbiol. 2013, 59, 627–640. [Google Scholar] [CrossRef]
- Moura-Alves, P.; Puyskens, A.; Stinn, A.; Klemm, M.; Guhlich-Bornhof, U.; Dorhoi, A.; Furkert, J.; Kreuchwig, A.; Protze, J.; Lozza, L.; et al. Host monitoring of quorum sensing during Pseudomonas aeruginosa infection. Science 2019, 366, eaaw1629. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Q.; Jia, Z.; Zhang, S.; Wang, J.; Song, S.; Jia, Y. N-3-Oxo-octanoyl homoserine lactone primes plant resistance against necrotrophic pathogen Pectobacterium carotovorum by coordinating jasmonic acid and auxin-signaling pathways. Front. Plant Sci. 2022, 14, 886268. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef] [Green Version]
- Packiavathy, I.A.S.V.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin–an anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef]
- Zhou, J.W.; Chen, T.T.; Tan, X.J.; Sheng, J.Y.; Jia, A.Q. Can the quorum sensing inhibitor resveratrol function as an aminoglycoside antibiotic accelerant against Pseudomonas aeruginosa? Int. J. Antimicrob. Agents 2018, 52, 35–41. [Google Scholar] [CrossRef]
- Sheng, J.Y.; Chen, T.T.; Tan, X.J.; Chen, T.; Jia, A.Q. The quorum-sensing inhibiting effects of stilbenoids and their potential structure–activity relationship. Bioorganic Med. Chem. Lett. 2015, 25, 5217–5220. [Google Scholar] [CrossRef]
- Wu, J.; Fan, Y.; Wang, X.; Jiang, X.; Zou, J.; Huang, R. Effects of the natural compound, oxyresveratrol, on the growth of Streptococcus mutans, and on biofilm formation, acid production, and virulence gene expression. Eur. J. Oral Sci. 2020, 128, 18–26. [Google Scholar] [CrossRef]
- Stervbo, U.; Vang, O.; Bonnesen, C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem. 2007, 101, 449–457. [Google Scholar] [CrossRef]
- Mattio, L.M.; Catinella, G.; Dallavalle, S.; Pinto, A. Stilbenoids: A natural arsenal against bacterial pathogens. Antibiotics 2020, 9, 336. [Google Scholar] [CrossRef]
- Jadhav, S.; Shah, R.; Bhave, M.; Palombo, E.A. Inhibitory activity of yarrow essential oil on Listeria planktonic cells and biofilms. Food Control 2013, 29, 125–130. [Google Scholar] [CrossRef]
- Pham, D.Q.; Ba, D.T.; Dao, N.T.; Choi, G.J.; Vu, T.T.; Kim, J.C.; Giang, T.P.L.; Vu, H.D.; Le Dang, Q. Antimicrobial efficacy of extracts and constituents fractionated from Rheum tanguticum Maxim. ex Balf. rhizomes against phytopathogenic fungi and bacteria. Ind. Crops Prod. 2017, 108, 442–450. [Google Scholar] [CrossRef]
- Norizan SN, M.; Yin, W.F.; Chan, K.G. Caffeine as a potential quorum sensing inhibitor. Sensors 2013, 13, 5117–5129. [Google Scholar] [CrossRef]
- Truchado, P.; Tomás-Barberán, F.A.; Larrosa, M.; Allende, A. Food phytochemicals act as quorum sensing inhibitors reducing production and/or degrading autoinducers of Yersinia enterocolitica and Erwinia carotovora. Food Control 2012, 24, 78–85. [Google Scholar] [CrossRef]
- Roberti, M.; Pizzirani, D.; Simoni, D.; Rondanin, R.; Baruchello, R.; Bonora, C.; Buscemi, F.; Grimaudo, S.; Tolomeo, M. Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents. J. Med. Chem. 2003, 46, 3546–3554. [Google Scholar] [CrossRef]
- Chen, T.; Sheng, J.; Fu, Y.; Li, M.; Wang, J.; Jia, A.Q. 1H NMR-based global metabolic studies of Pseudomonas aeruginosa upon exposure of the quorum sensing inhibitor resveratrol. J. Proteome Res. 2017, 16, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Pacheco, M.M.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Lizardi-Mendoza, J.; Madera-Santana, T.J.; Bernal-Mercado, A.T.; Ayala-Zavala, J.F. Carvacrol inhibits biofilm formation and production of extracellular polymeric substances of Pectobacterium carotovorum subsp. carotovorum. Food Control 2018, 89, 210–218. [Google Scholar] [CrossRef]
- Hossain, M.M.; Shibata, S.; Aizawa, S.I.; Tsuyumu, S. Motility is an important determinant for pathogenesis of Erwinia carotovora subsp. carotovora. Physiol. Mol. Plant Pathol. 2005, 66, 134–143. [Google Scholar] [CrossRef]
- Wang, C.; Pu, T.; Lou, W.; Wang, Y.; Gao, Z.; Hu, B.; Fan, J. Hfq, a RNA Chaperone, Contributes to Virulence by Regulating Plant Cell Wall-Degrading Enzyme Production, Type VI Secretion System Expression, Bacterial Competition, and Suppressing Host Defense Response in Pectobacterium carotovorum. Mol. Plant Microbe Interact. 2018, 31, 1166–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttenplan, S.B.; Kearns, D.B. Regulation of flagellar motility during biofilm formation. FEMS Microbiol. Rev. 2013, 37, 849–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.; Wang, M.; Zhang, B.; Liu, Y.; Fan, J.; Wang, Z.; Song, L.; Mohamed Abdul, P.; Zhang, H. Effects of Natural Rheum tanguticum on the Cell Wall Integrity of Resistant Phytopathogenic Pectobacterium carotovorum subsp. carotovorum. Molecules 2022, 27, 5291. [Google Scholar] [CrossRef]
- García-Contreras, R.; Maeda, T.; Wood, T.K. Can resistance against quorum-sensing interference be selected? ISME J. 2016, 10, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Zhang, L.; Liu, Y.; Zhou, S.; Yang, Y.; Xu, N.; Yang, Q.; Ai, X. Resveratrol influences the pathogenesis of Aeromonas hydrophila by inhibiting production of aerolysin and biofilm. Food Control 2021, 126, 108083. [Google Scholar] [CrossRef]
- Singh, A.A.; Singh, A.K.; Nerurkar, A. Disrupting the quorum sensing mediated virulence in soft rot causing Pectobacterium carotovorum by marine sponge associated Bacillus sp. OA10. World J. Microbiol. Biotechnol. 2021, 37, 5. [Google Scholar] [CrossRef]
- Manefield, M.; Welch, M.; Givskov, M.; Salmond, G.P.; Kjelleberg, S. Halogenated furanones from the red alga, Delisea pulchra, inhibit carbapenem antibiotic synthesis and exoenzyme virulence factor production in the phytopathogen Erwinia carotovora. FEMS Microbiol. Lett. 2001, 205, 131–138. [Google Scholar] [CrossRef]
- Corbett, M.; Virtue, S.; Bell, K.; Birch, P.; Burr, T.; Hyman, L.; Lilley, K.; Poock, S.; Toth, I.; Salmond, G. Identification of a new quorum-sensing-controlled virulence factor in Erwinia carotovora subsp. atroseptica secreted via the type II targeting pathway. Mol. Plant-Microbe Interact. 2005, 18, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Chatterjee, A.; Liu, Y.; Dumenyo, C.K.; Chatterjee, A.K. Identification of a global repressor gene, rsmA, of Erwinia carotovora subsp. carotovora that controls extracellular enzymes, N-(3-oxohexanoyl)-L-homoserine lactone, and pathogenicity in soft-rotting Erwinia spp. J. Bacteriol. 1995, 177, 5108–5115. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kong, J.; Huang, F.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Hexanal as a QS inhibitor of extracellular enzyme activity of Erwinia carotovora and Pseudomonas fluorescens and its application in vegetables. Food Chem. 2018, 255, 1–7. [Google Scholar] [CrossRef]
- Vasilchenko, A.S.; Poshvina, D.V.; Sidorov, R.Y.; Iashnikov, A.V.; Rogozhin, E.A.; Vasilchenko, A.V. Oak bark (Quercus sp. cortex) protects plants through the inhibition of quorum sensing mediated virulence of Pectobacterium carotovorum. World J. Microbiol. Biotechnol. 2022, 38, 184. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Essential oil components inhibit biofilm formation in Erwinia carotovora and Pseudomonas fluorescens via anti-quorum sensing activity. Lwt 2018, 92, 133–139. [Google Scholar] [CrossRef]
- Kang, J.E.; Han, J.W.; Jeon, B.J.; Kim, B.S. Efficacies of quorum sensing inhibitors, piericidin A and glucopiericidin A, produced by Streptomyces xanthocidicus KPP01532 for the control of potato soft rot caused by Erwinia carotovora subsp. atroseptica. Microbiol. Res. 2016, 184, 32–41. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Huang, J.; Yi, Y.; Liu, S.; Liu, R.; Xiao, Z.; Li, C. Effects of Rhapontigenin as a Novel Quorum-Sensing Inhibitor on Exoenzymes and Biofilm Formation of Pectobacterium carotovorum subsp. carotovorum and Its Application in Vegetables. Molecules 2022, 27, 8878. https://doi.org/10.3390/molecules27248878
Li B, Huang J, Yi Y, Liu S, Liu R, Xiao Z, Li C. Effects of Rhapontigenin as a Novel Quorum-Sensing Inhibitor on Exoenzymes and Biofilm Formation of Pectobacterium carotovorum subsp. carotovorum and Its Application in Vegetables. Molecules. 2022; 27(24):8878. https://doi.org/10.3390/molecules27248878
Chicago/Turabian StyleLi, Bincheng, Jiaoli Huang, Youjin Yi, Sisi Liu, Rukuan Liu, Zhihong Xiao, and Changzhu Li. 2022. "Effects of Rhapontigenin as a Novel Quorum-Sensing Inhibitor on Exoenzymes and Biofilm Formation of Pectobacterium carotovorum subsp. carotovorum and Its Application in Vegetables" Molecules 27, no. 24: 8878. https://doi.org/10.3390/molecules27248878
APA StyleLi, B., Huang, J., Yi, Y., Liu, S., Liu, R., Xiao, Z., & Li, C. (2022). Effects of Rhapontigenin as a Novel Quorum-Sensing Inhibitor on Exoenzymes and Biofilm Formation of Pectobacterium carotovorum subsp. carotovorum and Its Application in Vegetables. Molecules, 27(24), 8878. https://doi.org/10.3390/molecules27248878





