MALDI-TOF Mass Spectrometry for the Diagnosis of Citrus Canker Caused by Xanthomonas citri subsp. citri
Abstract
1. Introduction
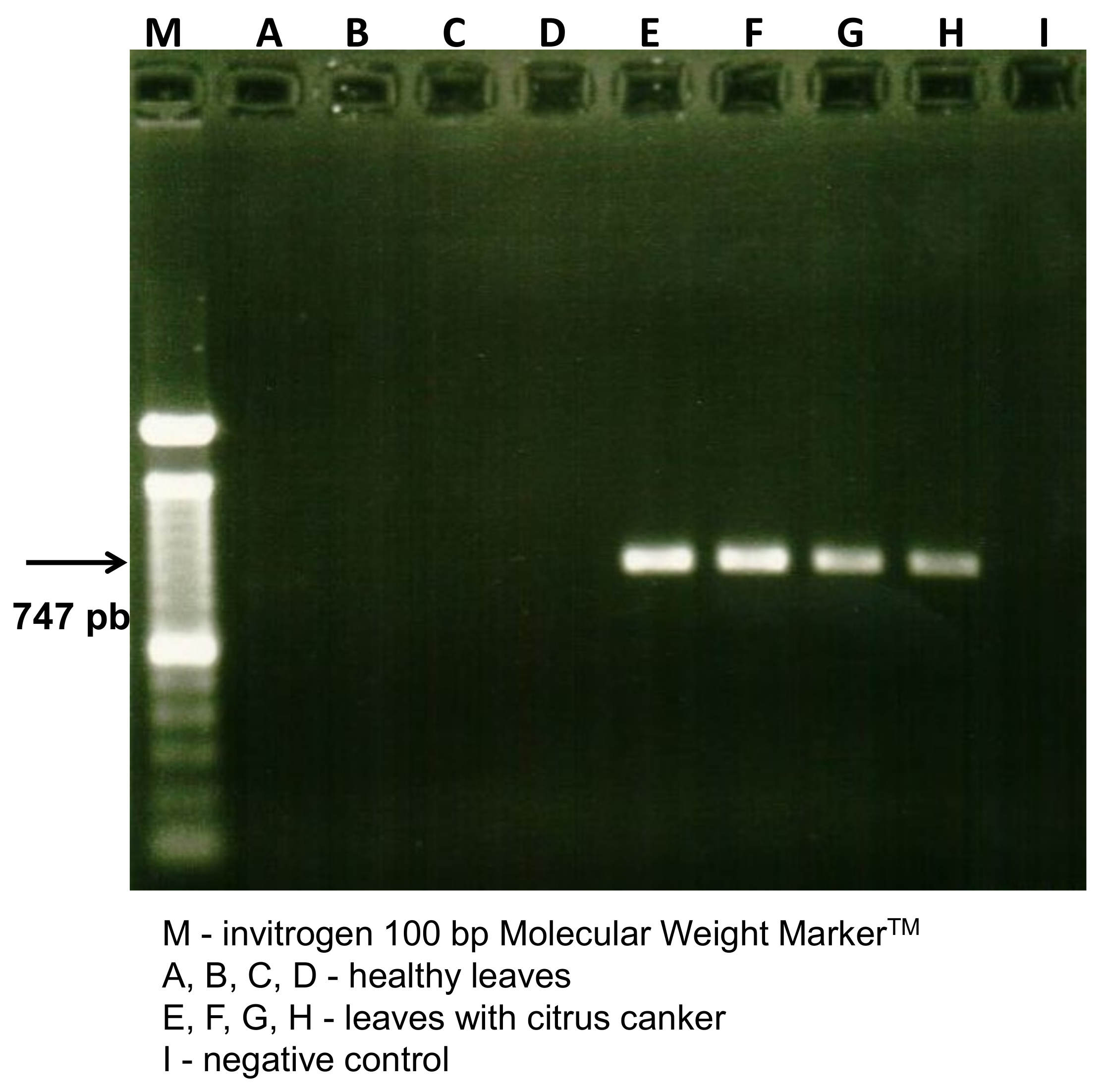
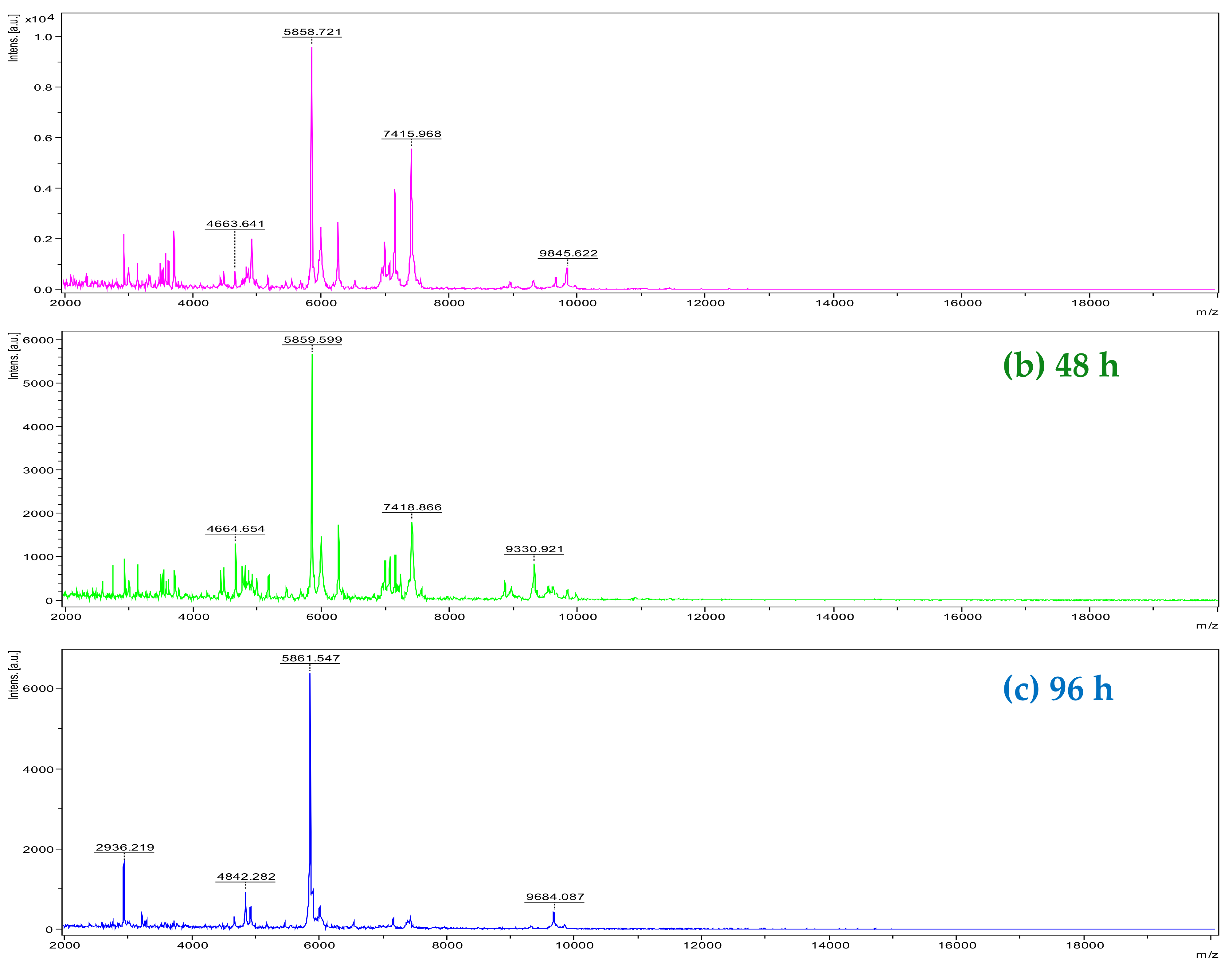
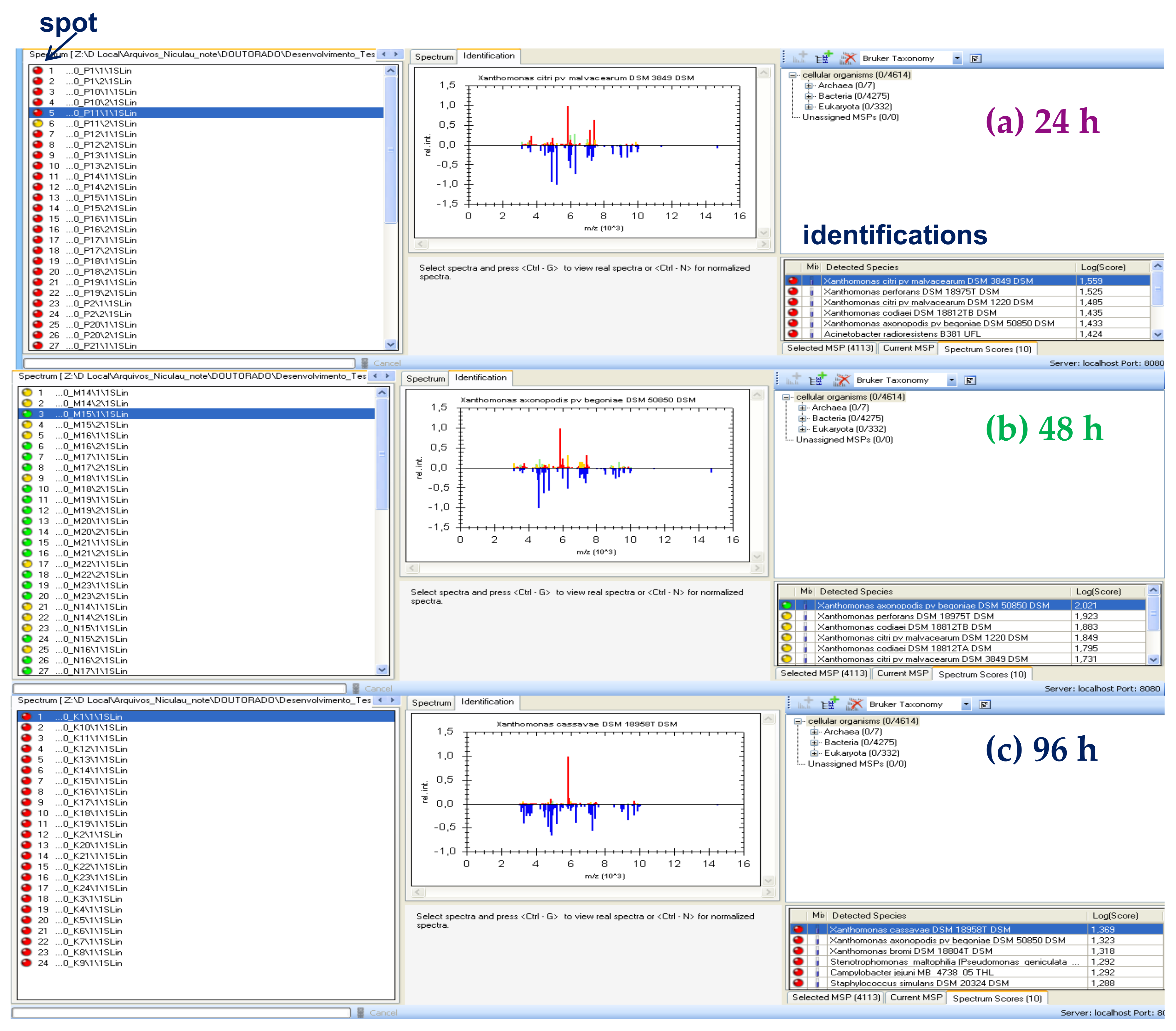
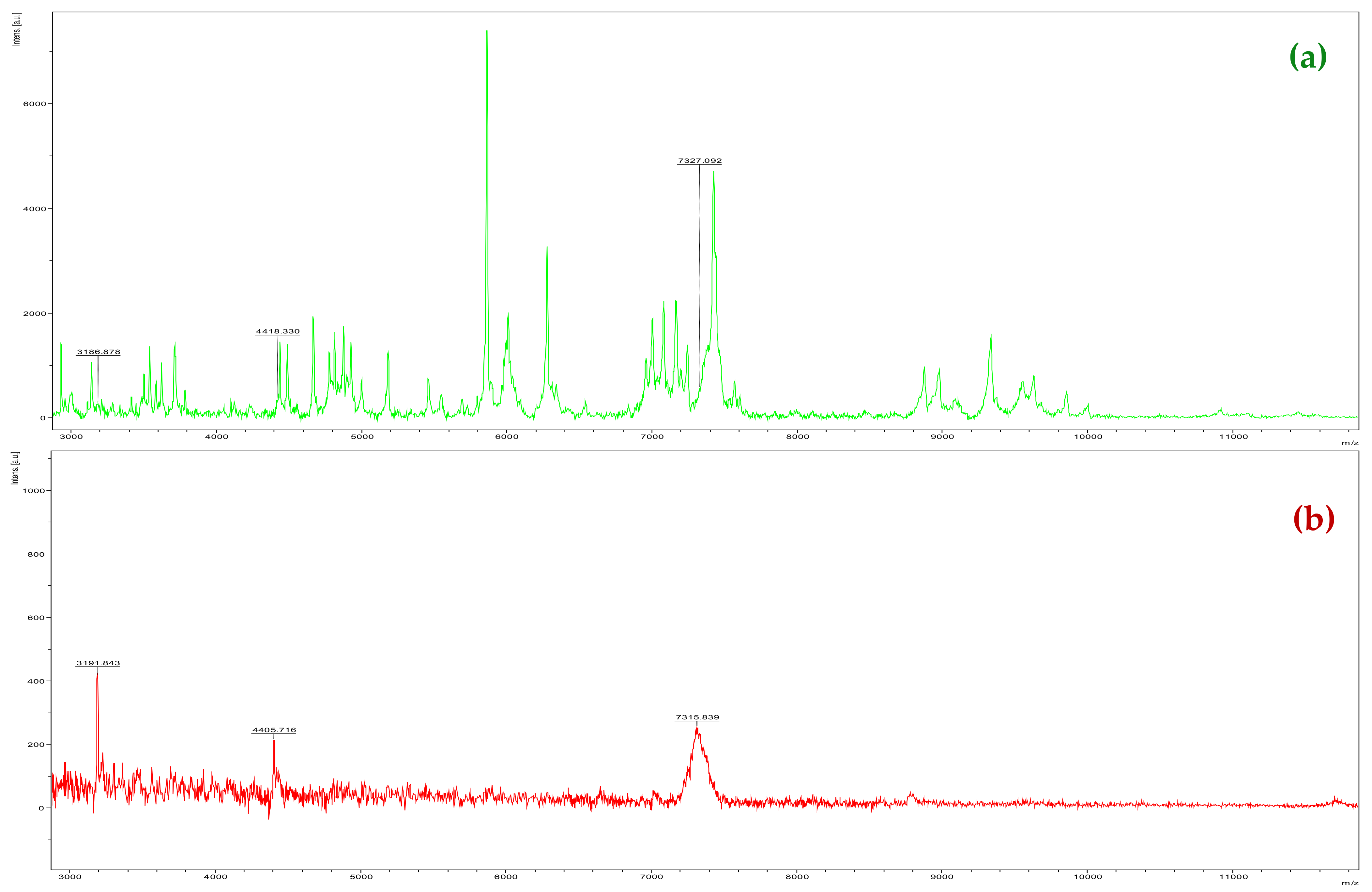
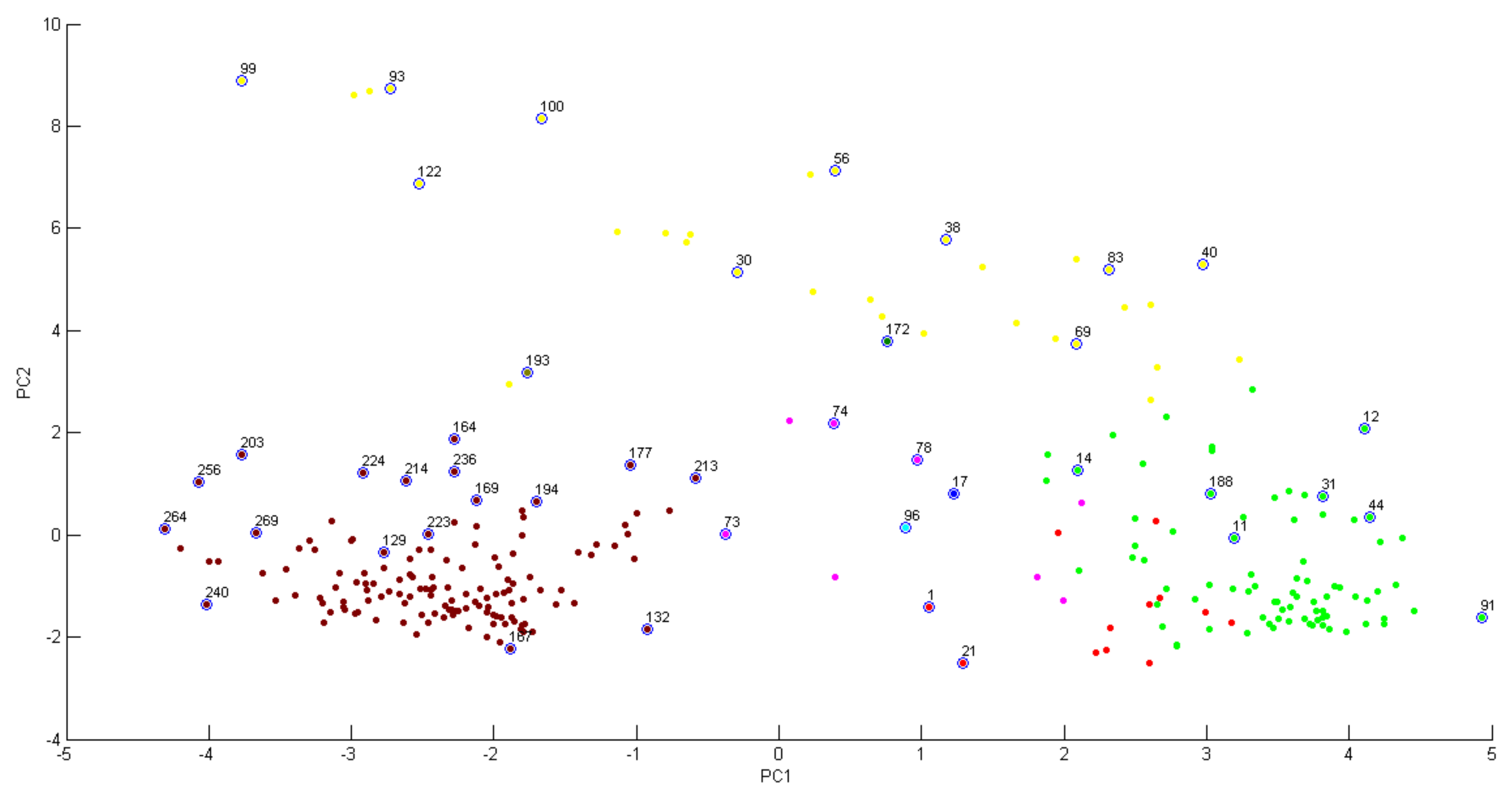
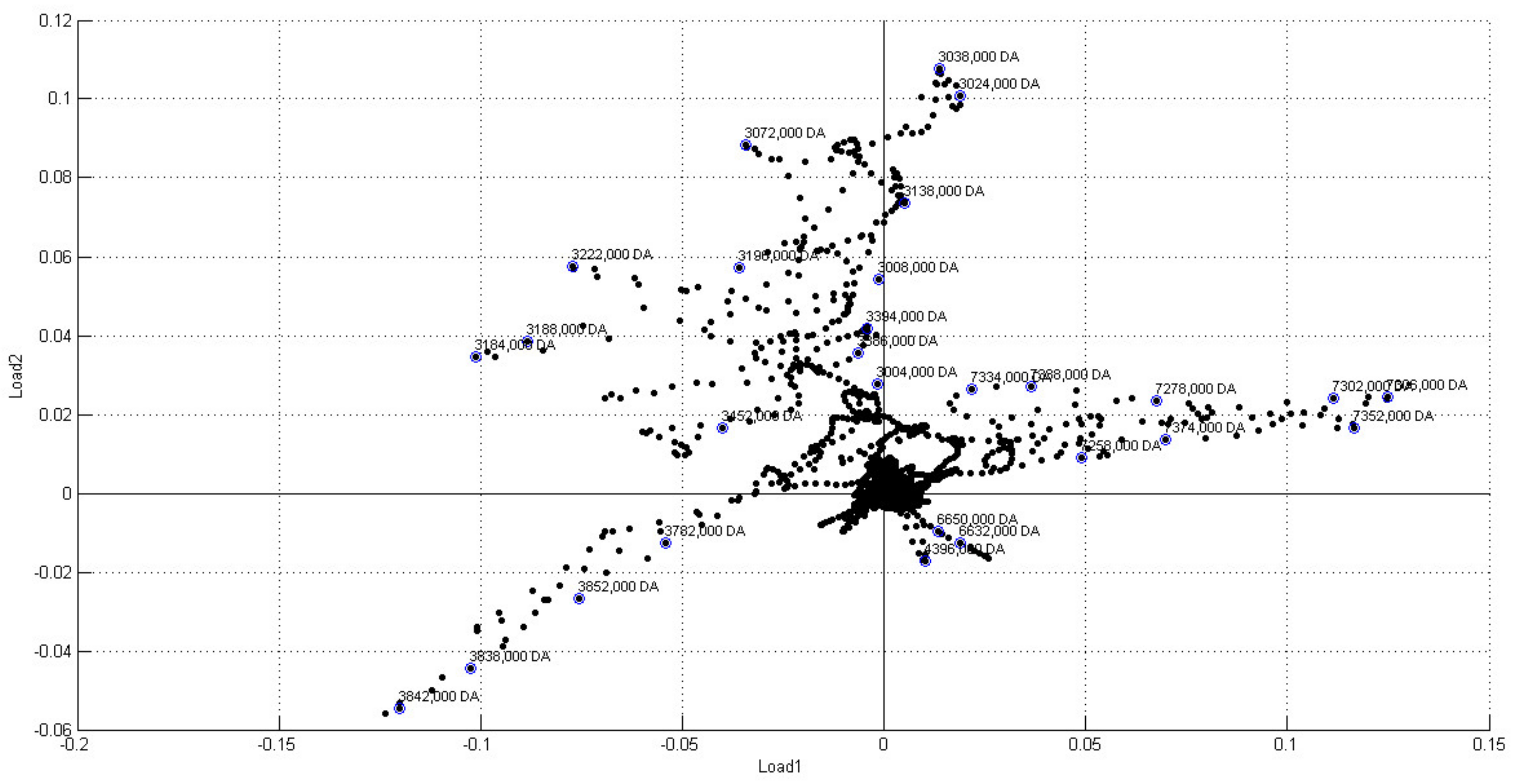
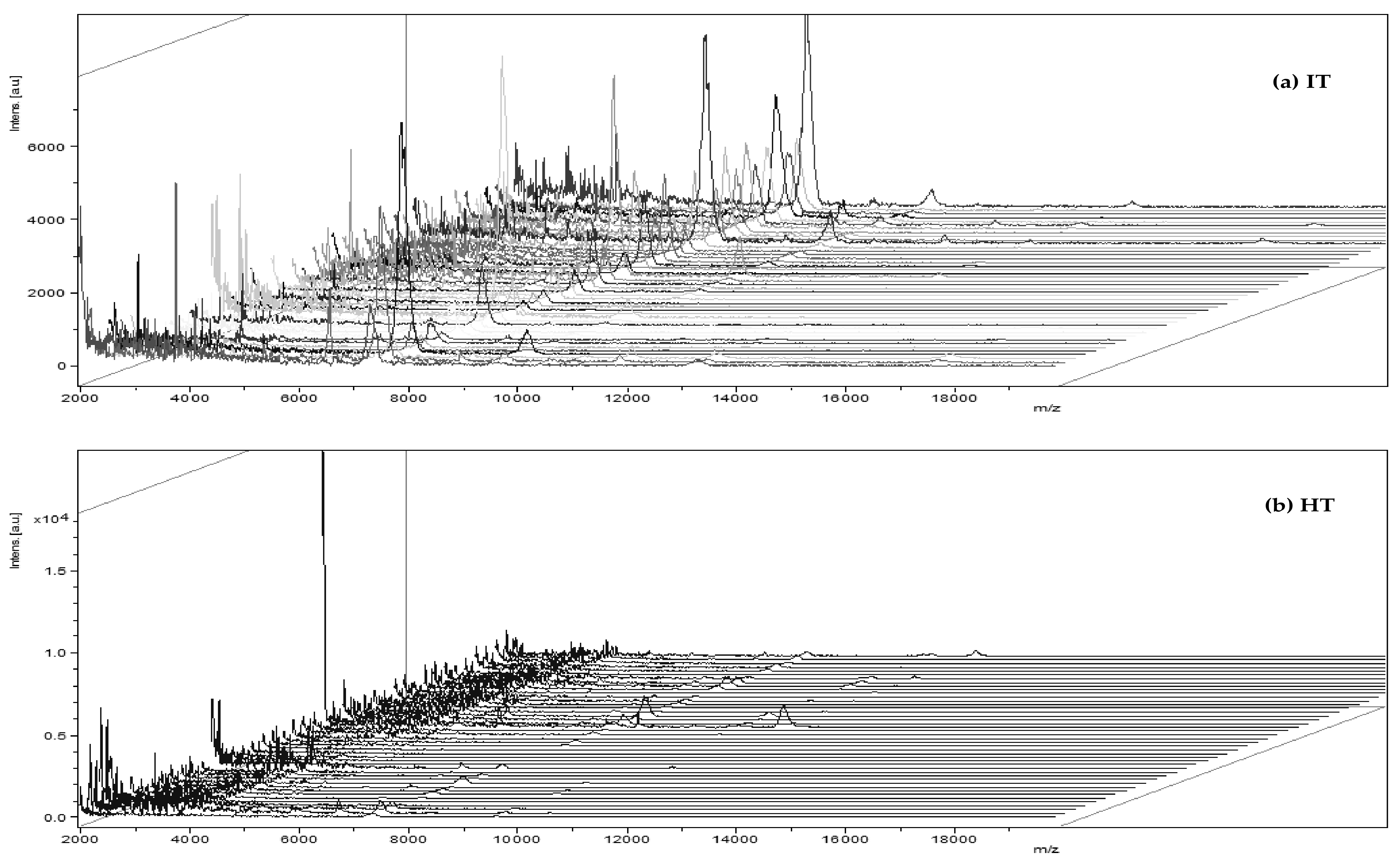
2. Results and Discussion
3. Materials and Methods
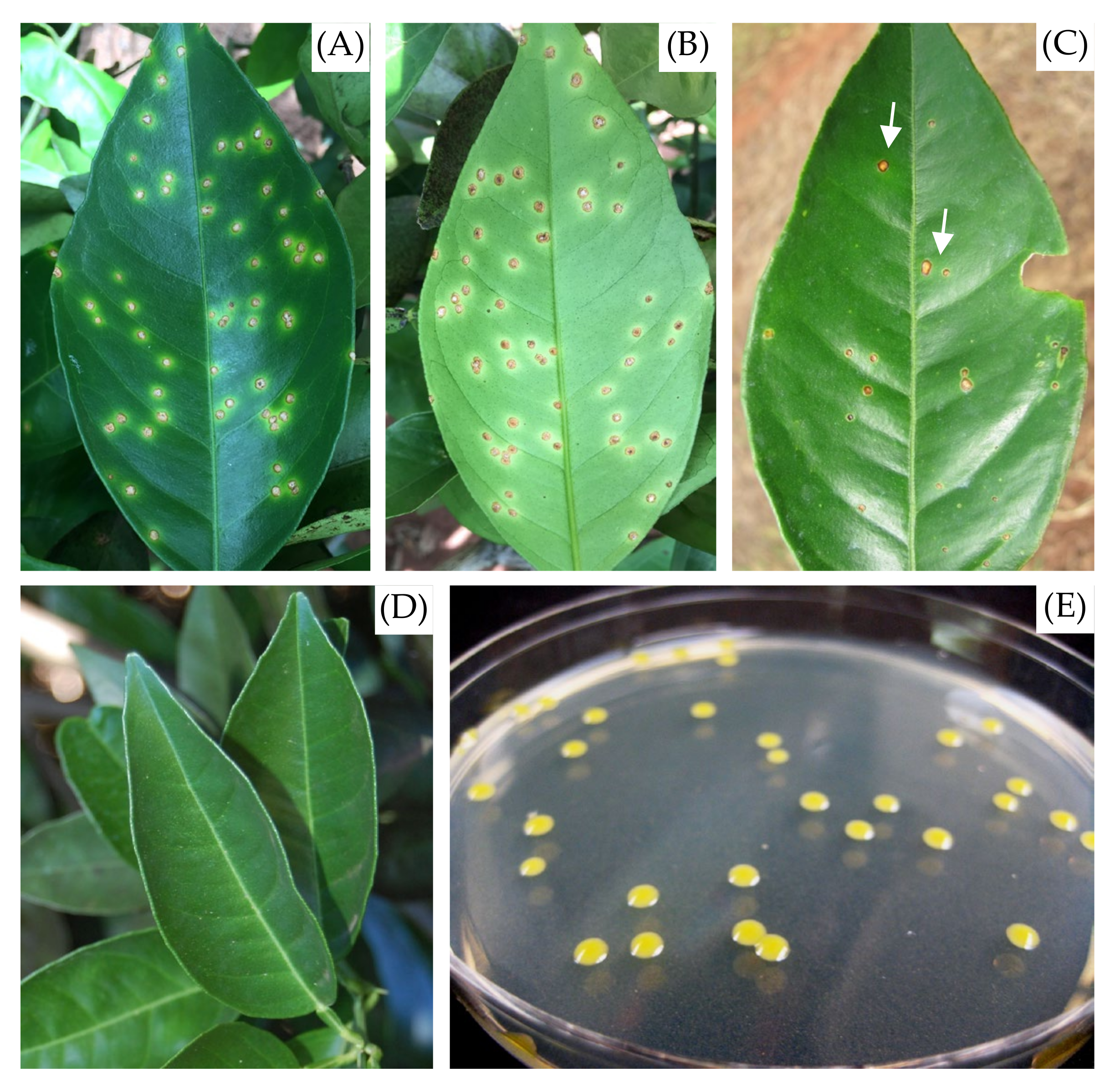
3.1. Sampling Groups
3.2. MALDI-TOF Mass Spectrometry Analysis
3.2.1. Extraction, Sample Preparation of Plant, and Analysis by MALDI-TOF MS
3.3. Construction of Main Spectra (MSPs)
3.4. Statistical Analysis
3.5. Diagnosis of Citrus Canker by Polymerase Chain Reaction (PCR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Behlau, F. An overview of citrus canker in Brazil. Trop. Plant Pathol. 2021, 46, 1–12. [Google Scholar] [CrossRef]
- Lanza, F.E.; Marti, W.; Silva, G.J., Jr.; Behlau, F. Characteristics of citrus canker lesions associated with premature drop of sweet orange fruit. Phytopathology 2019, 109, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Dalla, P.M.; Christiano, R.C.S.; Furtado, E.L.; Amori, L.; Bergamin, F.A. Effect of temperature and leaf wetness duration on infection of sweet oranges by Asiatic citrus canker. Plant Pathol. 2006, 55, 657–663. [Google Scholar] [CrossRef]
- Christiano, R.S.C.; Dalla, P.M.; Jesus, J.W.C.; Amorim, L.; Bergamin, F.A. Modelling the progress of Asiatic citrus canker on Tahiti lime in relation to temperature and leaf wetness. Eur. J. Plant Pathol. 2009, 124, 1–7. [Google Scholar] [CrossRef]
- Behlau, F. Fonseca, A.E.; Belasque, J. A comprehensive analysis of the Asiatic citrus canker eradication program in São Paulo State, Brazil, from 1999 to 2009. Plant Pathol. 2016, 65, 1390–1399. [Google Scholar] [CrossRef]
- Cubero, J.; Graham, J.H. Genetic Relationship among Worldwide Strains of Xanthomonas Causing Canker in Citrus Species and Design of New Primers for Their Identification by PCR. Appl. Environ. Microbiol. 2002, 68, 1257–1264. [Google Scholar] [CrossRef]
- Robène, I.; Maillot-Lebon, V.; Chabirand, A.; Moreau, A.; Becker, N.; Moumène, A.; Rieux, A.; Campos, P.; Gagnevin, L.; Gaudeul, M.; et al. Development and comparative validation of genomic-driven PCR-based assays to detect Xanthomonas citri pv. citri in citrus plants. BMC Microbiol. 2020, 20, 296. [Google Scholar] [CrossRef]
- Sun, X.; Stall, R.E.; Jones, J.B.; Cubero, J.; Gottwald, T.R.; Graham, J.H.; Dixon, W.N.; Schubert, T.S.; Chaloux, P.H.; Stromberg, V.K.; et al. Detection and Characterization of a New Strain of Citrus Canker Bacteria from Key/Mexican Lime and Alemow in South Florida. Plant Dis. 2004, 88, 1179–1188. [Google Scholar] [CrossRef]
- Naqvi, S.A.H.; Wang, J.; Malik, M.T.; Umar, U.U.D.; Rehman, A.U.; Hasnain, A.; Sohail, M.A.; Snakeel, M.T.; Nauman, M.; Rehman, H.U.; et al. Citrus Canker-Distribution, Taxonomy, Epidemiology, Diesease Cycle, Pathogen Biology, Detection, and Management: A Critical Review and Future Research Agenda. Agronomy 2022, 12, 1075. [Google Scholar] [CrossRef]
- Schubert, T.S.; Rizvi, S.A.; Sun, X.; Gottwald, T.R.; Graham, J.H.; Dixon, W.N. Meeting the challenge of Eradicating Citrus Canker in Florida-Again. Plant Dis. 2001, 85, 340–356. [Google Scholar] [CrossRef]
- Gottwald, T.; Graham, J.; Bock, C.; Bonn, G.; Civerolo, E.; Irey, M.; Leite, R.; McCollum, G.; Parker, P.; Ramalho, J.; et al. The epidemiological significance of post-packinghouse survival of Xanthomonas citri subsp. citri for dissemination of Asiatic citrus canker via infected fruit. Crop Prot. 2009, 28, 508–524. [Google Scholar] [CrossRef]
- Bock, C.H.; Parker, P.E.; Gottwald, T.R. Effect of Simulated Wind-Driven Rain on Duration and Distance of Dispersal of Xanthomonas axonopodis pv. citri from Canker-Infected Citrus Trees. Plant Dis. 2005, 89, 71–80. [Google Scholar]
- Bock, C.H.; Gottwald, T.R.; Graham, J.H. A Comparison of Pathogen Isolation in Culture and Injection-infiltration Bioassay of Citrus Leaves for Detecting Xanthomonas citri subsp. citri. J. Phytopathol. 2014, 162, 291–301. [Google Scholar] [CrossRef]
- Cubero, J.; Graham, J.H.; Gottwald, T.R. Quantitative PCR method for diagnosis of citrus bacterial canker. Appl. Environ. Microbiol. 2001, 67, 2849–2852. [Google Scholar] [CrossRef][Green Version]
- Wilson, I.G. Inhibition and facilitation of nucleic acid amplification. Appl. Environ. Microbiol. 1997, 63, 3741–3751. [Google Scholar] [CrossRef]
- Alvarez, A.M.; Benedict, A.A.; Mizumoto, C.Y.; Pollard, L.W.; Civerolo, E.L. Analysis of Xanthomonas campestris pv citri and X. c. citrumelo with monoclonal-antibodies. Phytopathol. 1991, 81, 857–865. [Google Scholar] [CrossRef]
- Cardinali, M.C.D.B.; Villas Boas, P.R.; Milori, D.M.B.P.; Ferreira, E.J.; França e Silva, M.; Machado, M.A.; Bellete, B.S.; Da Silva, M.F.G.F. Infrared spectroscopy: A potential tool in huanglongbing and citrus variegated chlorosis diagnosis. Talanta 2012, 91, 1–6. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Futch, D.B.; Shilts, T.; Folimonova, S.Y.; Reyes-De-Corcuera, J.I. GC-MS metabolomic differentiation of selected citrus varieties with different sensitivity to citrus huanglongbing. Plant Physiol. Biochem. 2012, 53, 69–76. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Grayer, R.J.; Harborne, J.B. A survey of antifungal compounds from higher plants, 1982–1993. Phytochemistry 1994, 37, 19–42. [Google Scholar] [CrossRef]
- Stintzi, A.; Heitz, T.; Prasad, V.; Wiedemann-Merdinoglu, S.; Kauffmann, S.; Geoffroy, P.; Legrand, M.; Fritig, B. Plant ‘pathogenesis-related’ proteins and their role in defense against pathogens. Biochimie 1993, 75, 687–706. [Google Scholar] [CrossRef] [PubMed]
- Vanetten, H.D.; Matthews, D.E.; Matthews, P.S. Phytoalexin detoxification: Importance for pathogenicity and practical implications. Annu. Rev. Phytopathol. 1989, 27, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Stump, M.J.; Black, G.; Fox, A.; Fox, K.F.; Turick, C.E.; Matthews, M. Identification of marker proteins for Bacillus anthracis using MALDI-TOF MS and ion trap MS/MS after direct extraction or electrophoretic separation. J. Sep. Sci. 2005, 28, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Demirev, P.A.; Fenselau, C. Mass spectrometry for rapid characterization of microorganisms. Annu. Rev. Anal. Chem. 2008, 1, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Bizzini, A.; Greub, G. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry, a revolution in clinical microbial identification. Clin. Microbiol. Infect. 2010, 16, 1614–1619. [Google Scholar] [CrossRef]
- Ahmad, F.; Babalola, O.O.; Tak, H.I. Potential of MALDI-TOF mass spectrometry as a rapid detection technique in plant pathology: Identification of plant-associated microorganisms. Anal. Bioanal. Chem. 2012, 404, 1247–1255. [Google Scholar] [CrossRef]
- Lopes, A.A.; Pina, E.S.; Silva, D.B.; Pereira, A.M.S.; da Silva, M.F.G.F.; Da Costa, F.B.; Lopes, N.P.; Pupo, M.T. A biosynthetic pathway of sesquiterpene lactones in Smallanthus sonchifolius and their localization in leaf tissues by MALDI imaging. Chem. Commun. 2013, 49, 9989–9991. [Google Scholar] [CrossRef]
- Ernst, M.; Silva, D.B.; Silva, R.; Monge, M.; Semir, J.; Vêncio, R.Z.N.; Lopes, N.P. A metabolomic protocol for plant systematics by matrix-assisted laser-desorption/ionization time-of flight mass spectrometry. Anal. Chim. Acta 2015, 859, 46–58. [Google Scholar] [CrossRef]
- Valenzuela, A.D.S. In-Field molecular diagnosis od plant pathogens: Recent trends and future perspectives. Plant Pathol. 2018, 67, 1451–1461. [Google Scholar]
- Padliya, N.D.; Garret, W.M.; Campbell, K.B.; Tabb, D.L.; Cooper, B. Tandem mass spectrometry for the detection of plant patrhogenic fungi and the effects of database composition on protein inferences. Proteomics 2007, 7, 3932–3942. [Google Scholar] [CrossRef]
- Sindt, N.M.; Robison, F.; Brick, M.A.; Schwartz, H.F.; Heuberger, A.L.; Prenni, J.E. MALDI-TOF-MS with PLS modeling enables strain typing of the bacterial plant pathogen Xanthomonas axonopodis. J. Am. Soc. Mass Spectrom. 2018, 29, 413–421. [Google Scholar] [CrossRef]
- Tiku, A.R. Antimicrobial Compounds (Phytoanticipins and Phytoalexins) and Their Role in Plant Defense; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2020; pp. 845–868. [Google Scholar]
- Ge, M.; Li, B.; Wang, L.; Tao, Z.; Mao, S.; Wang, Y.; Xie, G.; Sun, G. Differentiation in MALDI-TOF MS and FTIR spectra between two pathovars of Xanthomonas oryzae. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 730–734. [Google Scholar] [CrossRef]
- Božik, M.; Mrázková, M.; Novotná, K.; Hrabětová, M.; Maršik, P.; Klouček, P.; Černý, K. MALDI-TOF MS as a method for rapid identification of Phytophthora de Bary, 1876. PeerJ 2021, 9, e11662. [Google Scholar] [CrossRef]
- Chun, S.; Gopal, J.; Muthu, M. A consolidative synopsis of the MALDI-TOF MS accomplishments for the rapid diagnosis of microbial plant disease pathogens. TrAC Trends Anal. Chem. 2022, 156, 116713. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Coletta-Filho, H.D.; Takita, M.A.; Souza, A.A.; Neto, J.R.; Destéfano, S.A.L.; Hartung, J.S.; Machado, M.A. Primers based on the rpf gene region provide improved detection of Xanthomonas axonopodis pv. citri in naturally and artificially infected citrus plants. J. Appl. Microbiol. 2006, 100, 279–285. [Google Scholar] [CrossRef]
- Ah-you, N.; Gagnevin, J.; Grimont, P.A.D.; Brisse, S.; Nesme, X.; Chiroleu, F.; Bui Thi Ngoc, L.; Jouen, E.; Lefeuvre, P.; Vernière, C.; et al. Polyphasic characterization of xanthomonads pathogenic to members of the Anacardiaceae and their relatedness to species of Xanthomonas. Int. J. Syst. Evol. Microbiol. 2009, 59, 306–318. [Google Scholar] [CrossRef]



| MS Code | Strain Identification | Origin | Year of Isolation |
|---|---|---|---|
| PC 1646 | Xcc-03-1622 | Bella Vista, Corrientes, Argentina | 2003 |
| PC 1644 | 1645 | Tres Lagunas, Formosa, Argentina | 2010 |
| PC 1647 | Xcc-07-3180 | Monte Caseros, Corrientes, Argentina | 2007 |
| PC A44 | A44 | Bella Vista, Corrientes, Argentina | 2003 |
| PC 1648 | FDC 1648 | Santa Rosa, Corrientes, Argentina | 2010 |
| PC 1666 | FDC 1666 | Rondon, Paraná, Brazil | 2011 |
| PC 1670 | FDC 1670 | Alto Paraná, Paraná, Brazil | 2011 |
| PC 1705 | FDC 1705 | Paranavaí, Paraná, Brazil | 2013 |
| PC 1707 | FDC 1707 | Alto Paraná, Paraná, Brazil | 2013 |
| PC 1733 | FDC 1733 | Guairaçá, Paraná, Brazil | 2014 |
| PC 306 | 306 | Paranavaí, Paraná, Brazil | 1997 |
| PC 75 | FDC 75 | Casa Branca, São Paulo, Brazil | 1998 |
| PC 316 | FDC 316 | Presidente Prudente, São Paulo, Brazil | 2002 |
| PC 621 | FDC 621 | Chapecó, Santa Catarina, Brazil | 2001 |
| PC 10 | FDC 10 | Guararapes, São Paulo, Brazil | 1980 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculau, E.d.S.; Ferreira, D.; Rodrigues-Filho, E.; Behlau, F.; Magnani, R.F.; Toffano, L.; Prieto, E.L.; Fernandes, J.B.; Silva, M.F.d.G.F.d. MALDI-TOF Mass Spectrometry for the Diagnosis of Citrus Canker Caused by Xanthomonas citri subsp. citri. Molecules 2022, 27, 8947. https://doi.org/10.3390/molecules27248947
Niculau EdS, Ferreira D, Rodrigues-Filho E, Behlau F, Magnani RF, Toffano L, Prieto EL, Fernandes JB, Silva MFdGFd. MALDI-TOF Mass Spectrometry for the Diagnosis of Citrus Canker Caused by Xanthomonas citri subsp. citri. Molecules. 2022; 27(24):8947. https://doi.org/10.3390/molecules27248947
Chicago/Turabian StyleNiculau, Edenilson dos Santos, Douglas Ferreira, Edson Rodrigues-Filho, Franklin Behlau, Rodrigo Facchini Magnani, Leonardo Toffano, Evandro Luis Prieto, João Batista Fernandes, and Maria Fátima das Graças Fernandes da Silva. 2022. "MALDI-TOF Mass Spectrometry for the Diagnosis of Citrus Canker Caused by Xanthomonas citri subsp. citri" Molecules 27, no. 24: 8947. https://doi.org/10.3390/molecules27248947
APA StyleNiculau, E. d. S., Ferreira, D., Rodrigues-Filho, E., Behlau, F., Magnani, R. F., Toffano, L., Prieto, E. L., Fernandes, J. B., & Silva, M. F. d. G. F. d. (2022). MALDI-TOF Mass Spectrometry for the Diagnosis of Citrus Canker Caused by Xanthomonas citri subsp. citri. Molecules, 27(24), 8947. https://doi.org/10.3390/molecules27248947







